ABSTRACT
Angiogenesis is a physiological procedure during which the new blood vessels develop from the pre-existing vessels. Uncontrolled angiogenesis is related to various diseases including cancers. Clinical inhibition of undesired angiogenesis is still under investigation. We utilized nicotinic acid, a family member of the B-vitamin niacin (vitamin B3) that has been used in the prevention and treatment of atherosclerosis or other lipid-metabolic disorders, to treat human umbilical vein endothelial cells (HUVECs) and chick chorioallantoic membrane (CAM), and investigated its influence on angiogenesis in vitro and in vivo. We found that nicotinic acid could obviously inhibit HUVEC proliferation induced by vascular endothelial growth factor. Both the in vitro and in vivo assays showed that nicotinic acid could significantly inhibit the process of angiogenesis. To further investigate the mechanism underlying the effect of nicotinic acid on angiogenesis, we found that it might function via regulating the cytoskeleton arrangements, especially the rearranging the structures of F-actin and paxillin. In summary, we discovered that nicotinic acid could obviously inhibit the process of angiogenesis by changing the angiogenesis factor expression levels and inducing the cytoskeleton rearrangement of endothelial cells.
INTRODUCTION
Angiogenesis is a physiological procedure during which the new blood vessels develop from the pre-existing vessels.Citation1,2 Angiogenesis differs from vasculogenesis, which defines the de novo formation of endothelial cells and neovascularization,Citation3 and is the first vessels in the developing embryo. While angiogenesis is responsible for most vascular growth during embryonic development and in diseases,Citation4 the first identified form of angiogenesis is sprouting angiogenesis, which occurs in different stages. Endothelial cells present in pre-existing blood vessels express angiogenic growth factor receptors, which could be activated by angiogenic growth factors acting as biological signals. By binding the ligands, the angiogenic growth factor receptors activate the downstream pathways to activate endothelial cells. The activated endothelial cells then release proteases degrading the basement membrane to facilitate the migration of endothelial cells though the original vessel walls in order to proliferate in the extracelluar matrix and form solid sprouts connecting the surrounding vessels. Upon angiogenic stimulus, endothelial cells use integrins to maintain cell-cell adhesion and migrate in sequence.
Cancer cells have the ability to undergo uncontrolled proliferation, thus tumors need sophisticated blood vessel system for the oxygen and nutrient supply to facilitate their rapid growth.Citation5,6 Normally, tumors secret growth factors such as fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF) to promote angiogenesis. Different from the normal blood vessels, tumor blood vessels are irregularly shaped.Citation7 VEGF plays an essential role in tumor angiogenesis. It was reported that the anti-VEGF enzyme PKG, which functions to restrict β-catenin that promotes angiogenesis, was inhibited in cancer cells. Metastatic tumor cells could depart from the original solid tumor, invade into the blood vessel and migrate to a distant site, where they can initiate a secondary tumor. It has been reported that in solid tumor, blood vessel contains both endothelial cells and tumor cells, and this mosaic construction allows circulating cancer cells to shed into the blood vascular system and results in the malignancies.Citation8
As blood vessels are indispensable for tumor growth and metastasis, and endothelial cells are widely considered genetically more stable than tumor cells, it is theoretically possible to target angiogenesis for anti-tumor therapy. In this context, endothelial cells could be an optimal target for cancer therapies.Citation9 It has been reported that in mouse xenografts, targeting tumor stem cell-derived endothelial cells could result in reduction of tumor.Citation10 The potential of targeting angiogenesis in tumor therapy suggests the importance of developing efficient method to inhibit the growth of tumor blood vessels.
Nicotinic acid is the common form of the B-vitamin niacin (vitamin B3). As it has the ability to reduce the plasma level of low-density lipoprotein cholesterol, nicotinic acid has been used in the prevention and treatment of atherosclerosis or other lipid-metabolic disorders.Citation11,12 However, its side effect, mainly cutaneous flushing, has been recently recognized, while its effects in other aspects of physiology or cell biology remain unclear.
Previous studies have showed that nicotinic acid regulates the intracellular calcium levels in a concentration and exposure time-dependent manner. They reported that a relatively high concentration of nicotinic acid could result in cytoskeleton disassembly by inducing β-tubulin degradation. As the cytoskeleton plays an important role in cell migration, the disassembly of cytoskeleton by nicotinic acid might interrupt the metastasis process of tumor development. This indicated that nicotinic acid has promising potential for clinical therapy of various diseases in the future.Citation13
To investigate the effects of nicotinic acid on cell proliferation and angiogenesis induced by VEGF, human umbilical vein endothelial cells (HUVEC) and chick chorioallantoic membrane (CAM) were used in the current study. HUVECs are cells derived from the endothelium of veins from the umbilical cord. They are very suitable for the purpose of our study, as they are widely used as a laboratory model for the study of the pathology and function of endothelial cells (e.g., angiogenesis).
RESULTS
Nicotinic acid inhibits HUVEC proliferation induced by VEGF
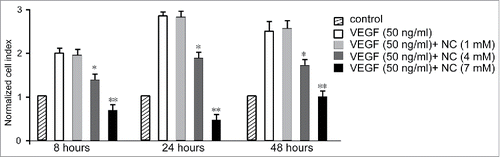
To explore the effects of nicotinic acid on VEGF-induced HUVEC proliferation, we co-administrated HUVEC with 50 ng/ml VEGF, and increasing concentrations of nicotinic acid (1, 4 and 7 mM). By investigating the cell proliferation at 8, 24, and 48 hours after treatment, we found that the cell proliferation inhibition was positively related to the concentration of nicotinic acid (). 7 mM nicotinic acid induced the most significant cell proliferation inhibition, while cells treated with 1 mM nicotinic acid showed no inhibition of cell proliferation.
Nicotinic acid inhibits HUVEC migration
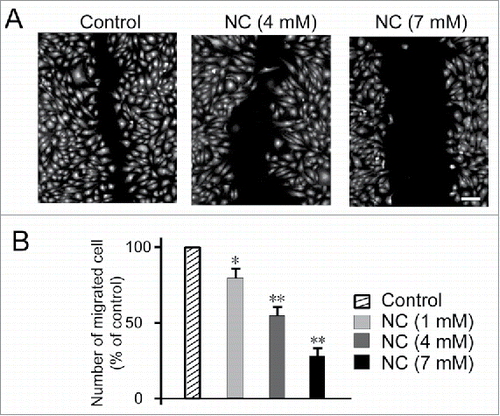
Next, we conducted the scratch-wound healing assay to study whether nicotinic acid could also exhibit effect on HUVEC migration. We wounded the confluent monolayer of HUVEC and treated them with either nicotinic acid (1, 4, 7 mM) or medium alone (untreated control) (). After 8 h exposure to indicated concentrations of nicotinic acid, we found that, when treated with 7 mM nicotinic acid, cells showed lowest migration ability ().
FIGURE 2. Effects of NC on HUVECs migratory ability as determined by scratch-wound assay. (A) Confluent monolayer of HUVECs was wounded and treated with either NC (1, 4, 7 mM) or medium alone (untreated control) for 8 h. The cells were then fixed and stained with Hoechst 33342 and Cellomics® whole cell stain green (B) Quantification of the number of migrated cells after 8 h exposure to indicated concentrations of NC. For each monolayer sample, three measurements were taken in three independent wounds. Percentage of inhibition was expressed using untreated wells at 100%. Data are expressed as means ± SD of three independent experiments. Statistical significance is expressed as **, P<0.001; *, P<0.05 versus untreated control. Scale bar indicates 200 µm.
VEGF-induced angiogenesis in vivo is attenuated by nicotinic acid
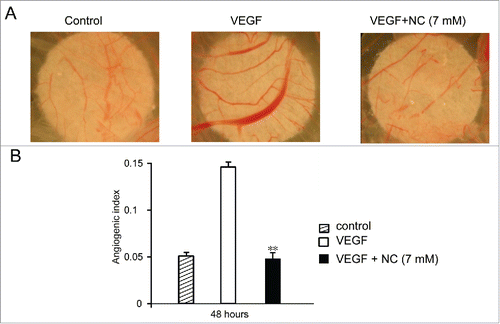
We then conducted the chick chorioallantoic membrane (CAM) assay to investigate the effect of nicotinic acid on angiogenesis in vivo. The fertilized eggs were incubated and windowed. On day 7 of development, VEGF, with or without 7 mM nicotinic acid was applied to the CAMs, and the treated CAMs were photographed 48 hours later (). Consistent with the hypothesis, VEGF significantly promoted the vascular growth of CAMs, which showed obvious overdevelopment of blood vessels. However, when the CAMs were administrated with both VEGF and nicotinic acid, they showed similar vascular conditions as the vehicle control CAMs (). The above results indicated that nicotinic acid was able to inhibit vascular development in vivo.
FIGURE 3. VEGF-induced angiogenesis in vivo is attenuated by NC. Fertilized eggs were incubated and windowed. On day 7 of development sterile filters soaked with vehicle or VEGF in the absence or presence of NC were applied to the CAMs (see Methods) which were photographed 48 hours later; representative images are shown. Pooled data from 12–15 eggs per treatment are given as angiogenic index (mean ± SD). Statistical significance is expressed as **, P<0.001 versus VEGF treated.
Nicotinic acid inhibits VEGF-induced angiogenesis through decreasing transcription of angiogenesis factors and interrupting the cytoskeleton
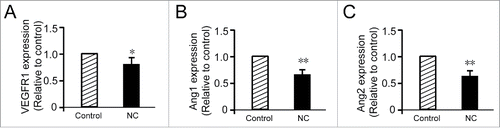
As we have demonstrated that nicotinic acid could inhibit vascular development both in vitro and in vivo, we further investigated the mechanisms of nicotinic acid functions. We examined the mRNA expression levels of several angiogenesis factors in HUVEC cells, and found a dramatic decrease in the transcriptional levels of the VEGF receptor-1 (VEGFR1) and angiopoietins Ang1, Ang2, upon the treatment of 7 mM nicotinic acid ().
FIGURE 4. NC decreases VEGFR1 and angiopoietins (Ang1, Ang2) gene transcription. (A) RT-PCR analysis of VEGFR1 demonstrated that the treatment with 7 mM NC (48 h) reduces VEGFR1 mRNA in HUVEC. Data are expressed as fold increase versus control cells treated with PBS only and are the mean ± SD of three experiments. NC significantly decreased Ang1 (B) and Ang2 (C) gene expression. The data are representative of three independent experiments performed in triplicate. Statistical significance is expressed as **, P<0.001; *, P<0.05 versus untreated control.
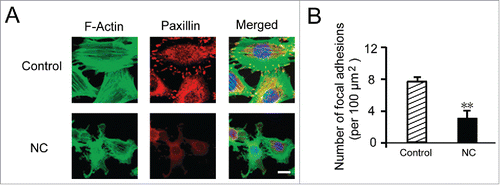
It has been reported that nicotinic acid could affect the cytoskeleton, we next conducted experiment to study the changes in cytoskeleton upon administration of nicotinic acid. We treated the HUVEC with nicotinic acid or medium alone for 16 hours, and then fixed the cells and stained F-actin and paxillin with fluorescent dyes (). The F-actin and paxillin were observed by microscopy, and the adhesion sites were analyzed. We found that both F-actin and paxillin expression levels were decreased upon nicotinic acid treatment compared to the control group. Furthermore, the cell adhesion sites of nicotinic acid treated group were significantly reduced compared to the control group (). This indicated that nicotinic acid could interrupt the cytoskeletal system in the HUVEC.
FIGURE 5. Effects of NC on cytoskeletal system of HUVECs. (A) HUVECs were treated with 7 mM NC or medium alone (untreated control) for 16 h. HUVECs were fixed and stained with phalloidin for F-actin, and anti-paxillin antibody for paxillin, respectively. (B) Number of adhesion sites were analyzed. Data are expressed as means ± SD of three independent experiments. Statistical significance is expressed as **, P<0.001; versus untreated control. Scale bar indicates 50 µm.
DISCUSSION
Tumors require a sophisticated blood vessel system to provide tumor cells with oxygen and other nutrients. Thus, tumor cells constantly release diverse growth factors, such as VEGF and FGF, which could induce angiogenesis. It was reported that expression of PKG, an anti-VEGF enzyme, was downregulated in tumor cells. While in normal cells, PKG functions to inhibit angiogenesis by limiting the expression of β-catenin. It has been postulated that targeting angiogenesis could stop or delay tumor progress. Various studies have since focused on investigating the underlying mechanisms and discovering novel drugs to inhibit tumor associated angiogenesis.
Various anti-tumor drugs targeting angiogenesis have been approved in clinical therapies. Bevacizumab, a humanized monoclonal antibody against VEGF-A, is the first approved anti-angiogenesis drug for colorectal cancer (CRC) therapy, and has been shown to be clinically beneficial in cooperation with first- and second-line chemotherapy.Citation14-19 The ML18147 study first reported the continuation of bevacizumab on standard bevacizumab-based first-line therapy.Citation19 Studies have also shown that after induction chemotherapy, bevacizumab-dependent maintenance treatment with a fluoropyrimidine was effective for cancer patients.Citation20,21 In CRC, the standard chemotherapy plus inhibition of VEGF with bevacizumab have exhibited significant clinical efficacy, and this application has now been expanded to other solid tumor types.
In addition, there are other compounds targeting angiogenesis used in clinical trials. For example, Ziv-aflibercept is a soluble fusion protein which efficiently targets VEGF and placental growth factor (PlGF). For CRC patients who acquire resistance to chemotherapy, Ziv-aflibercept is approved to be used in combination with FOLFIRI.Citation22 Ramucirumab is a human VEGFR-2 monoclonal antibody, and has been used to treat CRC patients with FOLFIRI, and study showed that ramucirumab together with FOLFIRI could dramatically increase overall survival of patients.Citation23
Furthermore studies have shown that angiogenesis is associated with innate immunity. Development processes, such as embryogenesis, requires the generation of new blood vessels.Citation24 Under stress conditions such as tissue injury, functions including reconstructing blood flow at the injured site, initiating the innate immune response to pathogens, and repairing the wound are essential.Citation25 These processes require close cooperation between the angiogenesis and innate immunity. For example, NOD-like receptors (NLRs) function in tumorigenesis, development and pathology of various cancers such as colon cancer, lung cancer, breast cancer, prostate cancer, glioblastoma and melanoma. The downstream components of NLR signaling pathway, such as caspase-1, IL-18 and IL-1β, are able to directly or indirectly regulate angiogenesis by affecting the expression levels of angiogenesis factors including Ang1, Ang2, HIF-1α, FGF and VEGF.Citation26 In line with the above notion, anti-angiogenesis therapies by modulating innate immunity in combination with other therapies are presently utilized for glioma treatment.Citation27,28 These above studies have indicated the importance of inhibiting tumor angiogenesis.
In our study, we aimed to use nicotinic acid to inhibit angiogenesis and investigated the underlying mechanisms. Nicotinic acid is a member of the water-soluble vitamin B family, and has the ability to reduce plasma lipids. Nicotinic acid has been widely used to prevent and treat atherosclerosis.Citation11,12 Recently, nicotinic acid has been reported to efficiently protect against cardiovascular disease.Citation29 Although nicotinic acid has been wildly studied and utilized in the field of lipid metabolism, its functions on the other cellular biology remain unclear.
In the present study, we first found that inhibition in HUVEC proliferation was positively related to the concentration of nicotinic acid. 7 mM nicotinic acid resulted in the most dramatic cell proliferation inhibition, whereas cells treated with 1 mM nicotinic acid displayed no inhibition in cell proliferation. The scratch-wound healing assay showed that, when treated with 7 mM nicotinic acid, cells showed lowest migration ability. These results have indicated that nicotinic acid is able to inhibit the growth, proliferation and metastasis of HUVEC. The CAM assay also indicated that VEGF significantly promoted the vascular growth of CAMs, which showed obvious overdevelopment of blood vessel. However, when the CAMs were administrated with both VEGF and nicotinic acid, they showed similar vascular conditions as the vehicle control CAMs, indicating that nicotinic acid has the ability to inhibit vascular development in vivo.
Previous studies have shown that nicotinic acid could affect the intracellular calcium levels in dose and time-dependent manners. They found that high concentration of nicotinic acid could result in the disassembly of cytoskeleton and induce degradation of β-tubulin. As the cytoskeleton plays an important role in cell migration, the disassembly of cytoskeleton by nicotinic acid might interrupt the metastasis process in tumor development.Citation13 In our study, we found that nicotinic acid inhibited VEGF-induced angiogenesis through repressing transcription of angiogenesis factors such as VEGFR1, Ang1 and Ang2, and interrupting the cytoskeleton by reducing expressions of F-actin and paxillin in HUVEC.
In conclusion, we hereby report that nicotinic acid could inhibit angiogenesis by changing the expressions of angiogenesis factors and inducing the cytoskeleton rearrangement in endothelial cells. Our results indicate that nicotinic acid might be a potent agent to exert its functions in anti-tumor therapy by targeting angiogenesis.
METHODS
Cell culture
HUVECs were purchased from ScienCell (CA, USA), while the human hepatic epithelial cell line (WRL-68) and human fibroblast-like fetal lung cells (WI-38) were purchased from American Type Culture Collection (ATCC; VA, USA). HUVECs were cultured in Endothelial Cell Medium (ECM; ScienCell, USA) supplemented with 5% heat-inactivated fetal bovine serum (FBS; ScienCell, USA), 1% penicillin/streptomycin (ScienCell, USA) and 1% Endothelial Cell Growth Supplement (ECGS; ScienCell, USA). WI-38 and WRL-68 were maintained in Dulbecco's Modified Eagle Medium (DMEM; Gibco, CA, USA) and Roswell Park Memorial Institute medium 1640 (RPMI; Gibco, USA), respectively, supplemented with 10% heat inactivated FBS (Sigma-Aldrich, MO, USA) and 1% penicillin/streptomycin (Gibco, USA). All cells were incubated at 37°C in humidified 5% CO2, 95% air. Nicotinic acid was dissolved in culture medium directly, and culture medium group was employed as untreated control.
Scratch-wound directional migration assay
HUVECs were seeded at the cell density of 1 × 105 cells/well in a 96-well microtiter plate and allowed to grow into a confluent monolayer overnight. Then, the monolayer was scraped using a sterile 20–200 µl micropipette tip to create a wound of ±1 mm width. The cells were washed twice with Hanks’ Balanced Salt Solution (HBSS; Sigma-Aldrich, USA) and replaced with fresh medium supplied with indicated concentrations of NC. After 8 h, the cells were stained with Hoechst 33342 and Cellomics® whole cell stain green (Thermo Fisher Scientific, Waltham, MA, USA). Cell migration was estimated by measuring the number of endothelial cells that had migrated from the edge of the wounded monolayer. An area of 512 × 512 pixels of the wounded area was acquired using Cellomics Array Scan HCS Reader and the number of migrated cells was calculated by the HCS automated algorithm. Inhibition of migration was represented by a decrease in the number of cells in the image acquired relative to the untreated control. For each monolayer sample, three measurements were taken for three independent wounds.
CAM assay
Fertilized white Leghorn eggs were incubated at 37°C in a humidified incubator and windowed. On day 7 of development, sterile filters soaked with either vehicle or VEGF (100 ng/disk) in the presence or absence of NC (9 µg/disk) were applied to relatively avascular regions of the CAM. CAMs were fixed (4% paraformaldehyde in PBS) in vivo on day 9 and photographed in the localized area of the filter. The newly capillarised area in the region of each filter was quantified using Leica QWin Lite software and neovascularisation is expressed as an angiogenic index (n = 12–15 eggs per treatment).
Real-time PCR
HUVEC were treated with 7 mM NC for 16 h, PBS was used as control. Brief, RNA extraction from sub-confluent treated or non-treated cells was performed using 1.0 ml of Trizol (Invitrogen, Carlsbad, CA, USA) for each Huvecs 1 × 106 cells of sample according to manufacturer's recommendation. RNA integrity was assayed by agarose gel electrophoresis and treated with DNAse (RQ1 RNAse free DNAse – Promega, Madison, WI, USA). cDNA and PCR were performed using SuperScript III Platinum one-step qRT-PCR Systems (Invitrogen, USA). Gene expression was measured in 7500 Fast (Applied Biosystems, Waltham, MA, USA) using GAPDH (Hs99999905_m1) as endogenous gene. Taqman gene expression assay from Applied Biosystems were performed for VEGFR1 and Ang1/2 genes, respectively.
Immunocytofluorescence study
The effects of PA on the actin and tubulin cytoskeletal systems of HUVECs were investigated by immunofluorescence. Briefly, HUVECs at ∼80% confluency were treated with PA for 16 h and stained with phalloidin (Abcam, Cambridge, MA) for F-actin and anti-paxillin antibody (Abcam) for paxillin, respectively. Images were acquired on conventional fluorescence microscope and the effects on F-actin and paxillin were analyzed by Morphology BioApplication Algorithm (Thermo Fisher Scientific, USA).
Statistics
Data were presented as mean ± SD. Data were analyzed for statistical significance by Prism. One-way or two way ANOVA analysis followed by Tukey's tests were performed as appropriate, with significances when p value was less than 0.05.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
FUNDING
This study was supported by a grant from Natural Science Foundation of Zhejiang Province of China (LY16H160048).
REFERENCES
- Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Pericytes at the intersection between tissue regeneration and pathology. Clin Sci (Lond). 2015;128(2):81-93; https://doi.org/10.1042/CS20140278
- Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A, Delbono O. Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol. 2014; 307(1):C25-38; https://doi.org/10.1152/ajpcell.00084.2014
- Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;1173-91.
- Flamme I, Frolich T, Risau W. Molecular mechanisms of vasculogenesis and embryonic angiogenesis. J Cell Physiol. 1997; 173(2):206-10; https://doi.org/10.1002/(SICI)1097-4652(199711)173:2%3c206::AID-JCP22%3e3.0.CO;2-C
- McDougall SR, Anderson AR, Chaplain MA. Mathematical modelling of dynamic adaptive tumour-induced angiogenesis: clinical implications and therapeutic targeting strategies. J Theor Biol. 2006; 241(3):564-89; https://doi.org/10.1016/j.jtbi.2005.12.022
- Spill F, Guerrero P, Alarcon T, Maini PK, Byrne HM. Mesoscopic and continuum modelling of angiogenesis. J Math Biol. 2015; 70(3):485-532; https://doi.org/10.1007/s00285-014-0771-1
- Khurana R, Simons M. Insights from angiogenesis trials using fibroblast growth factor for advanced arteriosclerotic disease. Trends Cardiovasc Med. 2003; 13(3):116-22; https://doi.org/10.1016/S1050-1738(02)00259-1
- Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004; 10(20):6897-904; https://doi.org/10.1158/1078-0432.CCR-04-0378
- Bagri A, Kouros-Mehr H, Leong KG, Plowman GD. Use of anti-VEGF adjuvant therapy in cancer: Challenges and rationale. Trends Mol Med. 2010; 16(3):122-32; https://doi.org/10.1016/j.molmed.2010.01.004
- Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G, Larocca LM, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010; 468(7325):824-8; https://doi.org/10.1038/nature09557
- Carlson LA. Nicotinic acid: The broad-spectrum lipid drug. A 50th anniversary review. J Intern Med. 2005; 258(2):94-114; https://doi.org/10.1111/j.1365-2796.2005.01528.x
- Figge HL, Figge J, Souney PF, Mutnick AH, Sacks F. Nicotinic acid: A review of its clinical use in the treatment of lipid disorders. Pharmacotherapy. 1988; 8(5):287-94; https://doi.org/10.1002/j.1875-9114.1988.tb04085.x
- Li J, Li Y, Zhang P, Niu H, Shi Y. Nicotinic acid modulates intracellular calcium concentration and disassembles the cytoskeleton. Mol Med Rep. 2014; 10(6):2805-10; https://doi.org/10.3892/mmr.2014.2576
- Cao Y, Tan A, Gao F, Liu L, Liao C, Mo Z. A meta-analysis of randomized controlled trials comparing chemotherapy plus bevacizumab with chemotherapy alone in metastatic colorectal cancer. Int J Colorectal Dis. 2009; 24(6):677-85; https://doi.org/10.1007/s00384-009-0655-9
- Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, Wong L, Fehrenbacher L, Abubakr Y, Saif MW, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol. 2008; 26(21):3523-9; https://doi.org/10.1200/JCO.2007.15.4138
- Petrelli F, Borgonovo K, Cabiddu M, Ghilardi M, Lonati V, Maspero F, Sauta MG, Beretta GD, Barni S. FOLFIRI-bevacizumab as first-line chemotherapy in 3500 patients with advanced colorectal cancer: A pooled analysis of 29 published trials. Clin Colorectal Cancer. 2013; 12(3):145-51; https://doi.org/10.1016/j.clcc.2013.04.006
- Komatsu Y, Ishioka C, Shimada K, Yamada Y, Gamoh M, Sato A, Yamaguchi T, Yuki S, Morita S, Takahashi S, et al. Study protocol of the TRICOLORE trial: A randomized phase III study of oxaliplatin-based chemotherapy versus combination chemotherapy with S-1, irinotecan, and bevacizumab as first-line therapy for metastatic colorectal cancer. BMC Cancer. 2015; 15:626; https://doi.org/10.1186/s12885-015-1630-1
- Cunningham D, Lang I, Marcuello E, Lorusso V, Ocvirk J, Shin DB, Jonker D, Osborne S, Andre N, Waterkamp D, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol. 2013; 14(11):1077-85; https://doi.org/10.1016/S1470-2045(13)70154-2
- Bennouna J, Sastre J, Arnold D, Osterlund P, Greil R, Van Cutsem E, von Moos R, Vieitez JM, Bouche O, Borg C, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol. 2013; 14(1):29-37; https://doi.org/10.1016/S1470-2045(12)70477-1
- Diaz-Rubio E, Gomez-Espana A, Massuti B, Sastre J, Abad A, Valladares M, Rivera F, Safont MJ, Martinez de Prado P, Gallen M, et al. First-line XELOX plus bevacizumab followed by XELOX plus bevacizumab or single-agent bevacizumab as maintenance therapy in patients with metastatic colorectal cancer: The phase III MACRO TTD study. Oncologist. 2012; 17(1):15-25; https://doi.org/10.1634/theoncologist.2011-0249
- Simkens LH, van Tinteren H, May A, ten Tije AJ, Creemers GJ, Loosveld OJ, de Jongh FE, Erdkamp FL, Erjavec Z, van der Torren AM, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): A phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015; 385(9980):1843-52; https://doi.org/10.1016/S0140-6736(14)62004-3
- Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausova J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko V, Ferry D, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012; 30(28):3499-506; https://doi.org/10.1200/JCO.2012.42.8201
- Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouche O, Mineur L, Barone C, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013; 381(9863):303-12; https://doi.org/10.1016/S0140-6736(12)61900-X
- Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993; 362(6423):841-4; https://doi.org/10.1038/362841a0
- Frantz S, Vincent KA, Feron O, Kelly RA. Innate immunity and angiogenesis. Circ Res. 2005; 96(1):15-26; https://doi.org/10.1161/01.RES.0000153188.68898.ac
- Saxena S, Jha S. Role of NOD- like receptors in glioma angiogenesis: insights into future therapeutic interventions. Cytokine Growth Factor Rev. 2017; 34:15-26; https://doi.org/10.1016/j.cytogfr.2017.02.001
- Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007; 8(8):610-22; https://doi.org/10.1038/nrn2175
- Multhoff G, Radons J, Vaupel P. Critical role of aberrant angiogenesis in the development of tumor hypoxia and associated radioresistance. Cancers (Basel). 2014; 6(2):813-28; https://doi.org/10.3390/cancers6020813
- Offermanns S. The nicotinic acid receptor GPR109A (HM74A or PUMA-G) as a new therapeutic target. Trends Pharmacol Sci. 2006; 27(7):384-90; https://doi.org/10.1016/j.tips.2006.05.008