ABSTRACT
Cryopreservation is the only long-term storage option for the storage of vessels and vascular constructs. However, endothelial barrier function is almost completely lost after cryopreservation in most established cryopreservation solutions. We here aimed to improve endothelial function after cryopreservation using the 2D-model of porcine aortic endothelial cell monolayers. The monolayers were cryopreserved in cell culture medium or cold storage solutions based on the 4°C vascular preservation solution TiProtec®, all supplemented with 10% DMSO, using different temperature gradients. After short-term storage at −80°C, monolayers were rapidly thawed and re-cultured in cell culture medium. Thawing after cryopreservation in cell culture medium caused both immediate and delayed cell death, resulting in 11 ± 5% living cells after 24 h of re-culture. After cryopreservation in TiProtec and chloride-poor modifications thereof, the proportion of adherent viable cells was markedly increased compared to cryopreservation in cell culture medium (TiProtec: 38 ± 11%, modified TiProtec solutions ≥ 50%). Using these solutions, cells cryopreserved in a sub-confluent state were able to proliferate during re-culture. Mitochondrial fragmentation was observed in all solutions, but was partially reversible after cryopreservation in TiProtec and almost completely reversible in modified solutions within 3 h of re-culture. The superior protection of TiProtec and its modifications was apparent at all temperature gradients; however, best results were achieved with a cooling rate of −1°C/min. In conclusion, the use of TiProtec or modifications thereof as base solution for cryopreservation greatly improved cryopreservation results for endothelial monolayers in terms of survival and of monolayer and mitochondrial integrity.
Introduction
To ensure the availability of vascular grafts for vascular reconstruction/replacement surgery, as well as to allow the storage of products of tissue engineering containing vascular structures,Citation1 of biohybrid prostheses and of organs-on-chips,Citation2 adequate storage options have to be provided. For short or intermediate storage, vessels are usually kept at 4°C in buffered salt solutions or in cell culture media. For long-term storage, the only option is cryopreservation. The current gold standard used in vessel banking is cryopreservation in various serum-containing cell culture media (M 199,Citation3 RPMICitation4,Citation5) with addition of cryoprotective agents (mostly DMSO) and sometimes other additives like human albumin.Citation5 However, very modest results are achieved with most current freezing protocols in terms of muscular and especially endothelial function and integrity.Citation6–Citation9 In the clinical setting, an impaired endothelial lining induces platelet adhesion and clot formation, so it is highly desirable to preserve the endothelial layer of cryopreserved vessels for transplantation purposes. For vascular constructs in tissue engineering, very little experience exists in the field of storage/cryopreservation.
The vascular storage solution TiProtec®, which has been developed for cold (4°C) storage of vessels and is based on mechanistic studies, provided marked improvement for cold storage of porcine aortic segments,Citation10 rat mesenteric arteries and aortae,Citation11,Citation12 and human arteries.Citation13 TiProtec contains iron chelators to inhibit cold-induced iron-dependent cell injury,Citation14,Citation15 glycine and alanine to prevent hypoxic injury,Citation16,Citation17 and high chloride and potassium concentrations, which both proved favorable for cold storage of vessels.Citation10,Citation11
Recent results showed that cryopreservation in TiProtec with 10% DMSO – as compared to supplemented cell culture medium with 10% DMSO – improved viability and function of rat hepatocytes after thawing; even better cryopreservation results for hepatocytes, however, were achieved in a chloride-poor modification of TiProtec with balanced sodium/potassium concentrations.Citation18 TiProtec solution (and modifications thereof) have the additional advantage that they are serum-free and contain no albumin. In contrast to porcine aortic endothelial cells,Citation10 rat hepatocytes display a chloride-dependent cold-induced cell injury,Citation19,Citation20 i.e. the chloride-poor TiProtec modification is superior to TiProtec for both, cold storageCitation19 and cryopreservationCitation18 of rat hepatocytes. Since porcine aortic endothelial cells are better protected in chloride-rich TiProtec at 4°C cold storage,Citation10 the question arises whether, for these cells, better cryopreservation results can be achieved in the original TiProtec or in chloride-poor modifications.
In this study, we therefore used monolayers of aortic endothelial cells as a simplified 2D-tissue-model, and tested whether TiProtec or the chloride-poor TiProtec modification, which showed best results for rat hepatocyte cryopreservation, are also suitable as base solution for endothelial cryopreservation. In a second step, we transferred the results to porcine aortic segments to assess the effect of cryopreservation in the new solution on (the endothelial lining of) complete vessels.
Results
Cell viability after cryopreservation
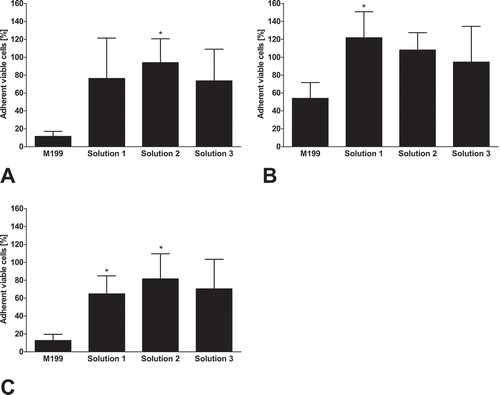
In the initial monolayer cultures, virtually no dead cells could be observed (data not shown). After slow (−0.1°C/min) freezing in serum-containing cell culture medium (M 199) with 10% DMSO, cell viability directly after thawing was decreased to around 50% (; PI-negative cells). During subsequent re-culture, cell death and cell detachment progressed further, resulting in about 10% viable cells after 3 h of re-culture (). While control cultures formed confluent monolayers (), hardly any attached and intact cells were left after freezing in cell culture medium and 3 h re-culture (). Viability after freezing in solution 1 was only slightly higher than after freezing in cell culture medium directly after thawing (), but delayed cell death was markedly lower () and an intact monolayer with only few detached cells was observed after 3 h of re-culture (). In the chloride-poor solution 2 (with balanced sodium and potassium concentrations), a solution that yielded good results in the cryopreservation of rat hepatocytesCitation18 and in solution 3, the chloride-poor analogue of TiProtec (potassium-rich), initial viability after thawing was higher than in the other solutions (). Decrease of viability during re-culture was similar to that of cells frozen in solution 1 yielding a final viability of around 50% (). Few cells detached and a morphologically normal monolayer was observed at the end of 3 h re-culture (, ).
FIGURE 1. Cell viability directly after thawing and after 3 h re-culture.Porcine aortic endothelial cell monolayers were cryopreserved (−0.1°C/min) in serum-containing cell culture medium (M 199), or in solution 1 (chloride-rich), solution 2 (chloride-poor, balanced Na+/K+ concentrations) or solution 3 (chloride-poor, potassium-rich), all supplemented with 10% DMSO. Cell viability was determined by propidium iodide (PI) staining directly after thawing (A; n = 7) or after 3 h of re-culture in cell culture medium (B; n = 7). * percentage of PI-negative cells significantly different from M199.
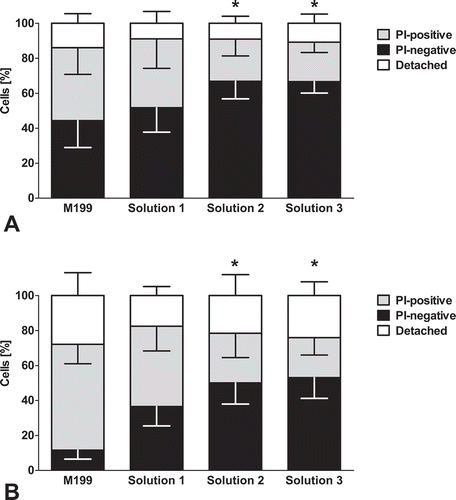
FIGURE 2. Monolayer integrity after 3 h re-culture.Porcine aortic endothelial cell monolayers (control, A) were cryopreserved (−0.1°C/min) in cell culture medium (M 199; B), solution 1 (chloride-rich; C), solution 2 (chloride-poor, balanced Na+/K+ concentrations; D) or solution 3 (chloride-poor, potassium-rich; E), all supplemented with 10% DMSO. Phase contrast micrographs (original magnification: 200x) were taken after 3 h of re-culture in cell culture medium.
Adherent viable cells and metabolic activity after cryopreservation
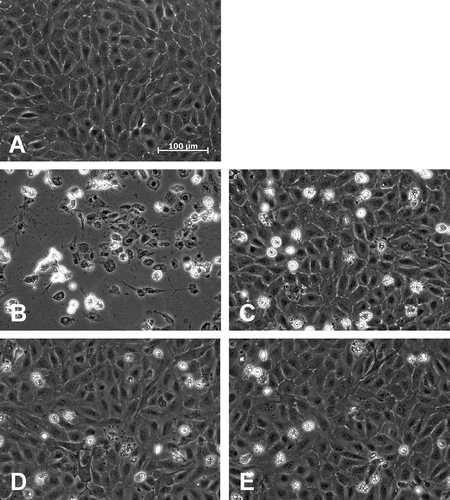
After 24 h of re-culture, the percentage of attached viable cells, as assessed by (residual) intracellular LDH (), was similar to the percentage of viable cells after 3 h rewarming in all solutions (compare and ) and significantly differed between cells frozen in cell culture medium and cells frozen in solutions 2 or 3 (). Metabolic activity (resazurin reduction; ) corresponded to the percentage of viable cells and was significantly higher in cells cryopreserved in solution 3 as compared to cell culture medium.
FIGURE 3. Adherent viable cells after 24 h re-culture.Porcine aortic endothelial cell monolayers were cryopreserved in cell culture medium (M 199), solution 1 (chloride-rich), solution 2 (chloride-poor, balanced Na+/K+ concentrations) or solution 3 (chloride-poor, potassium-rich), all supplemented with 10% DMSO, at a cooling rate of −0.1°C/min. After rapid thawing and 24 h re-culture in cell culture medium, the number of adherent viable cells (as percentage of control cultures; A, n = 6; P ≤ .05) and resazurin reduction (as percentage of control cultures; B, n = 4) was quantified. * significantly different from M199.
Mitochondrial integrity/membrane potential after thawing
Mitochondria were stained with two dyes: MitoTracker Green (green fluorescence), a dye that binds covalently and remains in the mitochondria even after the loss of mitochondrial membrane potential, and TMRM (red fluorescence), which leaks out of the mitochondria when their membrane potential decreases.
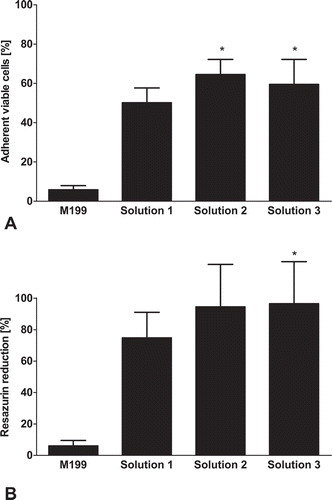
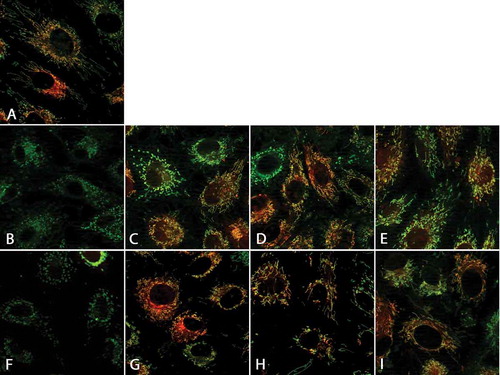
Control cells displayed long filamentous mitochondria with intact mitochondrial membrane potential (). Directly after thawing, mitochondrial fragmentation could be observed in all solutions (-E). Cells frozen in cell culture medium almost completely lost their mitochondrial membrane potential () and did not recover normal mitochondrial morphology after 1 h of re-culture (). Of cells frozen in solution 1, about half of cells retained the mitochondrial membrane potential () and some regained normal mitochondrial morphology after 1 h of re-culture (), while the proportion of cells with normal mitochondrial morphology and intact membrane potential was larger in cells frozen in solutions 2 and 3 both, directly after thawing (, ) and after rewarming (, ).
FIGURE 4. Mitochondrial alterations after cryopreservation and after 1 h of re-culture.Porcine aortic endothelial cell monolayers on glass coverslips were stained with the mitochondrial dyes MitoTracker green (green, binds covalently to mitochondrial proteins) and TMRM (red, leaks out after loss of mitochondrial membrane potential; A: control cells). Afterwards, cells were cryopreserved at a cooling rate of −0.1°C/min in cell culture medium (M 199; B, F), solution 1 (chloride-rich; C, G), solution 2 (chloride-poor, balanced Na+/K+; D, H), or solution 3 (chloride-poor, potassium-rich; E, I), all supplemented with 10% DMSO. After rapid thawing (B-E) and 1 h re-culture in cell culture medium (F-I), confocal fluorescence micrographs were taken (original maginification 630x).
Cell proliferation after thawing
Sub-confluent cell cultures frozen in cell culture medium showed hardly any proliferation during 24 h re-culture after thawing (9 ± 10% (mean ± SD) of control cultures; ). Cultures frozen in solution 1 displayed a significantly higher proliferation capacity than those frozen in cell culture medium (about 40% of control cultures; ) after re-culture; proliferation rate of cell cultures frozen in solution 2 exceeded that of cell frozen in solution 1 and reached almost 90% of the value of control cultures ().
FIGURE 5. Cell proliferation after thawing.Sub-confluent porcine aortic endothelial cell monolayers (24 h after sub-cultivation) were cryopreserved at a cooling rate of −0.1°C/min in cell culture medium (M 199; A), solution 1 (chloride-rich; A, B) or solution 2 (chloride-poor, balanced Na+/K+; B), all supplemented with 10% DMSO. Control cultures were kept for another 24 h at 37°C before quantification of adherent viable cells (intracellular LDH). After rapid thawing and 24 h re-culture in cell culture medium (total culture time: 48 h), cell proliferation was compared to control cultures. A: n = 10, B: n = 4; *significantly different from M199.
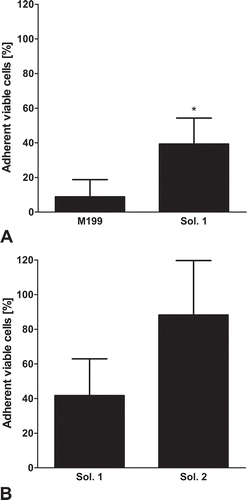
Protective effect of solutions 1–3 for different cooling rates
After cryopreservation in cell culture medium and 24 h re-culture, massive cell loss/loss of proliferation ability was observed for all temperature gradients (). The amount of living adherent cells after thawing and 24 h of re-culture was markedly improved in all new cryopreservation solutions (solutions 1–3, ); no significant difference was observed between the different variants of the new solution. Freezing at a rate of −1°C/min produced the best results in all solutions ().
FIGURE 6. Different cooling rates.Confluent (early confluent) porcine aortic endothelial cell monolayers were cryopreserved in cell culture medium (M 199), solution 1, solution 2 (chloride-poor, balanced Na+/K+ concentrations) or solution 3 (chloride-poor, potassium-rich), all supplemented with 10% DMSO at cooling rates of 0.1°C/min (A; n = 7; P = .0077), 1°C/min (B; n = 7; P = .0077) and 5°C/min (C; n = 7; P = .0041). After rapid thawing and 24 h re-culture in cell culture medium, the number of adherent viable cells (as percentage of control cultures) was determined. *significantly different from M 199.
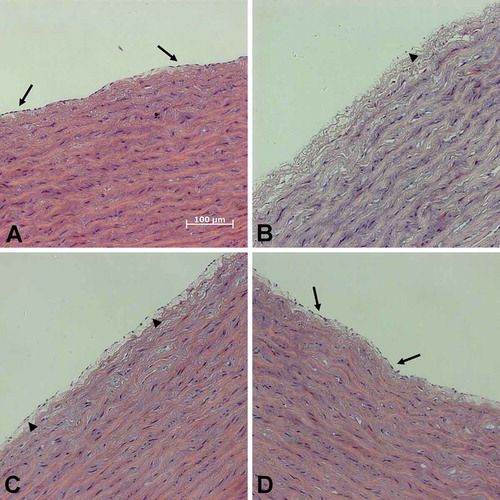
Integrity of porcine aortic segments after cryopreservation
Control segments without cryopreservation displayed a continuous endothelial monolayer and regular wall structures (). After cryopreservation in cell culture medium, the endothelial monolayer was either detached or widely disrupted with large gaps (). The endothelium of aortic segments frozen in solution 1 appeared partially detached, but less gaps in the endothelium could be observed. After freezing in solution 3, the endothelial monolayer was preserved best ().
FIGURE 7. Integrity of porcine aorta segments after thawing.Segments of porcine aortae (control; A) were cryopreserved in cell culture medium (B), solution 1 (C) or solution 3 (chloride-poor; D), all supplemented with 10% DMSO. After quick thawing in a 37°C water bath segments were fixed. Sections were stained with haematoxylin and eosin. Arrows: intact endothelial monolayer; arrowheads: detached endothelium.
Discussion
Our results show that post-thaw results of endothelial monolayers and aortic segments can be greatly improved by the use of the serum- and albumin-free vascular storage solution TiProtec (and modifications thereof) as compared to standard cell culture medium (, , , , and ).
Classical view of cryoinjury
Following the classical two-factor hypothesis, cryoinjury is attributed to purely physical processes, ice crystal formation and osmotic imbalance during the freezing/thawing process. A slow cooling rate is supposed to favour freezing of extracellular water, leading to extracellular hyperosmolarity, cellular water loss and resulting in osmotic stress, while a fast decrease in temperature is regarded to promote intracellular ice crystal formation associated with mechanical disruption of the cell membrane.Citation21–Citation25 Also, much emphasis is placed on potential cell damage caused by osmotic stress and volume excursion inflicted by the addition/removal of cryoprotectants, used in high concentrations, such as DMSO.Citation25,Citation26 These mechanisms are also considered for endothelial cells/vessels, so most approaches to optimize vascular cryoprotection are conducted along these lines.Citation26–Citation30 Little attention, however, is paid to the actual cryopreservation base solution; usually, cells and tissues are simply frozen in supplemented cell culture medium with addition of cryoprotectants.Citation3–Citation6,Citation9,Citation27,Citation28,Citation31
Cell death and proliferation delay
In addition to immediate cell death caused by the freezing/thawing process, delayed cell death following functional impairment of the cells is discussed.Citation25 In our experiments, early cell death directly after thawing was most pronounced in standard cell culture medium (). Also, most delayed cell death occurred in cell culture medium (additional loss of > 30% viability; ), while modified cold storage solutions protected against this delayed cell death (~ 15% additional cell death during 3 h of re-culture; ). This protective effect of the new solutions was similar in solutions 1, 2 and 3, thus being largely independent of anion and Na+/K+ composition.
Besides cell death, reduced proliferation rates in cryopreserved cells add to the overall cryopreservation injury. Reduced proliferation rates, at least for the first 24 h of re-culture, were described for human umbilical cord cellsCitation32,Citation33 and human saphenous vein endothelial cells.Citation34 In our study, cell proliferation could be improved by use of solutions 1 or 2 compared to M199 ().
Mitochondrial impairment during cryopreservation
For cold (4°C) storage injury, loss of mitochondrial membrane potential followed by mitochondrial permeability transition was identified as main pathway for cold-induced cell injury.Citation14,Citation35,Citation36 Loss of mitochondrial membrane potential was also described after cryopreservation, for example in sperm,Citation37–Citation39 mouse embryos,Citation40 muscle,Citation41,Citation42 hepatocytesCitation43 and in isolated mitochondria.Citation44,Citation45 Also, ultrastructural changes in mitochondria, like mitochondrial swelling and rupture have been described to occur in endothelial cells after vascular cryopreservation,Citation46,Citation47 however, little is known about mitochondrial membrane potential in this cell type. Here, almost complete loss of mitochondrial membrane potential was observed after cryopreservation in cell culture medium, while mitochondrial membrane potential was widely preserved in solutions 1, 2 and 3.
Another phenomenon described for various cell types during cold (4°C) storage is mitochondrial fragmentation, which is itself not necessarily associated with cell injury and is partially reversible during rewarming.Citation48–Citation50 Mitochondrial fragmentation, thought to be irreversible, was also described in cryopreserved porcine iliac arteries after rapid thawing.Citation46 In our study, in addition to loss of membrane potential, marked mitochondrial fragmentation after thawing was observed in all solutions, but was partially reversible during rewarming, if cells were cryopreserved in solutions 2 or 3 ().
Influence of chloride/lactobionate
For cold (4°C) storage, porcine aortic endothelial cells prefer chloride- and potassium-rich solutions.Citation10 Both, the high potassium concentration and a physiological extracellular chloride concentration increased survival of aortic endothelial cells during cold storage.Citation10 The high potassium concentration protected against the loss of the mitochondrial membrane potential.Citation10 For the cold storage of rat renal proximal tubules, extracellular chloride has recently been shown to protect mitochondria,Citation51 although the exact mechanism of mitochondrial protection is still unclear. In contrast to porcine aortic endothelial cells and rat renal proximal tubules, rat hepatocytes display a chloride-dependent cell injury after cold (4°C) storage in chloride-rich solutions and are therefore significantly better protected in chloride-poor solutions.Citation19,Citation20 This chloride-dependent cold-induced injury appears to involve lysosomal permeabilization.Citation52 In this setting, substitution of chloride by lactobionate might favour the maintenance of a slightly alkaline cytosolic pH (via inhibition of bicarbonate extrusion by the HCO3−/Cl− antiporter), thus limiting the adverse effects of released lysosomal enzymes. In contrast to the findings at 4°C, after cryopreservation, cell survival of both, rat hepatocytesCitation18 and porcine aortic endothelial cell monolayers ( and ) was higher in the chloride-poor solutions compared to their chloride-rich counterparts. Cryopreservation in chloride-poor solutions also seemed to improve protection of the endothelial layer of aortic segments (), while high potassium concentrations had no beneficial effect (, , , similar results in solutions 2 and 3). For cryopreservation, in contrast to cold storage,Citation19 this protection by the chloride-poor solutions could in part be caused by the presence of the fairly high concentration (95 mM) of the large impermeable anion lactobionate rather than by the mere absence of chloride. Lactobionate is a disaccharide derivative, and other disaccharides are known to act as cryoprotective agents in various cell typesCitation53; the presence of the disaccharide sucrose in combination with DMSO has also been shown to improve functionality of cryopreserved vessels.Citation54 Lactobionate might stabilize the plasma membrane during cryopreservation as known for other disaccharides and/or the low concentration of chloride might avoid chloride influx via a destabilized plasma membrane/fluid-to-gel phase transition during freezing/thawing.Citation55 Chloride fluxes in the frozen state, in contrast, are unlikely. Although the mechanism of lactobionate protection during cryopreservation is not resolved yet, it is interesting that for porcine aortic endothelial cells lactobionate-rich, chloride-poor solutions are favourable during cryopreservation while they are unfavourable during hypothermic storage (where, however, ion fluxes can occur during the whole storage period). Further experiments will have to show whether this is a general principle for cryopreservation (irrespective of the need for high or low chloride concentrations during hypothermic storage) or whether other cell types react differently.
Serum/albumin in cryopreservation
Addition of albumin or serum is generally thought to improve cryopreservation results.Citation5,Citation56 Also, since cryopreservation is often performed in supplemented cell culture media, high concentrations of serum are often already present in the cryopreservation solution.Citation6,Citation46,Citation57 However, especially for the use in tissue banking, serum-free cryopreservation is desirable, as serum is i) undefined and ii) of animal origin. Here, better results were achieved in serum-free cryopreservation solutions than in completed M 199 with 20% foetal calf serum (, , ).
Influence of different temperature gradients
The chosen freezing protocols with a short temperature dip to initiate crystallization and absorb the heat release, which result in a more or less linear decrease of sample temperature, showed good results in other cell types.Citation58,Citation59 Optimum cooling rates not only differ between different cell types, but also between cells in suspension and attached cells.Citation60,Citation61 Cells in suspension seem less affected by changes of cooling rates than attached cells, which show increasing cryopreservation damage with increasing cooling rates and meet their optimum cooling rates well below the standard temperature slope of −1°C/min.60,Citation61 We here compared three different cooling rates (0.1°C, 1°C and 5°C/min) and observed better results for solutions 1–3 than for cell culture medium with all three. Surprisingly, in contrast to previous publications on immortalized human endothelial cell monolayersCitation60 and corneal keratinocytes,Citation62 best results for porcine aortic endothelial cell monolayers were not achieved with the slowest cooling rate (0.1°C/min; ), but with a standard cooling rate of −1°C/min (). Von Bomhard et al. also produced good results with endothelial cell suspensions in a modified TiProtec solution in vitrification experiments (plunging in liquid nitrogen).Citation63
Monolayer model
In this study, we used endothelial cell monolayers as 2D-model of vascular endothelium that features cell-cell-contact between endothelial cells. Compared to cell suspensions, monolayers seem more susceptible to cryo-injury, especially at higher cooling rates,Citation61 which might be caused by intracellular ice crystal formation progressing via gap junctions.Citation61,Citation64 However, in cryopreserved vessels, more variables, like different requirements of endothelial cells and smooth muscle cellsCitation26 and fracturing of the vascular tissue after cryopreservationCitation65,Citation66 have also to be taken into account. As a simplified 3D-model, we here used aortic segments. First results indicate that the modified solutions also have a protective effect on endothelium and wall integrity in this compound tissue (). Clearly, further experiments with other vascular cells, with endothelial cells of other vascular provinces (differing in chloride conductivity) and with other vessel types will be required in the future.
Outlook
In a next step, the results of our experiments in endothelial monolayers and aortic segments have to be verified in 3D-models such as tissue-engineered vascular constructs and complete vessels, especially in small vessels in which endothelial integrity is particularly important.
Materials & methods
Chemicals and cryopreservation solutions
Freezing solutions are based on solution 8 of Wille et al.Citation10, which is potassium- and chloride-rich and contains iron chelators (solution 1, ; commercially available (with minor modifications) as the vascular preservation solution TiProtec®), since these components showed to be protective in cold storage of porcine aortic segments. Solution 2 is a chloride-poor variant with balanced Na+/K+ concentrations, as used earlier for cryopreservation of rat hepatocytes.Citation18 For direct comparison to TiProtec in terms of chloride concentration, solution 3 was used, which resembles solution 1 with respect to cation composition (high potassium concentration) but is chloride-poor (). N-acetylhistidine and the iron chelator LK 614 were kindly provided by Dr. F. Köhler Chemie; all solutions (composition see ) were prepared in our laboratory. All other chemicals were obtained from Sigma-Aldrich or Merck KGaA.
TABLE 1. Composition of cryopreservation solutions.
Cell culture
Porcine aortic endothelial cells were isolated as described by Peters et al. by gently scraping the endothelial cells off the intima of porcine aortae.Citation67 Cells were cultured in medium 199 (M 199) with Earle’s salt, supplemented with 100 IU/mL penicillin G, 100 µg/mL streptomycin, and 20% (vol/vol) fetal bovine serum. Subcultures were obtained by trypsinization (0.25% trypsin). For the experiments cells were split 1:3 and seeded onto collagen-coated six-well-plates (Sarstedt, #83.3920) or, for fluorescence microscopy, onto collagen-coated 28 mm glass coverslips (Thermo Scientific). The cells were used for experiments 2 days after subcultivation.
Experimental procedure – freezing/thawing
At the beginning of the experiments, cell cultures were washed three times with Hanks’ balanced salt solution (HBSS; 37°C), covered with supplemented cell culture medium, TiProtec or modified solutions (; all supplemented with 10% DMSO) at room temperature, and subsequently incubated at 4°C for 10 min to allow DMSO to enter the cells. Cell cultures were then transferred to the pre-cooled (4°C) cooling chamber of a controlled-rate freezer and cooled at a linear temperature gradient (−0.1°C/min for most experiments, −1°C/min and −5°C/min in additional series) to −4°C sample temperature. Then a short, rapid temperature drop to −40°C chamber temperature and immediately back to the approximate sample temperature was inserted to initialize crystallization, followed by linear decrease to −80°C at the initial cooling rate. For the slowest protocol (−0.1°/min), cooling rate was increased to −1°C/min at a sample temperature of −20°C. After 18–24 h storage at −80°C the monolayers were rapidly thawed in a shaking water bath (3 min, 37°C) and re-cultured in supplemented cell culture medium (M 199) for 1 to 24 hours.
Assessment of viability
Directly after thawing and after 3 h of re-culture, cells were stained with propidium iodide (PI; 5 µg/mL). Phase contrast micrographs and red fluorescence micrographs were taken using a Zeiss Observer.Z1 (Zeiss; λexc = 546 ± 6 nm/λem ≥ 590 nm). Dead cells (red fluorescent nuclei) and total cells were counted in nine fields of vision and viability was calculated as percentage of non-frozen control cells, thus taking into account the cell loss caused by cell detachment.
Determination of adherent viable cells
After 24 h of re-culture, adherent viable cells were quantified by assessment of (residual) intracellular lactate dehydrogenase (LDH) as described previously.Citation20
Metabolic activity
Cells were washed with HBSS, and 40 µM resazurin in warm HBSS + 10 mM D-glucose was added to the wells. Reductive conversion of resazurin to resorufin was followed in a plate reader (FluoStar Optima, BMG Labtec) at λexc. = 560 ± 10 nm and λem. ≥ 590 nm at 37°C for 10 min and conversion rate was calculated as percentage of that of control cultures.
Proliferation capacity
Sub-confluent endothelial cell cultures (24 h after subcultivation) were frozen at −0.1°C/min in M199, solution 1 or solution 2, each with 10% DMSO. Control cultures were cultured for another 24 h at 37°C (48 h total culture time after subcultivation) before lysis and determination of intracellular LDH. After 18–24 h storage at −80°C, frozen monolayers were rapidly thawed in a shaking water bath (3 min, 37°C) and re-cultured in supplemented cell culture medium (M 199) for 24 hours. Adherent viable cell density was then compared to control cultures (48 h after subcultivation) to compare proliferation rates.
Fluorescence microscopy of the mitochondria
For determination of mitochondrial integrity by fluorescence microscopy, cells were seeded onto collagen-coated glass coverslips in six-well plates. Before cryopreservation, cells were loaded for 40 min with 500 nM MitoTracker Green (molecular probes, #M7514) and 20 min with 200 nM tetramethyl rhodamine methyl ester (TMRM) in HBSS at 37°C. For TMRM, a maintenance concentration of 20 nM was used after loading. After thawing, the cryopreservation solutions were exchanged for Krebs-Henseleit buffer with 10 mM D-glucose and 20 nM TMRM. Fluorescence was assessed by confocal fluorescence microscopy using a Zeiss Observer.Z1 with ApoTome module (Zeiss) with filter sets for λexc = 546 ± 6 nm/λem ≥ 590 nm (TMRM) and λexc = 470 ± 20 nm/λem = 525 ± 25 nm (MitoTracker Green).
Studies in aortic segments
Porcine aortic segments were frozen in cell culture medium, solution 1 or solution 3 (50 mL, all supplemented with 10% DMSO) in 100-mL freezing bags at −1°C/min. After thawing (5 min in a water bath at 37°C), segments were fixed in formalin, embedded in paraffin blocks, cut and stained with haematoxylin and eosin.
Statistical analysis
Cell culture experiments were performed in triplicates 4–10 times (see respective figure legends). The Friedman test with post-hoc Dunn’s multiple comparison test was used to compare M199 with solutions 1–3 and solution 1 with solution 3. To limit the influence of inter-experiment variability, only sets from the same cell isolation were compared. P ≤ .05 was considered statistically significant.
Disclosure of interest
U. Rauen obtained consulting fees from Dr. Franz Köhler Chemie GmbH, Bensheim, Germany and the work was partially funded by a research grant by this company. Dr. F. Köhler Chemie GmbH holds a patent on TiProtec®, also covering the modified solutions used in this study. However, the study design and data interpretation has never been influenced by the company, which was also agreed upon in the funding contract.
Acknowledgments
We thank Ms. S. Enge and Mr. P. Haßenkamp for his excellent technical support.
Additional information
Funding
References
- Traore MA, George SC. Tissue engineering the vascular tree. Tissue Eng Part B Rev. 2017;23(6):505–514.
- Kimura H, Sakai Y, Fujii T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab Pharmacok. 2017. DOI:10.1016/j.dmpk.2017.11.003
- Jashari R, Van Hoeck B, Ngakam R, Goffin Y, Fan Y. Banking of cryopreserved arterial allografts in Europe: 20 years of operation in the European Homograft Bank (EHB) in Brussels. Cell Tissue Bank. 2013;14(4):589–599.
- Rendal Vazquez ME, Rodriguez Cabarcos M, Fernandez Mallo RO, Sanchez Ibanez J, Segura Iglesias R, Bermudez Gonzalez T, Matheu Capo G, Filgueira Fernandez P, Pertega Diaz S, Andion Nunez C. Functional assessment of human femoral arteries after cryopreservation. Cryobiology. 2004;49(1):83–89.
- Buzzi M, Mirelli M, Vaselli C, Tazzari PL, Terzi A, Stella A, Conte R. Vascular tissue banking: state of the art. Transplant Proc. 2005;37(6):2428–2429.
- Ku DD, Winn MJ, Grigsby T, Caulfield JB. Human coronary vascular smooth muscle and endothelium-dependent responses after storage at −75 degrees C. Cryobiology. 1992;29(2):199–209.
- Wusteman MC, Pegg DE, Warwick RM. The banking of arterial allografts in the United kingdom. A technical and clinical review. Cell Tissue Bank. 2000;1(4):295–301.
- Stanke F, Riebel D, Carmine S, Cracowski JL, Caron F, Magne JL, Egelhoffer H, Bessard G, Devillier P. Functional assessment of human femoral arteries after cryopreservation. J Vasc Surg. 1998;28(2):273–283.
- Langerak SE, Groenink M, van der Wall EE, Wassenaar C, Vanbavel E, van Baal MC, Spaan JA. Impact of current cryopreservation procedures on mechanical and functional properties of human aortic homografts. Transpl Int. 2001;14(4):248–255.
- Wille T, de Groot H, Rauen U. Improvement of the cold storage of blood vessels with a vascular preservation solution. Study in porcine aortic segments. J Vasc Surg. 2008;47(2):422–431.
- Zatschler B, Dieterich P, Muller B, Kasper M, Rauen U, Deussen A. Improved vessel preservation after 4 days of cold storage: experimental study in rat arteries. J Vasc Surg. 2009;50(2):397–406.
- Veres G, Hegedus P, Barnucz E, Schmidt H, Radovits T, Zoller R, Karck M, Szabo G. TiProtec preserves endothelial function in a rat model. J Surg Res. 2016;200(1):346–355.
- Garbe S, Zatschler B, Muller B, Dieterich P, Ebner A, Rauen U, Matschke K, Deussen A. Preservation of human artery function following prolonged cold storage with a new solution. J Vasc Surg. 2011;53(4):1063–1070.
- Rauen U, Kerkweg U, Weisheit D, Petrat F, Sustmann R, de Groot H. Cold-induced apoptosis of hepatocytes: mitochondrial permeability transition triggered by nonmitochondrial chelatable iron. Free Radic Biol Med. 2003;35(12):1664–1678.
- Rauen U, Petrat F, Li T, de Groot H. Hypothermia injury/cold-induced apoptosis – evidence of an increase in chelatable iron causing oxidative injury in spite of low O2−/H2O2 formation. FASEB J. 2000;14(13):1953–1964.
- Brecht M, de Groot H. Protection from hypoxic injury in cultured hepatocytes by glycine, alanine, and serine. Amino Acids. 1994;6(1):25–35.
- Frank A, Rauen U, de Groot H. Protection by glycine against hypoxic injury of rat hepatocytes: inhibition of ion fluxes through nonspecific leaks. J Hepatol. 2000;32(1):58–66.
- Pless-Petig G, Rauen U. Serum-free cryopreservation of primary rat hepatocytes in a modified cold storage solution – improvement of cell attachment and function. Biopreserv Biobank. 2018;in press. doi:10.1089/bio.2018.0002.
- Rauen U, Kerkweg U, de Groot H. Iron-dependent vs. iron-independent cold-induced injury to cultured rat hepatocytes: a comparative study in physiological media and organ preservation solutions. Cryobiology. 2007;54(1):77–86.
- Pless-Petig G, Singer BB, Rauen U. Cold storage of rat hepatocyte suspensions for one week in a customized cold storage solution – preservation of cell attachment and metabolism. PLoS ONE. 2012;7(7):e40444.
- Pegg DE. The relevance of ice crystal formation for the cryopreservation of tissues and organs. Cryobiology. 2010;60(3 Suppl):S36–44.
- Mazur P, Leibo SP, Chu EH. A two-factor hypothesis of freezing injury. Evidence from Chinese hamster tissue-culture cells. Exp Cell Res. 1972;71(2):345–355.
- Mazur P. A biologist’s view of the relevance of thermodynamics and physical chemistry to cryobiology. Cryobiology. 2010;60(1):4–10.
- Fuller B, Paynter S. Fundamentals of cryobiology in reproductive medicine. Reprod Biomed Online. 2004;9(6):680–691.
- Armitage WJ. Cryopreservation of animal cells. Symp Soc Exp Biol. 1987;41:379–393.
- Wusteman MC, Pegg DE. Differences in the requirements for cryopreservation of porcine aortic smooth muscle and endothelial cells. Tissue Eng. 2001;7(5):507–518.
- Arnaud F. Endothelial and smooth muscle changes of the thoracic and abdominal aorta with various types of cryopreservation. J Surg Res. 2000;89(2):147–154.
- Brockbank KG. Effects of cryopreservation upon vein function in vivo. Cryobiology. 1994;31(1):71–81.
- Lehle K, Hoenicka M, Jacobs VR, Schmid FX, Birnbaum DE. Identification and reduction of cryoinjury in endothelial cells: a first step toward establishing a cell bank for vascular tissue engineering. Tissue Eng. 2006;12(12):3439–3447.
- Rigol M, Heras M, Martinez A, Zurbano MJ, Agusti E, Roig E, Pomar JL, Sanz G. Changes in the cooling rate and medium improve the vascular function in cryopreserved porcine femoral arteries. J Vasc Surg. 2000;31(5):1018–1025.
- Krs O, Burkert J, Slizova D, Kobylka P, Spatenka J. Allograft semilunar cardiac valves processing and cryopreservation - morphology in scanning electron microscope. Cell Tissue Bank. 2006;7(3):167–173.
- Lehle K, Hoenicka M, Jacobs VR, Schmid FX, Birnbaum DE. Cryopreservation of human endothelial cells for vascular tissue engineering. Cryobiology. 2005;50(2):154–161.
- Polchow B, Kebbel K, Schmiedeknecht G, Reichardt A, Henrich W, Hetzer R, Lueders C. Cryopreservation of human vascular umbilical cord cells under good manufacturing practice conditions for future cell banks. J Transl Med. 2012;10:98.
- Bambang LS, Mazzucotelli JP, Moczar M, Beaujean F, Loisance D. Effects of cryopreservation on the proliferation and anticoagulant activity of human saphenous vein endothelial cells. J Thorac Cardiovasc Surg. 1995;110(4 Pt 1):998–1004.
- Rauen U, de Groot H. New insights into the cellular and molecular mechanisms of cold storage injury. J Invest Med. 2004;52(5):299–309.
- Salahudeen AK, Huang H, Joshi M, Moore NA, Jenkins JK. Involvement of the mitochondrial pathway in cold storage and rewarming-associated apoptosis of human renal proximal tubular cells. Am J Transplant. 2003;3(3):273–280.
- Martin G, Sabido O, Durand P, Levy R. Cryopreservation induces an apoptosis-like mechanism in bull sperm. Biol Reprod. 2004;71(1):28–37.
- Paasch U, Sharma RK, Gupta AK, Grunewald S, Mascha EJ, Thomas AJ, Jr., Glander HJ, Agarwal A. Cryopreservation and thawing is associated with varying extent of activation of apoptotic machinery in subsets of ejaculated human spermatozoa. Biol Reprod. 2004;71(6):1828–1837.
- Forero-Gonzalez RA, Celeghini EC, Raphael CF, Andrade AF, Bressan FF, Arruda RP. Effects of bovine sperm cryopreservation using different freezing techniques and cryoprotective agents on plasma, acrosomal and mitochondrial membranes. Andrologia. 2012;44(Suppl 1):154–159.
- Sohn IP, Ahn HJ, Park DW, Gye MC, Jo DH, Kim SY, Min CK, Kwon HC. Amelioration of mitochondrial dysfunction and apoptosis of two-cell mouse embryos after freezing and thawing by the high frequency liquid nitrogen infusion. Mol Cells. 2002;13(2):272–280.
- Kuznetsov AV, Kunz WS, Saks V, Usson Y, Mazat JP, Letellier T, Gellerich FN, Margreiter R. Cryopreservation of mitochondria and mitochondrial function in cardiac and skeletal muscle fibers. Anal Biochem. 2003;319(2):296–303.
- Larsen S, Wright-Paradis C, Gnaiger E, Helge JW, Boushel R. Cryopreservation of human skeletal muscle impairs mitochondrial function. Cryo Letters. 2012;33(3):170–176.
- Stephenne X, Najimi M, Ngoc DK, Smets F, Hue L, Guigas B, Sokal EM. Cryopreservation of human hepatocytes alters the mitochondrial respiratory chain complex 1. Cell Transplant. 2007;16(4):409–419.
- De Loecker P, Fuller BJ, De Loecker W. The effects of cryopreservation on protein synthesis and membrane transport in isolated rat liver mitochondria. Cryobiology. 1991;28(5):445–453.
- Fuller BJ, Rubinacci A, Geboes K, De Loecker W. The bioenergetics of mitochondria after cryopreservation. Cryobiology. 1989;26(4):333–340.
- Pascual G, Rodriguez M, Corrales C, Turegano F, Garcia-Honduvilla N, Bellon JM, Bujan J. New approach to improving endothelial preservation in cryopreserved arterial substitutes. Cryobiology. 2004;48(1):62–71.
- Song YC, Hunt CJ, Pegg DE. Cryopreservation of the common carotid artery of the rabbit. Cryobiology. 1994;31(4):317–329.
- Rauen U, Kerkweg U, Wusteman MC, de Groot H. Cold-induced injury to porcine corneal endothelial cells and its mediation by chelatable iron - Implications for corneal preservation. Cornea. 2006;25(1):68–77.
- Pless G, Sauer IM, Rauen U. Improvement of the cold storage of isolated human hepatocytes. Cell Transplant. 2012;21(1):23–37.
- Kerkweg U, Jacob M, de Groot H, Mannherz HG, Rauen U. Cold-induced apoptosis of rat liver endothelial cells: contribution of mitochondrial alterations. Transplantation. 2003;76(3):501–508.
- Bienholz A, Walter B, Pless-Petig G, Guberina H, Kribben A, Witzke O, Rauen U. Characterization of injury in isolated rat proximal tubules during cold incubation and rewarming. PLoS One. 2017;12(7):e0180553.
- Knoop S. Untersuchungen zur Beteiligung der Lysosomen und des Cytoskeletts an der kälteinduzierten Zell- und Gewebeschädigung [dissertation]. Essen (Germany): Fakultät für Chemie, Universität Duisburg-Essen; 2011.
- Elliott GD, Wang S, Fuller BJ. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology. 2017;76:74–91.
- Muller-Schweinitzer E, Ellis P. Sucrose promotes the functional activity of blood vessels after cryopreservation in DMSO-containing fetal calf serum. Naunyn Schmiedebergs Arch Pharmacol. 1992;345(5):594–597.
- Glafke C, Akhoondi M, Oldenhof H, Sieme H, Wolkers WF. Cryopreservation of platelets using trehalose: the role of membrane phase behavior during freezing. Biotechnol Prog. 2012;28(5):1347–1354.
- Stevenson DJ, Morgan C, Goldie E, Connel G, Grant MH. Cryopreservation of viable hepatocyte monolayers in cryoprotectant media with high serum content: metabolism of testosterone and kaempherol post-cryopreservation. Cryobiology. 2004;49(2):97–113.
- Ku DD, Willis WL, Caulfield JB. Retention of endothelium-dependent vasodilatory responses in canine coronary arteries following cryopreservation. Cryobiology. 1990;27(5):511–520.
- Lee JH, Jung DH, Lee DH, Park JK, Lee SK. Slow cooling rate with a shock cooling program can effectively cryopreserve pig hepatocytes. Transplant Proc. 2012;44(4):1002–1004.
- Seth G. Freezing mammalian cells for production of biopharmaceuticals. Methods. 2012;56(3):424–431.
- Pegg DE. Cryopreservation of vascular endothelial cells as isolated cells and as monolayers. Cryobiology. 2002;44(1):46–53.
- Armitage WJ, Juss BK. The influence of cooling rate on survival of frozen cells differs in monolayers and in suspension. Cryo Letters. 1996;17:213–218.
- Armitage WJ. Preservation of human cornea. Transfus Med Hemother. 2011;38(2):143–147.
- von Bomhard A, Elsasser A, Ritschl LM, Schwarz S, Rotter N. Cryopreservation of endothelial cells in various cryoprotective agents and media - vitrification versus slow freezing methods. PLoS One. 2016;11(2):e0149660.
- Armitage WJ, Juss BK. Freezing monolayers of cells without gap junctions. Cryobiology. 2003;46(2):194–196.
- Pegg DE, Wusteman MC, Boylan S. Fractures in cryopreserved elastic arteries. Cryobiology. 1997;34(2):183–192.
- Hunt CJ, Song YC, Bateson EA, Pegg DE. Fractures in cryopreserved arteries. Cryobiology. 1994;31(5):506–515.
- Peters S, Reis A, Noll T. Preparation of endothelial cells from micro- and macrovascular origin. In: Dhein S, Mohr F, Delmar M, editors. Practical methods in cardiovascular research. Heidelberg, Germany: Springer; 2005. p. 610–629.
