ABSTRACT
Limb regeneration is a complex yet fascinating process observed to some extent in many animal species, though seen in its entirety in urodele amphibians. Accomplished by formation of a morphologically uniform intermediate, the blastema, scientists have long attempted to define the cellular constituents that enable regrowth of a functional appendage. Today, we know that the blastema consists of a variety of multipotent progenitor cells originating from a variety of tissues, and which contribute to limb tissue regeneration in a lineage-restricted manner. By continuing to dissect the role of stem cells in limb regeneration, we can hope to one day modulate the human response to limb amputation and facilitate regrowth of a working replacement.
INTRODUCTION
The ability to autonomously replace lost or damaged appendages with fully functional equivalents is an enviable trait shared by many animal species, though unfortunately not by homo sapiens. Some of the most primitive organisms, such as hydra and the planarian, have the most striking regenerative capabilities, possessing the ability to regrow large portions of their bodies: an entire hydra can be regenerated from a segment comprising 1% of the original organism's total volume.Citation1-3 Amphibians are perhaps the most the well-known vertebrates that possess regenerative capabilities. Salamanders have been studied in this capacity for centuries, beginning with the observations of the 18th century Italian philosopher, Larazzo Spallanzani, who meticulously documented the phenomena of urodele tail and limb regrowth.Citation4,5 In recent decades, studies in Urodela have been largely driven by the hypothesis that regeneration is dependent upon cellular machinery that is highly conserved between both regenerative and non-regenerative species.Citation6 Indeed, recent novel evidence from fossil records suggests that salamander-like regeneration is an ancient feature of vertebrate tetrapods that is presently conserved in the modern salamander.Citation7 As a result of studies in salamander regeneration, much progress has been made toward elucidating the process by which the blastema, a morphologically similar mass of cells, transforms into a completely regenerated limb. Advances in stem cell biology and the generation of transgenic animal strains have particularly facilitated discoveries in this area.
Among vertebrates, mammals and amphibians both display regenerative capacity (). Mammals are particularly known for embryonic regeneration, from scarless fetal wound healing to embryonic mouse digit regeneration, though the adult mouse digit tip is also capable of regenerating.Citation8-11 However, unlike amphibious species, both embryonic and adult murine limb regeneration is restricted to the distal region of the terminal phalanx: proximal amputations do not result in regeneration. Also unlike the regeneration seen in amphibians, the newly formed digit is not identical to that lost, and successful regeneration is facilitated by the presence of a nail bed.Citation12,13 This limited capacity is similar to what is seen in humans, as digit tip regeneration has been observed after some pediatric fingertip amputations.Citation12,14-16
TABLE 1. Regenerative properties across vertebrate models of whole limb and digit tip regeneration after amputation.
Amphibians, specifically urodele amphibians (newts and salamanders), possess more striking regenerative abilities, as they are able to regenerate entire limbs, regardless of the site of amputation. Known as epimorphic regeneration, the contrast between the apparent cellular homogeneity of the blastema and the heterogeneity of a fully-formed limb has raised many important questions, most notably on the origin of blastema cells.Citation17 While it has been speculated that pluripotent stem cells contribute to formation of all tissues within the regenerated limb, this has now been primarily disproved. Instead, 3 main theories regarding the origin of blastema cells exist: dedifferentiation of resident cells into multipotent progenitors; transdifferentiation of resident cells into cells of a new lineage; and the differentiation and proliferation of local, tissue-specific stem cells.Citation18 Recent studies have found limited support for the transdifferentiation hypothesis, with most experiments showing lineage-restriction during blastema formation and limb regeneration. However, stem cells, whether produced by dedifferentiation or drawn from a resident population, play a large role in the process.Citation17,19
While recent reviews have addressed the complex topic of limb regeneration and its cellular players,Citation18,20 we will focus on what is currently known regarding the participation of stem cells in blastema formation. The role of stem cells in cases of natural tissue regeneration remains a fascinating topic, precisely because it gives us clues as to how human stem cells may be manipulated to potentially accomplish the same goal.
STAGES OF LIMB REGENERATION
Wound healing
Immediately after amputation, the damaged tissues of a limb are characterized by erythema and edema, consistent with the initial phases of a wound healing response to injury. This wound healing stage, which includes visible signs of nerve degeneration at the amputation site, persists for approximately one week post-injury in newts.Citation21 The inflammatory reaction induced by amputation injury is thought to be closely linked to the ability for regeneration. Anuran amphibians such as Xenopus lose their entire capacity for tail regeneration by metamorphosis, an outcome thought to be due to maturation of the larvae's immune system.Citation22 A prolonged inflammatory response to injury due to aging has been linked to decreased expression of genes required for limb repatterning,Citation23 and cell-mediated immunity of Urodeles tends to be suppressed.Citation23,24 Conversely, macrophage activity has been demonstrated to be critical for limb regeneration in both axolotls and newts: both species of salamander manifest highly efficient, macrophage-dependent clearance of senescent cells after limb injury.Citation25 In mammals, decreased clearance of senescent cells has been implicated not only in failure of regeneration, but in poor wound healing in aged organisms as well.Citation26,27 This differential regenerative response to the presence or absence of innate or adaptive immunity may be explained by the complement system, a possible signaling link between the immune system and the stem/progenitor populations of the blastema, as C3 expression uniquely identifies a subset of myogenic blastema cells.Citation28
As with any wound, keratinocyte migration over the amputation site begins within hours of injury. In the case of an amputated limb, this culminates in the formation of the apical epidermal cap (AEC), which serves a key role in facilitating regeneration.Citation29 The AEC is structurally and functionally distinct from mature epidermis. It lacks a normal basement membrane, thus facilitating uninterrupted contact between epithelial and mesenchymal cells.Citation21,29 It also serves as a signaling center, secreting a variety of growth factors necessary to recapitulate embryonic limb development, including members of the WNT and FGF signaling pathways.Citation20 Perhaps most interestingly, the AEC interacts with the axons present in the remaining limb tissue to facilitate limb regrowth: without the presence of both AEC and nerves, dedifferentiated cells exit the cell cycle and do not contribute to blastema formation.Citation30 The AEC itself has been hypothesized to form due to nerve-induced dedifferentiation of keratinocytes.Citation31
Blastema formation
Approximately one week after amputation, blastema cells begin to aggregate under the AEC, marking the second phase of limb regeneration.Citation21,29 Morphologically homogenous, the origins of these cells have long been questioned.Citation18 A key study by Kragl et al. utilized transplantation of GFP-labeled embryonic cells to trace the contributions of dermis, muscle, cartilage, and Schwann cells to the regenerated axolotl limb. Their findings revealed a lack of pluripotency, in that cells do not regenerate tissues of a different germ layer, and in most cases seem to be limited to replacing their tissue of origin.Citation32 Lineage-tracing experiments in transgenic mice have drawn the same conclusion: limb regeneration is accomplished by lineage-restricted cells that replenish tissues of their same germ layer.Citation33 The lineage-restricted cells effecting regeneration seem to be derived from local populations of dedifferentiated cells and resident stem cells, though within each tissue the relative contributions of each group have yet to be fully elucidated.Citation29,34 As the tissues of the limb are gradually regenerated and
Peripheral nerves
In amphibians, formation of the blastema is intimately linked to the presence of nerves at the amputation site.Citation35 This was first noted over a century ago when it was found that denervated newt limbs were unable to regenerate.Citation36 In the 1970s, Smith and Wolpert concluded that the effects of denervation on blastema formation were primarily due to inhibition of angiogenesis.Citation37 Since then, our knowledge has been refined such that we now know that while denervation does not preclude the aggregation of dedifferentiated cells to form the early blastema, it does affect the ability of these cells to subsequently proliferate and form a complete limb.Citation38 These effects are primarily due to biochemical interactions between axons and the AEC, and have facilitated development of the axolotl accessory limb model (ALM), where a nerve is rerouted to the site of a cutaneous wound that has been skin grafted.Citation39 The interplay between axons and the AEC creates the appropriate biochemical niche for blastema formation and development into a mature limb. Axons specifically contribute the mitogenic factor newt anterior gradient protein (nAG), which is differentially expressed between normal and aneurogenic/denervated limbs.Citation40,41
While most limb innervation/denervation studies have utilized amphibian models, key insights have been made regarding the effects of denervation in a mouse model of digit tip regeneration. Multiple studies have demonstrated a similar dependence of effective digit tip regeneration on innervation.Citation42,43 Generalized hypoplasia, and, at the cellular level, decreased mitosis, is seen in denervated salamander limbs.Citation36,38 In contrast, mice demonstrate hypertrophy and histological disorganization of the regenerated bone and nail upon amputation of denervated digit tips. Notably, Rinkevich et al. found that denervation produced no impairment in functioning of the resident stem/progenitor cell populations of ectodermal and mesodermal tissues, suggesting that nerves influence cellular/tissue patterning, rather than proliferative abilities.Citation43 Subsequent studies by Takeo et al. demonstrated a critical role for Wnt-responsive nail stem cells (NSCs) in the mouse digit tip: regeneration of the mouse digit tip requires an intact proximal nail bed containing NSCs for regeneration of denervated bone.Citation13 Though the regeneration ability of murine digit tips would seem to be a remnant of an evolutionarily conserved process still seen in salamanders today, such differences hint at the complex and largely unknown nature of limb regeneration.
Connective tissue and bone
By far the largest contributors to blastema formation, dermal fibroblasts can be seen migrating from the dermis of the wound stump toward the center of the blastema between 5 and 10 d post-amputation.Citation44 These cells have been found to make up 43% of blastema cells, in contrast to chondrocytes, which form only 2%.Citation45 Experiments using the ALM for focused assessment of the role of dermal fibroblasts in limb regeneration found that the cells dedifferentiated to regenerate cartilage, in addition to connective tissue fibroblasts.Citation46 The ability of dermal fibroblasts to de- and redifferentiate has been linked to the presence of peripheral nerves. Nerve-mediated inhibition of the transcription factor AmTwist, a marker for fibroblast redifferentiation during limb regeneration, perpetuates the dedifferentiated state of fibroblasts in the early blastema.Citation47
The mechanisms by which bone is formed in cases of both amphibian and mammalian regeneration remain somewhat less investigated. Though lineage-tracing experiments show regenerated bone having lineage-restricted origins,Citation32,33 in some cases such as ALM where no skeletal remnants are present, skeletal tissues can still be regenerated, likely from subpopulations of dedifferentiated dermal fibroblasts.Citation29 Cells from post-amputation skeletal tissue are also able to contribute to blastema formation and skeletal regeneration in a nerve-independent manner, through formation of a cartilaginous callous.Citation48 Once again, experiments by Rinkevich et al. highlight differences between the effects of denervation on limb regeneration in amphibians and mammals (mice): the regenerated phalanges of denervated mouse digits were histologically abnormal.Citation43 Said et al. linked the poor digit tip regeneration seen in proximal mouse digit amputations to the presence of a more extensive network of remaining blood vessels.Citation49
Lineage-restricted regeneration of skeletal tissues has been shown in an additional model of blastema formation: zebrafish fin regeneration. Sousa et al. observed proliferation, migration into the blastema, and eventual dedifferentiation of mature skeletal cells in response to fin amputation.Citation50 While beyond the scope of this review, we refer the reader to several other reviews focused on zebrafish fin regeneration, as this organism has been particularly useful in studying bone regeneration.Citation51-53
Muscle
While mammals are significantly limited in their ability to regenerate whole limbs, they are able to regenerate muscle tissue. Due to the presence of muscle satellite cells, skeletal muscle has the capacity for growth and repair throughout an animal's lifetime.Citation54 This normally quiescent group of cells is characterized by the expression of paired box 7 (Pax7), a transcription factor that not only defines the population, but is necessary for satellite cell-mediated muscle growth and response to injury.Citation55 While not traditional stem cells in the sense that there is limited in vivo evidence for multipotency, satellite cells both self renew and give rise to differentiated daughter cells (myoblasts).Citation56,57
Satellite cells are present in salamanders, enabling lineage-tracing experiments to determine their contribution to the regeneration blastema. Remarkably, recent experiments have shown an interspecies discrepancy between the contributions of Pax7+ cells and dedifferentiated myocytes to blastema formation. Sandoval-Guzmán et al.Citation58 found that dedifferentiated muscle cells had no contribution to the blastemas or regenerated limbs of axolotls; rather, this was accomplished primarily by muscle satellite cells. Conversely, newt blastemas and regenerated muscle were composed solely of Pax7− cells – cells derived from dedifferentiated myofibers.Citation58 This divergence in salamander regenerative mechanisms raises questions regarding the potential similarities and differences between mouse and human digit tip regeneration.
Patterning and redifferentiation
Much information regarding the patterning of the regenerating limb has been determined via blastema transplant experiments.Citation59-61 The re-expression of developmentally conserved genes such as HoxA9 and HoxA13 occurs within the distal stump one to 2 d post-amputation in axolotls, marking cells as dedifferentiated.Citation29,62 As regeneration continues, HoxA9 expression corresponds with the regenerated distal arm and hand, while HoxA13 correlates with the regenerated hand alone.Citation62 Establishment of Hox-expression in the distal region of the blastema is crucial for the final phase of limb regeneration: patterning/redevelopment.Citation29 Early on in this phase, a distal region of proliferating, dedifferentiated cells has been established, while proximally-located cells are still dedifferentiating.Citation29 The phase culminates in the establishment of a pattern where the proximal region of the blastema contains cells that have redifferentiated to acquire a stable identity of their position along the limb axis, whereas cells of the distal blastema are less differentiated.Citation59
The eventual repatterning of the limb into a functional replacement depends on intercalation, or the restoration of continuity between cells with different positional information along the limb axis, by the formation of intermediary cells.Citation63 This is facilitated by the undifferentiated nature of blastema cells, which enables them to be “reprogrammed” to cells of a different positional identity in the limb.Citation59 Multiple factors have been implicated in the development of positional identity in blastema cells, including retinoic acid, which has been shown to endogenously modulate the activity of several cell types during limb regeneration, including a subpopulation of fibroblasts.Citation63,64 The amount of positional information inherent in blastema cells is unclear, though the early expression of HoxA genes suggests that in spite of their “lability,” or ability to remain undifferentiated and open to acquiring novel positions within the blastema/limb, they do have a baseline positional identity.Citation59,63
STEM CELLS AND LIMB REGENERATION
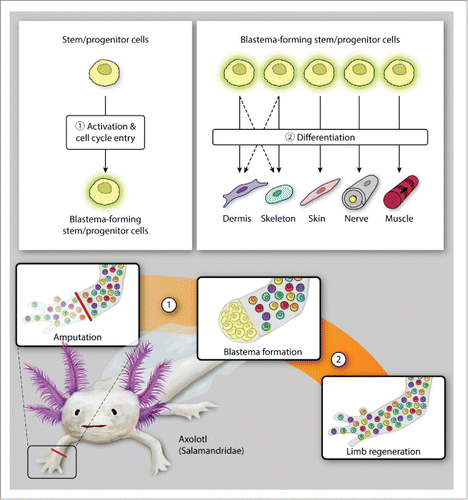
While a full discussion of the intricacies of each of the stages of limb regeneration is beyond the scope of this review, stem cells undoubtedly play a role in each part of the process. The precise nature of the stem cells involved in regeneration within each animal model of limb regeneration differs, however. Among the amphibian species known to undergo epimorphic regeneration, the axolotl appears to best recapitulate embryonic limb development as the dermal fibroblasts implicated in limb regeneration via the ALM demonstrate a clear capacity for lineage switching (). Interestingly, this ability is limited in that, while a large proportion of the cells making up the blastema are dermal fibroblasts, they do not contribute to nerve and muscle regeneration.Citation45,46 In contrast, neural stem cells have been found to contribute to both muscle and cartilage formation, in addition to nerve regeneration, after axolotl tail amputation.Citation65 It is worth considering that the populations of neural stem cells available for regeneration may differ between tails and limbs due to differences between the central and peripheral nervous systems, respectively, perhaps accounting for the less prominent role of neural stem cells in limb regeneration. Further investigations on this topic would be both interesting and warranted.
FIGURE 1. Limb regeneration in axolotls. The axolotl follows a cellular mechanism of dedifferentiation and tissue-resident stem/progenitor cell activation for blastema formation (A) and subsequent differentiation to regenerate amputated limb (B).
While both axolotls and newts rely primarily on dedifferentiation for blastema formation, we have mentioned that the newt utilizes stem cells derived from dedifferentiated mature myocytes to accomplish muscle regeneration (). This is in contrast to both axolotls and humans, which utilize muscle satellite cells, a pre-existing pool of progenitor cells, for muscle regeneration.Citation58 As the majority of limb regeneration experiments seem to be done on axolotls, one wonders whether further lineage tracing experiments in newts might uncover additional, alternate regeneration strategies. Such strategies would undoubtedly be useful as we progress toward translating epimorphic regeneration into the mammalian world. As it stands, mouse digit tip regeneration appears to be primarily lineage restricted, with no demonstrated ability for lineage switching as in axolotls (). Given the limited numbers of stem/progenitor populations in adult mammals, the additional ability to induce dedifferentiation of fully differentiated adult cells is a desirable goal.
FIGURE 2. Limb regeneration in newts. Newt amphibians utilize a diverse array of cellular mechanisms for blastema formation (A), demonstrating both dedifferentiation and activation of both tissue-resident stem/progenitor cells and mature myocytes (muscle regeneration only). (While dedifferentiated dermal fibroblasts likely contribute to both dermal and skeletal regeneration, this has not been specifically shown in a newt model.) The cells forming the blastema then differentiate or re-differentiate to reconstitute the missing tissues of the amputated limb (B).
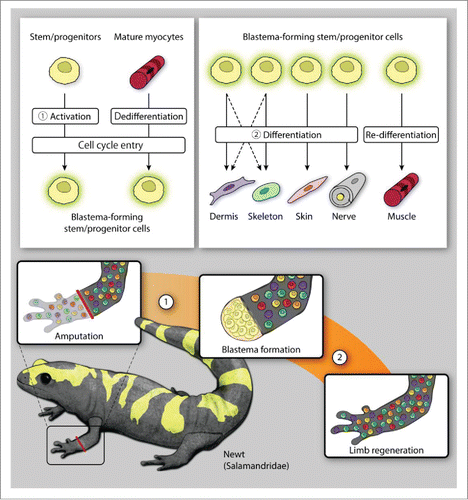
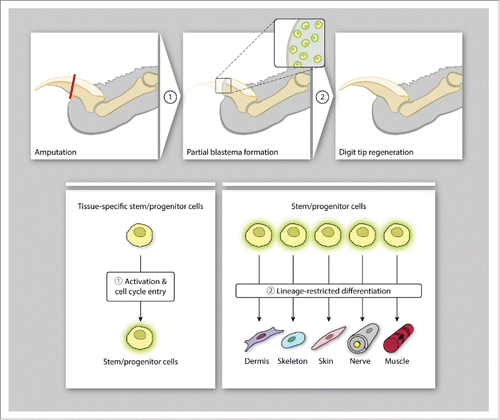
FIGURE 3. Digit tip regeneration in mammals. Mice employ cellular mechanisms that activate tissue-specific stem/progenitor cells for the formation of a blastema-like structure (A) which undergo germ-layer and lineage-restricted differentiation to reconstitute the distal digit tip (B).
The ability of axolotls and newts to successfully regenerate limbs is accompanied by the ability for scarless wound healing. This is only conserved in fetal mice and humans: it is lost after embryonic day 18 in mice, and during the third trimester in humans.Citation66 Salamanders have been shown to have a decreased immune response after injury, similar to mammalian fetuses, which may in part account for this phenomenon.Citation67 Alternatively, as our lab has recently identified a specific fibroblast lineage responsible for scar formation in the ventral dermis of adult mice, a specialized type of fibroblast capable of both dedifferentiation into blastema cells as well as skin regeneration may exist in salamanders.Citation68 Regardless of the subtype of fibroblast involved, salamander skin regeneration is also linked to the presence of nerves. In the ALM, repeated denervation of the graft site leads to a wound healing response instead of limb regeneration. Satoh et al. found that the increased collagen deposition seen during ALM wound healing inversely correlated with upregulation of AEC genes, suggesting that diffusion of nerve-secreted growth factors such as FGF was inhibited by the presence of denser connective tissue, ultimately resulting in decreased maintenance of the undifferentiated state of epidermal cells.Citation69
CONCLUSIONS
Representing the ultimate outcome in tissue response to injury, whole limb regeneration in humans currently seems an intangible goal. However, the fact that humans have some small limb regenerative capacity, as seen in cases of digit tip regeneration, gives hope that we may one day be capable of larger scale regeneration. In spite of over a century's worth of research, we are only beginning to scratch the surface of how mammalian and amphibian regeneration is accomplished. Transplantation experiments and lineage-tracing experiments using transgenic animals have revealed a complex environment where multiple cell types contribute to the rebuilding of tissues and coordinate to recapitulate the architecture of a lost appendage. Stem cells are integral to this process, whether arising from dedifferentiated tissues or quiescent resident progenitor cell populations. Their multipotent nature is not only linked to the regeneration of individual tissues, but is tied to the proximal-distal patterning of the regenerating limb. Though the relative participation of stem cell subpopulations may differ across species, as our knowledge of the interplay between these cells and the tissues of an amputated limb grows, we will gain clues as to how human limb regeneration may one day be accomplished.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- Bode HR. Head regeneration in Hydra. Dev Dyn 2003; 226:225-36; PMID:12557201; http://dx.doi.org/10.1002/dvdy.10225
- Rink JC. Stem cell systems and regeneration in planaria. Dev Genes Evol 2013; 223:67-84; PMID:23138344; http://dx.doi.org/10.1007/s00427-012-0426-4
- Trembley M. Observations and Experiments upon the Freshwater Polypus, by Monsieur Trembley, at the Hague. Translated from the French by PHZFRS. Philosophical Transactions 1742; 42:iii-xi; http://dx.doi.org/10.1098/rstl.1742.0005
- Dinsmore CE. Urodele limb and tail regeneration in early biological thought: an essay on scientific controversy and social change. Int J Dev Biol 1996; 40:621-7; PMID:8877433
- Spallanzani L. Nouvelles Recherches, Part 1-2: Sur Les Decouvertes Microscopiques Et La Generation Des Corps Organises (1769). 2009 Apr 30; pg 680.
- Frobisch NB, Bickelmann C, Witzmann F. Early evolution of limb regeneration in tetrapods: evidence from a 300-million-year-old amphibian. Proc Biol Sci 2014; 281:20141550; PMID:25253458; http://dx.doi.org/10.1098/rspb.2014.1550
- Frobisch NB, Bickelmann C, Olori JC, Witzmann F. Deep-time evolution of regeneration and preaxial polarity in tetrapod limb development. Nature 2015; 527:231-4; PMID:26503047; http://dx.doi.org/10.1038/nature15397
- Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plast Reconstr Surg 2010; 126:1172-80; PMID:20885241; http://dx.doi.org/10.1097/PRS.0b013e3181eae781
- Yokoyama H. Initiation of limb regeneration: the critical steps for regenerative capacity. Dev Growth Differ 2008; 50:13-22; PMID:17986260; http://dx.doi.org/10.1111/j.1440-169X.2007.00973.x
- Schotte OE, Smith CB. Wound healing processes in amputated mouse digits. Biol Bull 1959; 117:546-61; http://dx.doi.org/10.2307/1538866
- Borgens RB. Mice regrow the tips of their foretoes. Science 1982; 217:747-50; PMID:7100922; http://dx.doi.org/10.1126/science.7100922
- Han M, Yang X, Lee J, Allan CH, Muneoka K. Development and regeneration of the neonatal digit tip in mice. Dev Biol 2008; 315:125-35; PMID:18234177; http://dx.doi.org/10.1016/j.ydbio.2007.12.025
- Takeo M, Chou WC, Sun Q, Lee W, Rabbani P, Loomis C, et al. Wnt activation in nail epithelium couples nail growth to digit regeneration. Nature 2013; 499:228-32; PMID:23760480; http://dx.doi.org/10.1038/nature12214
- Illingworth CM. Trapped fingers and amputated finger tips in children. J Pediatr Surg 1974; 9:853-58; PMID:4473530; http://dx.doi.org/10.1016/S0022-3468(74)80220-4
- Rosenthal LJ, Reiner MA, Bleicher MA. Nonoperative management of distal fingertip amputations in children. Pediatrics 1979; 64:1-3; PMID:450537
- Vidal P, Dickson MG. Regeneration of the distal phalanx. A case report. J Hand Surg Br 1993; 18:230-3; PMID:8501382; http://dx.doi.org/10.1016/0266-7681(93)90116-W
- Tamura K, Ohgo S, Yokoyama H. Limb blastema cell: a stem cell for morphological regeneration. Dev Growth Differ 2010; 52:89-99; PMID:19891640; http://dx.doi.org/10.1111/j.1440-169X.2009.01144.x
- Hyun JS, Chung MT, Wong VW, Montoro D, Longaker MT, Wan DC. Rethinking the blastema. Plast Reconstr Surg 2012; 129:1097-103; PMID:22544093; http://dx.doi.org/10.1097/PRS.0b013e31824a2c49
- Tanaka EM. Cell differentiation and cell fate during urodele tail and limb regeneration. Curr Opin Genet Dev 2003; 13:497-501; PMID:14550415; http://dx.doi.org/10.1016/j.gde.2003.08.003
- Simon A, Tanaka EM. Limb regeneration. Wiley Interdiscip Rev Dev Biol 2013; 2:291-300; PMID:24009038; http://dx.doi.org/10.1002/wdev.73
- Iten LE, Bryant SV. Forelimb regeneration from different levels of amputation in the newt,Notophthalmus viridescens: Length, rate, and stages. Wilhelm Roux' Archiv für Entwicklungsmechanik der Organismen 1973; 173:263-82; http://dx.doi.org/10.1007/BF00575834
- Fukazawa T, Naora Y, Kunieda T, Kubo T. Suppression of the immune response potentiates tadpole tail regeneration during the refractory period. Development 2009; 136:2323-7; PMID:19515697; http://dx.doi.org/10.1242/dev.033985
- Mescher AL, Neff AW, King MW. Changes in the inflammatory response to injury and its resolution during the loss of regenerative capacity in developing Xenopus limbs. PloS One 2013; 8:e80477; PMID:24278286; http://dx.doi.org/10.1371/journal.pone.0080477
- Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci U S A 2013; 110:9415-20; PMID:23690624; http://dx.doi.org/10.1073/pnas.1300290110
- Yun MH, Davaapil H, Brockes JP. Recurrent turnover of senescent cells during regeneration of a complex structure. Elife 2015; 4; PMID:25942455; http://dx.doi.org/10.7554/eLife.05505
- Sousa-Victor P, Gutarra S, Garcia-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, Jardí M, Ballestar E, González S, Serrano AL, et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 2014; 506:316-21; PMID:24522534; http://dx.doi.org/10.1038/nature13013
- Telgenhoff D, Shroot B. Cellular senescence mechanisms in chronic wound healing. Cell Death Differ 2005; 12:695-8; PMID:15861190; http://dx.doi.org/10.1038/sj.cdd.4401632
- Mastellos DC, Deangelis RA, Lambris JD. Complement-triggered pathways orchestrate regenerative responses throughout phylogenesis. Semin Immunol 2013; 25:29-38; PMID:23684626; http://dx.doi.org/10.1016/j.smim.2013.04.002
- Bryant SV, Endo T, Gardiner DM. Vertebrate limb regeneration and the origin of limb stem cells. Int J Dev Biol 2002; 46:887-96; PMID:12455626
- Heber-Katz E, Zhang Y, Bedelbaeva K, Song F, Chen X, Stocum DL. Cell cycle regulation and regeneration. Curr Top Microbiol Immunol 2013; 367:253-76; PMID:23263201
- Satoh A, Graham GM, Bryant SV, Gardiner DM. Neurotrophic regulation of epidermal dedifferentiation during wound healing and limb regeneration in the axolotl (Ambystoma mexicanum). Dev Biol 2008; 319:321-35; PMID:18533144; http://dx.doi.org/10.1016/j.ydbio.2008.04.030
- Kragl M, Knapp D, Nacu E, Khattak S, Maden M, Epperlein HH, Tanaka EM. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature 2009; 460:60-5; PMID:19571878; http://dx.doi.org/10.1038/nature08152
- Rinkevich Y, Lindau P, Ueno H, Longaker MT, Weissman IL. Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature 2011; 476:409-13; PMID:21866153; http://dx.doi.org/10.1038/nature10346
- Lehoczky JA, Robert B, Tabin CJ. Mouse digit tip regeneration is mediated by fate-restricted progenitor cells. Proc Natl Acad Sci U S A 2011; 108:20609-14; PMID:22143790; http://dx.doi.org/10.1073/pnas.1118017108
- Tanaka EM, Reddien PW. The cellular basis for animal regeneration. Dev Cell 2011; 21:172-85; PMID:21763617; http://dx.doi.org/10.1016/j.devcel.2011.06.016
- Maden M. Neurotrophic control of the cell cycle during amphibian limb regeneration. J Embryol Exp Morphol 1978; 48:169-75; PMID:744947
- Smith AR, Wolpert L. Nerves and angiogenesis in amphibian limb regeneration. Nature 1975; 257:224-5; PMID:1161022; http://dx.doi.org/10.1038/257224a0
- Stocum DL. The role of peripheral nerves in urodele limb regeneration. Eur J Neurosci 2011; 34:908-16; PMID:21929624; http://dx.doi.org/10.1111/j.1460-9568.2011.07827.x
- Satoh A, Gardiner DM, Bryant SV, Endo T. Nerve-induced ectopic limb blastemas in the Axolotl are equivalent to amputation-induced blastemas. Dev Biol 2007; 312:231-44; PMID:17959163; http://dx.doi.org/10.1016/j.ydbio.2007.09.021
- Kumar A, Delgado JP, Gates PB, Neville G, Forge A, Brockes JP. The aneurogenic limb identifies developmental cell interactions underlying vertebrate limb regeneration. Proc Natl Acad Sci U S A 2011; 108:13588-93; PMID:21825124; http://dx.doi.org/10.1073/pnas.1108472108
- Kumar A, Godwin JW, Gates PB, Garza-Garcia AA, Brockes JP. Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science 2007; 318:772-7; PMID:17975060; http://dx.doi.org/10.1126/science.1147710
- Mohammad KS, Neufeld DA. Denervation retards but does not prevent toetip regeneration. Wound Repair Regen 2000; 8(4):277-81; PMID: 11013019
- Rinkevich Y, Montoro DT, Muhonen E, Walmsley GG, Lo D, Hasegawa M, Januszyk M, Connolly AJ, Weissman IL, Longaker MT. Clonal analysis reveals nerve-dependent and independent roles on mammalian hind limb tissue maintenance and regeneration. Proc Natl Acad Sci U S A 2014; 111:9846-51; PMID:24958860; http://dx.doi.org/10.1073/pnas.1410097111
- Gardiner DM, Muneoka K, Bryant SV. The migration of dermal cells during blastema formation in axolotls. Dev Biol 1986; 118:488-93; PMID:379-2618; http://dx.doi.org/10.1016/0012-1606(86)900-20-5
- Muneoka K, Fox WF, Bryant SV. Cellular contribution from dermis and cartilage to the regenerating limb blastema in axolotls. Dev Biol 1986; 116:256-60; PMID:3732605; http://dx.doi.org/10.1016/0012-1606(86)90062-X
- Hirata A, Gardiner DM, Satoh A. Dermal fibroblasts contribute to multiple tissues in the accessory limb model. Dev Growth Differ 2010; 52:343-50; PMID:20148925; http://dx.doi.org/10.1111/j.1440-169X.2009.01165.x
- Satoh A, Bryant SV, Gardiner DM. Regulation of dermal fibroblast dedifferentiation and redifferentiation during wound healing and limb regeneration in the Axolotl. Dev Growth Differ 2008; 50:743-54; PMID:19046162; http://dx.doi.org/10.1111/j.1440-169X.2008.01072.x
- Egawa S, Miura S, Yokoyama H, Endo T, Tamura K. Growth and differentiation of a long bone in limb development, repair and regeneration. Dev Growth Differ 2014; 56:410-24; PMID:24860986; http://dx.doi.org/10.1111/dgd.12136
- Said S, Parke W, Neufeld DA. Vascular supplies differ in regenerating and nonregenerating amputated rodent digits. Anat Rec A Discov Mole Cell Evol Biol 2004; 278:443-9; PMID:15103739; http://dx.doi.org/10.1002/ar.a.20034
- Sousa S, Afonso N, Bensimon-Brito A, Fonseca M, Simoes M, Leon J, Roehl H, Cancela ML, Jacinto A. Differentiated skeletal cells contribute to blastema formation during zebrafish fin regeneration. Development 2011; 138:3897-905; PMID:21862555; http://dx.doi.org/10.1242/dev.064717
- Watson CJ, Kwon RY. Osteogenic programs during zebrafish fin regeneration. Bonekey Rep 2015; 4:745; PMID:26421148; http://dx.doi.org/10.1038/bonekey.2015.114
- Wehner D, Weidinger G. Signaling networks organizing regenerative growth of the zebrafish fin. Trends Genet 2015; 31:336-43; PMID:25929514; http://dx.doi.org/10.1016/j.tig.2015.03.012
- Duran I, Ruiz-Sanchez J, Santamaria JA, Mari-Beffa M. Holmgren's principle of delamination during fin skeletogenesis. Mech Dev 2015; 135:16-30; PMID:25460362; http://dx.doi.org/10.1016/j.mod.2014.11.002
- Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J 2004; 23:3430-9; PMID:15282552; http://dx.doi.org/10.1038/sj.emboj.7600346
- von Maltzahn J, Jones AE, Parks RJ, Rudnicki MA. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc Natl Acad Sci U S A 2013; 110:16474-9; PMID:24065826; http://dx.doi.org/10.1073/pnas.1307680110
- Relaix F, Zammit PS. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development 2012; 139:2845-56; PMID:22833472; http://dx.doi.org/10.1242/dev.069088
- Morrison JI, Borg P, Simon A. Plasticity and recovery of skeletal muscle satellite cells during limb regeneration. FASEB J 2010; 24:750-6; PMID:19887652; http://dx.doi.org/10.1096/fj.09-134825
- Sandoval-Guzman T, Wang H, Khattak S, Schuez M, Roensch K, Nacu E, Tazaki A, Joven A, Tanaka EM, Simon A. Fundamental differences in dedifferentiation and stem cell recruitment during skeletal muscle regeneration in two salamander species. Cell stem Cell 2014; 14:174-87; PMID:24268695; http://dx.doi.org/10.1016/j.stem.2013.11.007
- McCusker CD, Gardiner DM. Positional information is reprogrammed in blastema cells of the regenerating limb of the axolotl (Ambystoma mexicanum). PloS One 2013; 8:e77064; PMID:24086768; http://dx.doi.org/10.1371/journal.pone.0077064
- Stocum DL, Melton DA. Self-organizational capacity of distally transplanted limb regeneration blastemas in larval salamanders. J Exp Zool 1977; 201:451-61; PMID:908916; http://dx.doi.org/10.1002/jez.1402010312
- Echeverri K, Tanaka EM. Proximodistal patterning during limb regeneration. Dev Biol 2005; 279:391-401; PMID:15733667; http://dx.doi.org/10.1016/j.ydbio.2004.12.029
- Gardiner DM, Blumberg B, Komine Y, Bryant SV. Regulation of HoxA expression in developing and regenerating axolotl limbs. Development 1995; 121:1731-41; PMID:7600989
- McCusker CD, Gardiner DM. Understanding positional cues in salamander limb regeneration: implications for optimizing cell-based regenerative therapies. Dis Model Mech 2014; 7:593-9; PMID:24872456; http://dx.doi.org/10.1242/dmm.013359
- Monaghan JR, Maden M. Visualization of retinoic acid signaling in transgenic axolotls during limb development and regeneration. Dev Biol 2012; 368:63-75; PMID:22627291; http://dx.doi.org/10.1016/j.ydbio.2012.05.015
- Echeverri K, Tanaka EM. Ectoderm to mesoderm lineage switching during axolotl tail regeneration. Science 2002; 298:1993-6; PMID:12471259; http://dx.doi.org/10.1126/science.1077804
- Walmsley GG, Hu MS, Hong WX, Maan ZN, Lorenz HP, Longaker MT. A mouse fetal skin model of scarless wound repair. J Vis Exp 2015; 95:52297; PMID:25650841; http://dx.doi.org/10.3791/52297
- Denis JF, Levesque M, Tran SD, Camarda AJ, Roy S. Axolotl as a Model to Study Scarless Wound Healing in Vertebrates: Role of the Transforming Growth Factor Beta Signaling Pathway. Adv Wound Care 2013; 2:250-60; PMID:24527347; http://dx.doi.org/10.1089/wound.2012.0371
- Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, Januszyk M, Krampitz GW, Gurtner GC, Lorenz HP, et al. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 2015; 348:aaa2151; PMID:25883361; http://dx.doi.org/10.1126/science.aaa2151
- Satoh A, Hirata A, Makanae A. Collagen reconstitution is inversely correlated with induction of limb regeneration in Ambystoma mexicanum. Zoolog Sci 2012; 29:191-7; PMID:22379987; http://dx.doi.org/10.2108/zsj.29.191
