ABSTRACT
Alopecia has several causes, but its relationship with ischemia/hypoxia has not yet been investigated in detail. In this study, we studied the changes of hair follicles induced by ischemia and potential effects of normobaric hyperoxygenation (NBO) on the hair cycle and growth. We found that skin ischemia reduced hair growth rate, hair shaft size, and its pigmentation in the anagen phase of mice, which may reflect an aspect of pathophysiology of hair loss (alopecia) and depigmentation (gray/white hairs). Hyperoxygenation increased hair growth rate in organ culture of both human and murine hair follicles. Systemic NBO promoted hair growth in early anagen and mid-anagen, and delayed catagen onset in mice. However, telogen-to-anagen transition was not affected by NBO as far as non-ischemic skin is concerned. The results of this study indicated that the hair follicle is very sensitive to oxygen tension and oxygen tension affects the regulation of hair growth and cycle in vitro and in vivo. It was suggested that systemic NBO can be safely applied for a long period and can be a noninvasive therapeutic approach to alter hair growth and cycle by manipulating the microenvironment of hair follicles.
Introduction
Alopecia, otherwise known as hair thinning or baldness, has several contributing factors including genetics, hormone metabolism disorders, trauma, infections, chemotherapy, autoimmune.Citation1 However, ischemia has not yet been investigated in detail as an influential factor on hair growth. The reduced blood flow that is characteristic of ischemia causes both hypoxia and undernutrition. The effects of O2 supply on hair growth rate and the hair cycle have not been carefully examined.
Among the limited studies conducted to date, one reports that areas of alopecia are associated with ischemia and hypoxia.Citation2 In this report of androgenic alopecia cases, the partial pressure of O2 in the forehead tissue (where hair loss was occurring) was significantly lower than that in the temporal hairy region, and the difference in partial oxygen pressure between forehead and temporal area was greater in androgenic alopecia than those exhibiting normal hair growth. This finding suggests that transcutaneous oxygen pressure was significantly lower in the frontal area than in temporal area of hair loss subjects. This may be due to the lower vascularity and/or high mechanical tension of the skin. However, how ischemia or hypoxia relates to hair follicle growth and the hair cycle has not been examined in details before.
When performing basic research on hair growth rate and the hair cycle, the most commonly used experimental animal is the C57BL/6JJcl mouse, because correlations between the hair cycle and age, the accompanying histological changes, and other details have been thoroughly investigated.Citation3 Citation4 Stimuli, signals, and tissue changes have been investigated using these mice at 7 weeks of age, which are known to be in telogen phage.Citation5 Using the same strain, we investigated the effects of tissue partial pressure of O2 on hair growth and hair cycle.
If ischemia or hypoxia can cause alopecia, then it may be possible to treat or prevent alopecia by correcting the ischemic condition or improving tissue oxygen tension. Minoxidil, an approved hair-restoring agent, has a variety of effects (e.g., increasing the proportion of anagen-phase follicles, extending the anagen phase, and increasing the rate of hair growth by promoting the proliferation of dermal papilla and hair matrix cells) that are mediated by the vasodilatory activity of minoxidil and the production of adenosine after binding to the sulfonylurea receptors of cell membrane-bound potassium channels of dermal papilla cells.Citation6 Other methods of improving ischemia that have been attempted previously include local injection of growth factors or platelet-rich plasma,Citation7 fat grafts, or adipose-derived stem cells; and, in recent years, improving blood flow using botulinum toxin,Citation8 which showed clinical improvement of hair growth.
We sought to examine if oxygenation induces anagen and promotes hair growth.
Hyperbaric oxygenation has been clinically used for some pathological conditions, but this approach can lead to cellular dysfunction due to oxygen toxicity and cannot be applied for more than 2 hours.Citation9–Citation11 The relation between oxidative stress and androgenic alopecia is also reported.Citation12 Therefore, rather than hyperbaric hyperoxygenation, we used normobaric hyperoxygenation (NBO) as a treatment to improve hair growth and cycles. We previously compared the partial pressure of O2 in various organs and tissues by housing mice under 20% or 60% O2 and found that long-term application of 60% NBO is safe and maintain partial pressure of O2 in skin at 178% compared to room air.Citation13 In this study, we sought to clarify the influence of ischemia and oxygenation on hair growth and hair cycle.
Results
Organ culture of hair follicles in different O2 concentrations
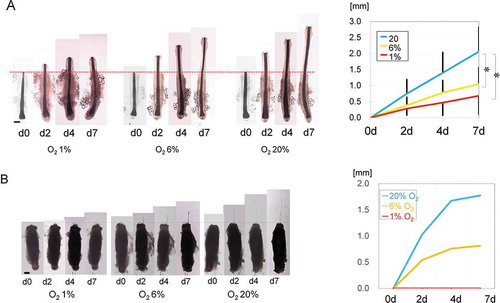
Human scalp hair follicles were organ cultured in 1% (hypoxia), 6% (normoxia), and 20% (hyperoxia) O2. The physiological partial O2 tension of human subcutaneous fat tissue is around 50–60 mmHg, which corresponds to 6% O2 at 1 atmosphere. In all groups, anagen-phase follicles continued to grow for 7 days (). On Day 7, the 1% O2 hair averaged 0.7 ± 0.6 mm (n = 5), the 6% O2 hair averaged 1.0 ± 0.5 mm (n = 5), and the 20% O2 hair averaged 2.1 ± 0.8 mm (n = 5) in length. Similar results were also seen in hairs in mice (). On Day 7, growth of the 1% O2 strands was not observed. Nonetheless, hairs maintained under 6% O2 were 0.8 mm and those under 20% O2 were 1.8 mm.
Figure 1. Organ culture of human hair follicles under different oxygen tensions.
Influence of ischemia on mouse hair follicles
After ischemic and non-ischemic skin flaps were elevated in the groin region on each side, the tissue O2 partial pressure was measured over time and compared. Values stayed at approximately 60 mmHg on the control side, whereas the oxygen pressure reached approximately 30 mmHg on the ischemic side until 2 weeks after flap elevation and depilation for anagen induction. Blood flow recovered starting from Week 3 and stayed relatively stable until Week 5 ().
Figure 2. Effects of ischemia on hair follicles in mice.
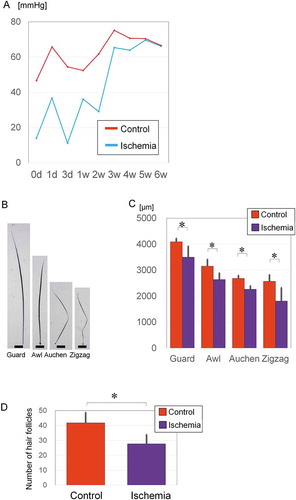
C57BL/6JJcl mice have four types of body hair; guard, awl, auchen, and zigzag (). The guard hair type is the longest and straight, awl is short and straight, auchen has one bend, and zigzag has two bends. Hairs on the ischemic side were significantly shorter than those on the control side (). Guard hairs were 4,096 ± 127.9 µm (n = 7) on the control side and 3,504 ± 420.8 µm (n = 10) on the ischemic side (p = .0015). Awl hairs were 3,156 ± 261.0 µm (n = 21) on the control side and 2,642 ± 249.9 µm (n = 21) on the ischemic side (p = .000009). Auchen hairs were 2,683 ± 118.9 µm on the control side (n = 15) and 2,267 ± 130.1 µm on the ischemic side (n = 20) (p = .000003). Zigzag hairs were 2,574 ± 249.4 µm (n = 7) on the control side and 1,812 ± 512.2 µm (n = 7) on the ischemic side (p = .006).
The number of anagen-phase follicles per section was significantly higher on the control side (41.8 ± 7.0, n = 8) than on the ischemic side (27.7 ± 6.2, n = 8) (p = .0084) (). These findings suggest that ischemia makes the hair shafts thinner, decreases hair density, delays hair growth and affects the hair cycle in mice.
Effect of NBO on hair follicles in early anagen, mid-anagen, and late-anagen phases ()
i) NBO in early anagen phase
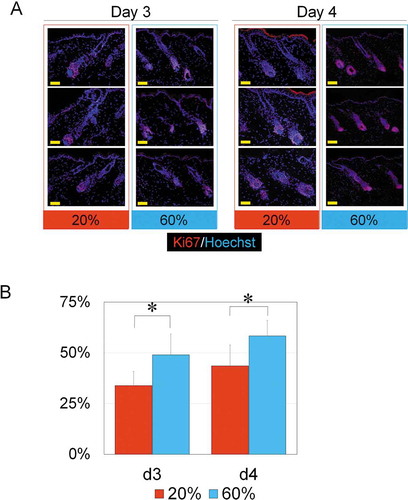
After a 7-day treatment of NBO starting from Day 0, hair was harvested on Day 7. Several days after anagen induction, the dorsal skin started to turn gray in color in both 20% and 60% O2 groups. Immunohistology for Ki67 indicated that a large number of Ki67-positive hair matrix cells were confirmed in both 20% and 60% O2 groups on Days 3 and 4 (). The proportion of Ki67-positive cells among all hair matrix cells in the follicles was 49.0 ± 9% in the 60% O2 group (n = 5) and 33.9 ± 7% in the 20% O2 group (n = 5) on Day 3, whereas the proportion was 58.3 ± 8% in the 60% O2 group (n = 5) and 43.6 ± 10% in the 20% O2 group (n = 5) on Day 4 (). On both days, there were a significantly larger number of Ki67-positive hair matrix cells in the 60% O2 group (Day 3, p = .008; Day 4, p = .037), indicating that NBO promoted hair growth in early anagen phase.
Figure 3. Treatment protocols of systemic normobaric hyperoxygenation (NBO) for hair follicles in early, mid, and late-anagen stages.
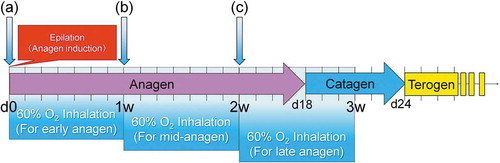
Figure 4. NBO effects on hair follicles in early anagen phase.
ii) NBO in mid-anagen phase
In mid-anagen, the hair length increases rapidly. After a 7-day treatment of NBO starting from Day 7, hair was harvested on Day 14. The hair length of NBO-treated mice was 7.7 ± 0.50 mm (n = 64) and significantly longer than that from 20% O2-treated mice was 6.9 ± 0.42 mm (n = 64), indicating that NBO promotes the hair shaft growth in mid-anagen.
iii) NBO in late anagen to catagen phase
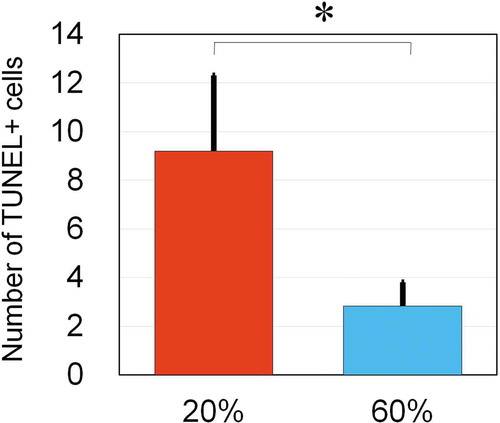
After a 7-day treatment of NBO starting from Day 14, hair was harvested on Day 21. In control mice, anagen-catagen transition occurred on Day 17 to 19. Differences in macroscopic views on either the external or subcutaneous side were not apparent. In catagen-phase follicles, apoptotic epithelial cells, which are terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)-positive, were found in the hair matrix. On Day 19, histological sections of NBO-treated mice showed 2.8 ± 1.0 (n = 5) TUNEL-positive cells per section, which was significantly (p = .007) smaller in number compared to control mice (9.2 ± 3.1, n = 5) (), suggesting that NBO may delay anagen-to-catagen transition and extend the anagen phase.
Effect of NBO on telogen hair follicles
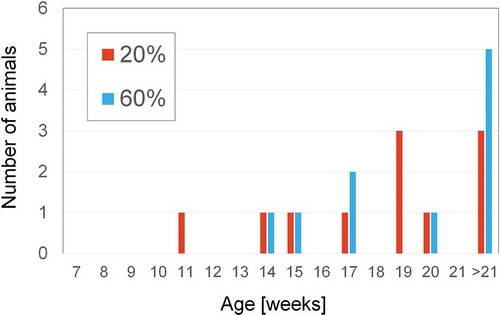
In contrast to depilation, shaving of the back hairs of 7-week-old C57BL/6JJcl mice does not induce anagen phase, and the telogen phase continues. To verify whether NBO affects the length of telogen phase, the mice were maintained under 20% or 60% O2 for 14 weeks after shaving. In one animal in the 20% O2 group and 2 animals in the 60% O2 group, anagen phase was induced probably by mechanical injury at the time of shaving. Eleven animals in the 20% O2 group and 10 animals in the 60% O2 group were evaluated. Among animals in which the next anagen occurred between 35 and 98 days after shaving, anagen was observed after 47.3 ± 14.4 days and 45.5 ± 9.65 days in the 20% and 60% O2 group, respectively. However, the other animals in both groups did not show anagen onset until 98 days (). Between 7 and 21 weeks, anagen induction occurred in 8 mice at 20% O2 and 5 mice at 60% O2 with no significant difference ().
Discussion
This study is the first to quantify how much induction of ischemia and hyper-or hypo-oxygenation may affect on hair cycle and hair shaft growth. Regenerated hairs in ischemic flaps presented not only slower rate of hair growth but also some typical features of alopecia such as thinner hair shafts and lesser hair density. It is known that hair loss frequently occurs after temporary ischemia after facelift surgery or hair transplantation though followed by hair regeneration in part.Citation14 One study on frontal fibrosing alopecia showed increasing vascular flow in the deep plexus, while superficial tissue became ischemia or fibrosis.Citation15 Partial arterial occlusion by hyaluronic acid injection induced transient alopecia in the ischemic area.Citation16
We also observed depigmented hairs in some ischemic flaps of mice, which may suggest that melanocyte stem cells may be more sensitive to ischemia/hypoxia than epithelial ones. In the case of early-stage androgenic alopecia, subcutaneous blood flow to the forehead has been reported to be about 2.6 times lower than that in those exhibiting normal hair growth.Citation17 In our study, the O2 partial pressure of the ischemic side was about one-third of that on the control side, which may be similar to the condition of androgenic alopecia.
In organ culture, hair growth was slower under hypoxic conditions (1% O2) when compared to physiological (6% O2) or hyperoxic (20% O2) environments and there was no difference between the two latter treatments. It was shown that the rate of hair growth was highly dependent on O2 concentration. A previous study using human hair follicles organ-cultured in 20% or 95% O2 showed that hair matrix cell degeneration was suppressed and hair matrix degeneration was reduced and structural integrity of the matrix was maintained for a longer period of time when exposed to 95% O2.Citation18
NBO can be easily performed clinically, while hyperbaric hyperoxygenation cannot be applied for longer than 90 min due to its oxygen toxicity.Citation19–Citation23 In this study, 7-day NBO applied either in the early anagen or mid-anagen promoted matrix cell proliferation and consequently accelerated hair growth. Our results suggested that oxygen tension is a very influential microenvironment factor on hair follicles and hyperoxygenation may provide beneficial effects to promote hair growth and regulate hair cycles in vitro and in vivo.
Transition to the catagen phase is known to occur approximately 16 to 19 days after the anagen phase is induced in C57BL/6JJcl mice.Citation3 Mice maintained under NBO for 7 days during this catagen-phase transition showed fewer TUNEL-positive cells than those maintained under 20% O2. When transition to the catagen phase occurs, hair matrix cells are known to become apoptotic (TUNEL-positive), so this result indicates that NBO can delay the anagen-to-catagen transition.
In addition to NBO effects on anagen, we examined NBO effects on a therapeutic potential of NBO for treating hair loss by inducing telogen-to-anagen transition. As the dorsal hairs of 7-week-old C57BL/6JJcl mice are known to be in the telogen phase, systemic NBO or control treatment was started in mice models with hair shaving (not inducing anagen by depilation) since at the age of 7 weeks. Under physiological condition, the next anagen was detected 6–7 weeks later (at the age of 13–14 weeks). A long-period NBO therapy did not significantly promote the transition from the telogen to anagen phase when applied to non-ischemic telogen skin. As pathological alopecia appears to be clinically associated with ischemic/hypoxic conditions of the scalp, our results may not always indicate that NBO is ineffective for treating alopecia in human. Further analyses using animal models with ischemic/hypoxic telogen skin may help to investigate the potentials of NBO more precisely.
Some signaling pathways such as TGF-β, BMP, and Wnt pathways have been shown to regulate hair development and cycles.Citation18–Citation20 Our results suggested that oxygen tension is a very influential microenvironment factor on hair follicles and hyperoxygenation may provide beneficial effects to promote hair growth and regulate hair cycles in vitro and in vivo. One study of oxygen reports suggested that oxidative stress inhibits migration, proliferation, and secretory functions of dermal papilla cells from bald skin and may be related pathogenesis of alopecia.Citation24
In conclusion, our findings suggested that ischemia and/or low skin pO2 reduces hair fiber growth rate, pigmentation and may contribute to the onset of catagen. These effects could contribute to alopecia as well as hair graying that occurs naturally with aging. Hyperoxygenation treatments increased hair fiber growth during anagen and delayed the onset of catagen. These findings support the hypothesis that ischemia and/or the resulting reduction in skin pO2 and nutrient supply may contribute to impaired hair growth and hair loss.
Materials and methods
All experimental protocols involving human subjects in this study were performed in accordance with relevant government guidelines and approved by the institutional review board (IRB) of the University of Tokyo Hospital. Prior to the procedure, each patient provided her/his informed consent using an IRB approved protocol. The protocols of animal experiments followed the relevant guidelines and were approved by the animal experimental committee of the University of Tokyo School of Medicine.
Inguinal ischemic mouse model
Nine-week-old C57BL/6JJcl mice (male) were anesthetized by intraperitoneal injection of somnopentyl (Kyoritsu Seiyaku, Tokyo, Japan). A minimal amount of anesthesia was administered to avoid deep anesthesia and thereby ensure that the O2 partial pressure in the tissue did not decrease. The groin area was depilated using depilating tape (Veet, Reckitt Benckiser, United Kingdom), and a 1.5 × 1.5 cm skin flap was raised. To create an ischemic environment for the skin around the hair follicles in the skin flaps, the femoral artery was ligated and dissected proximal to the inferior epigastric artery so that blood supply to the skin flaps came only from the reverse flow of the femoral artery. The contralateral side served as the control. Both groin areas were monitored, and after 2 weeks, the body hair on the skin flaps was pulled out. The length of the hair was measured, and the skin flaps were evaluated histologically. In addition to hair and skin flap sample collection, a probe (200 µm in diameter) was inserted directly into the fat in the groin to measure O2 partial pressure over time using an O2 partial pressure monitor (Eiko Kagaku, Tokyo, Japan).
Organ cultures of mouse whiskers and human hair
Six-week-old C57BL/6JJcl mice (male) were placed under deep anesthesia and euthanized. Skin samples including whisker follicles were removed from the face, and only anagen-phase follicles that penetrated the subcutaneous tissue were isolated using a microscope.Citation25 Sections of human scalp containing hair that was to be discarded following facelift operations were used. Hair follicles that penetrated the subcutaneous tissue were isolated from the skin sample. The isolated mouse whiskers (n = 3) and human hair follicles (n = 5) were placed in a 24-well plate and immersed in 1 ml of Williams’ medium E (Gibco®, Life Technologies, Hamburg, Germany) to which 10 µg/ml insulin, 0.4 ng/ml hydrocortisone, 2.0 mM L-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin had been added.Citation26–Citation28 The length in which the hair shaft extended was measured every two days. The hair follicles were randomly divided into three groups to be cultured under 1% (n = 5), 6% (n = 5), and 20% (n = 5) normobaric O2, and hair growth rate was subsequently measured. To reproduce the physiological oxygen condition (40–60 mmHg), the oxygen content inside the incubator was set at 6% (= 46–50 mmHg).
Immunohistochemistry
The hair follicles were placed in OCT compound (Sakura Finetek, Tokyo, Japan), frozen with liquid nitrogen, and stored at −80°C until sectioning. Using a standard method, 10-µm thick, frozen, serial sections were cut and mounted on glass slides. Before staining, the samples were air dried at room temperature for 1 h, fixed with 4% paraformaldehyde, and washed with PBS for 5 min. All sections were then stained with HE using a standard method. The sections that underwent immunohistochemistry staining, were first incubated at room temperature for 30 min in 5% fetal bovine serum (FBS) to block nonspecific binding sites. Rabbit anti-human Ki67 (clone SP6, 1:200 dilution; Thermo Fisher Scientific, Fremont, CA) was used as the primary antibody to detect proliferating keratinocytes. Alexa Fluor 594-conjugated donkey anti-rabbit IgG (dilution 1:200; Invitrogen) was used as the second antibody. An isotope antibody was used as the negative control. Additionally, TUNEL was used to evaluate apoptotic cells using an In-Situ Cell Death Detection Kit (Roche Diagnostics, Mannheim, Germany). Hoechst 33342 was used as the nuclear stain.
In vivo experiments for NBO effects on the hair cycle
Seven-week-old C57BL/6JJcl mice (male) were anesthetized by intraperitoneal injection of somnopentyl, and hair was pulled out from approximately 2 × 2 cm dorsal sections. This age of mice was chosen because the dorsal hair of 7-week-old C57BL/6JJcl mice is in the telogen phase, and pulling out hair in the telogen phase is known to induce the anagen phase of hair follicles.Citation2 In the first experiment (), immediately after the hair was depilated, mice were kept in a 20% O2 environment (20% O2 group) or a 60% O2 environment (60% O2 group) for 7 days, which is equivalent to the early anagen phase. On Day 7, hair growth was compared between groups. In the second one (), to verify the effects of NBO on growing hair, mice were kept in a 20% O2 environment (20% O2 group) or a 60% O2 environment (60% O2 group) for 7 days from Day 7 (starting 1 week after hair removal), which is equivalent to the mid-anagen phase. On Day 14, hair growth was evaluated. Mice are known to have four variations of body hair (i.e., guard, awl, auchen, and zigzag),Citation29 Citation30 among which the guard type is the thickest and longest. To evaluate body hair length, strands of guard hair were selected from those randomly pulled out, and their length was measured under a microscope. In the third one (), we verified whether administering 60% O2 caused a delay in transition to the catagen phase thereby producing an extended anagen phase. To do so, mice were kept in a 20% O2 environment (20% O2 group) or a 60% O2 environment (60% O2 group) for 7 days from Day 14 (starting at 2 weeks after hair removal), which is equivalent to the late-anagen phase, through the catagen phase, to the telogen phase. On Day 21, hair growth was evaluated. When moving from the anagen to the catagen phase, TUNEL-positive cells in the hair matrix showing apoptosis were confirmed. Histological evaluations were performed to determine whether 60% O2 showed any effect. In all groups, the animals were anesthetized during sample collection and euthanized. Samples containing dorsal skin and subcutaneous tissue were taken en bloc.
In vivo experiment for NBO effects on physiological telogen-to-anagen transition
Seven-week-old C57BL/6JJcl mice (male) were anesthetized by intraperitoneal injection of somnopentyl, and 2.5 × 4.5 cm sections on the dorsa were shaved. Pulling hair out during the telogen phase induces the anagen phase, but anagen induction does not occur with shaving. After being shaved, the mice were randomly assigned to the 60% O2 or 20% O2 group and maintained for 8 weeks. Changes in the dorsal hair were evaluated every 7 days.
Statistical analyses
Data are presented as mean ± standard deviation, and p < .05 (two-tailed test) was considered a statistically significant difference. The ischemic groin models were compared using a paired t-test. The effects of NBO on the hair cycle, hair growth, and physiological anagen-phase induction were examined using the Mann–Whitney U test. A multiple comparison test (Tukey–Kramer method) was used for the organ cultures.
Competing financial interests
There are no competing financial interests to declare.
Acknowledgments
There were no grants, equipment, or research support.
References
- Jackson AJ, Price VH. How to diagnose hair loss. Dermatol Clin. 2013;31(1):21–28. doi:10.1016/j.det.2012.08.007.
- Goldman B, Fisher D, Ringler S. Transcutaneous PO2 of the scalp in male pattern baldness: a new piece to the puzzle. Plast Reconstr Surg. 1996;97(6):1109–1116. doi:10.1097/00006534-199605000-00003.
- Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, Stenn K, Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117(1):3–15. doi:10.1046/j.0022-202x.2001.01377.x.
- Paus R, Handjiski B, Czarnetzki B, Eichmuller S. A murine model for inducing and manipulating hair follicle regression (catagen): effects of dexamethasone and cyclosporin A. J Invest Dermatol. 1994;103(2):143–147. doi:10.1111/1523-1747.ep12392542.
- Stenn KS, Paus R..Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494.
- Li M, Marubayashi A, Nakaya Y, Fukui K, Arase S. Minoxidil-induced hair growth is mediated by adenosine in cultured dermal papilla cells: possible involvement of sulfonylurea receptor 2B as a target of minoxidil. J Invest Dermatol. 2001;117(6):1594–1600. doi:10.1046/j.0022-202x.2001.01570.x.
- Takikawa M, Nakamura S, Nakamura S, Ishirara M, Kishimoto S, Sasaki K, Yanagibayashi S, Azuma R, Yamamoto N, Kiyosawa T. Enhanced effect of platelet-rich plasma containing a new carrier on hair growth. Dermatol Surg. 2011;37(12):1721–1729. doi:10.1111/j.1524-4725.2011.02123.x.
- Freund B, Schwartz M. Treatment of male pattern baldness with botulinum toxin: a pilot study. Plast Reconstr Surg. 2010;126(5):246e–248e. doi:10.1097/PRS.0b013e3181ef816d.
- Friedman H, Fitzmaurice M, Lefaivre J, Vecchiolla T, Clarke D. An evidence-based appraisal of the use of hyperbaric oxygen on flaps and grafts. Plast Reconstr Surg. 2006;117(7Suppl):175S–190S. doi:10.1097/01.prs.0000222555.84962.86.
- Godman CA, Joshi R, Giardina C, Perdrizet G, Hightower L. Hyperbaric oxygen treatment induces antioxidant gene expression. Ann N Y Acad Sci. 2010;1197(1):178–183. doi:10.1111/j.1749-6632.2009.05393.x.
- Thom S. Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg. 2011;127(Suppl 1):131S–141S. doi:10.1097/PRS.0b013e3181fbe2bf.
- Prie BE, Voiculescu VM, Ionescu-Bozdog OB, Petrutescu B, Iosif L, Gaman LE, Clatici VG, Stoian I, Giurcaneanu C. Oxidative stress and Alopecia. J Med Life. 2015;8:43–46.
- Araki J, Kato H, Doi K, Kinoshita K, Mineda K, Kanayama K, Yoshimura K. Application of normobaric hyperoxygenation to an ischemic flap and a composite skin graft. Plast Reconst Surg Glob Open. 2014;2:e152. doi:10.1097/GOX.0000000000000029.
- Morillas CE, Sánchez HE, Pita JG. Alopecia prevention in rhytidoplasty. Aesthet Surg J. 2004;24:379–383. doi:10.1016/j.asj.2004.05.003.
- Vazquez-Herrera NE, Eber AE, Martinez-Velasco MA, Perper M, Cervantes J, Verne SH, Magno RJ, Nouri K, Tosti A. Optical coherence tomography for the investigation of frontal fibrosing alopecia. J Eur Acad Dermatol Venereol. 2018;32(2):318–322. doi:10.1111/jdv.14571.
- Yang Q, Qiu L, Yi C, Xue P, Yu Z, Ma X, Su Y, Guo S. Reversible alopecia with localized scalp necrosis after accidental embolization of the parietal artery with hyaluronic acid. Aesthetic Plast Surg. 2017;41(3):695–699. doi:10.1007/s00266-017-0841-z.
- Klemp P, Peters K, Hansted B. Subcutaneous blood flow in early male pattern baldness. J Invest Dermatol. 1989;92(5):725–726. doi:10.1016/0022-202X(89)90189-9.
- Imai R, Miura Y, Mochida K, Jindo T, Takamori K, Ogawa H. Organ culture conditions of human hair follicles. J Dermatol Sci. 1992;3(3):163–171. doi:10.1016/0923-1811(92)90031-6.
- Moore D, Weston A, Hughes J, Oakley C, Cleland J. Effects of increased inspired oxygen concentrations on exercise performance in chronic heart failure. Lancet. 1992;339(8797):850–853. doi:10.1016/0140-6736(92)90288-E.
- Lumb A, Nair S. Effects of increased inspired oxygen concentration on tissue oxygenation: theoretical considerations. Eur J Anaesthesiol. 2010;27(3):275–279.
- Qadan M, Akca O, Mahid S, Hornung C, Polk H Jr. Perioperative supplemental oxygen therapy and surgical site infection: a meta-analysis of randomized controlled trials. Arch Surg. 2009;144(4):359–366. doi:10.1001/archsurg.2009.1.
- Tretinyak AS, Lee E, Uema K, d’Audiffret A, Caldwell M, Santilli S. Supplemental oxygen reduces intimal hyperplasia after intraarterial stenting in the rabbit. J Vasc Surg. 2002;35(5):982–87. doi:10.1067/mva.2002.123090.
- Warltier DC, Pagel PS, Kersten JR. Approaches to the prevention of perioperative myocardial ischemia. Anesthesiology. 2000;92(1):253–259. doi:10.1097/00000542-200001000-00038.
- Upton JH, Hannen RF, Bahta AW, Farjo N, Farjo B, Philpott MP. Oxidative stress-associated senescence in dermal papilla cells of men with androgenetic alopecia. J Invest Dermatol. 2015;135(5):1244–1252. doi:10.1038/jid.2015.28.
- Williams D, Profeta K, Stenn K. Isolation and culture of follicular papillae from murine vibrissae: an introductory approach. Br J Dermatol. 1994;130(3):290–297. doi:10.1111/j.1365-2133.1994.tb02923.x.
- Buhl A, Waldon D, Kawabe T, Holland JM. Minoxidil stimulates mouse vibrissae follicles in organ culture. J Invest Dermatol. 1989;92(3):315–320. doi:10.1111/1523-1747.ep12277095.
- Philpott M, Green MR, Kealey T. Studies on the biochemistry and morphology of freshly isolated and maintained rat hair follicles. J Cell Sci. 1989;93:409–418.
- Philpott MP, Green MR, Kealey T. Human hair growth in vitro. J Cell Sci. 1990;97:463–471.
- Paus R, Muller-Rover S, Van Der Veen C, Maurer M, Eichmuller S, Ling G, Hofmann U, Foitzik K, Mecklenburg L, Handjiski B. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113(4):523–532. doi:10.1046/j.1523-1747.1999.00740.x.
- Sharov A, Sharova T, Mardaryev AN, Tommasi Di Vignano A, Atoyan R, Weiner L, Yang S, Brissette J, Dotto G, Botchkarev V. Bone morphogenetic protein signaling regulates the size of hair follicles and modulates the expression of cell cycle-associated genes. Proc Natl Acad Sci U S A. 2006;103(48):18166–71.v 17. doi:10.1073/pnas.0608899103.