ABSTRACT
Supernumerary teeth are common clinical dental anomalies. Although various studies have provided abundant information regarding genes and signaling pathways involved in tooth morphogenesis, which include Wnt, FGF, BMP, and Shh, the molecular mechanism of tooth formation, especially for supernumerary teeth, is still unclear. In the population, some cases of supernumerary teeth are sporadic, while others are syndrome-related with familial hereditary. The prompt and accurate diagnosis of syndrome related supernumerary teeth is quite important for some distinctive disorders. Mice are the most commonly used model system for investigating supernumerary teeth. The upregulation of Wnt and Shh signaling in the dental epithelium results in the formation of multiple supernumerary teeth in mice. Understanding the molecular mechanism of supernumerary teeth is also a component of understanding tooth formation in general and provides clinical guidance for early diagnosis and treatment in the future.
INTRODUCTION
Supernumerary teeth are defined as “Teeth, or tooh-like structures that have either erupted or remain unerupted in addition to the 20 primary and 32 permanent teeth.”Citation1 The morphology of supernumerary teeth can be similar to that of the normal teeth or quite different, they can be classified into following types: conical type, tuberculate type, supplemental teeth and odontomas. The extra teeth can occur as a single tooth or multiple teeth, in a unilateral or bilateral fashionCitation2 and in any region of the dentition, though they frequently occur in the premaxillary.Citation3-7 The most common region in which these teeth arise is in the middle of the maxillary between 2 middle incisors, and the most common form of mesiodens is the canine-like type, which comprises 60% of all mesiodens.Citation8
PippiCitation9 summarized the prevalence of supernumerary teeth of previous studies, the rates varied from 0.04% to 2.29%. That may due to the different evaluation of methods or samples. Recently, Japanese scientists report that the prevalence of supernumerary teeth in permanent dentition was 0.04%, which was lower than the other investigations.Citation10 It may because they only focus on the erupted ones but ignored the unerupted ones. ShilpaCitation11 reported that the prevalence of supernumerary teeth in the primary dentition is 0.21%, while in mixed dentition, the rate was 0.9%.Citation12 Additionally, the occurrence of supernumerary teeth varies depending on gender, as it appears to be more common in males than females.Citation10,11,13 Furthermore, supernumerary teeth occur in the midline region more often in males, while incisor region supernumerary teeth are more common in females.Citation14
Supernumerary teeth in the permanent dentition are usually accompanied by various dental anomalies, such as odontoloxia, impaction, rotated permanent teeth adjacent to the supernumerary teeth, delayed eruption, ectopic eruption, overcrowding, periapical resorption of permanent teeth and the formation of follicular cysts.Citation15-18 Usually, supernumerary teeth are extracted because of their influence on normal dentition and aesthetics factors.
DIAGNOSIS AND MANAGEMENT
In most cases, erupted supernumerary teeth could be diagnosed by general oral examination, and imaging methods could be helped in diagnosis of unerupted extra teeth. More significantly, doctors should pay attention to the distinction between real supernumerary with uerupted prematurely deciduous teeth.
The management of supernumerary teeth depends on their type, position, and possible complications, as detected clinically and radiographically, and there is no clear consensus on when is the best time to remove unerupted supernumerary teeth.Citation16 Under the following circumstances an immediate elimination of the supernumerary teeth should be considered: inhibition or delay of eruption, displacement of the adjacent tooth, interference with orthodontic appliances, presence of a pathologic condition, or spontaneous eruption of the supernumerary teeth.Citation19
THEORY REGARDING SUPERNUMERARY TEETH FORMATION
The factors driving the morphogenesis of supernumerary teeth have remained unclear until now. Sometimes extra teeth occur as a sporadic case, but usually occur with some syndromic disease and cluster within families. AnthonappaCitation2 et al. summarized the possible aetiologies for the initiation of supernumerary teeth, which included atavism, dichotomy, hyperactivity of the dental lamina, heredity, progress zone theory and unified etiology.
The theory regarding hyperactivity of the dental lamina is widely accepted, and it suggests that there is a localized, independent and conditional hyperactivity of the remaining epithelial cells of the dental lamina. Excessive activity of the dental lamina is associated with abnormal development of embryos caused by genetic factors.Citation20
All the theories mentioned above have opened the door to research in the field of supernumerary teeth. Until now, each theory has had its own advantages, however, each one can only partially explain the phenomenon. Although hyperactivity of dental lamina is the most acceptable reason, we still do not know what cause this hyperactivity: genes or environmental factors? The etiology of supernumerary teeth is multifactorial, resulting from both genetic and environmental factors. There is a long way to go if we want to gain clarity regarding supernumerary teeth and be able to manipulate the number and morphogenesis of teeth temporally and spatially.
SYNDROMES RELATED TO SUPERNUMERARY TEETH
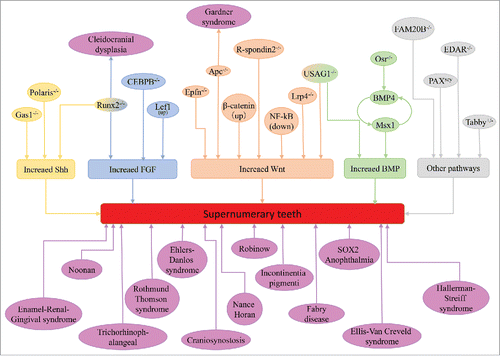
Supernumerary teeth can occur as sporadic cases in some conditions but usually occur in association with some syndromic disease and familiar tendency. We list the syndromes associated with supernumerary teeth (). Multiple supernumerary teeth with non-syndromic reasons are rarer and usually they are strongly associated with syndromic diseases, like cleidocranial dysplasia (CCD) and Gardner's syndrome. Runx2 and APC are the pathogenic genes of CCD and Gardner's syndrome, respectively. Clinical manifestations of some syndromes can be accompanied by supernumerary teeth, which have been identified are Enamel-Renal-Gingival syndrome,Citation21 craniosynostosis,Citation22 Crouzon syndrome,Citation23 Ehlers-Danlos syndromeCitation24 Incontinentia pigmenti,Citation25 Ellis-Van Creveld syndrome,Citation26 Hallerman-Streiff syndrome27(Ahn considered they are uerupted prematurely deciduous teeth rather than supernumerary teethCitation28), Nance-Horan syndrome,Citation29 Noonan syndrome,Citation30 Robinow syndrome,Citation31 SOX2 anophthalmia syndromeCitation32 and Trichorhinophalangea.Citation33
TABLE 1. Syndrome related supernumerary teeth.
Sequence similarity family 20 (FAM20) is a group of highly evolutionarily conserved molecules, including FAM20A, FAM20B and FAM20C. In human, mutations in FAM10A caused a supernumerary premolar, while mutations in FAM20C showed dental abnormalities and gingival hyperplasia but no supernumerary teeth, which was similar to the FAM10A null mice. However, FAM20B knock out mice occurred supernumerary incisors. Mutations in IL11RA in human appeared supernumerary teeth, but loss function of IL11RA in mice did not displayed supernumerary teeth,Citation22,34 so was the same as Elli-Van Creveld syndrome and EVC2 null mice.Citation35-37 These atypical findings suggested that the function of IL11 signaling pathway and EVC2 in dental development are different in human and mice. For Fabry syndrome, although there were cases of supernumerary teeth appeared, dental dysplasia was not detected in Aga−/− mice.Citation38,39 That might be a coincidence or the species differences. Supernumerary teeth in Hallerman-Streiff syndrome used to be a accompanied manifestation, but recently, scientists suggested that were prematurely unerupted teeth rather than extra teeth.Citation28,40
Thus, the early diagnosis of syndrome-related supernumerary teeth is quite important. For example, approximately 70% of all Gardner's syndrome patients had dental anomalies, including supernumerary teeth and others.Citation41 Without treatment, intestinal polyps have a 100% chance of becoming malignant.Citation42 Usually, finding the tooth anomalies occurs earlier than detecting osteoma and intestinal polyps, so dentists need to be acquainted with these anomalies to diagnose Gardner's syndrome as early as possible.
SIGNALING PATHWAYS INVOLVED IN SUPERNUMERARY TEETH FORMATION
The formation of the tooth is a result of a series of signal interactions between the epithelium and the neural crest-derived ectomesenchyme of the maxilla and mandible. Remarkable advancements in the genetic mechanisms have improved our understanding of supernumerary teeth formation and development. The initiation of tooth germs has a decisive effect on the number of teeth, and cells in the enamel knot, which forms at the center of the tooth germ at the onset of the cap stage, will produce several signaling molecules to control the development of tooth germs, including bone morphogenetic protein (BMP), fibroblast growth factor (FGF), tumor necrosis factor (TNF) and molecules of the sonic hedgehog (Shh) and wingless-related (Wnt) pathways.Citation20,43-45 The stable and accurate expression of signaling molecules is the foundation of tooth development, and up- or downregulation of any of these molecules leads to tooth and dentition anomalies. We summarized the signaling pathways and molecules involved in supernumerary teeth in .
The tooth morphogenesis of different vertebrates is similar, from thickened dental lamina to bud, cap and bell stage, then the hard tissue deposited and gradually become matured. Usually, the research of supernumerary teeth was performed in mice. A number of mouse mutant strains for supernumerary teeth have been established ().Citation43 In the mouse dentition, there is a tooth-less region, called diastema, between the incisor and molars in each quadrant. In the early stages of embryonic development, there are rudimentary tooth buds, and later in development, all of the tooth buds go through apoptosis, leading to the tooth-less regions. The general consensus is that signaling molecules in the mesenchyme arrest the development of the tooth at the bud stage.Citation46 However, the impropriated modulation of the signaling pathways may rescue the vestigial tooth rudiments and lead to supernumerary teeth.Citation47 Thus, the models of supernumerary teeth in mice can be classified into 2 categories, one is the extra tooth buds developed in the incisor or molar region, the other is the continues development of primary tooth buds in the diastema region.
TABLE 2. Mouse models associated with supernumerary teeth.
Supernumerary teeth formed in incisor and molar region
The development of supernumerary teeth was closely related to the upregulation or sensitivity to Wnt, Shh, BMP and FGF signaling. In the early stage of tooth germ development, the Shh signal is indispensable for dental placodes, and over activated Shh signal is an important premise for supernumerary teeth formation.Citation48,49 BMPs belong to the TGFβ family, and are expressed widely between the epithelium and mesenchyme in the developing tooth germs, BMP2, BMP4, BMP6 and BMP7 are all detected.Citation50 Among them, BMP4 in the dental mesenchyme have vital function in inducing the tooth germ develop from bud stage to bell stage. In the mesenchyme, BMP4 acts in a positive feedback loop with Msx1 to regulate the tooth development.Citation51,52 The transcription factor Osr2 and Dkk2 maintain this interation mainly act as an inhibitor to this positive feedback and restrict the area of presumptive odontogenic meshenchyme. In the deletion of Osr2 or Dkk2, BMP4, Msx1 and Shh signal were over activited and extra teeth came up.Citation53,54 USAG1 is a secreted BMP signal inhibitor, USAG1 null mice exhibit supernumerary teeth in the incisor region, and the other BMP signal inhibitor noggin could rescue the phenotype of extra teeth in vitro.Citation55 Further research showed the antagonistic interaction between USAG1 and BMP7.Citation56 Wnt is a secreted glycoprotein that can activate several intercellular signaling pathways, including canonical and non-canonical signaling pathways. Wnt4, Wnt5a, Wnt6, Wnt10a and Wnt10b are all detected during early stage of tooth development.Citation57,58 The stable expression of canonical Wnt signaling has great significance of tooth number, the over-activation of the Wnt/β-catenin signaling pathway in the dental epithelium results in the formation of supernumerary teeth. Apc is the inhibitor of canonical Wnt signaling.Citation59 Epithelial deletion of Apc in both mouse embryos and young mice resulted in continuous supernumerary teeth formation from multiple regions of the jaw. Additionally, the genetic deletion of Apc or the activation of β-catenin in the oral epithelium of old adult mice produced multiple supernumerary teeth in the incisor region.Citation60 Delete the other Wnt signaling antagonist USAG1 or Lrp4 also result supernumerary teeth.Citation61,62 Mouse model which overexpression of IKKβ, a major component of NF-κB signaling, showed supernumerary teeth in the lingual of incisor region, and the phenotype was similar to the model of Ectodin deletion.Citation63 Scientists reported that NF-κB signaling can activate Wnt signaling in colon cancer.Citation64 Thus, the supernumerary teeth in IKKβ overexpression mouse may due to the activation of Wnt signaling.
Supernumerary teeth in diastema region
In the embryonic period, there are rudimentary tooth primordia in the diastema region called R1 and R2 in maxillary and MS and R2 in mandible. From the embryonic day 13.5, these tooth buds became apoptosis and finally formed the tooth-less region.Citation65 The cyclin-dependent kinase inhibitor p21 is the marker for tooth bud apoptosis in diastema, and many molecules like FGFs and CCAAT/enhancer-binding protein β(CEBPB) are involved in the apoptosis procedure.Citation65,66 The supernumerary teeth in diastema region is result from the inhibit of apoptosis. In Tg737orpk mice, there are extra tooth and p21 expression is downregulated obviously.Citation49 Three of the 4 Sprouty (Spry) homologues that are expressed during tooth development are Spry1, Spry2 and Spry4, which are proteins that can negatively regulate FGF signaling.Citation67 Genetic deletion of Spry or CEBPB, cell proliferation instead of apoptosis and then came out extra teeth.Citation66,68,69 In Runx2 null mice, excess transcription factor Twist1 activated FGF signaling to rise supernumerary teeth, and that might be the mechanism of human cleidocranial dysplasia disease (CCD).Citation70 Wnt signaling also involved in the formation of diastema. Inactivation of USAG1, which binds to the extracellular domain of the Wnt co-receptors Lrp5 and Lrp6, can lead to the upregulation of Wnt signaling and therefore the development of supernumerary teeth.Citation61 Over expression of Lef1, a target gene of β-catenin, results in numerous tooth-like structures in the non-tooth region of the mouseCitation71; meanwhile, Lef1 can relay Wnt signal to cascade FGF4 activity.Citation72 R-spondins are Wnt signaling activator, and in R-spondin null mice, diastema teeth occurred. However, the Wnt signal was in low expression.Citation73 Ectopic Shh signal in dental lamina is an important reason for supernumerary teeth in diastema, and its expression is regulated by some molecules in dental mesenchyme. Inhibit the expression of Shh signal antagonistic molecules in mesenchyme leading the formation of supernumerary teeth, like Gas1, Polaris and Runx2.Citation49,74-76
There are some other mouse models for supernumerary teeth, but the exactly mechanisms were not clear. That ectodysplasin-A (EDA) molecule functions to suppress BMP signaling and up-regulate Shh signaling, and overexpression of EDA induces supernumerary teeth by counteracting BMP4 activity during teeth development.Citation77 Local loss Epiprofin Protein 6 (Sp6), a Kruppel-like family transcription factor, in the mesenchyme of the incisor region results in supernumerary incisors.Citation78,79 Pax6 gene homozygous mutant mice or Fam20B null mice showed extra teeth in incisor region.Citation80-82
CONCLUSIONS AND PERSPECTIVES
Supernumerary teeth could be isolated findings or developmental abnormalities with systemic syndromes. It can be important clue for early diagnosis in some distinctive disorders. In 2015, scientists find that human with Lrp6 mutant have supernumerary teeth but no systemic syndrome.Citation83 A Japanese research found some new genes may play role in human supernumerary teeth.Citation84 Thus, we believed that there must be genetic background of supernumerary teeth, even in non-syndromic cases. Although mouse dentitions are different from human being, the molecular mechanism research of supernumerary teeth in mouse is still meanful.
The field of tooth number interference is still in its infancy, lots of questions need to be answered. Animals more similar to human development patterns like chimpanzee need to be established to identified the genetic background of supernumerary teeth. How can researchers control or take advantage of the abnormal expression genes that found in supernumerary teeth into tooth regeneration?
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
AUTHOR CONTRIBUTIONS
X.L. and F.Y.: conception and design and manuscript writing; J.L. and W.C.: discussion and interpretation; Y.Z., S.Z. and S.L.: conception and design, manuscript editing, and final approval of manuscript.
FUNDING
This work was supported by the Ministry of Science and Technology of China (2013CB967500), the National Natural Science Foundation of China (81570965).
REFERENCES
- Anthonappa RP, King NM, Rabie ABM. Aetiology of supernumerary teeth: a literature review. Eur Arch Paediatr Dent 2013; 14:279-88; PMID:24068489; https://doi.org/10.1007/s40368-013-0082-z
- Garvey MT, Barry HJ, Blake M. Supernumerary teeth–an overview of classification, diagnosis and management. J Can Dent Assoc 1999; 65:612-6; PMID:10658390
- Bozkurt M, Bezgin T, Tüzüner Öncül A, Göçer R, Sarı Ş. Late developing supernumeraries in a case of nonsyndromic multiple supernumerary teeth. Case Rep Dent 2015; 2015:840460-6; PMID:25649422
- Dhull KS, Dhull RS, Panda S, Shweta Yadav SA, Mohanty G. Bilateral Mandibular Paramolars. IJCPD 2014; 7:38-40; https://doi.org/10.5005/jp-journals-10005-1231
- Mahabob MN, Anbuselvan GJ, Kumar BS, Raja S, Kothari S. Prevalence rate of supernumerary teeth among non-syndromic South Indian population: An analysis. J Pharm Bioallied Sci 2012; 4:S373-5; PMID:23066293; https://doi.org/10.4103/0975-7406.100279
- Mínguez-Martinez I, Ata-Ali J, Bonet-Coloma C, Peñarrocha-Oltra D, Peñarrocha-Diago MA, Minguez-Sanz JM. Management and outcome following extraction of 303 supernumerary teeth in pediatric patients. Pediatr Dent 2012; 34:136-9; PMID:23211898
- Rajab LD, Hamdan MAM. Supernumerary teeth: review of the literature and a survey of 152 cases. Int J Paediatr Dent 2002; 12:244-54; PMID:12121534; https://doi.org/10.1046/j.1365-263X.2002.00366.x
- Colak H, Uzgur R, Tan E, Hamidi MM, Turkal M, Colak T. Investigation of prevalence and characteristics of mesiodens in a non-syndromic 11256 dental outpatients. Eur Rev Med Pharmacol Sci 2013; 17:2684-9; PMID:24142619
- Pippi R. Odontomas and supernumerary teeth: is there a common origin? Int J Med Sci 2014; 11:1282-97; PMID:25419174; https://doi.org/10.7150/ijms.10501
- Hagiwara Y, Uehara T, Narita T, Tsutsumi H, Nakabayashi S, Araki M. Prevalence and distribution of anomalies of permanent dentition in 9584 Japanese high school students. Odontology 2016; 104:380-9; PMID:26612080; https://doi.org/10.1007/s10266-015-0225-2
- Shilpa G, Gokhale N, Mallineni SK, Nuvvula S. Prevalence of dental anomalies in deciduous dentition and its association with succedaneous dentition: A cross-sectional study of 4180 South Indian children. J Indian Soc Pedod Prev Dent 2017; 35:56-62; PMID:28139484; https://doi.org/10.4103/0970-4388.199228
- Laganà G, Venza N, Borzabadi-Farahani A, Fabi F, Danesi C, Cozza P. Dental anomalies: prevalence and associations between them in a large sample of non-orthodontic subjects, a cross-sectional study. BMC Oral Health 2017; 17(1):62: 1-7; PMID:28284207; https://doi.org/10.1186/s12903-017-0352-y
- Amarlal D, Muthu MS. Supernumerary teeth: review of literature and decision support system. Indian J Dent Res 2013; 24:117-22; PMID:23852244; https://doi.org/10.4103/0970-9290.114911
- Kuechler EC, da Costa AG, Costa M de C, Vieira AR, Granjeiro JM. Supernumerary teeth vary depending on gender. Braz Oral Res 2011; 25:76-9; PMID:21359454; https://doi.org/10.1590/S1806-83242011000100013
- Anthonappa RP, Omer RSM, King NM. Characteristics of 283 supernumerary teeth in southern Chinese children. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 105:e48-54; PMID:18417392; https://doi.org/10.1016/j.tripleo.2008.01.035
- Ata-Ali F, Ata-Ali J, Peñarrocha-Oltra D, Peñarrocha-Diago M. Prevalence, etiology, diagnosis, treatment and complications of supernumerary teeth. J Clin Exp Dent 2014; 6:e414-8; PMID:25593666; https://doi.org/10.4317/jced.51499
- Yassin OM, Hamori E. Characteristics, clinical features and treatment of supernumerary teeth. J Clin Pediatr Dent 2009; 33:247-50; PMID:19476099; https://doi.org/10.17796/jcpd.33.3.0j1227k74883531n
- Tanwar R, Jaitly V, Sharma A, Heralgi R, Ghangas M, Bhagat A. Non-syndromic multiple supernumerary premolars: Clinicoradiographic report of five cases. J Dent Res Dent Clin Dent Prospects 2017; 11:48-52; PMID:28413596
- Shah A, Gill DS, Tredwin C, Naini FB. Diagnosis and management of supernumerary teeth. Dent Update 2008; 35:510-2, 514-6, 519-20; PMID:19055087
- Fleming PS, Xavier GM, DiBiase AT, Cobourne MT. Revisiting the supernumerary: the epidemiological and molecular basis of extra teeth. Br Dent J 2010; 208:25-30; PMID:20057458; https://doi.org/10.1038/sj.bdj.2009.1177
- Kantaputra PN, Kaewgahya M, Khemaleelakul U, Dejkhamron P, Sutthimethakorn S, Thongboonkerd V, Iamaroon A. Enamel-renal-gingival syndrome and FAM20A mutations. Am J Med Genet 2014; 164A:1-9; PMID:24259279; https://doi.org/10.1002/ajmg.a.36187
- Nieminen P, Morgan NV, Fenwick AL, Parmanen S, Veistinen L, Mikkola ML, van der Spek PJ, Giraud A, Judd L, Arte S, et al. Inactivation of IL11 signaling causes craniosynostosis, delayed tooth eruption, and supernumerary teeth. Am J Hum Genet 2011; 89:67-81; PMID:21741611; https://doi.org/10.1016/j.ajhg.2011.05.024
- Torun GS, Akbulut A. Crouzon syndrome with multiple supernumerary teeth. Niger J Clin Pract 2017; 20:261-3; PMID:28091449; https://doi.org/10.4103/1119-3077.187332
- Ferreira O, Cardoso CL, Capelozza ALA, Yaedú RYF, da Costa AR. Odontogenic keratocyst and multiple supernumerary teeth in a patient with Ehlers-Danlos syndrome–a case report and review of the literature. Quintessence Int 2008; 39:251-6; PMID:18618041
- Van den Steen E, Bottenberg P, Bonduelle M. Dental anomalies associated with incontinentia pigmenti or Bloch-Sulzberger syndrome. Rev Belge Med Dent 2004; 59:94-9
- Cahuana A, Palma C, Gonzáles W, Geán E. Oral manifestations in Ellis-van Creveld syndrome: report of five cases. Pediatr Dent 2004; 26:277-82; PMID:15185812
- Robotta P, Schafer E. Hallermann-Streiff syndrome: case report and literature review. Quintessence Int 2011; 42:331-8; PMID:21516279
- Ahn BD, Kim JW. Hallermann-Streiff syndrome: Those are not supernumerary teeth. J Pediatr 2006; 148:415-1; PMID:16615982; https://doi.org/10.1016/j.jpeds.2005.07.035
- Gjørup H, Haubek D, Jacobsen P, Ostergaard JR. Nance-Horan syndrome-The oral perspective on a rare disease. Am J Med Genet 2016; 173:88-98; PMID:27616609; https://doi.org/10.1002/ajmg.a.37963
- Uloopi KS, Madhuri V, Gopal AS, Vinay C, Chandrasekhar R. Multiple unerupted permanent teeth associated with noonan syndrome. Ann Med Health Sci Res 2015; 5:317-20; PMID:26229724; https://doi.org/10.4103/2141-9248.160190
- Mazzeu JF, Pardono E, Vianna-Morgante AM, Richieri-Costa A, Ae Kim C, Brunoni D, Martelli L, de Andrade CEF, Colin G, Otto PA. Clinical characterization of autosomal dominant and recessive variants of Robinow syndrome. Am J Med Genet 2007; 143:320-5; PMID:17256787; https://doi.org/10.1002/ajmg.a.31592
- Numakura C, Kitanaka S, Kato M, Ishikawa S, Hamamoto Y, Katsushima Y, Kimura T, Hayasaka K. Supernumerary impacted teeth in a patient with SOX2 anophthalmia syndrome. Am J Med Genet 2010; 152A:2355-9; PMID:20803647; https://doi.org/10.1002/ajmg.a.33556
- Momeni P, Glöckner G, Schmidt O, Holtum von D, Albrecht B, Gillessen-Kaesbach G, Hennekam R, Meinecke P, Zabel B, Rosenthal A, et al. Mutations in a new gene, encoding a zinc-finger protein, cause tricho-rhino-phalangeal syndrome type I. Nat Genet 2000; 24:71-4; PMID:10615131; https://doi.org/10.1038/71717
- Keupp K, Li Y, Vargel I, Hoischen A, Richardson R, Neveling K, Alanay Y, Uz E, Elcioğlu N, Rachwalski M, et al. Mutations in the interleukin receptor IL11RA cause autosomal recessive Crouzon-like craniosynostosis. Mol Genet Genomic Med 2013; 1:223-37; PMID:24498618; https://doi.org/10.1002/mgg3.28
- Veena KM, Jagadishchandra H, Rao PK, Chatra L. Ellis-van Creveld syndrome in an Indian child: a case report. Imaging Sci Dent 2011; 41:167-70; PMID:22232726; https://doi.org/10.5624/isd.2011.41.4.167
- Mostafa MI, Temtamy SA, el-Gammal MA, Mazen IM. Unusual pattern of inheritance and orodental changes in the Ellis-van Creveld syndrome. Genet Couns 2005; 16:75-83; PMID:15844783
- Zhang H, Takeda H, Tsuji T, Kamiya N, Kunieda T, Mochida Y, Mishina Y. Loss of Function of Evc2 in Dental Mesenchyme Leads to Hypomorphic Enamel. J Dent Res 2017; 96:421-9; PMID:28081373; https://doi.org/10.1177/0022034516683674
- Brindley HP, Archard HO, Alling CC, Jurgens PE, Jurgens EH. Case 11, Part 2. Angiokeratoma corporis diffusum (Fabry's disease). J Oral Surg 1975; 33:199-205; PMID:163304
- Goldberga M, Septiera D, Limayeb A, Sreenathb T, Kulkarni AB. Dentin and Enamel Phenotype in Fabry Mice. Oral Biosci Med 2005; 4:265-271
- da Fonseca MA, Mueller WA. Hallerman-Streiff syndrome: case report and recommendations for dental care. J Dent Child 1994; 61:334-7
- Madani M, Madani F. Gardner's syndrome presenting with dental complaints. Arch Iran Med 2007; 10:535-9; PMID:17903064
- Panjwani S, Bagewadi A, Keluskar V, Arora S. Gardner's Syndrome. J Clin Imaging Sci 2011; 1:65; PMID:22347683; https://doi.org/10.4103/2156-7514.92187
- Cobourne MT, Sharpe PT. Making up the numbers: The molecular control of mammalian dental formula. Semin Cell Dev Biol 2010; 21:314-24; PMID:20080198; https://doi.org/10.1016/j.semcdb.2010.01.007
- Galluccio G, Castellano M, La Monaca C. Genetic basis of non-syndromic anomalies of human tooth number. Arch Oral Biol 2012; 57:918-30; PMID:22325622; https://doi.org/10.1016/j.archoralbio.2012.01.005
- Jernvall J, Kettunen P, Karavanova I, Martin LB, Thesleff I. Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation: non-dividing cells express growth stimulating Fgf-4 gene. Int J Dev Biol 1994; 38:463-9; PMID:7848830
- Lagronova-Churava S, Spoutil F, Vojtechova S, Lesot H, Peterka M, Klein OD, Peterkova R. The dynamics of supernumerary tooth development are differentially regulated by Sprouty genes. J Exp Zool B Mol Dev Evol 2013; 320:307-20; PMID:23606267; https://doi.org/10.1002/jez.b.22502
- Tummers M, Thesleff I. The importance of signal pathway modulation in all aspects of tooth development. J Exp Zool B Mol Dev Evol 2009; 312B:309-19; PMID:19156667; https://doi.org/10.1002/jez.b.21280
- Kangas AT, Evans AR, Thesleff I, Jernvall J. Nonindependence of mammalian dental characters. Nature 2004; 432:211-4; PMID:15538367; https://doi.org/10.1038/nature02927
- Ohazama A, Haycraft CJ, Seppala M, Blackburn J, Ghafoor S, Cobourne M, Martinelli DC, Fan CM, Peterkova R, Lesot H, et al. Primary cilia regulate Shh activity in the control of molar tooth number. Development 2009; 136:897-903; PMID:19211681; https://doi.org/10.1242/dev.027979
- Laurikkala J, Kassai Y, Pakkasjärvi L, Thesleff I, Itoh N. Identification of a secreted BMP antagonist, ectodin, integrating BMP, FGF, and SHH signals from the tooth enamel knot. Dev Biol 2003; 264:91-105; PMID:14623234; https://doi.org/10.1016/j.ydbio.2003.08.011
- Bei M, Maas R. FGFs and BMP4 induce both Msx1-independent and Msx1-dependent signaling pathways in early tooth development. Development 1998; 125:4325-33; PMID:9753686
- Zhang Z, Lan Y, Chai Y, Jiang R. Antagonistic actions of Msx1 and Osr2 pattern mammalian teeth into a single row. Science 2009; 323:1232-4; PMID: 19251632; https://doi.org/10.1126/science.1167418
- Tucker AS, Khamis Al A, Sharpe PT. Interactions between Bmp-4 and Msx-1 act to restrict gene expression to odontogenic mesenchyme. Dev Dyn 1998; 212:533-9; PMID:9707326; https://doi.org/10.1002/(SICI)1097-0177(199808)212:4%3c533::AID-AJA6%3e3.0.CO;2-I
- Torres CBB, Alves JB, Silva GAB, Goes VS, Nakao LYS, Goes AM. Role of BMP-4 during tooth development in a model with complete dentition. Arch Oral Biol 2008; 53:2-8; PMID:17803954; https://doi.org/10.1016/j.archoralbio.2007.07.005
- Murashima-Suginami A, Takahashi K, Sakata T, Tsukamoto H, Sugai M, Yanagita M, Shimizu A, Sakurai T, Slavkin HC, Bessho K. Enhanced BMP signaling results in supernumerary tooth formation in USAG-1 deficient mouse. Biochem Bioph Res Co 2008; 369:1012-6; https://doi.org/10.1016/j.bbrc.2008.02.135
- Kiso H, Takahashi K, Saito K, Togo Y, Tsukamoto H, Huang B, Sugai M, Shimizu A, Tabata Y, Economides AN, et al. Interactions between BMP-7 and USAG-1 (uterine sensitization-associated gene-1) regulate supernumerary organ formations. PLoS One 2014; 9:e96938; PMID:24816837; https://doi.org/10.1371/journal.pone.0096938
- Kettunen P, Løes S, Furmanek T, Fjeld K, Kvinnsland IH, Behar O, Yagi T, Fujisawa H, Vainio S, Taniguchi M, et al. Coordination of trigeminal axon navigation and patterning with tooth organ formation: epithelial-mesenchymal interactions, and epithelial Wnt4 and Tgfbeta1 regulate semaphorin 3a expression in the dental mesenchyme. Development 2005; 132:323-34; PMID:15604101; https://doi.org/10.1242/dev.01541
- Porntaveetus T, Ohazama A, Choi HY, Herz J, Sharpe PT. Wnt signaling in the murine diastema. Eur J Orthod 2012; 34:518-24; PMID:21531785; https://doi.org/10.1093/ejo/cjr049
- Seidensticker MJ, Behrens J. Biochemical interactions in the wnt pathway. Biochim Biophys Acta 2000; 1495:168-82; PMID:10656974; https://doi.org/10.1016/S0167-4889(99)00158-5
- Wang X-P, O'Connell DJ, Lund JJ, Saadi I, Kuraguchi M, Turbe-Doan A, Cavallesco R, Kim H, Park PJ, Harada H, et al. Apc inhibition of Wnt signaling regulates supernumerary tooth formation during embryogenesis and throughout adulthood. Development 2009; 136:1939-49; PMID:19429790; https://doi.org/10.1242/dev.033803
- Ahn Y, Sanderson BW, Klein OD, Krumlauf R. Inhibition of Wnt signaling by Wise (Sostdc1) and negative feedback from Shh controls tooth number and patterning. Development 2010; 137:3221-31; https://doi.org/10.1242/dev.054668
- Ohazama A, Johnson EB, Ota MS, Choi HY, Choi HJ, Porntaveetus T, Oommen S, Itoh N, Eto K, Gritli-Linde A, et al. Lrp4 modulates extracellular integration of cell signaling pathways in development. PLoS One 2008; 3:e4092; https://doi.org/10.1371/journal.pone.0004092
- Blackburn J, Kawasaki K, Porntaveetus T, Kawasaki M, Otsuka-Tanaka Y, Miake Y, Ota MS, Watanabe M, Hishinuma M, Nomoto T, et al. Excess NF-κB induces ectopic odontogenesis in embryonic incisor epithelium. J Dent Res 2015; 94:121-8; PMID:25376721; https://doi.org/10.1177/0022034514556707
- Kaler P, Godasi BN, Augenlicht L, Klampfer L. The NF-κB/AKT-dependent induction of wnt signaling in colon cancer cells by Macrophages and IL-1β. Cancer Microenviron 2009; 2:69-80; PMID:19779850; https://doi.org/10.1007/s12307-009-0030-y
- Klein OD, Oberoi S, Huysseune A, Hovorakova M, Peterka M, Peterkova R. Developmental disorders of the dentition: an update. Am J Med Genet C Semin Med Genet 2013; 163C:318-32; PMID:24124058; https://doi.org/10.1002/ajmg.c.31382
- Huang B, Takahashi K, Sakata-Goto T, Kiso H, Togo Y, Saito K, Tsukamoto H, Sugai M, Akira S, Shimizu A, et al. Phenotypes of CCAAT/enhancer-binding protein beta deficiency: hyperdontia and elongated coronoid process. Oral Dis 2013; 19:144-50; PMID:22849712; https://doi.org/10.1111/j.1601-0825.2012.01963.x
- Lan Y, Jia S, Jiang R. Molecular patterning of the mammalian dentition. Semin Cell Dev Biol 2014; 25-26:61-70; PMID:24355560; https://doi.org/10.1016/j.semcdb.2013.12.003
- Klein OD, Minowada G, Peterkova R, Kangas A, Yu BD, Lesot H, Peterka M, Jernvall J, Martin GR. Sprouty genes control diastema tooth development via bidirectional antagonism of epithelial-mesenchymal FGF signaling. Dev Cell 2006; 11:181-90; PMID:16890158; https://doi.org/10.1016/j.devcel.2006.05.014
- Lochovska K, Peterkova R, Pavlikova Z, Hovorakova M. Sprouty gene dosage influences temporal-spatial dynamics of primary enamel knot formation. BMC Dev Biol 2015; 15:21; PMID:25897685; https://doi.org/10.1186/s12861-015-0070-0
- Lu Y, Li Y, Cavender AC, Wang S, Mansukhani A, D'Souza RN. Molecular studies on the roles of Runx2 and Twist1 in regulating FGF signaling. Dev Dyn 2012; 241:1708-15; PMID:22972545; https://doi.org/10.1002/dvdy.23858
- Zhou P, Byrne C, Jacobs J, Fuchs E. Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev 1995; 9:700-13; PMID:7537238; https://doi.org/10.1101/gad.9.6.700
- Kratochwil K, Galceran J, Tontsch S, Roth W, Grosschedl R. FGF4, a direct target of LEF1 and Wnt signaling, can rescue the arrest of tooth organogenesis in Lef1(-/-) mice. Genes Dev 2002; 16:3173-85; PMID:12502739; https://doi.org/10.1101/gad.1035602
- kawasaki M, Porntaveetus T, Kawasaki K, Oommen S, Otsuka-Tanaka Y, Hishinuma M, Nomoto T, Maeda T, Takubo K, Suda T, et al. R-spondins/Lgrs expression in tooth development. Dev Dyn 2014; 243:844-51; PMID:24616052; https://doi.org/10.1002/dvdy.24124
- Lee CS, Buttitta L, Fan CM. Evidence that the WNT-inducible growth arrest-specific gene 1 encodes an antagonist of sonic hedgehog signaling in the somite. Proc Natl Acad Sci USA 2001; 98:11347-52; PMID:11572986; https://doi.org/10.1073/pnas.201418298
- Cobourne MT, Miletich I, Sharpe PT. Restriction of sonic hedgehog signalling during early tooth development. Development 2004; 131:2875-85; PMID:15151988; https://doi.org/10.1242/dev.01163
- Wang XP, Aberg T, James MJ, Levanon D, Groner Y, Thesleff I. Runx2 (Cbfa1) inhibits Shh signaling in the lower but not upper molars of mouse embryos and prevents the budding of putative successional teeth. J Dent Res 2005; 84:138-43; PMID:15668330; https://doi.org/10.1177/154405910508400206
- Pummila M, Fliniaux I, Jaatinen R, James MJ, Laurikkala J, Schneider P, Thesleff I, Mikkola ML. Ectodysplasin has a dual role in ectodermal organogenesis: inhibition of Bmp activity and induction of Shh expression. Development 2007; 134:117-25; PMID:17164417; https://doi.org/10.1242/dev.02708
- Fujimori S, Novak H, Weissenböck M, Jussila M, Gonçalves A, Zeller R, Galloway J, Thesleff I, Hartmann C. Wnt/β-catenin signaling in the dental mesenchyme regulates incisor development by regulating Bmp4. Dev Biol 2010; 348:97-106; PMID:20883686; https://doi.org/10.1016/j.ydbio.2010.09.009
- Ibarretxe G, Aurrekoetxea M, Crende O, Badiola I, Jimenez-Rojo L, Nakamura T, Yamada Y, Unda F. Epiprofin/Sp6 regulates Wnt-BMP signaling and the establishment of cellular junctions during the bell stage of tooth development. Cell Tissue Res 2012; 350:95-107; PMID:22868911; https://doi.org/10.1007/s00441-012-1459-8
- Lei H-H, LIU HE, Ge LH. PAX6 polymorphisms in 20 Chinese children with supernumerary teeth in the maxillary incisor area. Int J Paediatr Dent 2011; 21:271-7; PMID:21348901; https://doi.org/10.1111/j.1365-263X.2011.01119.x
- Tian Y, Ma P, Liu C, Yang X, Crawford DM, Yan W, Bai D, Qin C, Wang X. Inactivation of Fam20B in the dental epithelium of mice leads to supernumerary incisors. Eur J Oral Sci 2015; 123:396-402; PMID:26465965; https://doi.org/10.1111/eos.12222
- Lei HH, Liu H, Ge LH. PAX6 polymorphisms in 20 Chinese children with supernumerary teeth in the maxillary incisor area. Int J Paediatr Dent 2011; 21:271-7; PMID:21348901; https://doi.org/10.1111/j.1365-263X.2011.01119.x
- Massink MPG, Créton MA, Spanevello F, Fennis WMM, Cune MS, Savelberg SMC, Nijman IJ, Maurice MM, van den Boogaard M-JH, van Haaften G. Loss-of-Function Mutations in the WNT Co-receptor LRP6 cause Autosomal-Dominant Oligodontia. Am J Hum Genet 2015; 97:621-6; PMID:26387593; https://doi.org/10.1016/j.ajhg.2015.08.014
- Takahashi M, Hosomichi K, Yamaguchi T, Yano K, Funatsu T, Adel M, Haga S, Maki K, Tajima A. Whole-exome sequencing analysis of supernumerary teeth occurrence in Japanese individuals. Hum Gen Variation 2017; 4:16046-4; https://doi.org/10.1038/hgv.2016.46
- Minić S, Trpinac D, Gabriel H, Gencik M, Obradović M. Dental and oral anomalies in incontinentia pigmenti: a systematic review. Clin Oral Investig 2013; 17:1-8; PMID:22453515; https://doi.org/10.1007/s00784-012-0721-5
- Mundlos S. Cleidocranial dysplasia: clinical and molecular genetics. J Med Genet 1999; 36:177-82; PMID:10204840
- Melamed Y, Barkai G, Frydman M. Multiple supernumerary teeth (MSNT) and Ehlers-Danlos syndrome (EDS): a case report. J Oral Pathol Med 1994; 23:88-91; PMID:8164160; https://doi.org/10.1111/j.1600-0714.1994.tb00263.x
- Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M. Identification and characterization of the familial adenomatous polyposis coli gene. Cell 1991; 66:589-600; PMID:1651174; https://doi.org/10.1016/0092-8674(81)90021-0
- Burdon KP, McKay JD, Sale MM, Russell-Eggitt IM, Mackey DA, Wirth MG, Elder JE, Nicoll A, Clarke MP, FitzGerald LM, et al. Mutations in a novel gene, NHS, cause the pleiotropic effects of Nance-Horan syndrome, including severe congenital cataract, dental anomalies, and mental retardation. Am J Hum Genet 2003; 73:1120-30; PMID:14564667; https://doi.org/10.1086/379381
- Ferrante MI, Giorgio G, Feather SA, Bulfone A, Wright V, Ghiani M, Selicorni A, Gammaro L, Scolari F, Woolf AS, et al. Identification of the gene for oral-facial-digital type I syndrome. Am J Hum Genet 2001; 68:569-76; PMID:11179005; https://doi.org/10.1086/318802
- Kitao S, Lindor NM, Shiratori M, Furuichi Y, Shimamoto A. Rothmund-thomson syndrome responsible gene, RECQL4: genomic structure and products. Genomics 1999; 61:268-76; PMID:10552928; https://doi.org/10.1006/geno.1999.5959
- Seppala M, Depew MJ, Martinelli DC, Fan C-M, Sharpe PT, Cobourne MT. Gas1 is a modifier for holoprosencephaly and genetically interacts with sonic hedgehog. J Clin Invest 2007; 117:1575-84; PMID:17525797; https://doi.org/10.1172/JCI32032
- Zhang Q, Murcia NS, Chittenden LR, Richards WG, Michaud EJ, Woychik RP, Yoder BK. Loss of the Tg737 protein results in skeletal patterning defects. Dev Dyn 2003; 227:78-90; PMID:12701101; https://doi.org/10.1002/dvdy.10289
- Nakamura T, de-Vega S, Fukumoto S, Jimenez L, Unda F, Yamada Y. Transcription factor epiprofin is essential for tooth morphogenesis by regulating epithelial cell fate and tooth number. J Biol Chem 2008; 283:4825-33; PMID:18156176; https://doi.org/10.1074/jbc.M708388200
- Kuraguchi M, Wang X-P, Bronson RT, Rothenberg R, Ohene-Baah NY, Lund JJ, Kucherlapati M, Maas RL, Kucherlapati R. Adenomatous polyposis coli (APC) is required for normal development of skin and thymus. PLoS Genet 2006; 2:e146; PMID:17002498; https://doi.org/10.1371/journal.pgen.0020146
- Järvinen E, Salazar-Ciudad I, Birchmeier W, Taketo MM, Jernvall J, Thesleff I. Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc Natl Acad Sci USA 2006; 103:18627-32; PMID:17121988; https://doi.org/10.1073/pnas.0607289103
- Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, Yang SH, Lu M-M, Piccolo S, Schmidt-Ullrich R, et al. Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol 2008; 313:210-24; PMID:18022614; https://doi.org/10.1016/j.ydbio.2007.10.016
- Peterkova R, Churava S, Lesot H, Rothova M, Prochazka J, Peterka M, Klein OD. Revitalization of a diastemal tooth primordium in Spry2 null mice results from increased proliferation and decreased apoptosis. J Exp Zool B Mol Dev Evol 2009; 312B:292-308; PMID:19127536; https://doi.org/10.1002/jez.b.21266
- Munne PM, Tummers M, Järvinen E, Thesleff I, Jernvall J. Tinkering with the inductive mesenchyme: Sostdc1 uncovers the role of dental mesenchyme in limiting tooth induction. Development 2009; 136:393-402; PMID:19141669; https://doi.org/10.1242/dev.025064
- Mustonen T, Pispa J, Mikkola ML, Pummila M, Kangas AT, Pakkasjärvi L, Jaatinen R, Thesleff I. Stimulation of ectodermal organ development by Ectodysplasin-A1. Dev Biol 2003; 259:123-36; PMID:12812793; https://doi.org/10.1016/S0012-1606(03)00157-X
- Tucker AS, Headon DJ, Courtney JM, Overbeek P, Sharpe PT. The activation level of the TNF family receptor, Edar, determines cusp number and tooth number during tooth development. Dev Biol 2004; 268:185-94; PMID:15031115; https://doi.org/10.1016/j.ydbio.2003.12.019
- Peterkova R, Lesot H, Viriot L, Peterka M. The supernumerary cheek tooth in tabby/EDA mice-a reminiscence of the premolar in mouse ancestors. Arch Oral Biol 2005; 50:219-25; PMID:15721153; https://doi.org/10.1016/j.archoralbio.2004.10.020
- Kaufman MH, Chang HH, Shaw JP. Craniofacial abnormalities in homozygous Small eye (Sey/Sey) embryos and newborn mice. J Anat 1995; 186(Pt 3):607-17; PMID:7559133
- Sofaer JA. Aspects of the tabby-crinkled-downless syndrome. I. The development of tabby teeth. J Embryol Exp Morphol 1969; 22:181-205; PMID:5361554
- Danforth CH. The occurrence and genetic behavior of duplicate lower incisors in the mouse. Genetics 1958; 43:139-48; PMID:17247738