ABSTRACT
EphB and their ligands ephrin-B are an important family of protein tyrosine kinase receptors involved in thymocyte-thymic epithelial cell interactions known to be key for the maturation of both thymic cell components. In the present study, we have analyzed the maturation of cortical thymic epithelium in EphB-deficient thymuses evaluating the relative relevance of EphB2 and EphB3 in the process. Results support a relationship between the epithelial hypocellularity of mutant thymuses and altered development of thymocytes, lower proportions of cycling thymic epithelial cells and increased epithelial cell apoptosis. Together, these factors induce delayed development of mutant cortical TECs, defined by the expression of different cell markers, i.e. Ly51, CD205, MHCII, CD40 and β5t. Furthermore, although both EphB2 and EphB3 are necessary for cortical thymic epithelial maturation, the relevance of EphB3 is greater since EphB3−/− thymic cortex exhibits a more severe phenotype than that of EphB2-deficient thymuses.
INTRODUCTION
The thymus is a primary lymphoid organ in which bone marrow-derived lymphoid progenitors differentiate phenotypically and functionally into single positive (SP) (both CD4+CD8− and CD4−CD8+) thymocytes in a complex process in which thymic epithelial cells (TECs) play an essential role.Citation1 The organ consists of two compartments: an outer region, the cortex, which mainly contains immature DN (CD4−CD8−) lymphoid cells and DP (CD4+CD8+) thymocytes, and an inner region, the medulla, which contains mature SP cells.
The thymus derives embryologically from the third pharyngeal pouch which gives rise to a common primordium which contains parathyroid and thymus tissue at around 10–11 days post coitum (E).Citation2 At E12, the thymic area is individualized from the parathyroid, lymphoid precursors coming from the fetal liver seed the thymic primordium, and a three-dimensional epithelial network begins to be organized.Citation3 The maturation of epithelial phenotype from the appearance of the first epithelial cells and the mechanisms determining the cortex-medulla differentiation are not conclusively known, but there is a general consensus that the lymphoid progenitor cell seeding and the increased expression of the transcriptional factor, FoxN1, are essential for both processes.Citation4
Together with some transcriptional factors (i.e., Tbx1, Pax1, Pax3, Pax9, Hoxa3, Eya1, Six1)Citation5–8 that seem to govern the early stages of thymic development before FoxN1 appearance, several morphogens are involved in the maturation of pharyngeal endoderm to thymic primordium, including BMP4,Citation2,9 ShhCitation10 and Wnt,Citation11,12 both governing FoxN1 expression. Later, arrival of the first lymphoid progenitors into the thymus and the establishment of early thymocyte-TEC interactions affect the development of immature MTS20+ TECs,Citation13 although other studies have emphasized that mutual influences between thymocytes and TECs do not occur until E15.5 when DN3 thymocytes appear.Citation14
In previous studiesCitation15,16 we described that the absence of EphB2 and/or EphB3, two protein tyrosine kinase receptors important for regulating the epithelial organization,Citation17 results in profound morphological alterations of the thymic epithelium, including epithelial hypocellularity, altered phenotypes and down-regulated expression of keratins, etc.,Citation15 and speculated that they were largely related to altered EphB-mediated signaling between thymocytes and TECs.Citation16 Thus, we will quantify these morphological changes, evaluating their origin and determining whether the distinct thymic histological compartments or TEC subsets are similarly affected.
Eph are the largest family of tyrosine kinase receptors of animal cells including two families: A (10 members) and B (6 members) that largely interact with ephrin-A (6 components) and ephrin-B (3 components), respectively.Citation18 Eph/ephrin pathways activate after oligomerization sending signals through both receptors (forward) and ligands (reverse)Citation19 that modify the cellular behavior affecting numerous processes, including organogenesis and tissue homeostasis.Citation18
In the present study, we have evaluated the epithelial maturation in embryonic thymuses of EphB2- or EphB3-deficient mice, understood to mean as the pattern of appearance and evolution of specific marker expression,Citation14 by combining different approaches and using a battery of antibodies to study the appearance and development of distinct cortical TEC (cTEC) subsets. In a complementary study the condition of mutant medullary TEC (mTEC) has also been evaluated (submitted manuscript). Our results relate delayed maturation of distinct mutant cTEC subpopulations with the altered development of thymocytes previously reported,Citation13 increased proportions of apoptotic TECs and lower percentages of cycling TECs occurring in mutant thymuses. More importantly we demonstrate that both molecules, but principally EphB3, are involved in the formation and maturation of murine thymic cortex. Furthermore, by studying TECs expressing a truncated form of EphB2 (EphB2LacZ) that does not transmit forward signals but activates reverse ones in ephrin-B-expressing cells, we determined that forward signals mediated through Eph are more relevant for the process than those reverse signals transmitted by ephrins.
RESULTS
EphB-deficient thymuses exhibited delayed maturation of EpCAM+CD45− epithelial cells during fetal thymus development
Thymic epithelial cells (TECs) were defined as EpCAM+CD45− cells and studied comparatively from E12.5 to E17.5 in WT mice and EphB2- or EphB3-deficient mice as well as EphB2LacZ mice that allows the relevance of forward and reverse signals in TEC development to be analyzed.
The percentage of EpCAM+CD45− cells decreased significantly throughout fetal development (, Supplementary Fig. 1A). However, the reduction was different in WT and mutant thymuses. The proportion of EpCAM+CD45− cells from E12.5 to E15.5 in EphB2−/− thymuses and at E13.5 in EphB2LacZ ones was significantly higher than in WT ones, without differences with EphB3−/− thymuses (). Furthermore, in some stages studied the proportions of TECs in the EphB2−/− thymuses were significantly higher than in the other mutants ().
FIGURE 1. Percentages and numbers of total TECs (EpCAM+CD45− cells) in WT and EphB-deficient thymuses throughout fetal development. (A) Comparative analysis of the proportions of EpCAM+CD45− cells between WT and mutant fetal thymuses. (B) Comparative analysis of the numbers of total TECs between WT and mutant thymuses. The significance of the Student's t-test probability is indicated as *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.005; or #p ≤ 0.05; ##p ≤ 0.01; ###p ≤ 0.005. ns: non-significant.
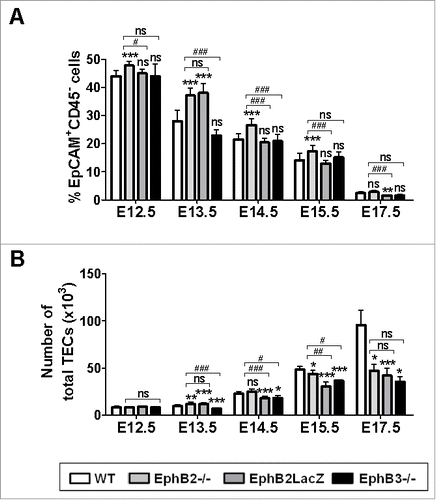
The absolute number of EpCAM+CD45− cells gradually increased (, Supplementary Fig. 1B) in both WT and mutant thymuses, although the important increase observed between E15.5-E17.5 in WT ones did not occur in mutant thymuses (). This reduction was also observed at E14.5 in EphB2LacZ and EphB3−/− thymuses, but not in EphB2−/− ones. On the contrary, at E13.5, EphB2−/− and EphB2LacZ values were higher than those of WT thymuses whereas at E12.5 there were no differences between WT and mutant mice ().
EphB-deficient thymuses exhibited delayed maturation of cortical Ly51+ TECs
To test whether TEC maturation coursed with alterations in cTECs we first analyzed in the total EpCAM+CD45− cell population between E12.5 and E17.5, the expression of Ly51 and UEA1 (Ulex europaeus agglutinin lectin 1), two cell markers that identify the two main TEC populations: the cortical (Ly51+UEA1−) one and the medullary (Ly51−UEA1+) one.Citation20,21 In E12.5 thymuses, most cells were immature Ly51−UEA1− cells (); gradually, this cell population disappeared, Ly51 expression was up-regulated and, at E17.5, most epithelial cells were Ly51med showing a variable expression of UEA1 (). At E12.5, there was no differences between any studied group but the EphB2−/− thymuses showed significant reduced proportions of the most immature Ly51−UEA1− (). From E13.5 onward the proportions of these cells were significantly higher in all mutants; only the E15.5 EphB2LacZ thymuses showed similar values to the WT ones (). When these proportions were compared between mutants, those of EphB2-deficient thymuses were significantly higher than those of EphB2LacZ ones at E14.5 and E15.5 and only at E14.5 compared with EphB3−/− ones ().
FIGURE 2. Maturation of the TEC subsets defined by Ly51/UEA1 expression during fetal development (E12.5-E17.5) in both WT and EphB-deficient mice. (A) Dot plots, representative of the performed analyses gating in total WT and mutant EpCAM+CD45− epithelial cells, show the maturation of distinct cell subsets defined by Ly51 and/or UEA1 expression. (B) Proportions of both WT and mutant thymic Ly51−UEA1− cells throughout embryonic development. (C) Proportions of both WT and mutant thymic Ly51loUEA1− cells during development. (D) Changes in the proportions of thymic Ly51medUEA1− cells in WT and mutant embryonic thymuses. Proportions of both Ly51medUEA1lo/med (E) and Ly51hiUEA1lo/med (F) cell subsets during embryonic development of WT and mutant thymuses. The significance of the Student's t-test probability is indicated as *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.005; or #p ≤ 0.05; ###p ≤ 0.005. ns: non-significant.
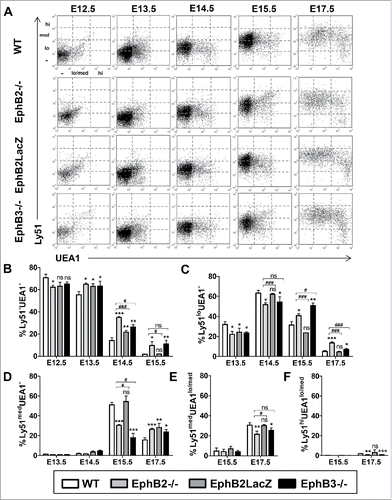
At E13.5 and E14.5, there was a significant reduction in the proportions of mutant Ly51lo UEA1− cells, except in E14.5 EphB2LacZ ones (). This cell population significantly accumulated from E15.5 (), except in EphB2LacZ thymuses that, following a similar pattern to the WT ones, matured to Ly51medUEA1− cells (). In the other mutant thymuses, the accumulation of Ly51loUEA1− cells () remained at E17.5 when the proportion of Ly51medUEA1− cells decreased in both WT and EphB2LacZ thymuses (). When EphB2−/− and EphB2LacZ thymuses were compared the proportions of these cells were lower at E14.5 and higher at E15.5 and E17.5 in EphB2−/− ones (). Differences between EphB2−/− and EphB3−/− thymuses appeared at E15.5 and E17.5. In the former stage, the proportions of Ly51loUEA1− cells were slightly lower in EphB2−/− thymuses, but significantly higher at E17.5 (). In this last stage, changes in the intermediate cell populations expressing both Ly51 and UEA1 confirmed the delayed maturation of EphB2−/− and EphB3−/− thymuses but not of EphB2LacZ ones. Both Ly51medUEA1lo/med () and Ly51hiUEA1lo/med () cells showed significantly reduced proportions in EphB2- and EphB3-mutant thymuses respect to WT values, without differences between them.
Other cortical TEC subsets defined by the expression of functional markers also undergo delayed maturation in EphB-deficient thymuses
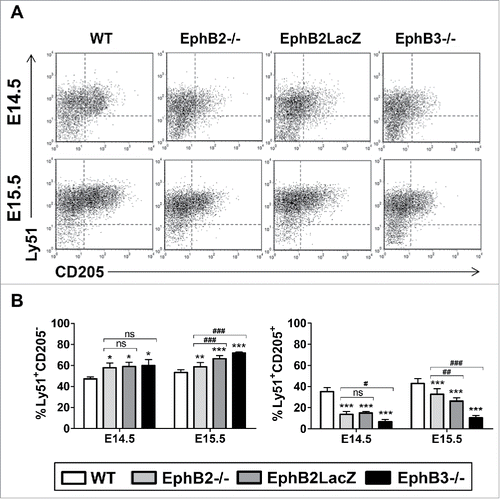
Together with Ly51, CD205, also called DEC205 has been frequently analyzed to characterize cTEC development.Citation14 Between E14.5 and E15.5, Ly51+ cells began to express CD205 and included both CD205− cells and CD205+ cells (). In all mutants, but particularly in the EphB3−/− thymuses, the proportions of Ly51+CD205+ cells were significantly lower than in the WT ones, in correlation with the significant accumulation of Ly51+CD205− cells ().
FIGURE 3. Maturation of cTEC subsets based on Ly51/CD205 expression in both WT and EphB-deficient thymuses. (A) Dot plots show the evolution of Ly51+CD205− and Ly51+CD205+ cTEC subpopulations at E14.5 and E15.5 in both WT and mutant thymuses. (B) Proportions of both Ly51+CD205− and Ly51+CD205+ cTECs in WT and mutant thymuses. The significance of the Student's t-test probability is indicated as *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.005; or #p ≤ 0.05; ##p ≤ 0.01; ###p ≤ 0.005. ns: non-significant.
MHCII molecules are expressed in both cTECs and mTECs appearing earlier in the first ones and undergoing a rapid up-regulation (). MHCII began to be expressed weakly at E13.5 in Ly51lo cells () up-regulating rapidly (, ) in both Ly51lo cells () and Ly51med cells (, ), although a low proportion of MHCIIlo remained throughout thymic development, mainly in the mutant thymuses (). At E12.5, an immature MHCII−Ly51− cell population predominated in all studied thymuses but disappeared rapidly by means of the aforementioned upregulated expression of MHCII. Remarkably, this differentiation from MHCII− cells to MHCII-expressing Ly51+ cells was delayed in the three mutants studied, including EphB2LacZ thymuses, but particularly in EphB3−/− mice that showed almost 10% of MHCII− cells at E15.5, and significantly higher values than those of EphB2−/− mice at both E13.5 and E14.5 ().
FIGURE 4. cTEC subsets defined by the expression of Ly51 and MHCII cell markers during fetal development (E12.5-E17.5) of both WT and EphB-deficient thymuses. (A) Dot plots show different TEC subsets defined by Ly51/MHCII expression throughout thymus development. They are representative of different analyses of TEC subsets gated in the total EpCAM+CD45− epithelial cell population. (B) Proportions of MHCII−Ly51− cells in WT and EphB-deficient embryonic thymuses. Proportions of both MHCIIloLy51lo (C) and MHCIImedLy51lo (D) cells in WT and mutant thymuses. (E) Proportions of MHCIImedLy51med cells in WT and mutant embryonic thymuses. (F, G) Percentage of mature MHCIIhiLy51med (F) and MHCIIhiLy51hi (G) cTECs in WT and mutant embryonic thymuses. The significance of the Student's t-test probability is indicated as *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.005 or #p ≤ 0.05; ##p ≤ 0.01; ###p ≤ 0.005. ns: non-significant.
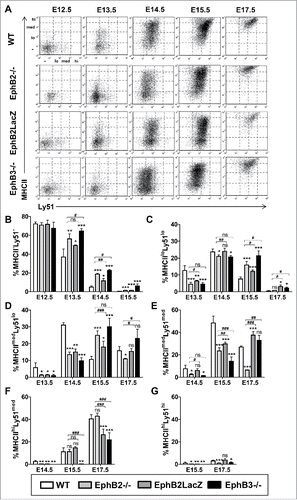
The proportion of MHCIIloLy51lo cells was significantly lower in both EphB2−/− and EphB3−/− thymuses at E13.5 and E14.5 than that of WT ones (). From E14.5, when WT values decreased rapidly, the mutant ones did so more slowly showing significantly higher proportions, particularly in EphB3−/− thymuses, confirming their severe phenotype compared to that of EphB2−/− ones (). More mature cTEC populations, both MHCIImedLy51lo cells () and MHCIImedLy51med cells (), behavior similarly with significantly lower values in the first studied stages and higher from E15.5, except in EphB2−/− thymuses in which the cells matured quickly into MHCIIhiLy51med cells (). In both MHCIIhiLy51med cells () and MHCIIhiLy51hi cells () the delay in the development of mutant cTECs was particularly evident in EphB3−/− mice, although at E17.5 the proportions of MHCIIhiLy51hi cells were significantly lower in both EphB2- and EphB3-mutant thymuses than in WT ones ().
The evolution of CD40 expression was analyzed from E13.5 onward (), the stage at which this co-receptor appears.Citation14,22 The maturation of distinct cTEC populations from CD40loLy51lo cells showed also a significant delay in both EphB2−/− and EphB3−/− thymuses (). Thus, the proportions of CD40loLy51med cells were significantly lower in these mutant thymuses from E14.5 to E17.5 (). At this last stage, the proportions of more mature CD40med/hiLy51lo and CD40med/hiLy51med cells were remarkably higher in both EphB2−/− and EphB3−/− thymuses, especially in the latter, than in WT ones, presumably reflecting a faster reduction in the proportions of WT cells than of either EphB2- or EphB3-deficient cells (, ). On the contrary, few variations were observed in the maturation of CD40-expressing cells of EphB2LacZ thymuses that showed a remarkable resemblance to WT ones. Only at E14.5 and E15.5 did the proportions of CD40loLy51med cells () and those of CD40med/hiLy51lo cells at E14.5 () showed significantly lower values in the mutant than in the WT thymuses.
FIGURE 5. cTEC subsets defined by the expression of Ly51 and CD40 or β5t cell markers and epithelial cell apoptosis during fetal development in both WT and EphB-deficient mice. (A) Dot plots show different TEC subsets defined by CD40/Ly51 expression throughout thymus development. CD40 expression in thymic cortex defines three cell subsets: negative (−), low (lo) (B, C) and medium/high (med/hi) (D, E) whose maturation in WT and mutant thymuses is represented in figures A to E. (F) The histogram shows a representative example of β5t expression (black line) in cortical EpCAM+CD45−Ly51+ cells (R1) at E15.5 respect to the negative expression in thymocytes (grey line). (G) Proportions of β5t+ cells in the cTEC subset, at E13.5, E15.5 and E17.5, determined by flow cytometry. (H) Significantly lower proportions of apoptotic EpCAM+CD45− epithelial cells at E12.5 and higher at E13.5 in mutant thymuses. The significance of the Student's t-test probability is indicated as *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.005 or #p ≤ 0.05; ##p ≤ 0.01; ###p ≤ 0.005. ns: non-significant.
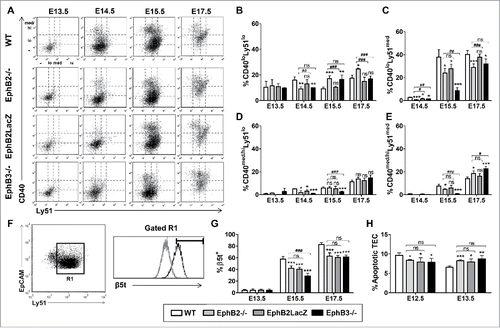
Finally, we studied the expression of the β5t subunit of thymoproteasome, that is specifically expressed by cTECs,Citation23 on histological thymic sections at E12.5 and E13.5 or by flow cytometric analysis of cTEC of E13.5, E15.5 and E17.5 thymuses. At E12.5 and E13.5, β5t was detected throughout the thymic sections with a stronger expression in the central area than in the periphery and slight differences between WT and mutant thymuses (Supplementary Fig. 2). By flow cytometry, we observed significantly reduced proportions of β5t+ cTECs at E15.5 and E17.5 in mutant thymuses respect to WT values, especially in E15.5 EphB3−/− thymuses as compared with EphB2−/− ones (, ).
EphB-deficient thymuses showed increased proportions of apoptotic TECs at E13.5 but not at E12.5
In order to determine the role played by other factors in the restricted expansion of mutant epithelial cell subsets, we studied the survival of total TECs in the thymic primordium (E12.5, E13.5) of both WT and EphB-mutant mice. Remarkably, whereas at E12.5 the proportions of apoptotic mutant TECs were lower than those of WT ones, at E13.5 the values were significantly higher in the EphB-deficient thymuses ().
EphB-deficient thymuses showed reduced proportions of cycling TECs
The proportions of cycling total EpCAM+CD45− WT TECs showed maximal values in the first stages of development (E12.5-E14.5), decreasing gradually from E13.5 (). The pattern followed by cycling mutant TECs was similar but exhibited an important delay respect to that of WT ones. Thus, their percentage increased between E12.5 and E14.5, and then began to diminish (). Furthermore, the proportion of cycling TECs was significantly lower at E12.5 and E13.5 in all mutants, even at E14.5 in EphB3−/− thymuses. Later, mutant and WT values became equal due to an important reduction in the last ones (). At E14.5 only the EphB3−/− TECs had significantly lower levels of proliferation than the EphB2−/− ().
FIGURE 6. Proportions of cycling cells in different epithelial cell subsets of both WT and EphB-mutant thymuses during fetal development (E12.5-E17.5). Figure represents the proportions of cycling cells defined as cells in S+G2/M phases. (A) Proportions of cycling total EpCAM+CD45− cells in both WT and mutant. (B) Changes in the proportions of cycling MTS20+ cells during thymus development are similar to those observed for the total EpCAM+CD45− TECs in both WT and mutant thymuses. (C) Proportions of both WT and mutant cycling MTS20− cells during thymus maturation. (D) Proportions of cycling Ly51−UEA1− cells in WT and mutant developing thymuses. (E) Percentage of cycling Ly51+UEA1− cTECs in mutant and WT thymuses. The significance of the Student's t-test probability is indicated as *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.005 or #p ≤ 0.05; ##p ≤ 0.01; ###p ≤ 0.005. ns: non-significant.
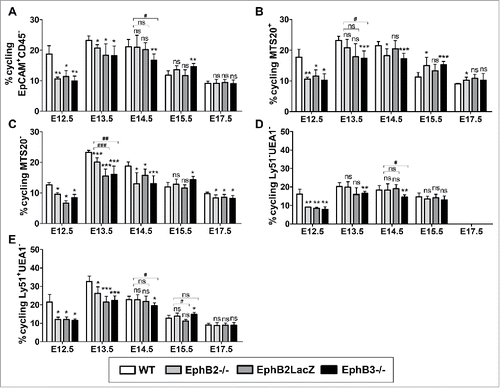
Next, we characterized phenotypically the cycling TECs analyzing the cycle in both MTS20+ cells and MTS20− cells gated on the EpCAM+CD45− cell population. The kinetics of proliferation of these two TEC subsets followed the same pattern as that described for the total TECs in both WT and mutant thymuses. Accordingly, the proportion of cycling MTS20+ cells was lower in the three studied mutants from E12.5 to E14.5, with significant differences particularly in EphB3−/− thymuses, whereas EphB2LacZ ones only showed low values at E12.5 (). From E14.5 onward the WT values diminished and the proportions of cycling cells resulted higher in EphB2−/− and EphB3−/− thymuses but not in EphB2LacZ ones (). The proportions of cycling MTS20− cells were significantly lower in all mutant thymuses from E12.5 to E17.5, except at E15.5 ().
We next analyzed the cell cycle in TEC subpopulations defined by the expression of Ly51 and UEA1 markers that, as mentioned above, permitted cTEC to be identified. Significantly reduced proportions of immature Ly51−UEA1− cells occurred at E12.5 in all studied mutants, as reported for both MTS20+ cells and MTS20− cells (). In other stages, only EphB3−/− thymuses showed lower values than the WT ones, with significant differences at E13.5 and E14.5 (). The evolution of cycling Ly51+UEA1− cTECs was notably similar to that found in the total EpCAM+CD45− TECs (), with lower proportions of cycling cells in all mutants at the first stages of development and no differences later, except for EphB3−/− TECs at E14.5 and E15.5 ().
Expression of EphB2 and EphB3, and their ligands, ephrin-B1 and ephrin-B2 in WT thymic cells
We also analyzed by flow cytometry the expression of EphB2 and EphB3 and their main ligands, ephrin-B1 and ephrin-B2 in both thymocytes and TECs of WT thymuses in order to establish possible correlations between their modulation throughout development and the alterations described in the maturation of mutant thymuses. The proportion of total EpCAM+CD45− TECs expressing either EphB2 or EphB3 was quite similar, with the highest values at E12.5 and a fast reduction in the following stages (). On the contrary, the percentage of ephrin-B2-expressing TECs was higher than that of ephrin-B1-expressing ones in all stages (). Indeed, the proportions of ephrin-B1+ epithelial cells did not change throughout development whereas those of ephrin-B2-expressing cells underwent a significant decline between E14.5 and E15.5 (). In all stages, the proportions of either EphB- or ephrin-B-expressing CD45+ thymocytes were significantly lower than those observed in total TECs, with the highest values in the case of EphB2 (). Indeed, the proportions of EphB-expressing thymocytes did not change throughout development whereas there was a reduction in the percentage of ephrin-B-expressing cells from E13.5 (). The expression of these molecules analyzed in terms of mean fluorescence intensity (MFI) (data not shown) in TECs was remarkably similar to the proportions of positive cells. Thus maximal values occurred at E12.5 for both EphB2 and EphB3 decreasing sharply in the next stages; on the contrary, ephrin-B2 expression remained high and relatively constant from E12.5 onward. The MFI in CD45+ thymocytes was significantly lower than in TECs throughout thymus development.
FIGURE 7. Expression of EphB and ephrin-B in WT thymic cells during early development (E12.5-E15.5). The figure shows the proportions of either TECs (A) or thymocytes (B) expressing EphB2, EphB3, ephrin-B1 and ephrin-B2 evaluated by flow cytometry. (C) Pattern of expression of EphB and ephrin-B in the WT and mutant Ly51−UEA1− cell population (D) Variations in the proportions of Eph/ephrin-B-expressing Ly51+UEA1− cTECs in WT and mutant embryonic thymuses. The significance of the Student's t-test probability is indicated between stages as *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.005.
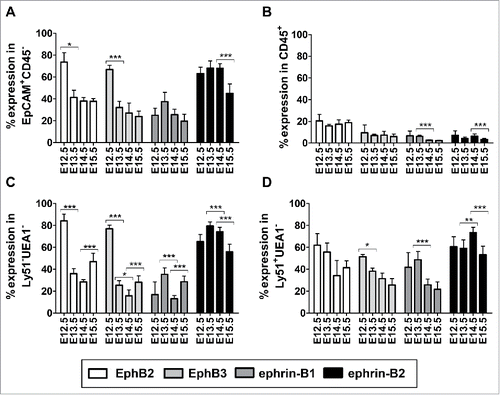
In addition, the pattern of expression of both EphB and ephrin-B in the immature Ly51−UEA1− TEC subset () was quite similar to that found in the total EpCAM+CD45− TECs (), with high proportions of EphB-positive cells at E12.5 and ephrin-B+ cells at E13.5 followed by a drop later on (). In addition, at E15.5 there was some recovery of the proportions of cells that expressed EphB2, EphB3 or ephrin-B1, but not of those of ephrin-B2-expressing cells, when each value was compared with that of the previous stage (). In the cortical Ly51+UEA1− cell population (), the differences between stages were less extreme. Thus, the proportions of EphB2+ cells did not change throughout development and the decline in the proportions of EphB3-expressing cTECs between E12.5 and E13.5 was smaller. On the contrary, the proportions of ephrin-B1-expressing cells were significantly higher at E13.5 than at E14.5 and those of ephrin-B2+ cells increased significantly between E13.5 and E14.5 ().
Changes in the expression of molecules involved in the maturation of thymic epithelium during the development of EphB-deficient thymuses
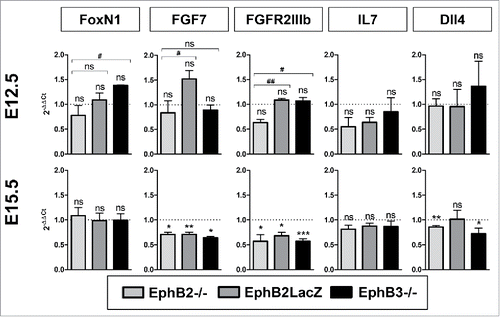
Although molecular mechanisms governing the maturation of thymic cortical epithelium are little known, some molecules have been identified that seem to be important for the maturation and proliferation of thymic epithelial progenitor cells. Accordingly, we studied their expression by quantitative PCR (qPCR) in E12.5 total thymic lobes, mainly containing TECs, and in both EpCAM+CD45− TECs and EpCAM−CD45− mesenchymal cells at E15.5. In each case, values observed in mutant thymuses were normalized to those of WT cells (). Our results did not show significant differences in the expression of FoxN1 between EphB-deficient samples and WT ones (). Fibroblast growth factor 7 (FGF7), a growth factor which is released by thymic mesenchyme and induces TEC proliferation after binding to the FGFR2IIIb receptor expressed on TECs,Citation24,25 showed at E12.5 no significance differences between mutant and WT thymuses (). On the contrary, at E15.5 the expression diminished significantly in mutant EpCAM−CD45− mesenchymal cells as compared to WT values (). Likewise, there were no differences in the expression of its receptor (FGFR2IIIb) between WT and mutant E12.5 TECs, although the expression in EphB2−/− thymic lobes was significantly lower than in the other mutant ones. However, at E15.5, the expression of FGFR2IIIb transcripts was significantly lower in the three mutant cells than in the WT ones. Finally, we evaluated whether, in turn, mutant cTECs were capable of producing two factors, IL7 and Dll4, necessary for the proper development of both DN and DP thymocytes that occurs in the thymic cortex.Citation26 IL7 expression was lower, but not significantly, in mutant TECs compared to WT values at both E12.5 and E15.5 (), whereas that of Dll4, a Notch ligand, was significantly lower in both EphB2−/− and EphB3−/− TECs at E15.5 but not at E12.5 ().
FIGURE 8. Expression of genes involved in thymic cell maturation in EphB-deficient thymuses. Figure shows the relative expression, RQ (2−ΔΔCt), of FoxN1, FGF7, FGFR2IIIb, IL7 and Dll4 transcripts in mutant cells relative to WT values (value 1, dotted line). At E12.5, the expression was determined on total thymic lobes whereas at E15.5 sorted EpCAM+CD45− TECs or, for FGF7 expression, EpCAM−CD45− mesenchymal cells were studied. The relative expression of mutant values was compared to the WT using the one-t test and between mutants according to Student's t-test. The significance is indicated as: *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.005 or #p ≤ 0.05; ##p ≤ 0.01. ns: non-significant.
DISCUSSION
In previous studies, we described the morphological changes undergone by the thymic epitheliumCitation15 as well as its survivalCitation27 in the absence of EphB receptors. Now, we quantify these phenotypical changes and evaluate the survival and cycling of cortical thymic epithelium during embryonic development, concluding that the lack of these molecules particularly of EphB3, although the presence of EphB2 is also necessary, results in delayed cortical epithelial maturation. Mutant mice with defects in other molecules but exhibiting a similar thymic phenotype to that of EphB-deficient mice also show delayed TEC maturation.Citation11,28
Our results confirm that the proportion of total EpCAM+CD45− TECs is higher in EphB2−/− thymuses than in WT ones, without significant differences in the case of EphB3−/− thymuses. In addition, whereas in the first stages studied (E12.5, E13.5) the number of mutant TECs and those of WT ones are only slightly different, the important increase in the number of total TECs found in WT thymuses from E14.5 onward does not occur in mutant thymuses. Other studies have pointed out that the lack of Eph or ephrins courses with thymic hypocellularity that affects both thymocytesCitation13,29 and TECs.Citation13,15 In addition, the blockade of Eph/ephrin-signaling reduces the thymic cell content.Citation30,31
The delayed maturation of TECs begins in the earliest stages of thymus development. At this time, the proportions of TECs expressing EphB2, EphB3 and ephrin-B2 expression, largely in Ly51−UEA1− cells and, to a lesser degree, in Ly51+UEA1− cells reaches maximal values, sharply decreasing later, except in the case of ephrin-B2 whose expression remains high until E14.5. All EphB are expressed in the thymus,Citation29,30 and we reported that the four molecules studied were already expressed in both total thymocytes and TECs of E15.5-old fetal thymuses.Citation29 In agreement with our results, Cejalvo found a similar pattern of expression in developing (EpCAM+CD45−) TECs in E12-E16-old thymuses by qPCR.Citation32 It is tentative to speculate that the lack of EphB provokes greater effects in those developmental stages in which their expression is maximal in WT thymuses. Furthermore, in these early stages of development the proportion of Eph/ephrin-B-expressing TECs is higher than that of Eph/ephrin-B+ thymocytes, which could explain the greater severity of the mutant alterations in the thymic epithelium (Citation15; our current results) than in the thymocytesCitation29 of mutant mice.
On the other hand, we recently demonstrated that low numbers of recent emigrants and their slow maturation after arriving at the mutant thymic primordium affected the development of immature MTS20+ epithelial cells.Citation13 In support, the blockade of Eph/ephrin-B signaling by soluble fusion proteins in fetal thymus organ cultures (FTOCs) induces impaired maturation of MTS20+ TECs that accumulate in the lobes, and 2′dGuo-treated alymphoid thymic lobes accumulated MTS20+ TECsCitation13 or exhibit reduced proportions of medullary EpCAM+CD80+ TECs.Citation33
In turn, delayed maturation and reduced proportions of cycling thymocytes found in mutant thymusesCitation13 could be due to an altered epithelial production of factors necessary for DN cell development. Our current results do not demonstrate changes in the IL7 values in mutant TECs, but preliminary studies show significantly reduced IL7 receptor α chain (IL7Rα) transcripts in both EphB2−/− and EphB3−/− FTOCs at E13.5 and E15.5 (data not shown), as previously reported in thymocytes with specific deletion of ephrin-B1 and ephrin-B2 genes.Citation34 On the other hand, the expression of Dll4, a ligand of Notch1, necessary for the differentiation of DN thymocytesCitation26 is reduced in EphB2- and EphB3-deficient thymuses, significantly at E15.5.
TEC subsets defined by Ly51 and UEA1 expression also show delayed maturation. Previously, we found altered expression of Ly51+ cells in thymuses with selective deletion of either ephrin-B1 and ephrin-B2 in thymocytes or ephrin-B2 in TECs.Citation21 In the first developmental stages, the immature Ly51−UEA1− cells show lower proportions in the EphB2−/− and EphB3−/− thymuses than in WT ones, but from E13.5 onward the mutant values increase, reducing the proportions of Ly51loUEA1− cells, which exhibit higher values than the WT ones from E15.5. The values of the most mature cTECs, Ly51medUEA1lo/med and Ly51hiUEA1lo/med, are also significantly reduced in both EphB2- and EphB3-deficient mice. On the other hand, the pattern of maturation of EphB2LacZ cTECs is quite similar to that of WT ones.
We confirmed this delayed epithelial maturation, particularly in EphB3−/− thymuses, by analyzing functional cell markers, such as CD205 a lectin involved in antigen uptake and processingCitation35 that, in agreement with previous studies,Citation36 is detected at E14.5. At E14.5 and E15.5 mutant thymuses accumulate immature Ly51+CD205− cells whereas the proportion of mature Ly51+CD205+ cells decreases, especially in EphB3−/− thymuses.
The pattern of CD40 throughout thymus development is quite similar: significantly lower values in the first stages and accumulation of different cell subsets (i.e., CD40loLy51lo cells, CD40loLy51med cells, CD40med/hiLy51med cells) later. Furthermore, a more severe phenotype occurs in all these cell subsets of EphB3−/− thymuses. Once again, there are few differences in CD40 expression when EphB2LacZ and WT thymuses are compared.
In agreement with other studies,Citation37 MHCII is weakly expressed at E13.5 but quickly up-regulates its expression in both Ly51lo cells and Ly51med cells. The maturation of MHCII−Ly51+ cells to MHCII+Ly51+ cells is delayed in the three mutants, particularly in EphB3−/− thymuses that show significant differences compared to EphB2−/− values at different stages in all studied cell subsets from MHCII−Ly51− to MHCIImedLy51med cTECs.
β5t, a specific component of thymoproteasomeCitation38 involved in generating the immune repertoire of CD8+ T cells in the context of MHCI,Citation39 is expressed specifically in the cTEC.Citation23 We detected, immunohistochemically, β5t expression at E12.5 and also one day later by flow cytometry. Other authors, using other antibody, detected β5t earlier.Citation38 In mutant thymuses, and particularly in EphB3−/− ones, the proportions of β5t+ cTECs are significantly lower than in WT ones, reflecting the delayed maturation of cTEC populations in EphB-deficient thymuses.
Factors regulating the maturation of thymic epithelium are largely unknown, except for the assumed relevance of the transcriptional factor FoxN1.Citation2 However, our results do not demonstrate significant changes in FoxN1 expression in mutant thymuses, presumably because EphB would act downstream to FoxN1 as occurs in the intestine, where FoxL1, another member of the Fox family, regulates the expression of EphB2 and EphB3.Citation40
The delayed epithelial maturation of EphB-deficient thymuses also seems to be related to the altered proportions of apoptotic cells and low percentages of cycling cTECs observed in these mutants. These changes are limited in some developmental stages but have a cumulative effect that would explain the enormous epithelial hypocellularity observed at E17.5 in mutant thymuses, contributing to the delayed development of cortical epithelium. Since TECs receive signals of survival/death from their cell environment in specific stages of their life-span, the lower proportions of apoptotic cells found in the E12.5-old mutant thymuses and their significantly higher values one day later, as compared to WT thymuses, would reflect the delay observed in the maturation of distinct mutant TECs. We had previously observed that from E13.5 onward the proportions of apoptotic TEC were higher in EphB-deficient thymusesCitation15 and the blockade of Eph/ephrin-B-signaling coursed with increased apoptotic TECs.Citation27 Importantly, the proportions of apoptotic TEC are higher in reaggregated thymus organ cultures (RTOCs) containing just TEC, indicating the relevance of EphB-mediated thymocyte-TEC interactions for TEC survival.Citation27
A similar condition could be affecting the cell cycle of mutant TECs. Both WT and EphB-deficient TECs cycle, but the last ones show some delay in the onset of proliferation, possibly due to the above explained gap (about 24 hours) in the lymphoid progenitor cell seeding into mutant thymic primordium.Citation13 In rats, the proliferation of TECs increases after lymphoid cell colonization of the primitive thymic primordium.Citation41 However, there are in fact very few data on the cell cycle of TECs and the results are controversial. As described herein, at E13.5 CookCitation42 reported a peak of proliferating TECs, whereas Jenkinson et alCitation43 indicated that maximal proportions of cycling TECs occur one day later, at E14.5. In a previous study, we observed slightly lower proportions of cycling CD45− cells in E15.5 EphB2−/− and/or EphB3−/− thymuses than those of WT onesCitation15 but an analysis of their changes throughout thymus ontogeny lacks. On the other hand, the immature MTS20+ cell subsetCitation44 largely divides in the first stages of development, as reported for Plet-1+ TECs recognized by the MTS20 and MTS24 antibodies,Citation42 with lower proportions in the mutant thymuses until E14.5, especially in those deficient in EphB3. Earlier, at E12.5, the low proportion of cycling MTS20+ cells would reflect an accumulation of primitive MTS20− cells that do not yet express the marker. The TECs defined by Ly51/UEA1 expression also show lower proportions of cycling cells associated with their delayed maturation in mutant thymuses, especially in EphB3−/− ones.
TEC proliferation has been related to neural crest-derived mesenchymeCitation25 that contributes to thymic capsule and trabeculae and its capability to produce proliferation-inducing molecules, including IGF1/245 and, particularly, those of the FGF family.Citation24,25 In our current study, we determined the relative values of both FGF7 and its receptor FGFR2IIIb transcripts in mutant thymuses compared to those observed in the WT ones. Whereas at E12.5 no significant differences appear in the expression of the two molecules, at E15.5 this is significantly lower in the three studied mutants, a result that could partially explain the decreased proportions of cycling TECs observed in the different mutant TEC subsets. Although we detect transcripts at E12.5, the FGFR2IIIb protein is expressed at E13.5,Citation24 when our results demonstrate a drastic decrease in the proportions of mutant cycling TECs, largely MTS20+ cells and Ly51−UEA1− cells. Whereas in other systems, Eph/ephrin-B signaling activates FGFR2IIIbCitation46 its lack in EphB-deficient mice could explain the observed fall in FGFR2IIIb transcripts. Furthermore, the lack of ephrin-B2 in neural crest-derived cells affects the behaviour of thymic mesenchyme.Citation47 Nevertheless, because earlier mutant thymuses also exhibit lower TEC proliferation than WT ones, when the expression of both FGF7 and FGFR2IIIb is similar in mutant and WT thymuses, other molecules, such as Wnt or β-catenin, that are related to EphB and in their absence thymuses show similar phenotypes to those deficient in EphB,Citation11 could be controlling TEC proliferation.
In summary, our results confirm the relevance of EphB2 and EphB3, not only in the organization of adult thymic epithelium but also for its maturation, demonstrating that defects in the number of colonizing lymphoid progenitor cells induce a delayed maturation of TEC that bears as well altered TEC survival and proliferation. Together, all these factors could explain the great hypocellularity exhibited by EphB-deficient thymuses. On the other hand, although both EphB2 and EphB3 are necessary for a proper maturation of TEC, since the absence of one is not compensated by the presence of the other, EphB3 is particularly important for the functional maturation of cortical epithelium, as evaluated by the appearance of cortical TEC markers such as MHCII, CD40 or β5t. Besides, EphB2LacZ thymic epithelium shows a quite similar, although not identical, phenotype to the WT one suggesting that the reverse signal provided by this molecule does not completely restore the EphB2−/− phenotype and supporting the relevance of EphB forward signals.
MATERIALS AND METHODS
Mice
EphB-deficient mice, EphB2−/−, EphB3−/− and EphB2LacZ, generated in a CD1 background were kindly provided by Dr. Mark Henkemeyer (University of Texas, Southwestern Medical Center, Dallas, Texas). EphB2LacZ mice expressed a truncated EphB2 molecule capable of stimulating ephrin-B-expressing cells (reverse signal), but unable to transmit EphB2 forward signals. For all the analyses performed, CD1 Wild Type (WT) and mutant mice were obtained from homozygous parents. The day of vaginal plug detection was designated as day 0.5 for determining the age of fetuses used in these studies and an accurate embryo staging was performed to avoid mistakes about the precise age of the studied fetuses. When individual thymuses (both WT and mutants) were studied, at least one embryo from five different litters was used. On the other hand, pooled thymic lobes were collected from one litter and, at least, five litters were used in the same experiment. All animals were bred and maintained under pathogen-free conditions in the animal housing of the Complutense University of Madrid.
Animal statement
This work has been carried out with all permissions from local authorities for using mice.
Cell suspensions and flow cytometry analysis
For thymic epithelial cell analysis, thymic cells were isolated from either WT or EphB-deficient fetal thymuses at different developmental stages (E12.5-E15.5 and E17.5). E12.5 pooled or E13.5-E17.5 individual thymic lobes were disaggregated using trypsin 0.25x (Thermo Fisher Scientific, Cat. 15090046) and DNaseI (0.1 mg/mL) (Roche, Cat. 11284932001) in RPMI 1640 for 20 minutes at 37°C and lobes gently pipetted to obtain a single-cell suspension. The obtained cell suspensions were washed in RPMI 1640 with 5% FBS and 10 mM ethylenediaminetetraacetic acid (EDTA) and stained for 15 minutes at 4°C in PBS 1% FBS with specific antibodies. The antibodies used corresponded to: EpCAM-Alexa488 (clone G8.8), CD45-PE or -PERCPCy5.5 or -Alexa647 or -APC/Cy7 (clone 30-F11), Ly51-PE (clone 6C3), MHCII-APC (clone M5/114.15.2), CD40-PE (clone 3/23) and CD205-PERCPCy5.5 (clone NLDC-145) from Biolegend. UEA1-Biotin (Ulex Europaeus Agglutinin lectin 1) from Vector Labs was detected by using streptavidin-PECy7 (Thermo Fisher Scientific). MTS20 supernatant (kindly provided by Dr. Richard Boyd) was detected using a goat anti-rat IgM-PE antibody (Jackson ImmunoResearch, Cat. 112-116-075). For β5t expression, cell suspensions were permeabilized using Fixation/Permeabilization solution (BD, Biosciences, Cat. 554722), incubated with anti-β5t polyclonal antibody (MBL, Cat. PD021) and washed using Perm/Wash™ Buffer (BD, Biosciences, Cat. 554723), and the primary antibody was detected using a donkey anti-rabbit IgG-AlexaFluor647 antibody (Thermo Fisher Scientific, Cat. A31573). Before flow cytometry analysis, stained cells were washed in PBS, suspended in PBS 1% FBS and analyzed either in a FACSCalibur device (BD Biosciences) equipped with CellQuest software or a FACSAriaIII (BD Biosciences) at the Cytometry and Fluorescence Microscopy Center of the Complutense University of Madrid. Thymic epithelial cells were identified as EpCAM+CD45− after gating in total thymic cells (Supplementary Fig. 3A). Non-viable cells were excluded by forward-side scatter in all cases and data were analyzed with FCS-Express 3 software (DeNovo Software).
Apoptosis assays
Cells from both pooled E12.5 and individual E13.5 WT and EphB-deficient thymic lobes were washed in Annexin buffer (10 mM HEPES, 140 mM NaCl and 2.5 mM CaCl2) with 1% FBS and incubated with AnnexinV (Biolegend, Cat. 640906), anti-EpCAM and anti-CD45 for 20 minutes at room temperature. After washing, 10 minutes before analysis, cells were resuspended in Annexin buffer with 1% FBS containing 1mg/mL of 7-Aminoactinomycin D (7-AAD) (Sigma-Aldrich, Cat. A9400). Apoptotic thymic epithelial cells were identified how AnnexinV+/7AAD− after gating in total EpCAM+CD45− cell subset (Supplementary Fig. 3B).
Cell cycle analysis
Both WT and EphB-deficient total thymic cells, isolated from E12.5-E13.5 pooled or E14.5-E17.5 individual thymic lobes as previously described, were incubated at 37°C for 90 minutes under stirring with a Hoechst Staining Buffer (HSB: HBSS 1x, 5.55 mM Glucose, 0.02 M HEPES, 0.025 mg/mL Verapamil) containing 5 μg/mL of Hoechst 33342 (Thermo Fisher Scientific, Cat. H1399). After washing, samples were incubated with anti-EpCAM, anti-MTS20, anti-Ly51, UEA1-Biotin and anti-CD45 diluted in HSB. MTS20 and UEA1-Biotin were identified as previously described. Cells were analyzed in a FACSAriaIII device (BD Biosciences) at the Cytometry and Fluorescence Microscopy Center at the Complutense University of Madrid. Cycling cells correspond to cells in S+G2/M cell cycle phase, as explained in Supplementary Fig. 3C. Analyses were carried out with the FCS-Express 3 software (DeNovo Software).
Immunofluorescence
12 μm thick thymic sections obtained from WT and EphB-deficient mice at E12.5 and E13.5 were fixed in acetone for 10 minutes, air dried and stained with anti-β5t antibody (MBL) for 1 hour at room temperature. After washing three times in cold PBS for 5 minutes, the primary antibody was detected using: donkey anti-rabbit IgG-Alexa488 (Thermo Fisher Scientific, Cat. A21206) for 45 minutes at room temperature. Sections were washed in cold PBS three times for 5 minutes, mounted with Prolong Gold antifade (Thermo Fisher Scientific, Cat. P36930) and analyzed and photographed in a Zeiss Axioplan microscope provided with a Spot 2 digital camera at the Cytometry and Fluorescence Microscopy Center (Complutense University of Madrid) equipped with Metamorph software (MDS Inc.).
Eph and ephrin expression
Total thymic cells isolated from E12.5-E13.5 pooled or E14.5-E15.5 individual WT thymic lobes as previously indicated, were fixed with formaldehyde 2% for 5 minutes at room temperature and washed with PBS 1x. Then, samples were incubated with either anti-EphB2, anti-EphB3, anti-ephrin-B1 or anti-ephrin-B2 (R&D Systems, Cat. AF467, AF432, AF473, AF496, respectively), and anti-Ly51, UEA1, anti-EpCAM and anti-CD45 antibodies, as previously indicated, and analyzed in a FACSAriaIII device (BD Biosciences) at the Cytometry and Fluorescence Microscopy Center at Complutense University of Madrid. EphB and ephrin-B were detected by using a donkey anti-goat IgG-Alexa488 antibody (Thermo Fisher Scientific, Cat. A11055). In all cases, non-viable cells were excluded by forward-side scatter and the analyses were carried out with the FCS-Express 3 software (DeNovo Software). The percentages of expression were calculated by interpolating the profiles of expression of each molecule in the different cell subpopulations with those obtained using a FMO (Fluorescence Minus One) control. In this control, the primary antibody that detects the molecule of interest (EphB or ephrin-B) was not added, but the remaining markers used in the labeling were incorporated (Supplementary Fig. 4). The program calculated a percentage of positivity represented as the expression of each molecule studied.
RNA extraction, RT-PCR and real-time PCR (qPCR)
Both WT and EphB-deficient total E12.5 thymic lobes and either TECs (EpCAM+CD45−) or mesenchymal cells (EpCAM−CD45−) isolated from E15.5 thymic lobes by sorting using the FACSAriaIII cell sorter (BD Biosciences, Cytometry and Fluorescence Microscopy Center, Complutense University of Madrid), were used for RNA extraction, using the RNAqueous®-Micro Kit (Thermo Fisher Scientific, Cat. AM1931), according to the manufacturer's instructions. 0.1 µg RNA was used to synthesize cDNA by RT-PCR using High-Capacity cDNA Reverse Transcription (Thermo Fisher Scientific, Cat. 4368813), according to the manufacturer's instructions. Real-time PCR (qPCR) was performed using Power SYBR® Green PCR Master Mix (Thermo Fisher Scientific, Cat. 4367659) together with specific primers for HPRT1 (Forward: cctcctcagaccgcttttt; Reverse: aacctggttcatcatcgctaa), FoxN1 (Forward: tgacggagcacttcccttac; Reverse: gacaggttatggcgaacagaa), FGF7 (Forward: tggctgacaccatgactagc; Reverse: ggctacaggctgtcgttttt), FGFR2IIIb (Forward: tgcatggttgacagttctgc; Reverse: tgcaggcgattaagaagacc), IL7 (Forward: ctgctgcagtcccagtcat; Reverse: tcagtggaggaattccaaagat) or Dll4 (Forward: aggtgccacttcggttacac; Reverse: gggagagcaaatggctgata). Primer sequences were identified using the Universal Probe Library Assay Design Center application (Roche) and produced by Sigma-Aldrich. Efficiency of amplification reaction and the Ct values were obtained from 7900HT Fast Real-Time PCR system with SDS2.3 software at the Genomic Center of the Complutense University of Madrid. The relative expression for each sample was normalized to HPRT1 values and represented as RQ (2−ΔΔCt). Data shown are the media of three independent experiments.
STATISTICAL ANALYSIS
The results were expressed as mean ± SD and the significance of differences was analyzed according to the Student's t-test with respect to control ones after analysis of f-test data. In the case of gene expression analyses, the relative expression of mutant values was compared to WT ones using the one-t test analysis. Significance probability between WT and mutant values or developmental stages is indicated as *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.005 or #p ≤ 0.05; ##p ≤ 0.01; ###p ≤ 0.005 when mutant values are compared between them.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors declare no financial or commercial conflict of interest.
FUNDING DETAILS
This work was supported by the Spanish Ministry of Economy and Competitiveness under grants BFU2013-41112-R and Cell Therapy Network (RD12/0019/0007 and RD16/0011/0002).
Supplementry_material.zip
Download Zip (1.7 MB)ACKNOWLEDGMENTS
We thank the Cytometry and Fluorescence Microscopy Center at Complutense University for the use of its facilities.
REFERENCES
- Anderson G, Takahama Y. Thymic epithelial cells: working class heroes for T cell development and repertoire selection. Trends Immunol. 2012;33:256–63. doi:10.1016/j.it.2012.03.005. PMID:22591984
- Blackburn CC, Manley NR. Developing a new paradigm for thymus organogenesis. Nat Rev Immunol. 2004;4:278–89. doi:10.1038/nri1331. PMID:15057786
- Itoi M, Kawamoto H, Katsura Y, Amagai T. Two distinct steps of immigration of hematopoietic progenitors into the early thymus anlage. Int Immunol. 2001;13:1203–11. doi:10.1093/intimm/13.9.1203. PMID:11526101
- Romano R, Palamaro L, Fusco A, Giardino G, Gallo V, Del Vecchio L, Pignata C. FOXN1: A Master Regulator Gene of Thymic Epithelial Development Program. Front Immunol. 2013;4:187. doi:10.3389/fimmu.2013.00187. PMID:23874334
- Su DM, Manley NR. Hoxa3 and pax1 transcription factors regulate the ability of fetal thymic epithelial cells to promote thymocyte development. J Immunol 2000;164:5753–60. doi:10.4049/jimmunol.164.11.5753. PMID:10820253
- Hetzer-Egger C, Schorpp M, Haas-Assenbaum A, Balling R, Peters H, Boehm T. Thymopoiesis requires Pax9 function in thymic epithelial cells. Eur J Immunol. 2002;32:1175–81. doi:10.1002/1521-4141(200204)32:4%3c1175::AID-IMMU1175%3e3.0.CO;2-U. PMID:11932925
- Arnold JS, Werling U, Braunstein EM, Liao J, Nowotschin S, Edelmann W, Hebert JM, Morrow BE. Inactivation of Tbx1 in the pharyngeal endoderm results in 22q11DS malformations. Development. 2006;133:977–87. doi:10.1242/dev.02264. PMID:16452092
- Zou D, Silvius D, Davenport J, Grifone R, Maire P, Xu PX. Patterning of the third pharyngeal pouch into thymus/parathyroid by Six and Eya1. Dev Biol. 2006;293:499–512. doi:10.1016/j.ydbio.2005.12.015. PMID:16530750
- Neves H, Dupin E, Parreira L, Le Douarin NM. Modulation of Bmp4 signalling in the epithelial-mesenchymal interactions that take place in early thymus and parathyroid development in avian embryos. Dev Biol. 2012;361:208–19. doi:10.1016/j.ydbio.2011.10.022. PMID:22057081
- Bain VE, Gordon J, O'Neil JD, Ramos I, Richie ER, Manley NR. Tissue-specific roles for sonic hedgehog signaling in establishing thymus and parathyroid organ fate. Development. 2016;143:4027–37. doi:10.1242/dev.141903. PMID:27633995
- Osada M, Ito E, Fermin HA, Vazquez-Cintron E, Venkatesh T, Friedel RH, Pezzano M. The Wnt signaling antagonist Kremen1 is required for development of thymic architecture. Clin Dev Immunol. 2006;13:299–319. doi:10.1080/17402520600935097. PMID:17162372
- Swann JB, Happe C, Boehm T. Elevated levels of Wnt signaling disrupt thymus morphogenesis and function. Sci Rep. 2017;7:785. doi:10.1038/s41598-017-00842-0. PMID:28400578
- Montero-Herradon S, Garcia-Ceca J, Sanchez Del Collado B, Alfaro D, Zapata AG. Eph/ephrin-B-mediated cell-to-cell interactions govern MTS20(+) thymic epithelial cell development. Histochem Cell Biol. 2016;146:167–82. doi:10.1007/s00418-016-1431-x. PMID:27060907
- Shakib S, Desanti GE, Jenkinson WE, Parnell SM, Jenkinson EJ, Anderson G. Checkpoints in the development of thymic cortical epithelial cells. J Immunol. 2009;182:130–7. doi:10.4049/jimmunol.182.1.130. PMID:19109143
- Garcia-Ceca J, Jimenez E, Alfaro D, Cejalvo T, Chumley MJ, Henkemeyer M, Munoz JJ, Zapata AG. On the role of Eph signalling in thymus histogenesis; EphB2/B3 and the organizing of the thymic epithelial network. Int J Dev Biol. 2009;53:971–82. doi:10.1387/ijdb.082702jg. PMID:19598115
- Garcia-Ceca J, Alfaro D, Montero-Herradon S, Tobajas E, Munoz JJ, Zapata AG. Eph/Ephrins-mediated thymocyte-thymic epithelial cell interactions control numerous processes of thymus biology. Front Immunol. 2015;6:333. doi:10.3389/fimmu.2015.00333. PMID:26167166
- Perez White BE, Getsios S. Eph receptor and ephrin function in breast, gut, and skin epithelia. Cell Adh Migr. 2014;8:327–38. doi:10.4161/19336918.2014.970012. PMID:25482622
- Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi:10.1016/j.cell.2008.03.011. PMID:18394988
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475–86. doi:10.1038/nrm856. PMID:12094214
- Seach N, Wong K, Hammett M, Boyd RL, Chidgey AP. Purified enzymes improve isolation and characterization of the adult thymic epithelium. J Immunol Methods. 2012;385:23–34. doi:10.1016/j.jim.2012.07.023. PMID:22910002
- Cejalvo T, Munoz JJ, Tobajas E, Fanlo L, Alfaro D, Garcia-Ceca J, Zapata A. Ephrin-B-dependent thymic epithelial cell-thymocyte interactions are necessary for correct T cell differentiation and thymus histology organization: relevance for thymic cortex development. J Immunol. 2013;190:2670–81. doi:10.4049/jimmunol.1201931. PMID:23408838
- Baik S, Jenkinson EJ, Lane PJ, Anderson G, Jenkinson WE. Generation of both cortical and Aire(+) medullary thymic epithelial compartments from CD205(+) progenitors. Eur J Immunol. 2013;43:589–94. doi:10.1002/eji.201243209. PMID:23299414
- Murata S, Sasaki K, Kishimoto T, Niwa S, Hayashi H, Takahama Y, Tanaka K. Regulation of CD8+ T cell development by thymus-specific proteasomes. Science. 2007;316:1349–53. doi:10.1126/science.1141915. PMID:17540904
- Revest JM, Suniara RK, Kerr K, Owen JJ, Dickson C. Development of the thymus requires signaling through the fibroblast growth factor receptor R2-IIIb. J Immunol. 2001;167:1954–61. doi:10.4049/jimmunol.167.4.1954. PMID:11489975
- Jenkinson WE, Jenkinson EJ, Anderson G. Differential requirement for mesenchyme in the proliferation and maturation of thymic epithelial progenitors. J Exp Med. 2003;198:325–32. doi:10.1084/jem.20022135. PMID:12860931
- Hirano K, Negishi N, Yazawa M, Yagita H, Habu S, Hozumi K. Delta-like 4-mediated Notch signaling is required for early T-cell development in a three-dimensional thymic structure. Eur J Immunol. 2015;45:2252–62. doi:10.1002/eji.201445123. PMID:25976373
- Garcia-Ceca J, Alfaro D, Montero-Herradon S, Zapata AG. Eph/ephrinB signalling is involved in the survival of thymic epithelial cells. Immunol Cell Biol. 2013;91:130–8. doi:10.1038/icb.2012.59. PMID:23146940
- Su DM, Navarre S, Oh WJ, Condie BG, Manley NR. A domain of Foxn1 required for crosstalk-dependent thymic epithelial cell differentiation. Nat Immunol. 2003;4:1128–35. doi:10.1038/ni983. PMID:14528302
- Alfaro D, Munoz JJ, Garcia-Ceca J, Cejalvo T, Jimenez E, Zapata A. Alterations in the thymocyte phenotype of EphB-deficient mice largely affect the double negative cell compartment. Immunology. 2008;125:131–43. doi:10.1111/j.1365-2567.2008.02828.x. PMID:18397270
- Yu G, Mao J, Wu Y, Luo H, Wu J. Ephrin-B1 is critical in T-cell development. J Biol Chem. 2006;281:10222–9. doi:10.1074/jbc.M510320200. PMID:16476740
- Alfaro D, Garcia-Ceca JJ, Cejalvo T, Jimenez E, Jenkinson EJ, Anderson G, Munoz JJ, Zapata A. EphrinB1-EphB signaling regulates thymocyte-epithelium interactions involved in functional T cell development. Eur J Immunol 2007; 37:2596–605. doi:10.1002/eji.200737097. PMID:17668899
- Cejalvo T. Role of ephrin B1 and ephrin B2 in the development and function of the thymus. PhD Thesis, Complutense University of Madrid, Madrid. 2011
- Rossi SW, Kim MY, Leibbrandt A, Parnell SM, Jenkinson WE, Glanville SH, McConnell FM, Scott HS, Penninger JM, Jenkinson EJ, et al. RANK signals from CD4(+)3(−) inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med. 2007;204:1267–72. doi:10.1084/jem.20062497. PMID:17502664
- Luo H, Wu Z, Qi S, Jin W, Han B, Wu J. Ephrinb1 and Ephrinb2 are associated with interleukin-7 receptor alpha and retard its internalization from the cell surface. J Biol Chem. 2011;286:44976–87. doi:10.1074/jbc.M111.316414. PMID:22069310
- Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–38. doi:10.1084/jem.20021598. PMID:12486105
- Saldana JI, Solanki A, Lau CI, Sahni H, Ross S, Furmanski AL, Ono M, Hollander G, Crompton T. Sonic Hedgehog regulates thymic epithelial cell differentiation. J Autoimmun. 2016;68:86–97. doi:10.1016/j.jaut.2015.12.004. PMID:26778835
- Nowell CS, Bredenkamp N, Tetelin S, Jin X, Tischner C, Vaidya H, Sheridan JM, Stenhouse FH, Heussen R, Smith AJ, et al. Foxn1 regulates lineage progression in cortical and medullary thymic epithelial cells but is dispensable for medullary sublineage divergence. PLoS Genet. 2011;7:e1002348. doi:10.1371/journal.pgen.1002348. PMID:22072979
- Ripen AM, Nitta T, Murata S, Tanaka K, Takahama Y. Ontogeny of thymic cortical epithelial cells expressing the thymoproteasome subunit beta5t. Eur J Immunol. 2011;41:1278–87. doi:10.1002/eji.201041375. PMID:21469133
- Nitta T, Murata S, Sasaki K, Fujii H, Ripen AM, Ishimaru N, Koyasu S, Tanaka K, Takahama Y. Thymoproteasome shapes immunocompetent repertoire of CD8+ T cells. Immunity. 2010;32:29–40. doi:10.1016/j.immuni.2009.10.009. PMID:20045355
- Takano-Maruyama M, Hase K, Fukamachi H, Kato Y, Koseki H, Ohno H. Foxl1-deficient mice exhibit aberrant epithelial cell positioning resulting from dysregulated EphB/EphrinB expression in the small intestine. Am J Physiol Gastrointest Liver Physiol. 2006;291:G163–70. doi:10.1152/ajpgi.00019.2006. PMID:16469829
- Brelinska R, Kaczmarek E, Ostalska D. Kinetics of thymic stroma development in the foetal period. Folia Histochem Cytobiol. 2001;39:195–6. PMID:11374822
- Cook AM. Proliferation and lineage potential in fetal thymic epithelial progenitor cells. PhD Thesis, The University of Edinburgh. 2010
- Jenkinson WE, Bacon A, White AJ, Anderson G, Jenkinson EJ. An epithelial progenitor pool regulates thymus growth. J Immunol. 2008;181:6101–8. doi:10.4049/jimmunol.181.9.6101. PMID:18941199
- Rossi SW, Chidgey AP, Parnell SM, Jenkinson WE, Scott HS, Boyd RL, Jenkinson EJ, Anderson G. Redefining epithelial progenitor potential in the developing thymus. Eur J Immunol. 2007;37:2411–8. doi:10.1002/eji.200737275. PMID:17694573
- Shinohara T, Honjo T. Studies in vitro on the mechanism of the epithelial/mesenchymal interaction in the early fetal thymus. Eur J Immunol. 1997;27:522–9. doi:10.1002/eji.1830270225. PMID:9045926
- Yokote H, Fujita K, Jing X, Sawada T, Liang S, Yao L, Yan X, Zhang Y, Schlessinger J, Sakaguchi K. Trans-activation of EphA4 and FGF receptors mediated by direct interactions between their cytoplasmic domains. Proc Natl Acad Sci U S A. 2005;102:18866–71. doi:10.1073/pnas.0509741102. PMID:16365308
- Foster KE, Gordon J, Cardenas K, Veiga-Fernandes H, Makinen T, Grigorieva E, Wilkinson DG, Blackburn CC, Richie E, Manley NR, et al. EphB-ephrin-B2 interactions are required for thymus migration during organogenesis. Proc Natl Acad Sci U S A. 2010;107:13414–9. doi:10.1073/pnas.1003747107. PMID:20616004
