ABSTRACT
Correct selection of an appropriate animal mode to closely mimic human extremity diseases or to exhibit desirable phenotypes of limb regeneration is the first critical step for all scientists in biomedical and regenerative researches. The commonly-used animals in limb regeneration and repairing studies, such as axolotl, mice, and rats, are discussed in the review and other models including cockroaches, dogs, and horses are also mentioned. The review weighs the general advantages, disadvantages, and precedent uses of each model in the context of limb and peripheral injury and subsequent regeneration. We hope that this review can provide the reader an overview of each model, from which to select one for their specific purpose.
INTRODUCTION
An estimated 2 million people in the United States alone are suffering from limb loss.Citation1 Among these, ∼38% patients are subjected to limb amputations due to a comorbid diagnosis of diabetes mellitus while ∼45% patients are attributed to the trauma of accidental, crime, or battlefield injuries.Citation1 Current solutions to treat patients with limb loss ostensibly rely on prosthetics and rehabilitative therapy; however these treatments have inherent inadequacies, such as biologically incompatible materials, limited ranges of motion, and lack of aesthetic appeal.Citation2 Organ and tissue regenerative medicine has demonstrated great promise to overcome these issues to potentially or even permanently treat patients with limb loss;Citation3 however, the complex nature of organ and tissue regeneration in the aspects of spatial temporal remodeling or reconstruction solely demands extensive animal models to understand the regenerative mechanisms and eventually transfer the findings to clinical practice in treatment of the patients with limb damages.
Modeling the regeneration of extremities using animal models serves multiple goals. In the case of animals, such as the axolotls, that exhibit ideal cases of regeneration, animal models serve to better our understanding of the mechanism by which regeneration is initiated and conducted.Citation4 Cross-phyla studies within the taxonomical animal kingdom have indicated that regeneration maintains an extensive conservation of developmental signaling pathways.Citation5 Research approaches using these animal models in limb regeneration often consist of the initial conditions of induced injury, and the manner in which these injuries are treated, and our abilities to understand the underlying mechanisms at the levels of molecular, cellular, organ and/or whole body. Usually after the morphological or functional abnormality in response to the specific injury for a given animal model is established, various analyses in pathohistological, biochemical, molecular biological and functional aspects will be utilized to explore the mechanisms at different levels following injury. Such approach often yields results on a spectrum varying from fibrosis of the injury site to the formation of a fully undifferentiated blastema on injured limb. However, researches with animal models do reveal enormous critical information with great physiological concordance to humans. These research animals, such as mice and rats, have been widely used as research platforms in all aspects of studies.Citation6
Ideally, the selection of animal model should consider the animal's analog close to a human in terms of body size, body weight, biochemical/structural/functional similarity, and closer responses to environment stimulus.Citation7 From small insect (e.g., cockroachCitation8) to large mammalian (e.g., dogCitation9) to non-human primates (e.g., monkeyCitation10) in limb regeneration or repairing, it seems the bigger the closer to human. By adhering to this weight-progressive animal model, we may have better chance to cost-efficiently understand the mechanisms of limb regeneration using simple (e.g., cockroach) or complex large animal (e.g., dog). The ultimate goal of regenerative medical research is to translate new findings to human clinical therapy, the weight-progressive use of animal models are likely to generate enormous useful data for human application.
This miniature review aims to briefly discuss the research application of the most commonly-used animal models, such as axolotls, mice, and rats in limb regeneration and wound-healing while the less common-used animal models, such as horses, dogs, and cockroaches, will be also addressed later on. Each animal model demonstrated a unique response and regenerative capability to certain type of injury, thus animal model should be carefully selected to meet the various purposes of the studies.
Axolotl model for limb regeneration
By far the most commonly-used vertebrae animal model in extremity regeneration and repairing is the axolotl.Citation11 The axolotl is widely recognized as one of the highly successful organisms known to regenerate an amputated body part (). It can completely regenerate near-perfect copies of a severed forelimb with an average occurrence of muscle tissue defects of merely 2.5% in regenerated limbs.Citation11 The surprising property of axolotl attributes to its neotonous (or pedomorphism) capability to form a blastema at the site of injury compared to other species. This ability is characterized as “epimorphic regeneration,” and is regarded both as the “most true/perfect regeneration,” and as the pinnacle goal of regenerative medicine.Citation12 It is a common assumption that the sequence in which regeneration occurs begins first with the formation of the fingertips. Once the fingertips have differentiated, a successive intercalation of the tissues between the damaged tissue and the leading fingertips would then occur.Citation13 The tissue's “positional identity,” essentially that dictates the type of differentiation in order for correct tissue development, was regarded as being derived from the interaction of the site of damage and the newly developed finger tips.Citation14 However, it was found that blastema cells acquire such positional identity via a proximal-distal sequence, as opposed to the assumed intercalation of tissue between fingertips and site of damage.Citation15 This successive, proximal-distal, near-flawless regeneration of a severed limb or damaged organ makes the axolotl such a desirable regenerative animal model in regenerative medicine.
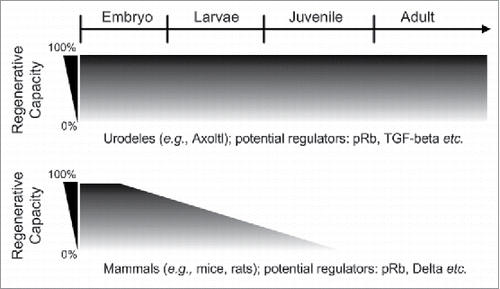
FIGURE 1. Regenerative capacity between urodeles and mammals during development. Urodeles (e.g., axolotl and newt) are the only known vertebrates that can perfectly regenerate different body parts throughout their life while mammals (e.g., mice and rats) are very limited in terms of epimorphic regeneration. It is known that the full regeneration of mammals limb is only limited to digit tips. During the first trimester of gestation, mammals are able to perfectly regenerate their digit tips, but this capacity seems to drop with aging.Citation12 The figure is modified from Roy S et al.Citation12 pRb: RetinSSSoblastoma protein; TGF-β: Transforming growth factor-β.
The main objective using axolotl model is to understand the basic cellular and molecular mechanisms of limb regeneration as well as to discern the injury conditions under which their ideal regeneration can be induced.Citation13 It is well known that axolotls respond to specific events from which regeneration is triggered. Interestingly, axolotls do not need to lose the entire limb to regenerate a new whole limb; partial loss of limb(s) would be enough to provoke a sufficient threshold of nerves, along with a skin wound, to regenerate an entire new limb.Citation16 By completing what is necessary from the site of injury, this regeneration process does not necessarily bear morphological continuity with the undamaged original portion of the limb. The critical question remains by what indication the ectopic additional limb determines the extent of its development. It is true that many studies utilizing the axolotl model have been focusing on identification of the signaling pathway within blastema during limb regeneration. The blastema is regarded as the critical characteristic first step in epimorphic regeneration. Such blastema can be formed in damaged limb, peripheral nerve tissue, or injured central nervous system (CNS) tissue.Citation17
Several factors have been found to be involved in blastema-related limb regeneration or repairing. For example, by deleting the sex determining region Y-box 2 (SOX2) gene using the clustered regularly-interspaced short palindromic repeats (CRISPR), Fei and colleagues found that the blastema formation was significantly inhibited in these transgenic axolotls.Citation17 Retinoblastoma protein (pRb) is a tumor suppressive homologous protein regulating cell cycling in both human and axolotl. Previous findings indicated that hypophosphorylated pRb represses cell cycle progression in mammals while the hyperphosphorylated pRb produced the opposite results in axolotls. Interestingly, cultured mammalian cells remain unresponsive to the treatment of the hyperphosphorylated pRb,Citation18 implying species or microenvironment dependence. Another impotent signaling protein, which was found to facilitate the dedifferentiation process in axolotls, is thrombin. Thrombin reverts the myotubes to the cell cycle following limb injury while it had no effect on mammalian cell cycling.Citation19
The mechanism-target researches for axolotls are quite new and the comprehensive picture of signaling pathway for blastema formation still remains unknown both in axolotls and mammals.Citation13,20 Although the axolotls demonstrate an impressive regenerative capacity, it is still a poor and seldom-used model to evaluate human disease, as its injury response will differ greatly from that of a high order mammal. Rather, the axolotl model could be primarily served as an optimal regeneration-capable model by which comparative studies can be conducted to compare other regeneration-deficient models.Citation12,13
Mouse model for limb regeneration
Mouse is one of the mostly used mammals in biomedical research due to their small size to easily handle, relatively mild temperament, costly efficiency for breeding/housing/experiment, fast growth and maturation rate; especially following the completion of sequence of mouse genomes, a wide range of transgenic strains, cell lines, mouse antibodies, growth factors, and various bioinformatics database is available. Thus, it is not surprising that the mouse is widely employed in the extremity regeneration studiesCitation21 (also see ). With the advanced innovation of gene editing techniques, there is a rising generation of transgenic mice, in which a specific genetic abnormity is induced to mimic human diseases. Among those transgenic mice, the Murphy Roths Large (MRL) mouse probably is specially drawn attention as the first-identified mouse with exceptional regenerative capacity to completely heal the through-ear hole punches in a study by Clark and colleague in 1998.Citation22 MRL mouse are able to heal complex tissue in an epimorphic fashion, i.e., to restore a damaged limb or organ to its normal structure and function.Citation21 In case of digit tip amputation, MRL mouse demonstrated the same ability of complete digit tip regeneration as the ear punches did.Citation23 Further studies indicate that such neotonous phenotype may be related with the 2 signaling pathways of limb regeneration and limb development.Citation24 Though the underlying mechanisms by which blastema formation during regeneration and development in the MRL mouse are still unknown completely, multiple unique responses at cellular and molecular levels may play important roles in MRL mouse after amputation.Citation21,25 It has been shown that the MRL mouse’ cells re-epithelialize over wound sites faster than other mouse (e.g., C57BL/6) strains, which is presumably to facilitates the blastema formation under the quickly covered tissue or though vascularization.Citation26 In addition, the altered immune system, progenitor cells, topography, and vascularization also plays important role in limb regrow of amputated mouse.Citation26,27 Interestingly, when cross-breeding between MRL and other breeds, such as the C57BL/6 mouse, are made, the resulting hybrid mice exhibit varying degrees of limb regeneration.Citation23 By comparing the hybrids, knockout (KO), and wild-type (WT) mice, it provides a powerful approach to identify single gene and potential signaling pathways involved in the blastema formation, a critical phenotype during mammalian limb regeneration. It is important to note that although MRL mice have been widely used in limb regeneration research; but the recent cardiac-specific studies using MRL mouse model exhibited conflicting results.Citation28,29 This has called into question the validity of the regenerative capability of the MRL in general. At the very least, the conflicting nature of the studies could indicate that the healing capacity for the MRL may vary drastically, depending upon the type of tissue injured. Thus, mouse model should be cautiously selected for a specific organ of interest.
Limb regeneration and repairing is obviously and highly clinical relevant and the mouse limb model mimicking human disease has been widely used in research. By far the largest plurality of the causes of limb loss in human is due to amputation with comorbid diagnosis of diabetes mellitus.Citation1 Therefore, studies that investigate potential therapies for diabetes-induced extremity damage and loss as well as other complications with similar pathophysiology are frequently performed using various strains of mice, including diabetic mice. Pretreatment utilizing angiogenesis-inducing agent prior to induced ischemia in, for example, normal or diabetic mice, often through ligation of the femoral artery, was a common approach to study limb repairing.Citation30-32 Congenital muscle dystrophy (e.g., Duchenne muscular dystrophy, DMD) is another common neuromuscular disorders in humans. The counterpart of transgenic mouse with DMD is an ideal model for studying this disease.Citation33 However, mice variants that model human DMD lack functional alleles of utrophin, a murine analog of dystrophin, the pivotal cytoskeletal protein which DMD patients lack. It should be noted that the mdx/utrn+/− breed of mouse is a useful variant for DMD modeling, as it correctly mimics the skeletal muscle fibrosis that occurs in humans with DMD, which other murine models of DMD lack.Citation34 An alternative method of modeling dystrophy was through the use of local intramuscular injection of toxins.Citation35
Rat model for limb regeneration
Due to its extensive documentation of rat genome, relatively fast reproduction, comparatively low cost, recent availability of transgenic variants, the rats has become the second most commonly-used mammalian models in various researches (including limb regeneration) compared to mice. Rats share many biological and physiological properties with mice, but its relative large size in addition of the above advantages similar to mice get it more preferable animal model in the studies of limb regeneration.Citation36 (also see ) The large size of rat makes it relatively easier to perform surgery (e.g., myocardial infarction), easier to conduct functional (e.g., echocardiography), and biological sampling (e.g., more blood withdrawal or tissue collection). The other similarity to mice makes the research feasible with availabilities of extensive resources.
In the disease-induced limb damage, diabetic rat model could be a priority model to choose simply because one of the most common diabetic-complications is the limb loss or damage.Citation37 In addition, diabetic rats display a greater degree of similarity to human diabetes, which include the ability of agents like toxins, diet, and stress to modify the disease effects.Citation36
However in limb regeneration studies, the rat model is predominantly used in nerve regeneration.Citation38 The rat model with sciatic nerve injury is widely used to evaluate surgical and pharmacological therapy following nerve injury.Citation39 Rats, compared to mice and other common mammal models, are able to maintain consistent longitudinal rates of nerve regeneration at approximately rate of 1 mm/day.Citation40 This might be the key reason for many laboratories which study nerve regeneration to choose the rat model. The underlying mechanisms are unknown. Cheng and colleague found that virus-mediated overexpression of the conserved dopamine neurotrophic factor (CDNF) in the transected distal sciatic nerve of rat significantly improved the regeneration of axonal and Schwann cells compared with the control groups at 4 and 8 weeks after injury.Citation41 Further study indicated that the limb muscle weight and functional recovery are closely related with muscle innervation. In a long-term (up to 12 months) denervation study using a rat hind limb model with the transected tibial nerve, Kobayashi and colleague observed a precipitous and profound decrease in the recovery of both muscle mass and integrated motor function in the experimental rats.Citation42 The progressive change seems proportional to the length of the denervation period; but significant discrete changes appeared occurring sometime after 1 month of denervation.Citation42 Another example in which the rat with transected sciatic nerve was used to examine the repairing efficiency of conventional nerve repair method vs the epineural sleeve techniques.Citation43 The results showed that the epineural sleeve technique was found to be superior to provide faster functional recovery and improved nerve regeneration compared with conventional nerve repair.Citation43
Aside from nerve regeneration research, the rat is utilized for many similar studies to that of mice, such as ischemia injury modeling.Citation44 The rat model is often considered an interchangeable model with that of the mouse; however this viewpoint is looked upon with a degree of criticism, as there are potentially significant differences between the 2 models. In comparison to prevalent mice models like the super-healing strain of MRL, the rat model is less widely used in limb and heart regeneration.Citation45 This is due to the MRL's popularity for exhibiting atypical healing rates, and the lack (until recently) of common and standard transgenic rat variants.
Other models (horses, dogs, and cockroach)
Aside from the models discussed above, there are clusters of circumstantially favorable animal models which have also been used in the limb regeneration or repairing. The larger animals, horses and dogs, are often used in limb cartilage or tendon damage and repairing.Citation46,47 The larger animal models can be easily utilized to focus on the site of damage to observe the regeneration. The horse model is one such directed animal model, as the high loading rate on and the weight distribution of the horse's mass on its bone structure makes it ideal for focused study of regeneration of relevant tissues. For example, Gaughan et al. reported that sodium hyaluronate, when administered intrathecally, appears to have a pharmaceutically beneficial action in this collagenase-induced tendinitis and adhesion model in horses.Citation48 Similarly, the dog, as an alternative model exhibiting heavy wear on its bones and ligaments, can also be used to easily focus on the site of damage due to its less expensive compared with horse and large size compared with rodents. This property also make the dog as a predominant model in other studies, such as mandibularCitation49 and dental,Citation50 and other osteological studies.Citation49 Although both dog and horse are the great animal models in the pre-clinic studies, but their highly costs for purchase, housing, and surgery as well as their long maturation rate renders them unsuitable for large scale of repeated studies.
For limb regeneration using marine animals, the zebrafish model stood out as one of the most favorable marine species due to its high regenerative capacity for “limb” (fin) and heart,Citation51-53 as well as well-documented genome.Citation54 In addition, populations of this model are quite cheap to maintain, and grow very quickly. Though the zebrafish does not model limb injury analogous to mammals well, it does provide a relatively simple model to study the basic mechanisms of organ regeneration, thus it has been widely used in many research laboratories.
The insect cockroach (e.g., Blatella germanica) is another animal model being used to study limb regeneration; however, there are not many studies reported recently using this model.Citation55 French et al. compared the stages and pattern of regeneration following hind limb amputation in a cockroach model to that of the axolotl.Citation8,56 The hope of this study is to establish a precedent to examine and compare the analogous injuries across phyla, thus providing a better approach to isolate the most primitive signaling pathways and mechanisms involved in limb regeneration.Citation8,56 Although previous studies using cockroach have found some interesting mechanisms in insect limb regeneration; but the results from this model appears not having direct transferable potential to human clinical trial due to its significant difference from mammalian. However, cross-phyla studies in limb regeneration using various animals, including cockroach, will still be important to delineate the commonalities of signaling pathways in organ/tissue regeneration among various species.
Concluding remarks
Most animal models are chosen by balancing their biological similarity to human disease of interest against the animal size, cost, ethic issue, housing/surgery convenience, availability of bioinformatics, as well as reagents specific to that model. This concise review briefly discussed the precedent studies of individual model in certain areas of limb research and summarized the general advantages and disadvantages of each model at the end (see ). It is our hope that this review can be useful to “standardize” the use of each model with a respective injury condition, so results of each model can be considered on a more comparable basis. By doing so, it may avoid or eliminate redundancy in research and allow resources to be allocated to progressively more translational study. It is our believe that axolotl will still be one of the most important models to study limb regeneration, especially for exploring the underlying mechanism at cellular and molecular level, in future simply because of its superior capability of organ regeneration. The mouse will continue to dominate limb regeneration, probably a better model for screening new drug and cell-based therapy for extremity disease as it is mammals and more close to human compared to axolotl; however other animal models (dogs or horses) may serve as better pre-clinical models to verification of mouse results before human clinical trial. The rat model may gain prominence as transgenic technology (e.g., CRISPR) enables to generate variant specific mutation in rats.
TABLE 1. Comparisons of advantages and disadvantages among various animal models of limb regeneration.
ABBREVIATIONS
| DMD | = | Duchenne muscular dystrophy |
| MRL | = | Murphy Roths Large |
| pRb | = | Retinoblastoma protein |
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest and financial statement were disclosed.
Funding
This work was supported by the PI's start-up fund and by the Institute for Critical Technology and Applied Science (ICTAS) at Virginia Tech #JFC2014_JIAHE958451025). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phy Med Rehabil 2008; 89:422-9; http://dx.doi.org/10.1016/j.apmr.2007.11.005
- Christensen J, Ipsen T, Doherty P, Langberg H. Physical and social factors determining quality of life for veterans with lower-limb amputation(s): a systematic review. Disabil Rehabil 2016; 38:1-9; http://dx.doi.org/10.3109/09638288.2015.1129446
- Hart CA, Tsui J, Khanna A, Abraham DJ, Baker DM. Stem cells of the lower limb: their role and potential in management of critical limb ischemia. Exp Biol Med (Maywood) 2013; 238:1118-26; PMID:23996960; http://dx.doi.org/10.1177/1535370213503275
- Stocum DL, Cameron JA. Looking proximally and distally: 100 years of limb regeneration and beyond. Dev Dynamics 2011; 240:943-68; http://dx.doi.org/10.1002/dvdy.22553
- Rinkevich B, Rinkevich Y. The “stars and stripes” metaphor for animal regeneration-elucidating two fundamental strategies along a continuum. Cells 2012; 2:1-18; PMID:24709641; http://dx.doi.org/10.3390/cells2010001
- Alvarado AS, Tsonis PA. Bridging the regeneration gap: genetic insights from diverse animal models. Nat Rev Genetics 2006; 7:873-84; PMID:17047686; http://dx.doi.org/10.1038/nrg1923
- Burkhardt GE, Spencer JR, Gifford SM, Propper B, Jones L, Sumner N, Cowart J, Rasmussen TE. A large animal survival model (Sus scrofa) of extremity ischemia/reperfusion and neuromuscular outcomes assessment: a pilot study. J Trauma 2010; 69 Suppl 1:S146-53; PMID:20622610; http://dx.doi.org/10.1097/TA.0b013e3181e6a09b
- French V. Leg regeneration in the cockroach, Blatella germanica. II. Regeneration from a non-congruent tibial graft/host junction. J Embryol Exp Morphol 1976; 35:267-301; PMID:939940
- Kazemi D, Fakhrjou A, Dizaji VM, Alishahi MK. Effect of autologous platelet rich fibrin on the healing of experimental articular cartilage defects of the knee in an animal model. Biomed Res Int 2014; 2014:486436; PMID:25028656; http://dx.doi.org/10.1155/2014/486436
- Hovius SE, Stevens HP, Van Nierop PW, Godschalk M, Kusuma A, Deelen G, Van de Bergh M, Van der Meulen JC. Replantation of the radial side of the hand in the rhesus monkey: anatomical and functional aspects. A preliminary study to composite tissue allografting. J Hand Surg Br 1992; 17:651-6; PMID:1484247; http://dx.doi.org/10.1016/0266-7681(92)90193-6
- Diogo R, Nacu E, Tanaka EM. Is salamander limb regeneration really perfect? Anatomical and morphogenetic analysis of forelimb muscle regeneration in GFP-transgenic axolotls as a basis for regenerative, developmental, and evolutionary studies. Anat Rec (Hoboken) 2014; 297:1076-89; PMID:24692358; http://dx.doi.org/10.1002/ar.22906
- Roy S, Gatien S. Regeneration in axolotls: a model to aim for! Exp Gerontol 2008; 43:968-73; PMID:18814845; http://dx.doi.org/10.1016/j.exger.2008.09.003
- McCusker C, Gardiner DM. The axolotl model for regeneration and aging research: a mini-review. Gerontology 2011; 57:565-71; PMID:21372551; http://dx.doi.org/10.1159/000323761
- Simon A, Tanaka EM. Limb regeneration. Wiley Interdiscip Rev Dev Biol 2013; 2:291-300; PMID:24009038; http://dx.doi.org/10.1002/wdev.73
- Roensch K, Tazaki A, Chara O, Tanaka EM. Progressive specification rather than intercalation of segments during limb regeneration. Science 2013; 342:1375-9; PMID:24337297; http://dx.doi.org/10.1126/science.1241796
- Endo T, Bryant SV, Gardiner DM. A stepwise model system for limb regeneration. Dev Biol 2004; 270:135-45; PMID:15136146; http://dx.doi.org/10.1016/j.ydbio.2004.02.016
- Fei JF, Schuez M, Tazaki A, Taniguchi Y, Roensch K, Tanaka EM. CRISPR-mediated genomic deletion of Sox2 in the axolotl shows a requirement in spinal cord neural stem cell amplification during tail regeneration. Stem Cell Reports 2014; 3:444-59; PMID:25241743; http://dx.doi.org/10.1016/j.stemcr.2014.06.018
- Tanaka EM, Gann AA, Gates PB, Brockes JP. Newt myotubes reenter the cell cycle by phosphorylation of the retinoblastoma protein. J Cell Biol 1997; 136:155-65; PMID:9008710; http://dx.doi.org/10.1083/jcb.136.1.155
- Tanaka EM, Drechsel DN, Brockes JP. Thrombin regulates S-phase re-entry by cultured newt myotubes. Curr Biol 1999; 9:792-9; PMID:10469562; http://dx.doi.org/10.1016/S0960-9822(99)80362-5
- Han M, Yang X, Taylor G, Burdsal CA, Anderson RA, Muneoka K. Limb regeneration in higher vertebrates: developing a roadmap. Anat Rec B New Anat 2005; 287:14-24; PMID:16308860; http://dx.doi.org/10.1002/ar.b.20082
- Heber-Katz E, Leferovich JM, Bedelbaeva K, Gourevitch D. Spallanzani's mouse: a model of restoration and regeneration. Curr Top Microbiol Immunol 2004; 280:165-89; PMID:14594211
- Clark LD, Clark RK, Heber-Katz E. A new murine model for mammalian wound repair and regeneration. Clin Immunol Immunopathol 1998; 88:35-45; PMID:9683548; http://dx.doi.org/10.1006/clin.1998.4519
- Chadwick RB, Bu L, Yu H, Hu Y, Wergedal JE, Mohan S, Baylink DJ. Digit tip regrowth and differential gene expression in MRL/Mpj, DBA/2, and C57BL/6 mice. Wound Repair Regeneration 2007; 15:275-84; PMID:17352761; http://dx.doi.org/10.1111/j.1524-475X.2007.00216.x
- Imokawa Y, Yoshizato K. Expression of Sonic hedgehog gene in regenerating newt limb blastemas recapitulates that in developing limb buds. Proc Natl Acad Sci U S A 1997; 94:9159-64; PMID:9256452; http://dx.doi.org/10.1073/pnas.94.17.9159
- Buckley G, Wong J, Metcalfe AD, Ferguson MW. Denervation affects regenerative responses in MRL/MpJ and repair in C57BL/6 ear wounds. J Anat 2012; 220:3-12; PMID:22066944; http://dx.doi.org/10.1111/j.1469-7580.2011.01452.x
- Heydemann A. The super super-healing MRL mouse strain. Frontiers Biol 2012; 7:522-38; http://dx.doi.org/10.1007/s11515-012-1192-4
- Kwiatkowski A, Piatkowski M, Chen M, Kan L, Meng Q, Fan H, Osman AH, Liu Z, Ledford B, He JQ. Superior angiogenesis facilitates digit regrowth in MRL/MpJ mice compared to C57BL/6 mice. Biochem Biophys Res Commun 2016; 473:907-12; PMID:27040769; http://dx.doi.org/10.1016/j.bbrc.2016.03.149
- Leferovich JM, Bedelbaeva K, Samulewicz S, Zhang XM, Zwas D, Lankford EB, Heber-Katz E. Heart regeneration in adult MRL mice. Proc Natl Acad Sci U S A 2001; 98:9830-5; PMID:11493713; http://dx.doi.org/10.1073/pnas.181329398
- Robey TE, Murry CE. Absence of regeneration in the MRL/MpJ mouse heart following infarction or cryoinjury. Cardiovasc Pathol 2008; 17:6-13; PMID:18160055; http://dx.doi.org/10.1016/j.carpath.2007.01.005
- Vaughan EE, Liew A, Mashayekhi K, Dockery P, McDermott J, Kealy B, Flynn A, Duffy A, Coleman C, O'Regan A, et al. Pretreatment of endothelial progenitor cells with osteopontin enhances cell therapy for peripheral vascular disease. Cell Transplant 2012; 21:1095-107; PMID:22304991; http://dx.doi.org/10.3727/096368911X623880
- Cunha FF, Martins L, Martin PK, Stilhano RS, Han SW. A comparison of the reparative and angiogenic properties of mesenchymal stem cells derived from the bone marrow of BALB/c and C57/BL6 mice in a model of limb ischemia. Stem Cell Res Ther 2013; 4:86-96; PMID:23890057; http://dx.doi.org/10.1186/scrt245
- Tan Y, Li Y, Xiao J, Shao H, Ding C, Arteel GE, Webster KA, Yan J, Yu H, Cai L, et al. A novel CXCR4 antagonist derived from human SDF-1beta enhances angiogenesis in ischaemic mice. Cardiovasc Res 2009; 82:513-21; PMID:19196827; http://dx.doi.org/10.1093/cvr/cvp044
- Watchko JF, O'Day TL, Hoffman EP. Functional characteristics of dystrophic skeletal muscle: insights from animal models. J Appl Physiol (1985) 2002; 93:407-17; PMID:12133845; http://dx.doi.org/10.1152/japplphysiol.01242.2001
- Gutpell KM, Hrinivich WT, Hoffman LM. Skeletal muscle fibrosis in the mdx/utrn+/− mouse validates its suitability as a murine model of Duchenne muscular dystrophy. PLoS One 2015; 10:e0117306; PMID:25607927; http://dx.doi.org/10.1371/journal.pone.0117306
- Salvini TF, Morini CC, Selistre de Araujo HS, Ownby CL. Long-term regeneration of fast and slow murine skeletal muscles after induced injury by ACL myotoxin isolated from Agkistrodon contortrix laticinctus (broad-banded copperhead) venom. Anat Rec 1999; 254:521-33; PMID:10203259; http://dx.doi.org/10.1002/(SICI)1097-0185(19990401)254:4%3c521::AID-AR7%3e3.0.CO;2-8
- Iannaccone PM, Jacob HJ. Rats! Dis Models Mech 2009; 2:206-10; http://dx.doi.org/10.1242/dmm.002733
- Lau TW, Sahota DS, Lau CH, Chan CM, Lam FC, Ho YY, Fung KP, Lau CB, Leung PC. An in vivo investigation on the wound-healing effect of two medicinal herbs using an animal model with foot ulcer. Eur Surg Res 2008; 41:15-23; PMID:18382110; http://dx.doi.org/10.1159/000122834
- Kaplan HM, Mishra P, Kohn J. The overwhelming use of rat models in nerve regeneration research may compromise designs of nerve guidance conduits for humans. J Materials Sci Materials Med 2015; 26:226-31; http://dx.doi.org/10.1007/s10856-015-5558-4
- Varejão AS, Melo-Pinto P, Meek MF, Filipe VM, Bulas-Cruz J. Methods for the experimental functional assessment of rat sciatic nerve regeneration. Neurological Res 2004; 26:186-94; http://dx.doi.org/10.1179/016164104225013833
- Gutmann E, Guttmann L, Medawar P, Young J. The rate of regeneration of nerve. J Exp Biol 1942; 19:14-44.
- Cheng L, Liu Y, Zhao H, Zhang W, Guo YJ, Nie L. Lentiviral-mediated transfer of CDNF promotes nerve regeneration and functional recovery after sciatic nerve injury in adult rats. Biochem Biophys Res Commun 2013; 440:330-5; PMID:24076387; http://dx.doi.org/10.1016/j.bbrc.2013.09.084
- Kobayashi J, Mackinnon SE, Watanabe O, Ball DJ, Gu XM, Hunter DA, Kuzon WM, Jr. The effect of duration of muscle denervation on functional recovery in the rat model. Muscle Nerve 1997; 20:858-66; PMID:9179158; http://dx.doi.org/10.1002/(SICI)1097-4598(199707)20:7%3c858::AID-MUS10%3e3.0.CO;2-O
- Tetik C, Ozer K, Ayhan S, Siemionow K, Browne E, Siemionow M. Conventional versus epineural sleeve neurorrhaphy technique: functional and histomorphometric analysis. Ann Plast Surg 2002; 49:397-403; PMID:12370646; http://dx.doi.org/10.1097/00000637-200210000-00011
- Ishikane S, Ohnishi S, Yamahara K, Sada M, Harada K, Mishima K, Iwasaki K, Fujiwara M, Kitamura S, Nagaya N, et al. Allogeneic injection of fetal membrane-derived mesenchymal stem cells induces therapeutic angiogenesis in a rat model of hind limb ischemia. Stem Cells 2008; 26:2625-33; PMID:18669910; http://dx.doi.org/10.1634/stemcells.2008-0236
- Sawa Y. Current status of myocardial regeneration therapy. Gen Thorac Cardiovasc Surg 2013; 61:17-23; PMID:23132738; http://dx.doi.org/10.1007/s11748-012-0153-9
- Gelberman RH, Khabie V, Cahill CJ. The revascularization of healing flexor tendons in the digital sheath. A vascular injection study in dogs. J Bone Joint Surg Am 1991; 73:868-81; PMID:1712787
- Estrada RJ, van Weeren PR, van de Lest CH, Boere J, Reyes M, Ionita JC, Estrada M, Lischer CJ. Comparison of healing in forelimb and hindlimb surgically induced core lesions of the equine superficial digital flexor tendon. Vet Comp Orthop Traumatol 2014; 27:358-65; PMID:25078543; http://dx.doi.org/10.3415/VCOT-13-11-0136
- Gaughan EM, Nixon AJ, Krook LP, Yeager AE, Mann KA, Mohammed H, Bartel DL. Effects of sodium hyaluronate on tendon healing and adhesion formation in horses. Am J Vet Res 1991; 52:764-73; PMID:1854104
- Costantino PD, Shybut G, Friedman CD, Pelzer HJ, Masini M, Shindo ML, Sisson GA, Sr. Segmental mandibular regeneration by distraction osteogenesis. An experimental study. Arch Otolaryngol Head Neck Surg 1990; 116:535-45; PMID:2328111; http://dx.doi.org/10.1001/archotol.1990.01870050035003
- Nakashima M, Iohara K. Regeneration of dental pulp by stem cells. Adv Dent Res 2011; 23:313-9; PMID:21677085; http://dx.doi.org/10.1177/0022034511405323
- Geraudie J, Monnot MJ, Brulfert A, Ferretti P. Caudal fin regeneration in wild type and long-fin mutant zebrafish is affected by retinoic acid. Int J Dev Biol 1995; 39:373-81; PMID:7669548
- Olsen AS, Sarras MP, Jr, Intine RV. Limb regeneration is impaired in an adult zebrafish model of diabetes mellitus. Wound Repair Regen 2010; 18:532-42; PMID:20840523; http://dx.doi.org/10.1111/j.1524-475X.2010.00613.x
- Jazwinska A, Sallin P. Regeneration versus scarring in vertebrate appendages and heart. J Pathol 2016; 238:233-46; PMID:26414617; http://dx.doi.org/10.1002/path.4644
- Varshney GK, Sood R, Burgess SM. Understanding and editing the Zebrafish genome. Adv Genet 2015; 92:1-52; PMID:26639914; http://dx.doi.org/10.1016/bs.adgen.2015.09.002
- Nakamura T, Mito T, Bando T, Ohuchi H, Noji S. Dissecting insect leg regeneration through RNA interference. Cell Mol Life Sci 2008; 65:64-72; PMID:18030418; http://dx.doi.org/10.1007/s00018-007-7432-0
- French V, Bryant PJ, Bryant SV. Pattern regulation in epimorphic fields. Science 1976; 193:969-81; PMID:948762; http://dx.doi.org/10.1126/science.948762
