ABSTRACT
Adipose-derived stromal cells have multilineage potential to differentiate into several specialized tissue types. Herein, we investigated whether ADSCs could differentiate into lymphoid node in vivo. Human ADSCs from routine liposuction were cultured in differentiation medium and were supplemented with transforming growth factor β1 (TGF)-β1 and basic fibroblast growth factor (bFGF). The induced hADSCs mixed with 13% (w/v) hydroxypropyl methylcellulose (HPMC) were injected into BALB/c nude mice subcutaneously. Eight weeks later, nodules were found under the injected sites. Histology, immunohistochemistry, and species identification analysis confirmed that the nodules were lymphoid nodes that were derived from the injected hADSCs. Our experiment demonstrated that the hADSCs could differentiate into lymphocyte-like cells and form lymphoid nodes in vivo. TGF-β1 and bFGF might play important roles in the differentiation of hADSCs into lymphocyte-like cells. Our study might present an alternative approach for engineering immune organs and thus offer potential treatment for immunodeficiency diseases.
1. INTRODUCTION
Diseases caused by primary or secondary immunodeficiencies are severe and sometimes fatal. Such diseases as malignancies metastasizing into lymph nodes also cause lymphatic injury and damage. Affected individuals would increase likelihood of infection, allergies, severe lymphedema, or even death.Citation1 More and more studies are focused on developing artificial lymphoid tissues using appropriate biomaterials. Daniel et al. engineered lymph nodes by using SDS to decellularize the lymph node of mice and repopulated it with leukocytes harvested from the spleen of another donor mouse.Citation2 Purwada et al. first engineered gelatin hydrogels combined with polyionic silicate nanoparticles to encapsulate murine germinal center B cell clusters in 2D cultures.Citation3 However, there are no widely accepted techniques at present. This may due to the complex structure of the lymphoid organ and constant movement of the immune cells.
To date, the use of multilineage stem cells combined with ideal scaffolds has gained significant favor for tissue engineering and has been used for decades.Citation4,Citation5 Adipose-derived stem cells (ADSCs) are a plentiful population of adult stem cells that are readily isolated from adipose tissue.Citation6 The characteristics of ease of isolation, high cell yields, immunomodulation, and multilineage potency qualify ADSCs as an ideal source for the repair or replacement of damaged tissues in clinical applications.Citation7 Scientists have successfully developed nerve, cartilage, muscle, liver, and adipose tissues, etc., using stem cell transplantation. But engineering lymphoid tissues still remain elusive. In this study, we tried to investigate the potential of human ADSCs as seeded cells combined with hydroxypropyl methylcellulose (HPMC) in constructing tissue engineered lymphoid nodes in vivo, and may offer a good technique to deliver immune cells after implantation against various diseases.
2. MATERIALS AND METHODS
2.1 Isolation of human ADSCs
hADSCs were obtained from human lipoaspirates from normal donors after informed consent was given. The Ethics Committee of the Fourth Military Medical University approved this study, and an informed consent was signed by the patient. The harvest liposuction aspirates (50 ml) were digested with 0.075% collagenase type II (Sigma–Aldrich, St. Louis, MO, USA) and the isolated cells were resuspended and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Sigma) containing fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA), 100 U/ml penicillin, and 100 ìg/ml streptomycin (Invitrogen). Briefly, the digested adipose tissue was filtered through a 150-μm cell strainer, and floating adipocytes were separated from the medium containing the stroma-vascular fraction by centrifugation at 3000 rpm for 10 min. After centrifugation, the solution was washed extensively with red blood cell lysis buffer (0.38% NH4Cl 0.05 mmol, Na2EDTA 0.1%, K2HCO3 pH 7.3) to remove the red cells, filtered through a 200-µm cell filter to remove cellular debris, and centrifuged again at 1800 rpm for 10 min. Then, the resultant cells were incubated at 37°C in a humidified atmosphere containing 5% CO2. Cell viability and proliferation were evaluated by an MTT assay.
2.2 hADSCs phenotypic analysis
hADSCs were detached using trypsin-EDTA (Invitrogen), washed in phosphate buffered saline (PBS), and immediately stained with mouse monoclonal antibodies against human CD14, CD31, and CD90 conjugated with fluorescein isothiocyanate (FITC); CD34, CD45, CD49d, CD29, CD56, and CD106 conjugated with PE (all from Immunotech Beckman Colter, San Jose, CA, USA). hADSCs were washed twice with ice-cold PBS supplemented with 1% bovine serum albumin (Invitrogen), followed by incubation with saturating concentrations of the appropriate antibodies for 15 min at room temperature. Thereafter, cells were washed twice in ice-cold PBS, 1% BSA and fixed with 1% paraformaldehyde in PBS. Samples were analyzed using a FACSCalibur (Becton-Dickinson, San Jose, CA, USA) and CellQuest analysis software.
2.3 Biocompatibility assessment of hydroxypropyl methyl cellulose to ADSCs
Preparation of HPMC hydrogels
HPMC powder was sterilized by CoCitation60 irradiation. Then, an appropriate amount of HPMC was mixed with the culture medium and stirred to dissolve. The final product was a viscous liquid at pH 7.4. A scanning electron microscope (SEM) assay was used to observe the morphological features of HPMC hydrogels at the following concentrations: 10% (w/v), 13% (w/v), and 15% (w/v)).Citation8
Cells seeded in HPMC
HPMC was prepared with culture medium and placed in 6-well plates for cell seeding. Subsequently, passage 6 hADSCs at a density of 1 × 104 cells/cm2 were gently mixed with the above HPMC hydrogel and incubated at 37°C, 5% CO2 for 12 h. Morphological assessments of the cells seeded with HPMC were performed using the SEM assay.
Cell proliferation in HPMC
The 10% (w/v), 13% (w/v), and 15% (w/v) HPMC hydrogels with passage 6 hADSCs were transferred to a 24-well plate and cultured for 24 h. Then, the cell viability of hADSCs from each group was measured using a Cell Counting Kit-8 (CCK-8; Beyotime, Shanghai, China). Next, 13% (w/v) HPMC hydrogel was selected to mix with passage 6 hADSCs. The mixtures were then transferred to a 24-well plate and cultured for 0, 12, or 24 h, at which time the cell viability of ADSCs from each group was measured using the CCK-8 assay (a similar amount of cells resuspended in culture medium alone was used as a control). For the CCK-8 assay, 50 μl CCK-8 reagent was added to each well and incubated at 37°C for 4 h. Then, 100 μL of the resulting solution was transferred to a 96-well plate, and six parallel replicates were prepared. The optical density (OD) of each sample was measured at 450 nm using a microplate reader (Multiskan MK33, Thermo Electron, China).Citation9
2.4 Lymph cyte-like cells transformation in vitro
Passage 6 hADSCs were seeded at a density of 5 × 105/cm2 in 6-well plates and cultured in cytokine-supplemented medium containing 10 ml/L insulin transferrin-sodium selenite medium supplement, 15 ìg/L TGF-â1, 25 ìg/L bFGF, 10−Citation7 M dexamethasone, 10% FBS, 100 U/ml penicillin, and 100 ìg/ml streptomycin. All cells were incubated at 37°C in a humidified atmosphere containing 5% CO2 and 95% air. The culture medium was changed every 2 to 3 days. When cells were grown to 90% confluence, they were then detached using trypsin/EDTA. Immunocytochemistry analysis of CD3, CD20, CD45RO, and CD79a (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used to assess the lymphocytic characteristics of the induced hADSCs after crawled on the coverslips.
2.5 In vivo assays
2.5.1 Preparation of HPMC hydrogels
HPMC powder (with gelation temperature: 37°C, hydroxypropyl content: 5–7%, methoxyl content: 26–28%, molecular weight: 243.96 g/M, sigma) was sterilized by CoCitation60 irradiation. An appropriate amount of HPMC was mixed with the DMEM/F12 (Gibco, Invitrogen) culture medium and stirred to dissolve into a viscous liquid. The final concentration was 13% (weight/volume) with a pH of 7.4Citation8 (The reason for choosing 13% was shown in the “supplements”).
2.5.2 hADSCs seeded in HPMC hydrogel
Passage 6 hADSCs at a density of 1 × 104 cells/cm2 were gently mixed with 13% (w/v) HPMC hydrogel in 6-well plates and incubated at 37°C, 5% CO2 for 12 h. Morphological assessments of the cells seeded with HPMC were performed using a scanning electron microscope (SEM) assay.
2.5.3 Lymphoid nodes generation in vivo
A total of eight male BALB/c nude mice (aged 6 to 8 weeks) were purchased from the Animal Center of the Fourth Military Medical University. Ethical approval was given by the medical ethics committee of the Fourth Military Medical University with the following reference number XJYYLL-2013022. As controls, three mixtures were used to administer the injections in the following experiment. Mixture 1 contained passage 6 hADSCs induced by 15 μg/L TGF-β1, 25 μg/L bFGF, and 13% w/v HPMC. Mixture 2 contained the same ingredients as mixture 1, except that is lacked cytokines of TGF-â1 and bFGF. Mixture 3 contained the same ingredients as mixture 1 except for HPMC. To improve comparability and reduce the variability, each mouse was injected at six sites in the dorsal subcutaneous tissue with the three mixtures (the sites of injection were distributed symmetrically along the middle line on the back of each mouse). Each recipient received an injection of mixtures 1 at two sites, respectively, and of mixtures 2 and 3 at one site, respectively. At each site, 0.3 ml mixture was injected. Specimens were harvested 8 weeks after implantation. Each specimen was divided into two parts, and one was used for histological analysis and the other was saved for species identification analysis. Samples used for histological analysis were fixed for 18–24 h in 10% buffered formalin and were routinely processed and embedded in paraffin. Hematoxylin and eosin staining was performed on 5-µm serial paraffin sections.
2.5.4 Species identification analysis
Samples were analyzed at the State Key Laboratory of the Ministry of Health for Forensic Sciences of Xi’an Jiao Tong University, Xi’an, China. Genomic DNA was extracted from both the subcutaneous nodes of experimental nude mice and hADSCs from the donor. A total of human STR loci specific to the donor were co-amplified in a fluorescence-based multiplex reaction using an ampFLSTR Identifier kit (Applied Biosystems, Foster City, CA, USA). The amplification reactions (20 μl total volume) contained 20 ng/μl genomic DNA template. Thermal cycling conditions were conducted according to the manufacturer’s protocols of the kit using GeneAmp PCR system 9600 (Applied Biosystems). Electrophoresis detection and genotyping of all PCR products were carried out using capillary electrophoresis on an ABI310 DNA Genetic Analyzer (Applied Biosystems). Allele designations were determined by comparing the sample PCR fragments with the allelic ladders provided with kit using GeneScan3.7 and Genotyper3.7. Alleles of all loci were named according to the number of repeat units detected as recommended by the DNA Commission of the International Society of Forensic Hemodgenetics.
2.5.5 Immunohistochemical analysis
Serial sections from each subcutaneous node were stained with mouse monoclonal antibodies specific to the following human T or B lymphocyte markers: CD3, CD20, CD45RO, and CD79α (1:50; DAKO, Glostrup, Denmark). Immunohistochemical staining was performed using a standard EnVision detection system (DAKO). Visualization was performed using diaminobenzidine for horseradish peroxidase. Sections were counter-stained with hematoxylin, dehydrated, cleared, and mounted. Specimens obtained from human spleen and lymph nodes were used as positive controls, and spleen tissues obtained from the experimental and non-experimental nude mice were used as negative controls.
2.6 Statistical analysis
Experimental data were analyzed using SPSS 11.0 statistical software (SPSS Predictive Analytics, Chicago, IL, USA). Pearson’s chi-square test was applied to detect statistically significant differences and correlations; p-values less than 0.05 were considered to indicate statistically significant differences.
3. RESULTS
3.1 Proliferation and differentiation of hADSCs
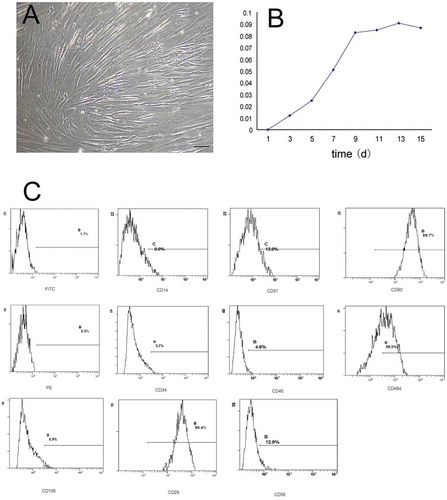
The hADSCs were obtained from human lipoaspirates of normal female donors, and then were isolated as previously described.Citation10 Most of hADSCs cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) maintained a fusiform shape with oval nuclei that were arranged in a swirl pattern ()). Cells were passaged every 5 to 9 days, and cells could reach passage 9. Subsequently, cells grew more slowly and became obviously aged ()).
FIGURE 1. Characteristics of hADSCs isolated by liposuction.
A, Morphological observation of hADSCs isolated by liposuction. The hADSCs cultured in 10% DMEM maintained a fusiform shape with oval nuclei that were arranged in a swirl pattern. Bar = 100 µm; B, Using the MTT assay, the proliferation of hADSCs cultured in DMEM with 10% FBS is shown. C, Flow cytometric analysis of CD markers expressed by hADSCs.
3.2 Phenotypic characteristics of isolated hADSCs
The hADSCs cultured at passage 6 were immunophenotyped by flow cytometry. After plastic adherence selection, antigenic phenotypes of hADSCs cultured at passage 6 were carefully confirmed by flow cytometry. We found that cells weakly expressed the hematopoietic lineage markers CD14, CD31, CD34, CD45, CD56, CD105, and CD106, while they highly expressed CD49d, CD29, and CD90, consistent with non-hematopoietic cells that resemble hADSCs ()), ).Citation11
TABLE 1. Flow cytometric analysis of CD markers for hADSCs showed that cells that weakly expressed hematopoietic lineage markers (CD14, CD31, CD34, CD45, CD56, and CD106) and highly expressed lineage markers (CD49d and CD90) were not expressed in hematopoietic lineage cells but were in hADSCs
3.3 Biocompatibility evaluation of HPMC to hADSCs
To select an HPMC hydrogel with appropriate physical properties, we compared a series of HPMC hydrogels with different concentrations. Our SEM results showed that a 10% (w/v) HPMC hydrogel exhibited tiny honeycomb-like features ()) and a 15% (w/v) HPMC hydrogel showed lamellar structures ()); both hydrogels lacked appropriate three-dimensional space for cell adhesion and development. However, a 13% (w/v) HPMC hydrogel showed clear cribriform patterns, and the void ratio was well-suited for cell proliferation and differentiation ()). Then, the cytocompatibility of the HPMC hydrogel was evaluated by cell attachment and proliferation of hADSCs. To assess cell attachment, an SEM assay showed that hADSCs adhered well to the fibers of 13% (w/v) HPMC, maintained a high vitality and a typical morphology with elongated processes, and a formed many cell-to-cell contacts ()).
FIGURE 2. Biocompatibility evaluation of HPMC to hADSCs.
The morphology of HPMC hydrogels at different concentrations analyzed using a scanning electron microscope (SEM). The SEM assay was used to observe morphological characteristics of HPMC hydrogels at different concentrations. A, 10% (w/v) HPMC hydrogel exhibited tiny honeycomb-like features. B, The 13% (w/v) HPMC hydrogel showed clear cribriform patterns, and the void ratio was suited for cell proliferation and differentiation. C, The 15% (w/v) HPMC hydrogel showed lamellar structures. D, An SEM assay of hADSCs seeded with 13% (w/v) HPMC showed that the hADSCs were strongly attached to the HPMC with cytoplasmic extensions and lamellipodia. E, The cell viability of hADSCs seeded alone (blue group) or with an HPMC hydrogel at different concentrations (red group) was measured by CCK-8 assays; *p < .05 (independent samples group t-test) F, The cell proliferation of hADSCs seeded alone (blue group) or with 13% (w/v) HPMC hydrogel (red group) was evaluated at different time points using CCK-8 assays; *p < .05 (independent samples group t-test).
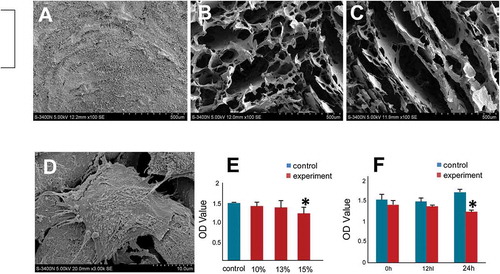
CCK-8 assays, which can be used as a quantitative indication for cell proliferation, showed that viability of hADSCs mixed with HPMC or incubated in culture medium alone increased over time. To investigate whether HPMC exhibited a dose-response to hADSCs, we tested the viability of hADSCs seeded with HPMC of different concentrations using the CCK-8 assay. We found that when mixed with hADSCs, only 15% (w/v) HPMC influenced the viability of hADSCs by a t-test of two independent samples (p < .05, )). Then, we evaluated the influence of HPMC on cell proliferation using a CCK-8 assay. We observed that the viability of hADSCs seeded alone increased with time, but when seeded with 13% (w/v) HPMC, the viability of hADSCs decreased significantly after 24 h (p < .05, )). Additionally, no significant difference between the two groups was observed at any time point by one-way ANOVA analysis (p > .05, )). All of these findings indicated that HPMC at a concentration of 13% (w/v) was the best at supporting attachment and proliferation for cell seeding.
3.4 hADSCs differentiated to lymphocyte-like cells in vitro after the inducement
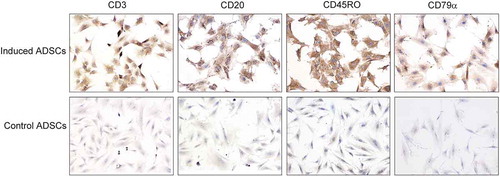
Immunocytochemistry results showed that passage 6 hADSCs, cultured in the induced medium for 14 days, were strongly positive for lymphocytic markers including CD3, CD20, CD45RO, and CD79a compared with the control groups (). It suggested that hADSCs could be stimulated and changed to lymphocytes, and TGF-β1 and bFGF might play a key role during the course.
FIGURE 3. hADSCs differentiation into lymphocyte-like cells in vitro.
Immunocytochemistry results showed that passage 6 hADSCs cultured in the lymphocytic inducement medium for 14 days were strongly positive for lymphocytic markers including CD3, CD20, CD45RO, and CD79a compared with the control groups. Bar = 100 µm.
3.5 hADSCs combined with HPMC could form lymph-like nodes in vivo
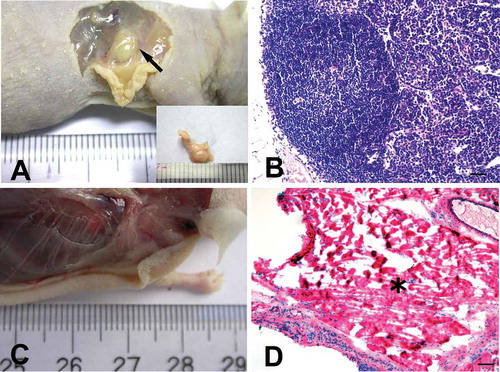
At 8 weeks after implantation, 10 nodules were found in 16 sites where mixture 1 was injected. On gross inspection, these nodules were typically grey–white, ovoid or bean-shaped, and ranged from 2 to 3 mm in diameter (black arrow, )). By contrast, no nodules were observed where mixtures 2 and 3 were injected (–)). These data are presented in .
TABLE 2. The probability of cartilage-like tissues or lymph nodes formed in nude mice of different mixtures after injection for 8 weeks. Overall, 10/16 nodes were formed in the mixture 1 group, which contained hADSCs, HPMC, 15 µg/L TGF-β1 and 25 µg/L bFGF. In contrast, no nodes were observed in either the mixture 2 or 3 groups, which contained the same ingredients as mixture 1 but without cytokines (mixture 2) or HPMCs (mixture 3) (chi-square test, χ2= 6.348, p = .012)
FIGURE 4. hADSCs formed lymphoid nodules in vivo.
A, At 8 weeks after injection, a round nodule had formed subcutaneously under the injection site (black arrow) where passage 6 hADSC after induction were mixed with 13% (w/v) HPMC hydrogel. B, Hematoxylin and eosin staining showed a lymphoid node in the newly formed nodule, with clearly lymphoid follicle, deputy cortex, sinuses and other typical lymphoid microstructures inside the nodule. Bar = 100 µm. C. No nodule was observed in nude mice that were injected with passage 6 hADSC after induction, but without HPMC hydrogel. D. Hematoxylin and eosin staining showed that only degenerated and necrotic muscle tissues (black asterisk) were found at the injection sites where mixture 2 or 3 (without cytokines or HPMC) was injected. Bar = 100 µm.
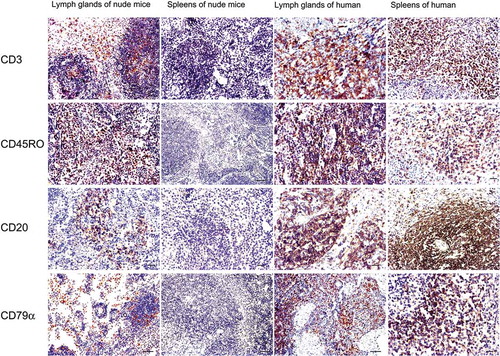
The histological features of these nodules were also evaluated under the microscope. Microscopically, the nodule was dominated by a population of small lymphocyte-like cells which just resembled a lymph node (characteristically contained cortical layer and inner medulla) even with the formation of lymphoid follicles, and such structures as sinuses and cords can also be observed in the medulla layer ()). Immunohistochemical results showed that the small dark-blue cells could be distinguished by four mouse monoclonal antibodies against the human T cell markers CD3 and CD45RO and the B cell markers CD20 and CD79α. The small lymphocyte-like cells were strongly positive for CD3 and CD45RO between the lymphoid follicles, while some of the cells inside the follicles were weakly positive for B cell markers including CD20 and CD79α. No immunoreactivity was detected in the spleens of the experimental nude mice or the control nude mice. In human spleen and lymph node tissues, the lymphocytes were positive for all four antigens ().
FIGURE 5. Immunohistochemical staining for T and B lymphocyte markers of the newly formed lymphoid nodules and spleens of the nude mouse compared with human markers.
In the newly formed node of the nude mice, lymphocyte-like cells were partly positive for the T cell markers CD3 and CD45RO between or inside the follicles, and some of the cells were weakly positive for B cell markers CD20 and CD79α that were mainly located inside the follicles. In human lymph node and spleen tissues, CD20- and CD79α-positive cells were mainly found inside the follicles, while CD3- and CD45RO-positive lymphocytes were mainly distributed throughout the interfollicular areas. No immunoreactivity was detected in spleens of the experimental nude mice. Bar = 50 µm.
3.6 Statistical analysis
Analysis of the lymph node formation rate of the mixture 1 versus mixture 2 groups showed significant differences (chi-square test, χ2= 6.348, p = .012).
3.7 Species identification of newly formed lymph nodes after hADSc transplantation
To determine whether the nodes that formed in the subcutaneous tissue of nude mice contained cells that differentiated from donor hADSCs, species identification was performed. Genomic DNA was extracted from both the subcutaneous nodes of experimental nude mice and donor hADSCs. A total of eight human-specific STR loci were used as human genetic markers. The species identification report (No.2007101) released confirmed that the lymph nodes from the nude mice implanted with hADSCs shared DNA markers with the human donor ().
TABLE 3. The species identification report (No. 2007101) showed that the lymph nodes formed in the nude mice shared the same human DNA genetic markers as the human adipose-derived stromal cells (hADSCs) of the human donor
4. DISCUSSION
Immunotherapy is a developing and promising approach to treat cancer and immunodeficiency disorders. In recent years, with an in-depth understanding of the immune system, immunotherapy has been developed to treat patients with a high efficacy. For examples, cytotoxic T cell therapies, such as chimeric antigen receptor T, CAR-T, have been highly acclaimed due to their direct killing property. However, a real problem is the difficulty in obtaining adequate number of cells.Citation12 Alternatively, several groups have focused energies in generating functional T cells from stem cells. Previous reports reported antigen-specific T lymphocytes can be generated from stem cells.Citation13,Citation14 Nevertheless, concerns have been raised on non-specific differentiation and/or extended in vivo development of stem cell-derived, antigen-specific mature lymphocytes such as T cells.
In this study, we showed that hADSCs isolated from human lipoaspirate could differentiate into chondrocytes, osteocytes, adipocytes and especially into lymphocyte-like cells in vitro. We established that the hADSCs induced by TGF-β1 and bFGF could form lymphoid nodules in vivo when mixed with 13% (w/v) HPMC and injected into the hypoderma of BALB/c nude mice. Our findings suggested that hADSCs might have the potential used in both tissue engineering and immunotherapy.
Many studies have testified that ADSCs have multilineage differentiation potential and can develop into nerve, cartilage, muscle, and adipose tissues under given conditions.Citation6 Rodriguez et al. described the isolation and culture of adipose tissue-derived stem cells with multipotent differentiation capacity at the single-cell level.Citation15 In one study, ADSCs might represent a potential cell-based therapeutic method for the treatment of PDAC (ductal adenocarcinoma of pancreas)Citation16 and facilitate lymphopoiesis.Citation17 Technau et al.Citation18 found that ADSCs exhibited both immunogenic and immunomodulatory properties after adding cytokine TGF-β3. It can be proposed that nude mice might provide an immunological environment similar to lethally irradiated mice. It is well known that both red and yellow bone marrow can be found in the medullary canal of bone. In yellow bone marrow, stroma of the reticular network is primarily filled with fat. Under certain conditions, such as anemia or severe blood loss, yellow marrow can turn into red marrow to generate more blood cells. Therefore, we propose that the multi-directional differentiation potential of hADSCs in adipose tissue might be evoked under certain conditions, which could explain why hADSCs might differentiate into the lymphoid lineage and become involved in local immune responses or the recovery process to accommodate the needs of the host.
An important question of this present study is that which types of cells were contained inside the lymphoid nodes formed in the nude mice and whether they were derived from the injected hADSCs. To address these questions, experiments were designed for two aims: using species identification to determine whether the newly formed lymphoid nodes in the nude mice were derived from the donor’s hADSCs and using immunohistochemistry method to identify the cell types inside the newly formed nodes. Species identification results confirmed that the lymphoid nodes formed in the nude mice after hADSC transplantation shared the same human DNA genetic markers with the female donor. Immunohistochemical analyses showed that human T and B cells could be distinguished by mouse monoclonal antibodies against human T cell markers (CD3 and CD45RO) and B cell markers (CD20 and CD79α), whereas no immunoreactive lymphocytes were observed in spleens of experimental or control nude mice using the same antibodies. These findings suggested that these antibodies were specific to human lymphocytes which had no cross reactivity with the mouse antigens, and that the newly formed lymphoid nodes contained human T and B cells that should be coming from the donor’s hADSCs.
In this study, such cytokines as TGF-β1 and bFGF may play important roles in the differentiation of hADSCs into lymphocyte-like cells. Of the results, analysis of the lymphoid node formation rate of the mixture 1 group (with 15 μg/l TGF-β1 and 25 μg/l bFGF) versus the mixture 2 group (without cytokines) showed significant differences. TGF-β1 and bFGF are both cytokines that have been reported to have effects on the regulation of cell differentiation and synthesis of the extracellular matrix. TGF-β1 has been implicated in the development and maintenance of various organs, and can evoke important signaling pathways to induce many types of cell differentiation in vitro.Citation19 For example, TGF-β1 can up-regulate several differentiation markers of chondrocytes such as collagen type II, aggrecan, and SRY-related high mobility group-box gene 9, as well as cartilage oligomeric matrix protein gene expression.Citation20 In one recently published study, the results showed that TGFβ signaling was required for hematopoietic stem cell emergence in embryos, and in detail, TGFβ1 and TGFβ3 could program endothelium to become hemogenic endothelium through regulating jag1 expression.Citation21 bFGF is one of the several heparin-binding growth factors that can affect the growth and differentiation of many cell types.Citation22–Citation24 Previous studies established that bFGF could increase both cell harvest during monolayer expansion in culture and the capacity of chondrocytes to maintain expression of cartilage-specific markers during successive three-dimensional cultures in vitro.Citation25 Chua et al.Citation26 found that under the common actions of insulin-like growth factor-1 with bFGF and TGF-β, the tissue engineering of cartilage formation could be accelerated. For hematopoietic lineage differentiation, several studies have found that bFGF combined with other factors like BMP4 and Activin A could promote hematopoietic fated mesodermal specification from pluripotent human cells, which may help to explain the development of the hematopoietic system for embryon research.Citation27,Citation28 Our findings also showed that the absence of bFGF and TGF-β1 could significantly reduce the formation of lymph nodes in nude mice after hADSC transplantation. Therefore, further work is needed to investigate the role of cytokines in the differentiation of hADSCs into lymphocytes.
Scaffold is another important factor for new tissue formation and remodeling, also helps to facilitate host tissue integration after implantation.Citation29 Good scaffold should possess appropriate physicochemical properties and biocompatibility for seeded-cell attachment, proliferation, and differentiation.Citation30,Citation31 A few of highly biocompatible materials have been testified effective in tissue engineering, including extra cellular matrix (ECM) components, polyglycolic acid (PGA), polylactic co-glycolic acid (PLGA), and hydrogelsCitation32, etc. In this study, we choose to use a hydrogel named hydroxypropyl methyl cellulose (HPMC), which has been mostly used as a delivery system in food, pharmaceutical, and other biomedical applications because of its cheap and nontoxic characters.Citation33 With high molecular weights and at higher concentrations, this hydrogel can form a moldable viscous mass that does not interact with proteins or most biological macromolecules.Citation34,Citation35 HPMC gels can also transform from a liquid to a solid at body temperature and have been shown to have robust mucoadhesiveness.Citation36 Specifically, HPMC solution is a type of low-viscosity liquid at room temperature, but can change to a solid gel at body temperature. However, the use of HPMC as a main scaffold material for tissue engineering has been less well characterized. In 2005, Trojani et al.Citation30 evaluated a silated HPMC-based hydrogel as a scaffold for three-dimensional culture of osteogenic cells. Vinatier et al.Citation37 later assessed the ability of this hydrogel to maintain a chondrocyte-specific phenotype in vitro using chondrocytes as seed cells. Additionally, Mathieu et al.Citation38 injected Si-HPMC seeded with mesenchymal stem cells (MSC) into the myocardium of rats and successfully preserved cardiac function and attenuated ventricular remodeling after myocardial infarction. As shown in the supplemental figure of this study, HPMC hydrogels of different concentrations exhibited different shapes and features. After carefully screening, we found that 13% w/v HPMC was an optimal ratio and can be a satisfying scaffold in this study.
5. CONCLUSIONS
In summary, this study demonstrated that hADSCs could differentiate into lymphocyte-like cells and form lymphoid nodules in vivo. TGF-β1 and bFGF might play important roles in the differentiation course. Although the precise mechanism remains to be elucidated, our findings present an alternative approach for engineering immune organs and offer adjunctive therapy for malignancies or offer potential treatment for immunodeficiency diseases.
Author contribution
Jing Zhang: Collection and assembly of data, manuscript writing
Yuqiao Xu: Collection and assembly of data, manuscript writing
Tao Liu: Provision of study material or patients, collection and/or assembly of data
Jie Min: collection and/or assembly of data, data analysis
Yu Ma: Provision of study material or patients
Yongli Song: Collection and/or assembly of data
Jianrong Lu: Collection and/or assembly of data
Wenjuan Mi: Collection and/or assembly of data
Yingmei Wang: Administrative support
Hang Li: Data analysis and interpretation
Wangzhou Li: Provision of study material of patients
Daqing Zhao: Conception and design, financial support, final approval of manuscript
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download Zip (4.9 MB)Acknowledgments
We thank Hongbo Zhang and Bin Yu from the State Key Laboratory of Ministry of Health for Forensic Sciences of Xi’an Jiao Tong University for species identification.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Reda SM, Afifi HM, Amine MM. Primary immunodeficiency diseases in Egyptian children: a single-center study. J Clin Immunol. 2009; 29:343–51. doi: 10.1007/s10875-008-9260-x
- Cuzzone DA, Albano NJ, Aschen SZ, Ghanta S, Mehrara BJ. Decelluarized lymph nodes as scaffolds for tissue engineered lymph nodes. Lymphatic Research and Biology. 2013;13:186–95.
- Purwada A, Jaiswal MK, Ahn H, Nojima T, Kitamura D, Gaharwar AK, Cerchietti L, Singh A. Ex vivo engineered immune organoids for controlled germinal center reactions. Biomaterials. 2015; 63:24–34. doi: 10.1016/j.biomaterials.2015.06.002
- Ho AD, Punzel M. Hematopoietic stem cells: can old cells learn new tricks? J Leukoc Biol. 2003;73:547–55. doi:10.1189/jlb.0902458.
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi:10.1089/107632701300062859.
- Du Y, Roh DS, Funderburgh ML, Mann MM, Marra KG, Rubin JP, Li X, Funderburgh JL. Adipose-derived stem cells differentiate to keratocytes in vitro. Mol Vis. 2010;16:2680–89.
- Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, Quarto N, Contag CH, Wu B, Longaker MT. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–67. doi:10.1038/nbt958.
- Dhote V, Skaalure S, Akalp U, Roberts J, Bryant SJ, Vernerey FJ. On the role of hydrogel structure and degradation in controlling the transport of cell-secreted matrix molecules for engineered cartilage. J Mech Behav Biomed Mater. 2012;19:61–74. doi:10.1016/j.jmbbm.2012.10.016.
- Sun F, Zhou K, Mi WJ, Qiu JH. Combined use of decellularized allogeneic artery conduits with autologous transdifferentiated adipose-derived stem cells for facial nerve regeneration in rats. Biomaterials. 2011;32:8118–28. doi:10.1016/j.biomaterials.2011.07.031.
- Xu Y, Zhang J, Ma Y, Han Y, Min J, Liang Y, Zhao D, Qiu J. The role of adipose-derived stromal cells and hydroxypropylmethylcellulose in engineering cartilage tissue in vivo. Cytotechnology. 2014;66:779–90. doi:10.1007/s10616-013-9627-6.
- Rodriguez AM, Elabd C, Amri EZ, Ailhaud G, Dani C. The human adipose tissue is a source of multipotent stem cells. Biochimie. 2005;87:125–28. doi:10.1016/j.biochi.2004.11.007.
- Lei F, Zhao B, Haque R, Xiong X, Budgeon L, Christensen ND, Wu Y, Song J. In vivo programming of tumor antien-specific T lymphocytes from pluripotent stem cells to promote cancer immunosurveillance. Cancer Res. 2011;71:4742–47. doi:10.1158/0008-5472.CAN-11-0359.
- Nishimura T, Kaneko S, Kawana-Tachikawa A, Tajima Y, Goto H, Zhu D, Nakayama-Hosoya K, Iriguchi S, Uemura Y, Shimizu T, et al. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell Stem Cell. 2013;12:114–26. doi:10.1016/j.stem.2012.11.002.
- Chen X, Lei F, Wang L, Xiong X, Song J. Generation of tumor antigen-specific cytotoxic T lymphocytes from pluripotent stem cells. Methods Mol Biol. 2019;1884:43–55. doi:10.1007/978-1-4939-8885-3_3.
- Rodriguez AM, Elabd C, Delteil F, Astier J, Vernochet C, Saint-Marc P, Guesnet J, Guezennec A, Amri EZ, Dani C, et al. Adipocyte differentiation of multipotent cells established from human adipose tissue. Biochem Biophys Res Commun. 2004;315(2), 255–263.
- Cousin B, Ravet E, Poglio S, De Toni F, Bertuzzi M, Lulka H, Touil I, André M, Grolleau J-L, Péron J-M, et al. Adult stromal cells derived from human adipose tissue provoke pancreatic cancer cell death both in vitro and in vivo. PLoS One. 2009;4:e6278. doi:10.1371/journal.pone.0006278.
- Cousin B, Andre M, Arnaud E, Penicaud L, Casteilla L. Reconstitution of lethally irradiated mice by cells isolated from adipose tissue. Biochem Biophys Res Commun. 2003;301:1016–22. doi:10.1016/s0006-291x(03)00061-5.
- Technau A, Froelich K, Hagen R, Kleinsasser N. Adipose tissue-derived stem cells show both immunogenic and immunosuppressive properties after chondrogenic differentiation. Cytotherapy. 2011;13:310–17. doi:10.3109/14653249.2010.504769.
- Watabe T, Miyazono K. Roles of TGF-beta family signaling in stem cell renewal and differentiation. Cell Res. 2009;19:103–15. doi:10.1038/cr.2008.323.
- Park KH, Na K. Effect of growth factors on chondrogenic differentiation of rabbit mesenchymal cells embedded in injectable hydrogels. J Biosci Bioeng. 2008;106:74–79. doi:10.1263/jbb.106.74.
- Monteiro R, Pinheiro P, Joseph N, Peterkin T, Koth J, Repapi E, Bonkhofer F, Kirmizitas A, Patient R. Transforming growth factor beta drives hemogenic endothelium programming and the transition to hematopoietic stem cells. Developmental Cell. 2016;38:358–70. doi: 10.1016/j.devcel.2016.06.024
- Bikfalvi A, Klein S, Pintucci G, Rifkin DB. Biological roles of fibroblast growth factor-2. Endocr Rev. 1997;18:26–45. doi:10.1210/edrv.18.1.0292.
- Zou H, Nie XH, Zhang Y, Hu M, Zhang YA. Effect of basic fibroblast growth factor on the proliferation, migration and phenotypic modulation of airway smooth muscle cells. Chin Med J (Engl). 2008; 121. 424–29.
- Solchaga LA, Penick K, Porter JD, Goldberg VM, Caplan AI, Welter JF. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2005;203:398–409. doi:10.1002/jcp.20238.
- Mandl EW, Jahr H, Koevoet JL, van Leeuwen JP, Weinans H, Verhaar JA, van Osch GJVM. Fibroblast growth factor-2 in serum-free medium is a potent mitogen and reduces dedifferentiation of human ear chondrocytes in monolayer culture. Matrix Biol. 2004;23:231–41. doi:10.1016/j.matbio.2004.06.004.
- Chua KH, Aminuddin BS, Fuzina NH, Ruszymah BH. Interaction between insulin-like growth factor-1 with other growth factors in serum depleted culture medium for human cartilage engineering. Med J Malaysia. 2004;59:7–8.
- Cerdan C, McIntyre BA, Mechael R, Levadoux-Martin M, Yang J, Lee JB, Bhatia M. Activin A promotes hematopoietic fated mesoderm development through upregulation of brachyury in human embryonic stem cells. Stem cells dev. 2012;21:2866–78. doi: 10.1089/scd.2012.0053
- Tara LH, Zhou Y, Paul EM, Leonard IZ. Cooperative effects of growth factors involved in the induction of hematopoietic mesoderm. Blood. 1998;11:4128–37.
- Sims CD, Butler PE, Casanova R, Lee BT, Randolph MA, Lee WP, Vacanti CA, Yaremchuk MJ. Injectable cartilage using polyethylene oxide polymer substrates. Plast Reconstr Surg. 1996;98:843–50. doi:10.1097/00006534-199610000-00015.
- Vacanti JP Beyond transplantation. Third annual Samuel Jason Mixter lecture. Arch Surg. 1988;123:545–49. doi:10.1001/archsurg.1988.01400290027003.
- Sterodimas A, de Faria J, Nicaretta B. Pitanguy I Tissue engineering with adipose-derived stem cells (ADSCs): current and future applications. J Plast Reconstr Aesthet Surg. 2010;63:1886–92. doi:10.1016/j.bjps.2009.10.028.
- Pabbruwe MB, Kafienah W, Tarlton JF, Mistry S, Fox DJ, Hollander AP. Repair of meniscal cartilage white zone tears using a stem cell/collagen-scaffold implant. Biomaterials. 2010;31:2583–91. doi:10.1016/j.biomaterials.2009.12.023.
- Trojani C, Weiss P, Michiels JF, Vinatier C, Guicheux J, Daculsi G, Gaudray P, Carle GF, Rochet N. Three-dimensional culture and differentiation of human osteogenic cells in an injectable hydroxypropylmethylcellulose hydrogel. Biomaterials. 2005;26:5509–17. doi:10.1016/j.biomaterials.2005.02.001.
- Merrill EW. Poly(ethylene oxide) star molecules: synthesis, characterization, and applications in medicine and biology. J Biomater Sci Polym Ed. 1993; 5. 1–11.
- Peppas NA, Langer R. New challenges in biomaterials. Science. 1994;263:1715–20. doi:10.1126/science.8134835.
- Yoo JW, Dharmala K, Lee CH. The physicodynamic properties of mucoadhesive polymeric films developed as female controlled drug delivery system. Int J Pharm. 2006;309:139–45. doi:10.1016/j.ijpharm.2005.11.020.
- Vinatier C, Magne D, Moreau A, Gauthier O, Malard O, Vignes-Colombeix C, Daculsi G, Weiss P, Guicheux J. Engineering cartilage with human nasal chondrocytes and a silanized hydroxypropyl methylcellulose hydrogel. J Biomed Mater Res A. 2007;80:66–74. doi:10.1002/jbm.a.30867.
- Mathieu E, Lamirault G, Toquet C, Lhommet P, Rederstorff E, Sourice S, Biteau K, Hulin P, Forest V, Weiss P, et al. Intramyocardial delivery of mesenchymal stem cell-seeded hydrogel preserves cardiac function and attenuates ventricular remodeling after myocardial infarction. PLoS One. 2012;7:e51991. doi:10.1371/journal.pone.0051991.
