ABSTRACT
Bioresorbable scaffolds have the potential to overcome several problems associated with metallic stents. Bioresorbable poly-L-lactic acid (PLLA) scaffold implantation for the treatment of peripheral artery disease has already been reported in animal models and clinical trials; however, no studies comparing PLLA scaffolds and bare metal stents (BMSs) with regard to early vascular morphological changes, identified using intravascular ultrasound (IVUS) analysis, have been reported. In this study, PLLA scaffolds and BMSs were implanted bilaterally in iliac arteries of five miniature pigs. Digital subtraction angiography and IVUS were performed before and immediately after stent implantation and at 6-week follow-up. All PLLA scaffolds and BMSs were patent at 6-week follow-up. Per IVUS analysis, the percent area stenosis did not significantly differ between PLLA scaffolds and BMSs (65.7% vs. 67.2%, P = .761). Furthermore, percent vessel lumen change also did not differ significantly. Neointima formation (the neointimal area plus medial area) was significantly less with PLLA scaffolds than with BMSs (15.65 mm2 vs. 25.69 mm2, P < .001). In conclusion, based on IVUS results, short-term results after stent implantation in porcine iliac arteries were comparable between PLLA scaffolds and BMSs. Therefore, PLLA scaffolds are safe and feasible for implantation in peripheral arteries.
INTRODUCTION
Percutaneous transluminal angioplasty has been reported to be associated with a high restenosis rate due to constrictive remodeling, elastic recoil, and neointimal hyperplasia,Citation1 and the use of stents helps prevent or resolve these complications. Bare metal stents (BMSs) have been shown to be useful in clinical practice, and they are currently being implanted in various vessels. Although sealing of the dissection flaps and prevention of acute recoil help significantly reduce acute vessel occlusion incidence, in-stent restenosis, which involves luminal re-narrowing in a stented segment, remains an important clinical issue. To overcome in-stent restenosis, drug-eluting stents have been introduced. The in-stent restenosis rate and target lesion revascularization rate have been shown to be significantly lower with drug-eluting stents than with BMSs.Citation2 However, drug-eluting stents are associated with risks of late and very late stent thrombosis,Citation3 necessitating long-term dual antiplatelet therapy. Furthermore, removal of an implanted BMS or drug-eluting stent requires surgery.
Bioresorbable scaffolds (BRSs) degrade over time, leaving only the remodeled vessel; they were developed to overcome the problems associated with metallic stents. Since BRSs leave no foreign body, additional treatment can be easily provided in the vessel segment that received a BRS, even if in-stent restenosis occurs. BRSs have the following advantages over current BMSs or drug-eluting stentsCitation1: (1) no foreign materials such as non-endothelialized struts and drug polymers remain in the vessel over a long period, as only temporary scaffolding is provided until the vessel has healed; (2) bioresorption facilitates return of vessel vasomotion, adaptive shear stress, late luminal enlargement, and late expansive remodeling; (3) long-term dual antiplatelet therapy can be discontinued once the BRS has been bioresorbed; and (4) imaging techniques such as computed tomography angiography and magnetic resonance imaging can be used for follow-up. As BRSs have less stiffness than metallic stents, they have the potential to overcome the problems related to local stiffening of the artery and compliance mismatch associated with metallic stents.Citation4
Current BRSs are made of either a polymeric scaffold or a bioresorbable metallic stent. Poly-L-lactic acid (PLLA) is the most frequently used polymer currently, and it has already been widely used in clinical devices and materials such as resorbable sutures, soft-tissue implants, orthopedic implants, and dialysis media. PLLA is metabolized into carbon dioxide and water over a period of approximately 12–18 months via Krebs' cycle.Citation1 This polymer has been shown to be biocompatible and well tolerated according to the results of both in vitro and in vivo studies.Citation5-7 The Igaki-Tamai stent (ITS, Kyoto Medical Planning Co., Ltd., Kyoto, Japan), which is made of PLLA, was the first BRS developed for use in humans in 2000, and it has shown promising results in coronary arteries and superficial femoral arteries (SFAs) in clinical trials.Citation6,8,9 This scaffold has been commercially available for treating peripheral artery disease in 15 countries of Europe since 2009. However, no previously published study has presented detailed in vivo experimental results, including intravenous ultrasound and histomorphometric analysis, on ITS implantation in peripheral arteries.
The aims of the present study were to compare the early vascular morphological changes between a BRS (ITS) and a BMS, and assess the feasibility and biocompatibility of the stents in porcine iliac arteries.
METHODS
Animals
This study included 15-month-old male miniature pigs (approximate weight, 40 kg). For anti-thrombotic therapy, the animals received oral acetylsalicylic acid (200 mg/day) and ticlopidine (200 mg/day) from 2 days before angioplasty till the end of follow-up. The study protocol was reviewed and approved by the local ethics committee for animal care and use. All pigs were cared for and used in accordance with the institutional guidelines, and animal care protocols complied with the Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. The pigs were allowed to acclimatize to the animal research laboratory for at least 3 days before they were used in the procedures, and they had free access to food and water before and after the procedure.
Characteristics of the bioresorbable scaffold
The bioresorbable PLLA scaffold used in this study was the ITS for implantation in peripheral arteries. Although previous PLLA scaffolds were prepared by laser cutting or braiding the PLLA monofilaments,Citation5,10 ITS, which was used in this study, had a new zigzag helical coil stent design, similar to that of recent metallic stents. The scaffold is radiolucent with two radio-opaque markers located 2.0 mm from each end. The scaffold is delivered using a balloon-expandable system, which is compatible with a 0.018-inch guidewire and a 7-French (Fr) sheath. Balloon inflation is performed for 60 s at 10 atm (the nominal pressure recommended by the manufacturer).
Stenting procedure
The animals were sedated with an intramuscular injection of a combination of midazolam (2 mg/kg) and medetomidine (0.1 mg/kg). They were placed in the supine position, intubated, and mechanically ventilated. Anesthesia was maintained with isoflurane using a respirator. An intravenous line was established, through which cefazolin sodium hydrate (1,000 mg) was administered for antibiotic prophylaxis. Electrocardiographic data and blood oxygen saturation were continuously monitored. After general anesthesia was administered, the right femoral artery was surgically exposed. Systemic heparinization was achieved with an intra-arterial injection of heparin (5,000 U). Digital subtraction angiography (DSA) with a 4-Fr pigtail catheter and intravascular ultrasound (IVUS) were performed before and immediately after stent implantation, and the results were recorded. The ITS was implanted in the right iliac artery by using balloon inflation at 10 atm (the nominal pressure recommended by the manufacturer) through a 7-Fr introducer sheath (7-Fr Brite Tip sheath, Cordis, Miami, FL). For comparison, the balloon-expandable BMS (Express LD, Boston Scientific, Natick, MA) was implanted in the left iliac artery by using balloon inflation at 8 atm (the nominal pressure recommended by the manufacturer) through a 6-Fr guiding sheath (SheathLess PV, Asahi Intecc Co., Nagoya, Japan). The size of each stent was determined based on the findings of the pre-implantation IVUS analysis, with a stent-to-artery ratio between 1.1:1 and 1.2:1.
Follow-up and harvesting
Specially trained laboratory animal care personnel continually monitored the animals' health. After 6 weeks of follow-up, the animals were sedated, and deep anesthesia was induced. The right internal carotid artery was exposed surgically, and a 5-Fr introducer sheath (5-Fr Catheter Introducer, Medikit Co., Tokyo, Japan) was placed. Immediately after DSA and IVUS analyses, the animals were euthanized with an intravenous injection of pentobarbital.
IVUS analysis
IVUS was performed with an IVUS system (Volcano s5 Imaging System; Volcano Corp., Rancho Cordova, CA) and a catheter (Visions PV; Volcano Corp.). The vessel lumen area and diameter were measured before and immediately after stent implantation. After 6 weeks of follow-up, the vessel lumen area and diameter were measured. The external elastic lamina (EEL) area and diameter were also measured. In this study, the vessel lumen area immediately after stent implantation was equal to the EEL area, as the medial layer was too thin to be visualized using IVUS. The vessel lumen area and EEL area were calculated as the average measurements from three parts of the stented vessel: the distal, middle, and proximal parts. The neointimal area plus medial area was calculated as the EEL area minus the vessel lumen area measured at 6-week follow-up.
Histomorphometry
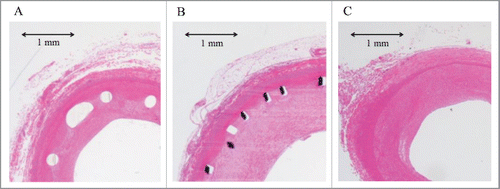
After the pigs were euthanized, each stented iliac artery was explanted and fixed in 10% formalin. Then, the stented vessel segment was embedded in methyl methacrylate; 2- to 4-μm-thick sections were obtained from the distal, middle, and proximal parts of each stented vessel. Three sections were obtained from each stented vessel and were analyzed histomorphometrically. The sections were stained with hematoxylin-eosin and Elastica van Gieson stains for histologic assessment. All stained sections were digitally exported onto a computer and analyzed using Image J software (version 1.48; National Institutes of Health, http://imagej.nih.gov/ij/). The neointimal area and medial area were measured.
Histopathology
Hematoxylin-eosin stained sections were evaluated by an experienced pathologist who was blinded to the section staining, and the injury and inflammation scores were determined. The mean injury score was calculated as described by Schwartz et al.Citation11 For each stent strut, the injury was scored from 0 to 3, and the mean injury score was calculated as the sum of the injury scores of the struts divided by the total number of struts. The definitions of the injury scores were as follows: 0, an intact internal elastic lamina (IEL) and medial compression without a laceration; 1, an IEL laceration and typical medial compression without a laceration; 2, visible IEL and medial lacerations and intact EEL compression; and 3, an EEL laceration and typical large medial lacerations extending through the EEL and occasional presence of coil wires in the adventitia.
For each stent strut, inflammation was scored from 0 to 3, and the mean inflammation score was calculated as the sum of the inflammation scores of the struts divided by the total number of struts. The definitions of the inflammation scores were as follows: 0, no inflammatory cells present around the strut; 1, few inflammatory cells present around the strut; 2, localized, moderate to dense cellular infiltration around the strut; and 3, circumferential, dense inflammatory cell infiltration around the strut.Citation12
Statistical analysis
Data are expressed as mean ± standard error of the mean. Differences between ITSs and BMSs were assessed using the unpaired t test. All statistical analyses were performed using SPSS software (version 22.0; IBM, Armonk, NY). A P-value <.05 was considered statistically significant.
RESULTS
Animals and interventions
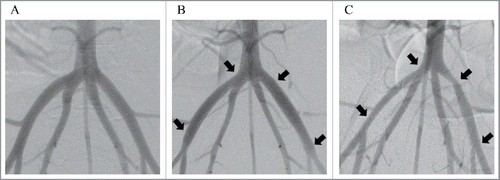
ITSs were implanted in the right iliac arteries, and BMSs were implanted in the left iliac arteries of 5 miniature pigs (). All five animals survived the scheduled follow-up period, and all stented arteries were angiographically patent with both devices at 6-week follow-up ().
FIGURE 1. Digital subtraction angiography images of the porcine iliac arteries before stent implantation (A), immediately after stent implantation (B) and 6 weeks after stent implantation (C). The Igaki-Tamai stent was placed in the right iliac artery, and a bare metal stent was placed in the left iliac artery. Each arrow shows an edge of the implanted stents.
IVUS analysis
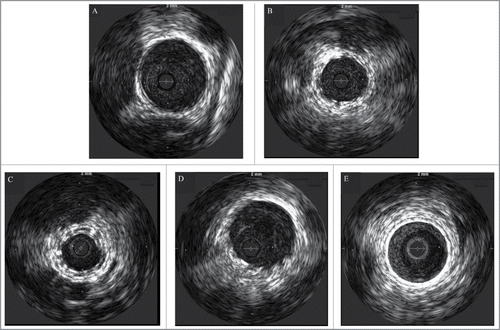
The IVUS data are presented in . Representative images of the IVUS analysis are shown in . No incidence of malapposition, thrombosis, or dissection was observed at 6-week follow-up with both devices.
TABLE 1. Comparison of the results of intravascular ultrasound analysis between Igaki-Tamai stents (ITSs) and bare metal stents (BMSs).
FIGURE 2. Intravascular images of the vessels before stent implantation (A), implanted with Igaki-Tamai stents (B, C) and bare metal stents (D, E) immediately after stent implantation (B, D) and 6 weeks after stent implantation (C, E).
There was no statistical difference in the vessel lumen area before implantation between ITSs and BMSs (22.55 mm2 vs. 24.36 mm2, P = .508). The vessel lumen area immediately after stent implantation was significantly smaller with ITSs than with BMSs (29.62 mm2 vs. 36.60 mm2, P = .001). The vessel lumen area (8.34 mm2 vs. 12.42 mm2, P = .041) and EEL area (24.00 mm2 vs. 38.11 mm2, P < .001) at 6-week follow-up were significantly smaller with ITSs than with BMSs.
Data regarding percent vessel lumen change are shown in . The percent vessel lumen change was calculated as follows: (vessel lumen area immediately after stent implantation or at 6-week follow-up / vessel lumen area before stent implantation) × 100. There was no significant difference in percent vessel lumen change immediately after implantation (154.2% vs. 159.0%, P = .831) and at 6-week follow-up (52.3% vs. 53.5%, P = .938).
FIGURE 3. Graph A shows percent vessel lumen change with Igaki-Tamai stents (ITSs) and bare metal stents (BMSs). Graph B shows percent area stenosis with ITSs and BMSs at 6-week follow-up. Graph C shows vascular remodeling with ITSs and BMSs at 6-week follow-up.
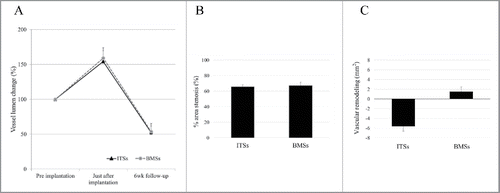
The neointimal area plus medial area was significantly smaller with ITS than with BMS (15.65 mm2 vs. 25.69 mm2, P < .001, ). shows the percent area stenosis at 6-week follow-up. The percent area stenosis was calculated as follows: (neointimal area plus medial area / EEL area) × 100. There was no significant difference in percent area stenosis at 6-week follow-up between ITSs and BMSs (65.7% vs. 67.2%, P = .761).
shows vascular remodeling at 6-week follow-up. Vascular remodeling was calculated as follows: EEL area at 6-week follow-up - vessel lumen area immediately after stent implantation. Vascular remodeling was significantly negative with ITSs than with BMSs (−5.63 mm2 vs. 1.52 mm2, P = .028).
Histomorphometry
Histomorphometric data are presented in , and representative photomicrographs are shown in . None of the evaluated arteries had incomplete stent apposition or an intraluminal thrombus. The neointimal area was significantly smaller with ITS than with BMS (5.83 mm2 vs. 16.85 mm2, P < .001). However, the medial area was significantly larger with ITS than with BMS (5.08 mm2 vs. 3.09 mm2, P = .002).
TABLE 2. Comparison of the results of histomorphometric analysis between Igaki-Tamai stents (ITSs) and bare metal stents (BMSs) at 6 weeks after stent implantation.
Histopathology
The injury and inflammation scores tended to be higher with ITSs than with BMSs (injury scores: 0.053 ± 0.014 vs. 0.023 ± 0.009, P = .085; inflammation scores: 0.073 ± 0.021 vs. 0.027 ± 0.010, P = .062; ), but there were no significant differences between ITSs and BMSs.
TABLE 3. Comparison of the results of histopathological analysis between Igaki-Tamai stents (ITSs) and bare metal stents (BMSs).
DISCUSSION
In the present study, all ITSs and BMSs were patent at 6-week follow-up, and acute stent thrombosis did not occur. Results of IVUS analysis showed that there was no significant difference in percent vessel lumen change and percent area stenosis between ITSs and BMSs. Additionally, the neointimal area plus medial area was significantly smaller with ITSs than with BMSs. In this study, ITSs and BMSs were analyzed mainly by using IVUS, whereas in previous studies on PLLA scaffolds other than ITSs that were implanted in porcine iliac arteries, the scaffolds were mainly analyzed using the histomorphometric method. As IVUS is performed in vivo, the results in this study are considered to represent the physiological state.
Several PLLA scaffolds have been reported to date. Some previous studies have reported that neointima formation after implantation was greater with PLLA scaffolds than with BMSs, and based on the results of the histomorphometric method,Citation4,9 inflammatory reaction of the vessel wall was suggested to promote neointima formation. Uurto et al.Citation5 reported significantly greater intimal thickness and larger lumen loss with bioresorbable poly-L/D-lactic acid self-expandable stents than with stainless steel stents after implantation in porcine iliac arteries, and a severe inflammatory reaction of the vessel wall was suggested to have resulted in the greater intimal thickness. Additionally, Bunger et al.Citation10 reported a significantly larger neointimal area and smaller residual lumen with blended polymeric stents of PLLA and poly-4-hydroxybutyrate than with stainless steel stents after implantation at iliac anastomotic sites, and a severe inflammatory reaction around the stent struts was noted with the polymeric stents. Unlike in previous studies, neointima formation was less with ITSs than with BMSs according to the results of IVUS analysis in this study. Additionally, according to the results of the histopathological analysis, there was no significant difference in the inflammation scores between ITSs and BMSs. The difference in the phenomenon between ITSs and other PLLA scaffolds may be due to improvement in the stent design. Previously reported PLLA scaffolds were prepared by laser cutting or simply braiding the PLLA monofilament.Citation5,9 However, ITS has a zigzag helical coil design, which is similar to that of recent metallic stents. This improvement in the stent design may have contributed to the decrease in inflammatory reaction of the vessel wall and hence in neointima formation. Curcio et al.Citation13 reported that neointima formation and endothelial regeneration was induced by vascular injury causing proliferation of vascular smooth muscle cells. In this study, there was no significant difference in the injury score between ITSs and BMSs, and neointima formation was significantly less with ITSs than BMSs.
The vessel lumen area immediately after implantation and the EEL area at 6-week follow-up were significantly less with ITSs than with BMSs, and vascular remodeling was significantly negative with ITSs than with BMSs according to IVUS results. These observations could be attributed to ITS having less radial force than the BMS. In histomorphometric analysis, the medial area at 6 weeks after implantation was significantly larger with ITS than with the BMS. This is considered to be because of the greater radial force of BMSs, as they stretch and press the medial area, whereas the less radial force of ITS leads to the medial area becoming larger after implantation. This study showed that the radial force of ITS is less than that of the BMS. Regarding the clinical use of ITS, Werner et al.Citation6 performed a prospective multicenter study on ITS implantation in human SFAs. They reported high restenosis rates at 6 and 12 months of follow-up (39.3% and 67.9%, respectively), and high target-lesion revascularization rates at 6 and 12 months of follow-up (25.0 and 57.1%, respectively). They demonstrated that the patency rates of ITS did not match those of the new nitinol stent, and they questioned whether an inflammatory reaction of the vessel wall after stent implantation and a poor radial force may affect long-term patency. In this study, a poor radial force of ITS was also suggested, but the difference in the severity of the inflammatory reaction between ITS and the BMS was not significant. ITS may not yield results comparable to those of BMS in all atherosclerotic lesions, especially in those with hard calcified plaque, for which a greater radial force is needed to sufficiently dilate the lesions. However, in this study, there was no significant difference in percent vessel lumen change and percent area stenosis between ITS and the BMS. Therefore, we think that ITS has the advantage of bioresorption in the treatment of select cases that do not require significant radial force, such as bail-out scaffolding for flow-limiting dissection after balloon angioplasty or during the intervention of atherosclerotic lesions with soft plaque.
To improve the patency of BRSs, the addition of drug elution properties has already been reported for decreasing neointima formation. Neointima formation has been reported to be reduced with drug-eluting polymeric scaffolds that contain sirolimus,Citation14 dexamethasone,Citation15 tyrosine kinase inhibitors,Citation16 or peroxisome proliferator-activator receptor (PPAR)-α/γ agonistsCitation17 than with BMSs or drug-free PLLA scaffolds. A BRS is expected to be useful as a vehicle to deliver antiproliferative agents for the suppression of neointimal hyperplasia, which is the primary contributor to restenosis. Currently, in the clinical setting, a drug-eluting BRS is not available for implantation in human peripheral arteries. Instead, ITS implantation in SFA lesions with pre-balloon dilation using a drug-eluting balloon has been reported. Werner et al.Citation18 studied ITS implantation in de novo SFA lesions with pre-balloon dilation using a paclitaxel-coated balloon. They reported a high restenosis rate (57.9%) and high target-lesion reconstruction rate (42.1%) at 12 months of follow-up. Unfortunately, using ITS with drug-eluting balloon angioplasty, it was impossible to achieve a result comparable to that with BMS for treating peripheral artery disease. Therefore, further research on drug-eluting BRSs is needed to improve patency.
For the future development of BRS, improvement in stent design or development of new bioresorbable materials could improve stent radial force and patency. Additionally, BRSs with drug-eluting technology are expected to improve patency after implantation. Refinement of the delivery system is also required for general use in the clinical setting. This study shows that the implantation of BRSs can be effective in select lesions. However, candidate lesions for BRS implantation should be clarified in future clinical or animal experiments.
The present study had some limitations. First, ITSs and BMSs were implanted in healthy vessels. The reaction of vessel walls with atherosclerotic lesions after stent implantation may differ from that of healthy vessel walls. Second, the follow-up period was 6 weeks. Neointima formation after stent implantation has been reported to peak around 1 month in pigs, whereas the peak neointimal thickness develops between 6 months and 1 year in humans.Citation19 Therefore, we decided to use a follow-up period of 6 weeks based on previous studies.Citation10,14 Only short-term patency and vessel wall reaction were analyzed. Thus, studies with a longer follow-up are needed.
CONCLUSIONS
Short-term results after stent implantation in porcine iliac arteries were comparable between ITSs and BMSs according to the results of IVUS analysis. The ITS is considered safe and feasible for implantation in peripheral arteries. Further studies on the long-term patency after implantation are needed in terms of atherosclerotic lesions.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
Yasuhito Sekimoto received funding from Kyoto Medical Planning Co., Ltd. The other co-authors have no competing interests to declare.
REFERENCES
- Onuma Y, Ormiston J, Serruys PW. Bioresorbable Scaffold Technologies. Circ J 2011; 75:509-20; PMID:21301138; http://dx.doi.org/10.1253/circj.CJ-10-1135
- Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. New Engl J Med 2002; 346:1773-80; PMID:12050336; http://dx.doi.org/10.1056/NEJMoa012843
- Lagerqvist B, James SK, Stenestrand U, Lindback J, Nilsson T, Wallentin L, Group SS. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. New Engl J Med 2007; 356:1009-19; PMID:17296822; http://dx.doi.org/10.1056/NEJMoa067722
- Brugaletta S, Gogas BD, Garcia-Garcia HM, Farooq V, Girasis C, Heo JH, van Geuns RJ, de Bruyne B, Dudek D, Koolen J, et al. Vascular compliance changes of the coronary vessel wall after bioresorbable vascular scaffold implantation in the treated and adjacent segments. Circ J 2012; 76:1616-23; PMID:22531596; http://dx.doi.org/10.1253/circj.CJ-11-1416
- Uurto I, Juuti H, Parkkinen J, Kellomaki M, Keski-Nisula L, Nevalainen T, Tormala P, Salenius JP. Biodegradable self-expanding poly-L/D-lactic acid vascular stent: a pilot study in canine and porcine iliac arteries. J Endovasc Ther 2004; 11:712-8; PMID:15615562; http://dx.doi.org/10.1583/04-127MR.1
- Werner M, Micari A, Cioppa A, Vadala G, Schmidt A, Sievert H, Rubino P, Angelini A, Scheinert D, Biamino G. Evaluation of the biodegradable peripheral Igaki-Tamai stent in the treatment of de novo lesions in the superficial femoral artery: the GAIA study. JACC Cardiovasc Interv 2014; 7:305-12; PMID:24529932; http://dx.doi.org/10.1177/1526602815620618
- Durand E, Sharkawi T, Leclerc G, Raveleau M, van der Leest M, Vert M, Lafont A. Head-to-head comparison of a drug-free early programmed dismantling polylactic acid bioresorbable scaffold and a metallic stent in the porcine coronary artery: six-month angiography and optical coherence tomographic follow-up study. Circ Cardiovasc Interv 2014; 7:70-9; PMID:24368820; http://dx.doi.org/10.1161/CIRCINTERVENTIONS.113.000738
- Tamai H, Igaki K, Kyo E, Kosuga K, Kawashima A, Matsui S, Komori H, Tsuji T, Motohara S, Uehata H. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation 2000; 102:399-404.
- Nishio S, Kosuga K, Igaki K, Okada M, Kyo E, Tsuji T, Takeuchi E, Inuzuka Y, Takeda S, Hata T, et al. Long-term (>10 years) clinical outcomes of first-in-human biodegradable poly-l-lactic acid coronary stents: Igaki-Tamai stents. Circulation 2012; 125:2343-2353; PMID:22508795; http://dx.doi.org/10.1161/CIRCULATIONAHA.110.000901
- Bunger CM, Grabow N, Sternberg K, Goosmann M, Schmitz KP, Kreutzer HJ, Ince H, Kische S, Nienaber CA, Martin DP, et al. A biodegradable stent based on poly(L-lactide) and poly(4-hydroxybutyrate) for peripheral vascular application: preliminary experience in the pig. J Endovasc Ther 2007; 14:725-733; PMID:17924740; http://dx.doi.org/10.1583/1545-1550(2007)14[725:ABSBOP]2.0.CO;2
- Schwartz RS, Huber KC, Murphy JG, Edwards WD, Camrud AR, Vlietstra RE, Vlietstra RE, Holmes DR. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol 1992; 19:267-274; PMID:1732351
- Kornowski R, Hong MK, Tio FO, Bramwell O, Wu H, Leon MB. In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol 1998; 31:224-230; PMID:9426044
- Curcio A, Torella D, Indolfi C. Mechanisms of smooth muscle cell proliferation and endothelial regeneration after vascular injury and stenting. Circ J 2011; 75:1287-1296; PMID:21532177; http://dx.doi.org/10.1253/circj.CJ-11-0366
- Bunger CM, Grabow N. Iliac anastomotic stenting with a sirolimus-eluting biodegradable poly-L-lactide stent: a preliminary study after 6 weeks. J Endovasc Ther 2006; 13:630-9; PMID:17042669; http://dx.doi.org/10.1583/06-1899R.1
- Uurto I, Mikkonen J, Parkkinen J, Keski-Nisula L, Nevalainen T, Kellomaki M, Tormala P, Salenius JP. Drug-eluting biodegradable poly-D/L-lactic acid vascular stents: an experimental pilot study. J Endovasc Ther 2005; 12:371-379; PMID:15943514; http://dx.doi.org/10.1583/05-1525.1
- Yamawaki T, Shimokawa H, Kozai T, Miyata K, Higo T, Tanaka E, Egashira K, Shiraishi T, Tamai H, Igaki K, et al. Intramural delivery of a specific tyrosine kinase inhibitor with biodegradable stent suppresses the restenotic changes of the coronary artery in pigs in vivo. J Am Coll Cardiol 1998; 32:780-786; PMID:9741527
- Uurto I, Hamalainen M, Suominen V, Laurila M, Kotsar A, Isotalo T, Tammela TL, Kellomaki M, Salenius JP. Muraglitazar-eluting bioabsorbable vascular stent inhibits neointimal hyperplasia in porcine iliac arteries. J Vasc Interv Radiol 2015; 26:124-130; PMID:25454655; http://dx.doi.org/10.1016/j.jvir.2014.10.005
- Werner M, Schmidt A, Scheinert S, Banning-Eichenseer U, Ulrich M, Bausback Y, Steiner S, Scheinert D. Evaluation of the biodegradable Igaki-Tamai scaffold after drug eluting balloon treatment of de novo superficial femoral artery lesions: the GAIA-DEB study. J Endovasc Ther 2016; 23:92-7; PMID:26620399; http://dx.doi.org/10.1177/1526602815620618
- Virmani R, Kolodgie FD, Farb A, Lafont M. Drug eluting stents: are human and animal studies comparable? Heart 2003; 89:133-138; PMID:12527658