ABSTRACT
An expressional lack of fibroblast growth factor 9 (FGF9) would cause male-to-female sex reversal in the mouse, implying the essential role of FGF9 in testicular organogenesis and maturation. However, the temporal expression of FGF9 and its receptors during testicular development remains elusive. In this study, immunohistochemistry was used to identify the localization of FGF9 and its receptors at different embryonic and postnatal stages in mice testes. Results showed that FGF9 continuously expressed in the testis during development. FGF9 had highest expression in the interstitial region at 17–18 d post coitum (dpc) and in the spermatocytes, spermatids and Leydig cell on postnatal days (pnd) 35–65. Regarding receptor expression, FGFR1 and FGFR4 were evenly expressed in the whole testis during the embryonic and postnatal stages. However, FGFR2 and FGFR3 were widely expressed during the embryonic testis development with higher FGFR2 expression in seminiferous tubules at 16–18 dpc and higher FGFR3 expression in interstitial region at 17–18 dpc. In postnatal stage, FGFR2 extensively expressed with higher expression at spermatids and Leydig cells on 35–65 pnd and FGFR3 widely expressed in the whole testis. Taken together, these results strongly suggest that FGF9 is correlated with the temporal expression profiles of FGFR2 and FGFR3 and possibly associated with testis development.
KEYWORDS:
INTRODUCTION
During gonad development, primordial germ cells migrate from the gut tube to the genital ridge. The primordial germ cells and supporting cells form the indifferent gonad, which undergoes through sex determination and gonad maturation steps to develop into a testis or an ovary.Citation1,2 At the sex determination step, the fibroblast growth factor (FGF) pathway and the Wnt signal pathway have been demonstrated to play reciprocally opposite roles, in which Wnt4 expression is inhibited in the male gonad after the FGF9 signal is up-regulated.Citation3 In fact, Colvin et al.Citation4 have shown that FGF9-knockout mice die after birth because of lung hypoplasia with various other phenomena from testicular hypoplasia to serious male-to-female sex reversion.Citation4 In the male gonad, supporting cells mainly differentiate into Sertoli and Leydig cells. Sertoli cells are enclosed within the germ cells inseminiferous tubule and can secret a Mullerian inhibiting substance to regress the female efferent ducts.Citation5 Leydig cells, which are located between the seminiferous tubule, produce testosterone to induce the maturation of the male efferent ducts, including the epididymis, ductus deferens, and seminal vesicle.Citation6 After birth, testis development continues until adolescence. Luteinizing hormone (LH), which is released from the pituitary gland, induces the Leydig cells to produce testosterone, induce the development of secondary sex characteristics, and promote sexual maturation in puberty.Citation7
FGFs have at least 18 members Citation8-10 with biological functions by interaction with its conjugate receptor, FGFRs.Citation11,12 Indeed, it has been well demonstrated that FGF signaling participates in gonad organogenesis.Citation8,13-17 Until now, four FGFRs were identified in the human genome participating in gonad functional regulations: FGFR1 maintains undifferentiated spermatogonia in mice; FGFR2 participates in sex determination and testis cord formation; FGFR3 is proven to be the pre-/spermatogonia maker associated with spermatogonial survival; and FGFR4 is involved in myogenesis and muscle regeneration with an expression level in the seminiferous epithelium.Citation18-22 In addition to genomic differences, mRNA of FGFRs could undergo alternative splicing to increase the protein diversity of FGFRs. Thus, different FGFs could interact with their specific FGFRs to pass different signal into cells.Citation11,23-25 In fact, FGF-FGFR signaling has been demonstrated to participate in mitogenesis, migration, embryogenesis, differentiation, tumorigenesis, and angio-genesis.Citation15,16,26-29
It has been shown that FGF9 participates in neuron development, bone formation, lens fiber differentiation, gap junction formation, sex determination, and steroidogenesis.Citation4,30-38 FGF9 could maintain a high SOX9 expression and induce testis cord formation during the sex determination period.Citation17,39 Other study showed that FGF9 inhibits the meiotic differentiation of spermatogonia.Citation40 Another study suggested that FGF9 could pass the signal through FGFR2 to regulate organogenesis of the testis.Citation19 According to previous studies, we know that FGF9 participates in sex determination and might regulate testis development.Citation3-5,19 However, the temporal expression profiles of FGF9 and FGFRs in testis development remain elusive. Thus, immunohistochemistry (IHC) was used to examine the localization/expression of FGF9 and FGFRs during embryonic and postnatal stages in mice testes.
MATERIALS AND METHODS
Animals
Male C57BL/6NCrj (B6) mice (5–6 weeks-old) were purchased from the Laboratory Animal Center of National Cheng Kung University, Tainan, Taiwan. All animals were housed under standard conditions in groups of four in 29 × 18 × 13 cm polyethylene cages maintained within 22–24°C on a constant 12 h light/dark cycle. Purina mouse chow (Ralston-Purina, St. Louis, Mo, USA) and water were available ad libitum. All procedures for maintaining and euthanizing the animals complied with the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of National Cheng Kung University.
Chemicals
Human FGF9 was purchased from PeproTech (Rocky Hill, NJ, USA). Xylene, sodium hydroxide (NaOH), and hydrochloric acid (HCl) were purchased from Merck (Darmstadt, Germany). Antibodies against FGF9 and FGFR4 were purchased from Abcam (Cambridge, UK). Primary antibodies against FGFR1, FGFR2, and FGFR3 and Mayer’s hematoxylin were purchased from Sigma (St. Louis, MO, USA). Streptavidin peroxidase complex was purchased from BioGenex (San Ramon, CA, USA). The 3′3-diaminobenzidine (DAB) was purchased from DakoCytomation (Carpinteria, CA, USA).
IHC
Testes were collected from male mice embryos at 14–18 d post coitum (dpc) and postnatal mice at 10, 20, 35, and 65 d of age, respectively. Embryonic testes were fixed by a 4% paraformaldehyde solution and dehydrated in a sucrose solution. Tissue was sectioned with a cryostat at 8 μm for further staining processes. The postnatal testes were immersed in Bouin’s fixative solution overnight and paraffin embedded. Four μm sections were cut by rotary microtome, floated in a water bath, and then picked up on poly-L-lysine coated slides. The sections were dewaxed in xylene, dehydrated in ethanol, and washed in tris-buffered saline and tween 20 (TBST). Endogenous peroxidase activity was blocked by incubation with 0.3% H2O2 followed with TBST. Trypsin (0.025%) was used to retrieve the antigen for 20 min. The sections were blocked with 5% fetal bovine serum for 1 hr. Primary antibodies against FGF9 (1:1000), FGFR1 (1:5000), FGFR2 (1:5000), FGFR3 (1:5000), and FGFR4 (1:5000) were incubated on the tissue sections overnight at 4°C. The next day, the biotinylated secondary antibody was added to the sections for 1 hr. After washing with TBST, the sections were incubated with a peroxidase-conjugated avidin-biotin complex (Vector Elite; Vector Laboratories, Burlingame, CA, USA) for 1 hr. The DAB tetrahydrochloride (DakoCytomation, Glostrup, Denmark) was used to reveal the signal. The sections were counterstained by hematoxylin, washed with TBST, dehydrated through a graded ethanol series, cleared in xylene, and cover slipped in mounting gel (Merck, Darmstadt, Germany). Blank controls were performed for each staining procedure by replacing the primary antibodies with a blocking buffer.
RESULTS
Expression of FGF9 and its receptors in mature mice testes
In this study, we first used mature mice testes to detect the possible expression sites of FGF9 and its conjugate, FGFR (). Results showed that the expressions of FGF9, FGFR2, and FGFR3, stained with the brown color, could be observed in mature mouse testis (). In addition, FGF9 was mainly expressed in Leydig cells (). FGFR2 had a strong expression in Leydig cells and a weak expression in the lumen of seminiferous tubules, including the spermatocytes and spermatids (). FGFR3 was also expressed in Leydig cells with a higher expression in the lumen of seminiferous tubules, particularly in the spermatids, compared with FGFR2 (). In fact, the expression of FGF9 in Leydig cells could be easily observed (). Given the preliminary confirmation of FGF9 and FGFR expression in mature mouse testis, the temporal expression patterns of FGF9 and FGFRs in mouse testis from the stages after sex determination () to adult () were further investigated.
FIGURE 1. FGF9 and its receptor expressions in adult mice testes. The expression patterns of FGF9 (A), FGFR2 (B), and FGFR3 (C) in 10–11 week-old mice testis were detected using immunohistochemistry. The cell nucleus was labeled using hematoxylin. The arrows indicate the interstitial regions between the seminiferous tubules of the testis (100 ×). (D) represents a higher magnification from (A) (400 ×). (E) represents control section without a primary antibody. LCs, Leydig cells; MCs, myoid cells; S, spermatids.
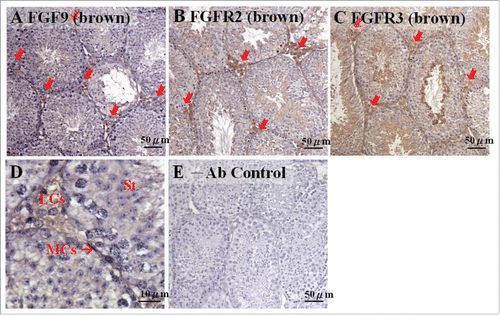
FIGURE 2. The expression pattern of FGF9 from 14 to 18 d post coitum (dpc) in embryonic mice testis. The expression patterns of FGF9 at 14 (A), 15 (B), 16 (C), 17 (D), and 18 (E) dpc in mice testis were detected using immunohistochemistry. The long arrow indicates the cell types as illustrated by the abbreviations: ST, seminiferous tubules and IT, interstitial region. The red arrowhead indicates the interstitial cells. The white arrowhead indicates the spermatogonia. Scale bar = 50 µm (A and B) (400 ×). Scale bar = 100 µm (C-E) (200 ×).
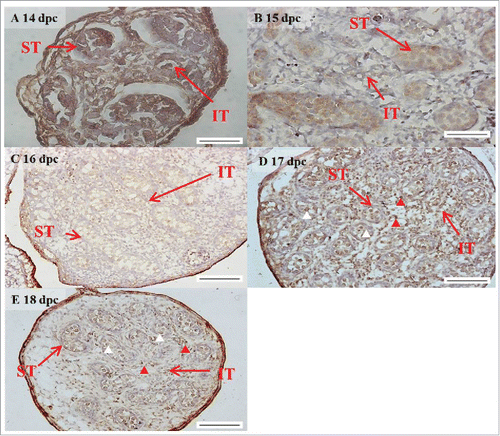
FIGURE 3. The expression patterns of FGFR1–4 at 14 d post coitum (dpc) in embryonic mice testis. The expression patterns of FGFR1 (A), FGFR2 (B), FGFR3 (C), and FGFR4 (D) at 14 dpc in mice testis were detected using immunohistochemistry. (E) represents control section without a primary antibody. The long arrow indicates the cell types as illustrated by the abbreviations: ST, seminiferous tubules and IT, interstitial region. The red arrowhead indicates the spermatogonia. Scale bar = 50 µm (A-D) (400 ×). Scale bar = 100 µm (E) (200 ×).
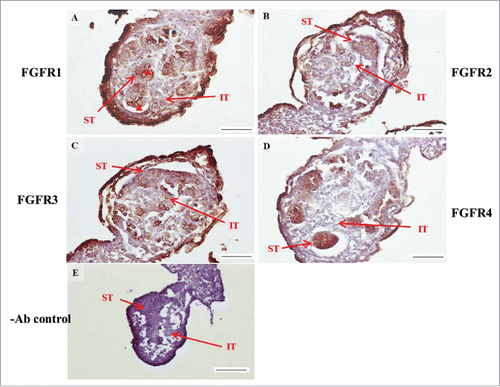
FIGURE 4. The expression patterns of FGFR1–4 at 15 d post coitum (dpc) in embryonic mice testis. The expression patterns of FGFR1 (A), FGFR2 (B), FGFR3 (C), and FGFR4 (D) at 15 dpc in mice testis were detected using immunohistochemistry. (E) represents control section without a primary antibody. The long arrow indicates the cell types as illustrated by the abbreviations: ST, seminiferous tubules and IT, interstitial region. The red arrowhead indicates the spermatogonia. Scale bar = 50 µm (400 ×) (A–D). Scale bar = 100 µm (E) (200 ×).
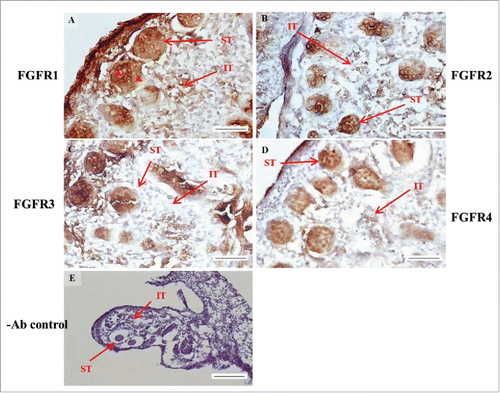
FIGURE 5. The expression patterns of FGFR1-4 at 16 d post coitum (dpc) in embryonic mice testis. The expression patterns of FGFR1 (A), FGFR2 (B), FGFR3 (C), and FGFR4 (D) at 14 dpc in mice testis were detected using immunohistochemistry. (E) represents control section without a primary antibody. The long arrow indicates the cell types as illustrated by the abbreviations: ST, seminiferous tubules and IT, interstitial region. Scale bar = 100 µm (200 ×).
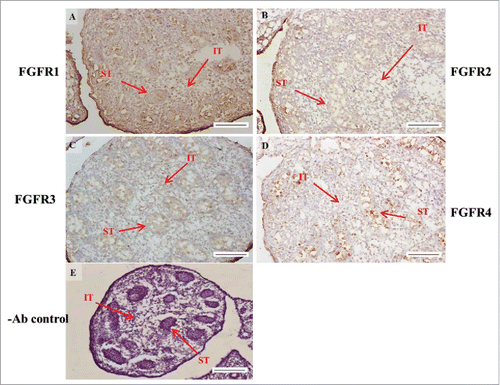
FIGURE 6. The expression patterns of FGFR1-4 at 17 d post coitum (dpc) in embryonic mice testis. The expression patterns of FGFR1 (A), FGFR2 (B), FGFR3 (C), and FGFR4 (D) at 17 dpc in mice testis were detected by immunohistochemistry. (E) represents control section without a primary antibody. The long arrow indicates the cell types as illustrated by the abbreviations: ST, seminiferous tubules and IT, interstitial region. Scale bar = 100 µm (200 ×).
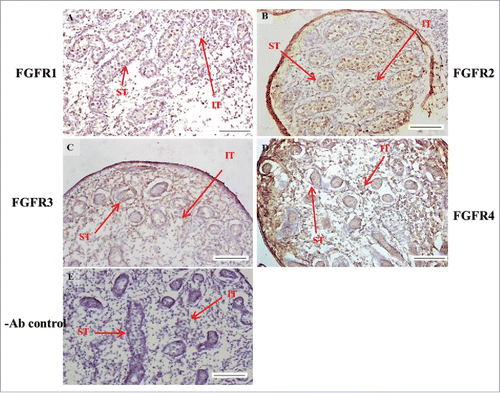
FIGURE 7. The expression patterns of FGFR1-4 at 18 d post coitum (dpc) in embryonic mice testis. The expression patterns of FGFR1 (A), FGFR2 (B), FGFR3 (C), and FGFR4 (D) at 18 dpc in mice testis were detected by immunohistochemistry. (E) represents control section without a primary antibody. The long arrow indicates the cell types as illustrated by the abbreviations: ST, seminiferous tubules and IT, interstitial region. Scale bar = 100 µm (200 ×).
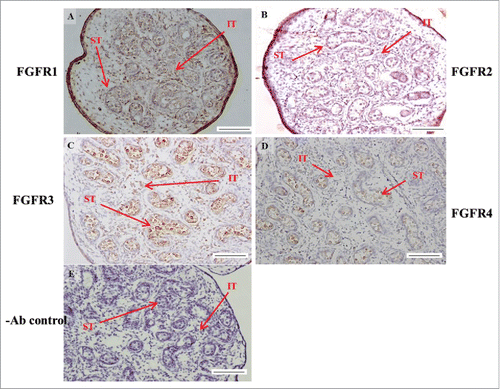
FIGURE 8. The expression pattern of FGF9 in postnatal mice testis. The expression pattern of FGF9 on postnatal days 10 (A and B), 20 (D and E), 35 (G and H), and 65 (J and K) in mice testis were detected using immunohistochemistry. The cell nucleus was labeled using hematoxylin. Control sections without a primary antibody treatment are arranged on right side of figures 8C, 8F, 8I, and 8L. The long arrow indicates the cell types as illustrated by the abbreviations: LCs, Leydig cells; MCs, myoid cells; SCs, Sertoli cells; Sg, spermatogonia; Sc, spermatocyte; and St, spermatids. Scale bar = 50 µm (200 ×) and 19 µm (400 ×). FGF9 pnd.
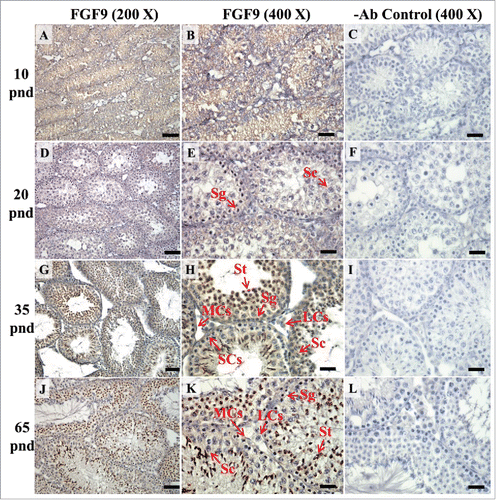
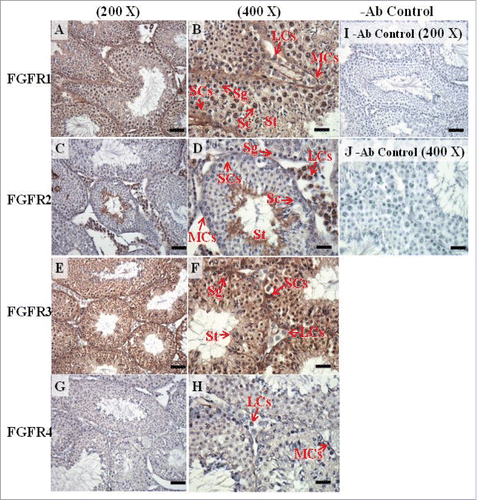
FIGURE 9. The expression patterns of FGFR1, FGFR2, and FGFR3 on postnatal day (pnd) 10 in mice testis. The expression pattern of FGFR1 (A and B), FGFR2 (C and D), FGFR3 (E and F), and FGFR4 (G and H) on pnd 10 mice testis were detected using immunohistochemistry. Control sections without a primary antibody treatment are arranged on right side of the figure (I and J). The long arrow indicates the cell types as illustrated by the abbreviations: MCs, myoid cells; SCs, Sertoli cells; and SGs, spermatogonia. Scale bar = 50 µm (200 ×) and 19 µm (400 ×).
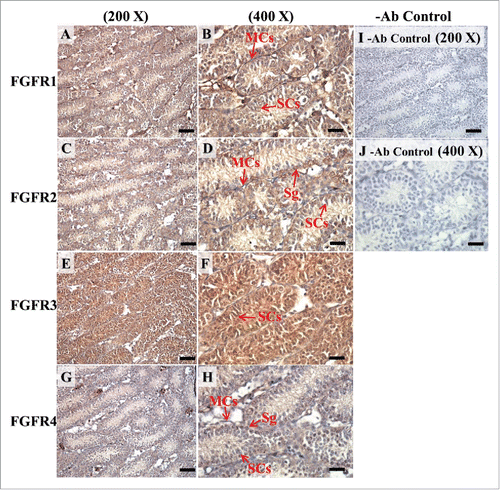
FIGURE 10. The expression patterns of FGFR1, FGFR2, and FGFR3 on postnatal day (pnd) 20 in mice testis. The expression patterns of FGFR1 (A and B), FGFR2 (C and D), FGFR3 (E and F), and FGFR4 (G and H) on pnd 20 mice testis were detected using immunohistochemistry. Control sections without a primary antibody treatment are arranged on right side of the figure (I and J). The long arrow indicates the cell types as illustrated by the abbreviation: MCs, myoid cells; SCs, Sertoli cells; Sg, spermatogonia; and Sc, spermatocyte. Scale bar = 50 µm (200 ×) and 19 µm (400 ×).
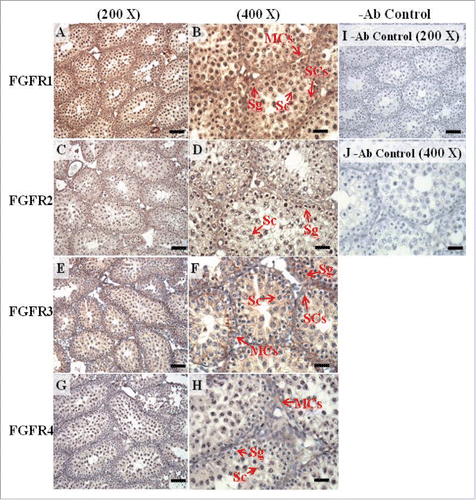
FIGURE 11. The expression patterns of FGFR1, FGFR2, and FGFR3 on postnatal day (pnd) 35 in mice testis. The expression patterns of FGFR1 (A and B), FGFR2 (C and D), FGFR3 (E and F), and FGFR4 (G and H) on pnd 35 mice testis were detected using immunohistochemistry. Control sections without a primary antibody treatment are arranged on right side of the figure (I and J). The long arrow indicates the cell types as illustrated by the abbreviations: LCs, Leydig cells; MCs, myoid cells; SCs, Sertoli cells; Sg, spermatogonia; Sc, spermatocyte; and St, spermatids. Scale bar = 50 µm (200 ×) and 19 µm (400 ×).
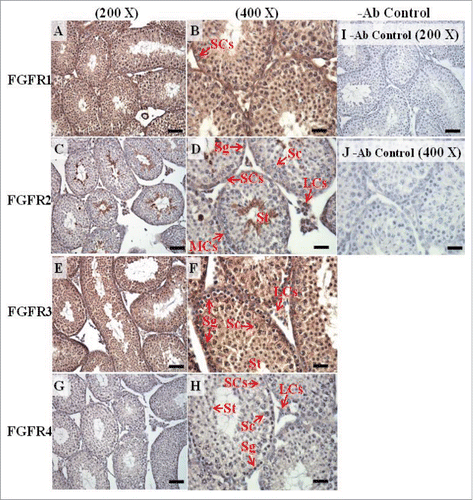
FIGURE 12. The expression patterns of FGFR1, FGFR2, and FGFR3 on postnatal day (pnd) 65 in mice testis. The expression patterns of FGFR1 (A and B), FGFR2 (C and D), FGFR3 (E and F), and FGFR4 (G and H) on pnd 65 mice testis were detected using immunohistochemistry. Control sections without a primary antibody treatment are arranged on right side of the figure (I and J). The long arrow indicates the cell types as illustrated by the abbreviations: LCs, Leydig cells; MCs, myoid cells; SCs, Sertoli cells; Sg, spermatogonia; Sc, spermatocyte; and St, spermatids. Scale bar = 50 µm (200 ×) and 19 µm (400 ×).
Expressions of FGF9 during testicular development and maturation in mice
In the embryonic stage, FGF9 expression could be observed in the seminiferous tubules and interstitial regions from 14–18 dpc (). However, interstitial cells had a stronger FGF9 expression at 17 and 18 dpc compared with the cells in the seminiferous tubules (). At 17 and 18 dpc, FGF9 was expressed in the nucleus and cytoplasm of the interstitial cells (). The cells, located in the seminiferous tubules, had a higher FGF9 protein expression in the membrane. In the postnatal stage, FGF9 was expressed in the lumen of the seminiferous tubules and Leydig cells on pnd 10 (), and in the interstitial region and seminiferous tubules on pnd 20 (), respectively. FGF9 was expressed in Leydig cells and seminiferous tubules on pnd 35 and 65 ( and J-K, respectively). Under 400 × resolutions, we observed that FGF9 was mainly expressed in the spermatid nucleus, spermatogonium cytoplasm and Leydig cell cytoplasm (, respectively).
Expressions of FGFRs during testicular development and maturation in mice
In the embryonic stage, the expression of FGFR1 (stained brown) could be observed in the whole testis at 14–17 dpc (, respectively). At 18 dpc, there were more FGFR1 stained cells in the interstitial region compared with cells in the seminiferous tubules (). During the embryonic stage, we found that FGFR1 was mainly expressed in the membrane of seminiferous tubule cells and interstitial cells at 14 and 15 dpc (). In the postnatal stage, FGFR1 had a strong expression in the seminiferous tubules and Leydig cells on pnd 10, 20, 35, and 65 (, respectively).
In FGFR2, we could observe the protein expression in the whole tissue at 14 and 15 dpc (, respectively). At 16–18 dpc, we noticed that FGFR2 had a higher expression level in the seminiferous tubules compared with the interstitial region (, respectively). With regard to the expression location in the cells, FGFR2 was mainly expressed at the membrane of seminiferous tubule cells at 14 dpc and in the whole cell from 15 to 18 dpc (, respectively). In the postnatal stage, FGFR2 had a strong expression level in the spermatogonia and at the cytoplasm of Leydig cells in the testis on pnd 10 (). On pnd 20, FGFR2 was expressed in the whole testis (). On pnd 35 and 65, FGFR2 was mainly expressed in Leydig cells and the tail part of the spermatid (, respectively).
For FGFR3, we could detect protein expression in the seminiferous tubules and interstitial regions from 14–17 dpc (, respectively). At 17 dpc, FGFR3 had a higher expression level in the interstitial region compared with the seminiferous tubules (). At 18 dpc, FGFR3 had a higher expression level in the seminiferous tubules and interstitial regions compared with 14–17 dpc (). In the postnatal stage, FGFR3 widely expressed on pnd 10 (). On pnd 20, FGFR3 was expressed in the cellular cytoplasm of spermatocytes and spermatids, and more strongly expressed in Leydig cells (). FGFR3 was expressed in the whole testis on pnd 35 and 65 (, respectively).
In FGFR4, we could observe protein expression in the seminiferous tubules and Leydig cells from 14–17 dpc (, respectively). FGFR4 had a stronger expression in the seminiferous tubules at 18 dpc (). In the postnatal stage, FGFR4 was expressed in spermatogonia and Leydig cells on pnd 10 (). On pnd 20, 35, and 65, FGFR4 was expressed in the whole testis (, respectively).
According to these data, we showed that FGF9 was continuously expressed in the mouse testis throughout testis development. Interestingly, FGF9 increased equivalently with FGFR2 and FGFR3 in the embryonic and postnatal stages (). These results suggest that FGF9 might function through FGFR2 and FGFR3 in the embryonic and postnatal stages, respectively, for testis development.
TABLE 1. FGF9 and FGFR1-4 expression intensity in embryonic stage.
TABLE 2. FGF9 and FGFR1-4 expression intensity in postnatal stage.
DISCUSSION
Many studies have reported that FGF9 participates in sex determination and gonad development. Colvin et al.Citation4 showed that FGF9 knockout mice had the phenotype from testis hypoplasia to male to female sex-reverse. FGF9 was also demonstrated to have a unique function in different cell lineages during testis development. In germ cells, FGF9 could increase NANOS2 expression levels to suppress meiosis in male mouse testis.Citation13,14 In FGF9 null mice, the number of germ cells was significantly decreased after 11.5 dpc of the XY genotype but there was no change to XX genotype.Citation41 These studies implied that FGF9 could suppress meiosis and promote survival in male germ cells. In embryonic Sertoli cells, FGF9 could induce cell proliferation in an in vitro culture system. At the same time, FGF9 and the extracellular matrix could induce core-like aggregations after 48 h stimulation.Citation5 FGF9 was also shown to induce cell proliferation and nuclear localization of FGFR2 in Sertoli precursor cells.Citation16 In fetal Leydig cells, there was no direct evidence that FGF9 could affect sex determination through Leydig cells. In other FGFs signaling, FGF1 and FGF2 were reported to increase testosterone production but had no effect on LH-induced maximal testosterone production on fetal Leydig cells.Citation42 However, FGF1 and FGF2 could not induce testosterone production in adult Leydig cells. In addition, FGF1 could inhibit testosterone production elicited by an LH treatment on adult Leydig cells.Citation43 Other studies showed that FGF2 could inhibit LH-induced androgen production in immature rat Leydig cells through decreased steroidogenesis related to protein expression levels, such as steroidogenic acute regulatory protein and steroidogenic factor 1.Citation44 In the present study, we observed that FGF9 and different FGFRs were continuously expressed in developing testis from 14 dpc to pnd 65. Our observations, combined with those from other studies, suggest that FGF9 could be continuously expressed in embryonic testis and regulate testicular development among different cell types. In the postnatal stage, we noticed that Leydig cells had an abundant FGFR2 expression in the adolescent period. In our previous study, we demonstrated that FGF9 could induce testosterone production in immature and tumor Leydig cells.Citation35,36 It is possible that FGF9 could use FGFR2 to regulate hormone production in Leydig cells during gonad maturation.
In this study, we demonstrated that FGF9 was continuously expressed in embryo mouse testis from 14 to 18 dpc. In fact, hormone regulation is an important process for gonad maturation of the testis. According to previous studies, fetal Leydig cells appeared at about 15.5 dpc to pnd 10 and increased steroidogenesis during embryo gonad development.Citation45,46 In embryo testis, Leydig cells and Sertoli cells could participate in testosterone synthesis. Because of an Hsd17b3 deficiency, fetal Leydig cells converted cholesterol into androstenedione. Fetal Sertoli cells produced testosterone by androdtenedione, which is offered by fetal Leydig cells.Citation47 Another study has shown that FGF9 deficiency would cause testicular hypoplasia to a complete sex reversal.Citation4 Our previous study indicated that FGF9 induced steroidogenesis in mice Leydig cells.Citation35 In this study, we observed that FGF9 was expressed in cells in the seminiferous tubules and interstitial regions. These results provide a possible participatory role of FGF9 in embryo testis organogenesis by hormone production and/or regulation.
The different isoforms and ligand-binding affinity of FGFRs created demonstrate that each FGF has a distinct function in each system. It has been demonstrated that FGF9 has a binding affinity with FGFR2IIIc, FGFR3IIIb, FGFR3IIIc, and FGFR4,Citation8 whereas FGFR1 and FGFR2IIIb are not receptors for FGF9. Recently, we showed that mouse Leydig cells expressed FGFR2IIIc, FGFR3 and FGFR4.Citation35 In the present study, we used general FGFR1–4 antibodies to detect the expression pattern in developing mouse testis. Our results did provide basal evidence regarding the different expressional profiles FGF9 and FGFRs in the developing testis. Further experiments and evidence are needed to support the direct interaction between FGF9 and FGFRs among different cell types in the developing testis.
The testes are the gamete production sites in male individuals. It has been shown that FGF2, secreted from spermatogonia, stimulate testosterone production in Leydig cells.Citation48 Testosterone can then activate Sertoli cells which release some unknown factors to stimulate the process of spermatogenesis, in which FGF2 forms a positive loop with testosterone during spermatogenesis.Citation48 Studies have also illustrated that the interaction between FGFs and FGFRs activating down-stream signaling pathways are indispensable for organ development and/or tumorigenesis.Citation46,47 In the present study, we found that Leydig cells had abundant FGF9, FGFR2, and FGFR3 expressions from pnd 35 to 65. It is highly possible that FGF9 might go through autocrine and paracrine pathways to activate the signal through FGFR2 and FGFR3 to regulate testosterone production, which could further regulate testicular development. In fact, there are no other studies regarding the roles of FGFs and FGFRs in spermatogenesis and steroidogenesis in developing and/or mature testis. The present study has provided the first evidence of different expressional profiles of FGF9 and FGFRs in the developing testis. Further experiments will clarify the real functions and mechanisms of FGF9 and FGFRs in the testis.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
ACKNOWLEDGMENTS
We appreciate the efforts from Pei-Rong Chen and Bo-Syong Pan on experiment execution.
Funding
This work was supported by Ministry Of Science and Technology Grants MOST 103-2320-B-006 -009 (BMH) and MOST 104-2320-B-006-041 (BMH), Taiwan, Republic of China.
REFERENCES
- McLaren A. Primordial germ cells in the mouse. Dev Biol 2003; 262:1-15; PMID:14512014; http://dx.doi.org/10.1016/S0012-1606(03)00214-8
- Saitou M, Barton S, Surani M. A molecular programme for the specification of germ cell fate in mice. Nature 2002; 418:293-300; PMID:12124616; http://dx.doi.org/10.1038/nature00927
- Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier MC, Poulat F, Behringer RR, Lovell-Badge R, Capel B. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLos Biol 2006; 4:e187; PMID:16700629; http://dx.doi.org/10.1371/journal.pbio.0040187
- Colvin J, Green R, Schmahl J, Capel B, Ornitz D. Male-to-female sex reversal in mice lacking fibroblast growth factor 9. Cell 2001; 104:875-89; PMID:11290325; http://dx.doi.org/10.1016/S0092-8674(01)00284-7
- Willerton L, Smith RA, Russell D, Mackay S. Effects of FGF9 on embryonic Sertoli cell proliferation and testicular cord formation in the mouse. Int J Dev Biol 2004; 48:637-43; PMID:15470636; http://dx.doi.org/10.1387/ijdb.031778lw
- Sajjad Y. Development of the genital ducts and external genitalia in the early human embryo. J Obstet Gynaecol Res 2010; 36:929-37; PMID:20846260; http://dx.doi.org/10.1111/j.1447-0756.2010.01272.x
- Dufau M, Catt K, Tsuruhara T. Gonadotrophin stimulation of testosterone production by the rat testis in vitro. Biochim Biophys Acta 1971; 252:574-9; PMID:4332841; http://dx.doi.org/10.1016/0304-4165(71)90161-9
- Cotton L, O'Bryan M, Hinton B. Cellular signaling by fibroblast growth factors (FGFs) and their receptors (FGFRs) in male reproduction. Endocrine Rev 2008; 29:193-216; http://dx.doi.org/10.1210/er.2007-0028
- Johnson D, Williams L. Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res 1993; 60:1-41; PMID:8417497; http://dx.doi.org/10.1016/S0065-230X(08)60821-0
- Wesche J, Haglund K, Haugsten Ellen M. Fibroblast growth factors and their receptors in cancer. Biochem J 2011; 437:199-213; PMID:21711248; http://dx.doi.org/10.1042/BJ20101603
- Powers C, McLeskey S, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocrine Related Cancer 2000; 7:165-97; PMID:11021964; http://dx.doi.org/10.1677/erc.0.0070165
- Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genetics 2004; 20:563-9; http://dx.doi.org/10.1016/j.tig.2004.08.007
- Barrios F, Filipponi D, Pellegrini M, Paronetto M, Di Siena S, Geremia R, Rossi P, De Felici M, Jannini E, Dolci S. Opposing effects of retinoic acid and FGF9 on Nanos2 expression and meiotic entry of mouse germ cells. J Cell Sci 2010; 123:871-80; PMID:20159962; http://dx.doi.org/10.1242/jcs.057968
- Bowles J, Feng C, Spiller C, Davidson T, Jackson A, Koopman P. FGF9 suppresses meiosis and promotes male germ cell fate in mice. Dev Cell 2010; 19:440-9; PMID:20833365; http://dx.doi.org/10.1016/j.devcel.2010.08.010
- Takeuchi Y, Molyneaux K, Runyan C, Schaible K, Wylie C. The roles of FGF signaling in germ cell migration in the mouse. Development 2005; 132:5399-409; PMID:16291796; http://dx.doi.org/10.1242/dev.02080
- Schmahl J, Kim Y, Colvin J, Ornitz D, Capel B. Fgf9 induces proliferation and nuclear localization of FGFR2 in Sertoli precursors during male sex determination. Development 2004; 131:3627-36; PMID:15229180; http://dx.doi.org/10.1242/dev.01239
- Hiramatsu R, Harikae K, Tsunekawa N, Kurohmaru M, Matsuo I, Kanai Y. FGF signaling directs a center-to-pole expansion of tubulogenesis in mouse testis differentiation. Development 2010; 137:303-12; PMID:20040496; http://dx.doi.org/10.1242/dev.04-0519
- Hasegawa K, Saga Y. FGF8-FGFR1 signaling acts as a niche factor for maintaining undifferentiated spermatogonia in the mouse. Biol Reprod 2014; 91:145; PMID:25359900; http://dx.doi.org/10.1095/biolreprod.114.121012
- Siggers P, Carre GA, Bogani D, Warr N, Wells S, Hilton H, Esapa C, Hajihosseini MK, Greenfield A. A novel mouse Fgfr2 mutant, hobbyhorse (hob), exhibits complete XY gonadal sex reversal. PLos One 2014; 9:e100447; PMID:24956260; http://dx.doi.org/10.1371/journal.pone.0100447
- Ewen KA, Olesen IA, Winge SB, Nielsen AR, Nielsen JE, Graem N, Juul A, Rajpert-De Meyts E. Expression of FGFR3 during human testis development and in germ cell-derived tumours of young adults. Int J Dev Biol 2013; 57:141-51; PMID:23784824; http://dx.doi.org/10.1387/ijdb.130022er
- Lesca E, Lammens A, Huber R, Augustin M. Structural analysis of the human fibroblast growth factor receptor 4 kinase. J Mol Biol 2014; 426:3744-56; PMID:25219510; http://dx.doi.org/10.1016/j.jmb.2014.09.004
- Steger K, Tentens F, Seitz J, Grothe C, Bergmann M. Localization of fibroblast growth factor 2 (FGF-2) protein and the receptors FGFR 1-4 in normal human seminiferous epithelium. Histo Chem Cell Biol 1998; 110:57-62; ; http://dx.doi.org/10.1007/s004180050-265
- Lee P, Johnson D, Cousens L, Fried V, Williams L. Purification and complementary DNA cloning of a receptor for basic fibroblast growth factor. Science 1989; 245:57-60; PMID:2544996; http://dx.doi.org/10.1126/science.2544996
- Schlessinger J, Plotnikov AN, Ibrahimi OA, Eliseenkov AV, Yeh BK, Yayon A, Linhardt RJ, Mohammadi M. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol Cell 2000; 6:743-50; PMID:11030354; http://dx.doi.org/10.101-6/S1097-2765(00)00073-3
- Sleeman M, Fraser J, McDonald M, Yuan S, White D, Grandison P, Kumble K, Watson JD, Murison JG. Identification of a new fibroblast growth factor receptor, FGFR5. Gene 2001; 271:171-82; PMID:11418238; http://dx.doi.org/10.1016/S0378-1119(01)00518-2
- Ornitz D, Yayon A, Flanagan J, Svahn C, Levi E, Leder P. Heparin is required for cell-free binding of basic fibroblast growth factor to a soluble receptor and for mitogenesis in whole cells. Mol Cell Biol 1992; 12:240-7; PMID:1309590; http://dx.doi.org/10.1128/MCB.12.1.240
- Sonvilla G, Allerstorfer S, Heinzle C, Stättner S, Karner J, Klimpfinger M, Wrba F, Fischer H, Gauglhofer C, Spiegl-Kreinecker S, et al. Fibroblast growth factor receptor 3-IIIc mediates colorectal cancer growth and migration. Br J Cancer 2010; 102:1145-56; PMID:20234367; http://dx.doi.org/10.1038/sj.bjc.6605596
- Jang E, Albadawi H, Watkins MT, Edelman ER, Baker AB. Syndecan-4 proteoliposomes enhance fibroblast growth factor-2 (FGF-2)-induced proliferation, migration, and neovascularization of ischemic muscle. Proc Natl Acad Sci U S A 2012; 109:1679-84; PMID: 22307630; http://dx.doi.org/10.1073/pnas.1117885109
- Behr B, Leucht P, Longaker M, Quarto N. Fgf-9 is required for angiogenesis and osteogenesis in long bone repair. Proc Natl Acad Sci U S A 2010; 107:11853-8; PMID:20547837; http://dx.doi.org/10.1073/pnas.1003317107
- Lovicu FJ, Overbeek PA. Overlapping effects of different members of the FGF family on lens fiber differentiation in transgenic mice. Development 1998; 125:3365-77; PMID:9693140
- Reuss B, Hertel M, Werner S, Unsicker K. Fibroblast growth factors-5 and -9 distinctly regulate expression and function of the gap junction protein connexin43 in cultured astroglial cells from different brain regions. Glia 2000; 30:231-41; PMID:10756073; http://dx.doi.org/10.1002/(SICI)1098-1136(200005)30:3<231::AID-GLIA3>3.0.CO;2-1
- Pirvola U, Zhang X, Mantela J, Ornitz DM, Ylikoski J. Fgf9 signaling regulates inner ear morphogenesis through epithelial-mesenchymal interactions. Dev Biol 2004; 273:350-60; PMID:15328018; http://dx.doi.org/10.1016/j.ydbio.2004.06.010
- Kanda T, Iwasaki T, Nakamura S, Kurokawa T, Ikeda K, Mizusawa H. Self-secretion of fibroblast growth factor-9 supports basal forebrain cholinergic neurons in an autocrine/paracrine manner. Brain Res 2000; 876:22-30; PMID:10973589; http://dx.doi.org/10.1016/S0006-8993(00)02563-4
- Cohen R, Chandross K. Fibroblast growth factor-9 modulates the expression of myelin related proteins and multiple fibroblast growth factor receptors in developing oligodendrocytes. J Neurosci Res 2000; 61:273-87; PMID:10900074; http://dx.doi.org/10.1002/1097-4547(20000801)61:3<273::AID-JNR5>3.0.CO;2-I
- Lin YM, Tsai CC, Chung CL, Chen PR, Sunny Sun H, Tsai SJ, Huang BM. Fibroblast growth factor 9 stimulates steroidogenesis in postnatal Leydig cells. Int J Androl 2010; 33:545-53; PMID:19508331; http://dx.doi.org/10.1111/j.1365-2605.2009.00966.x
- Lai M, Cheng Y, Chen P, Tsai S, Huang B. Fibroblast growth factor 9 activates akt and MAPK pathways to stimulate steroidogenesis in mouse leydig cells. Plos One 2014; 9:e90243; PMID:24603862; http://dx.doi.org/10.1371/journal.pone.0090243
- Hung IH, Schoenwolf GC, Lewandoski M, Ornitz DM. A combined series of Fgf9 and Fgf18 mutant alleles identifies unique and redundant roles in skeletal development. Dev Biol 2016; 411:72-84; PMID:26794256; http://dx.doi.org/10.1016/j.ydbio.2016.01.008
- Chaffee BR, Hoang TV, Leonard MR, Bruney DG, Wagner BD, Dowd JR, Leone G, Ostrowski MC, Robinson ML. FGFR and PTEN signaling interact during lens development to regulate cell survival. Dev Biol 2016; 410:150-63; PMID:26764128; http://dx.doi.org/10.1016/j.ydbio.2015.12.027
- Hiramatsu R, Matoba S, Kanai-Azuma M, Tsunekawa N, Katoh-Fukui Y, Kurohmaru M, Morohashi Ki, Wilhelm D, Koopman P, Kanai Y. A critical time window of Sry action in gonadal sex determination in mice. Development 2009; 136:129-38; PMID:19036799; http://dx.doi.org/10.1242/dev.029587
- Tassinari V, Campolo F, Cesarini V, Todaro F, Dolci S, Rossi P. Fgf9 inhibition of meiotic differentiation in spermatogonia is mediated by Erk-dependent activation of Nodal-Smad2/3 signaling and is antagonized by Kit Ligand. Cell Death Dis 2015; 6:e1688; PMID:25766327; http://dx.doi.org/10.1038/cddis.2015.56
- DiNapoli L, Batchvarov J, Capel B. FGF9 promotes survival of germ cells in the fetal testis. Development 2006; 133:1519-27; PMID:16540514; http://dx.doi.org/10.1242/dev.02303
- Laslett A, McFarlane J, Risbridger G. Developmental response by Leydig cells to acidic and basic fibroblast growth factor. J Steroid Biochem Mol Biol 1997; 60:171-9; PMID:9191974; http://dx.doi.org/10.1016/S0960-0760(96)00180-X
- Laslett AL, McFarlane JR, Risbridger GP. Developmental response by Leydig cells to acidic and basic fibroblast growth factor. J Steroid Biochem Mol Biol 1997; 60:171-9; PMID:9191974; http://dx.doi.org/10.1016/S0960-0760(96)00180-X
- Xiao YC, Hardy DO, Sottas CM, Li XK, Ge RS. Inhibition of LH-stimulated androgen production in rat immature Leydig cells: Effects on nuclear receptor steroidogenic factor 1 by FGF2. Growth Factors 2010; 28:1-9; PMID:19814654; http://dx.doi.org/10.3109/08977190903299379
- Wu X, Wan S, Lee M. Key factors in the regulation of fetal and postnatal Leydig cell development. J Cell Physiol 2007; 213:429-33; PMID:17674364; http://dx.doi.org/10.1002/jcp.21231
- O'Shaughnessy PJ, Fowler PA. Endocrinology of the mammalian fetal testis. Reproduction 2011; 141:37-46; PMID:20956578; http://dx.doi.org/10.1530/REP-10-0365
- Shima Y, Miyabayashi K, Haraguchi S, Arakawa T, Otake H, Baba T, Matsuzaki S, Shishido Y, Akiyama H, Tachibana T, et al. Contribution of Leydig and Sertoli cells to testosterone production in mouse fetal testes. Mol Endocrinol 2012; 27:63-73; PMID:23125070; http://dx.doi.org/10.1210/me.2012-1256
- Gonzalez-Herrera I, Prado-Lourenco L, Pileur F, Conte C, Morin A, Cabon F, Prats H, Vagner S, Bayard F, Audigier S, et al. Testosterone regulates FGF-2 expression during testis maturation by an IRES-dependent translational mechanism. FASEB J 2006; 20:476-8; PMID:16423876
