ABSTRACT
A film dressing is an easy and common wound management, which is flexible to cover many types of superficial injuries. In a recent study, we developed a scaffold from poly (1,8-octanediolco-citrate) incorporated decellularized amnion membrane (DAM-POC). The DAM-POC scaffold was biocompatible and could enhance soft and hard tissue regeneration when applied to repair the cleft palate in rat. The efficacy of the DAM-POC scaffold in oral repair had led us to hypothesize that it could be employed extensively in the medical field as a wound dressing. This study aimed to investigate the feasibility and efficacy of the DAM-POC scaffold as a film dressing in accelerating wound healing when applied in multiple tissue injuries. Our results demonstrated that both the DAM and DAM-POC scaffolds were biocompatible and anti-adhesive without causing severe foreign body reactions when covering wounds in abdominal wall, back muscle, tibia bone, and liver. In addition, the DAM-POC scaffold was superior to the DAM scaffold in reducing inflammation, preventing fibrosis, and regenerating tissues. In conclusion, the DAM-POC scaffold might potentially be adopted as a film dressing in a wide range of therapeutic applications and healing situations to protect the damaged tissues from the external environment and prevent infections.
Introduction
Wound healing is a complex biological process, which involves several distinct phases, such as hemostasis, inflammation, cell proliferation and migration, and tissue remodeling.Citation1 To achieve an effective wound healing with minimum scarring and maximum structure and function restoration, a proper wound management is necessary to maintain a moderate environment for nutrients exchanging, inhibiting inflammation, protecting the injured tissue from external factors, and stimulating wound closure and tissue regeneration.
A film dressing is an easy and most common wound care management, which is flexible to be applied as a physical barrier to cover many types of superficial injuries, such as burns, skin cuttings, traumatic or surgically induced wounds, and infected injuries, and provide a moist and protected environment during wound healing. Recent advances in biomaterials and regenerative medicine have shown a great potential for developing an ideal wound dressing material that can 1) be easily applied, 2) protect the wound bed from the external environment, 3) regulate the wound microenvironment, 4) prevent inflammation, 5) facilitate revascularization, and 6) shorten the duration of wound healing. Citation2–4
Many types of materials, such as collagen, gelatin, hydrogel, silk fibroin, fibrin, chitosan, and synthetic polymer, have been developed into a film dressing, each providing different biological, mechanical, and drug-releasing properties for wound healing.Citation5–8 Although many of these materials have shown satisfactory effects in promoting wound closing and preventing inflammation, their distinct characteristics (e.g., degradation rate, mechanical property, adhesion ability, and hemostasis capacity) may make a material suitable for one particular application but not another. Therefore, the search for a proper film dressing that ensures an optimal wound healing as well as helps treat and manage a wide variety of wound types and conditions is still ongoing.
The amniotic membrane (AM) is a thin, translucent, and fetal in origin material, with no nerves, vessels, muscles, or lymphatics.Citation9 AM has been increasingly used in regenerative medicine and clinical treatment due to the presence of abundant elementary biomolecules, such as fibronectin, laminins, elastin, nidogen, collagen types I, III, IV, V, and VI, growth factors, and cytokines., Citation1, Citation3, Citation10–12 In the field of wound care, AM is an ideal therapeutic in burns and acute trauma woundsCitation9 since AM has several unique properties that are beneficial for accelerating the tissue repair process, including low immunogenicity, anti-inflammatory, anti-fibrotic, anti-bacterial, and anti-tissue adhesion.Citation13–16
In our earlier work, we investigated the role of AM in bone defect repair. Our results identified that the cell-free AM graft could effectively prevent the invasion of fibrous tissues, stabilize implanted bone particles, and promote hard tissue regeneration. Citation17 As a natural biomaterial, AM is biocompatible, permeable, and has good bio-adhesive and hemostatic properties, while being mechanically weak, hard for handling and preservation, and prone to degradation. On the other hand, many properties of the synthetic polymers, such as mechanical features, thermal behavior, degradation rate, processability, diffusion, miscibility, et al., can be controlled and adjusted by varying the monomer units, reaction conditions, and ratios and concentrations of co-polymers.
Recent efforts have attempted to combine natural and synthetic materials to take advantages of the positive aspects of each type of material, which open up many possibilities for developing new biomaterials.Citation18,Citation19 In a recent study, we incorporated poly (1,8-octanediolco-citrate) (POC) with decellularized AM (DAM) to create a DAM-POC graft. The DAM-POC graft was cell-compatible and showed improved physical properties for wound healing and could further facilitate soft and hard tissue regeneration when applied to repair the large cleft palate in rat.Citation20
The efficacy of DAM-POC graft in oral defect repair has led us to hypothesize that the DAM-POC graft can be also employed extensively in the medical field, such as wound dressing, hemostasis, and prevention of post-operative adhesion. Therefore, the aim of this study is to investigate the feasibility and efficacy of the DAM-POC graft as a flexible film dressing for accelerating wound healing in multiple tissue wounds. Since the single-layer AM is thin (70–150 μm in thickness), soft, and difficult to handle, the AM is commonly processed into a multi-layered structure to enable an easy handling and better suit various clinical applications. We chose the 2-layer DAM and DAM-POC scaffolds for cleft palate surgery in our earlier work because we noticed that the 2-layer scaffold was easily handled and reliable in mechanical strength for maintaining the injured tissue during surgery. Therefore, in the current study, we continue to use the 2-layer DAM and DAM-POC scaffolds for wound covering.
Results
DAM-POC dressing
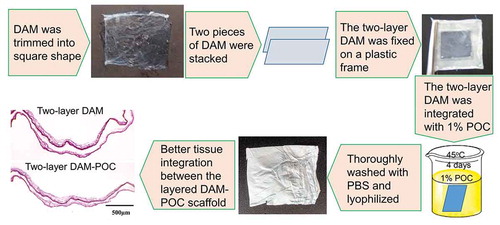
Our previous study had verified the POC integration with the DAM scaffold and some primary physical, mechanical, and biological properties of the DAM-POC dressing were also reported. Citation20 The basic procedure for preparing the 2-layer DAM-POC film dressing is shown in .
Wound healing in rats with abdominal wall wounds
Two weeks after surgery, all the rats with abdominal wall wounds were euthanized. Rats from the wound group showed mild to moderate tissue adhesion among the skin, abdomen wall, and intra-cavity from gross observation, and any adhesions could be separated by blunt or sharp dissection. Muscular defects and mild inflammation could be observed at the injury site ( arrows). Based on the Masson’s Trichrome staining, considerable fibrosis and scar formation were found at the injury site. Mononuclear inflammatory cells (, green arrow) were also identified in the defect area, invading the healthy muscle fibers at the edge of the muscle defect (, inside yellow dotted line).
Figure 2. (a) Gross view of the abdominal wounds setting up in the rat; (b) gross view and (e) Masson’s trichrome staining of the abdominal wounds after 2weeks without any film dressing covering (abdominal wound group); (c) gross view and (f) Masson’s trichrome staining of the abdominal wounds after 2-week repairing with DAM dressing (DAM group); (d) gross view and (f) Masson’s trichrome staining of the abdominal wounds after 2-week repairing with DAM-POC dressing (DAM-POC group). Abdominal wounds were indicated by arrows in A–D and in yellow dotted lines in E–G. Lower magnification (top) and higher magnification (bottom) of area in green rectangle were indicated in E–G. DAM and DAM-POC were indicated by red arrows in F and G. In E–G, inflammatory cell was indicated by the green arrow and the newborn myofiber was indicated by the yellow arrow
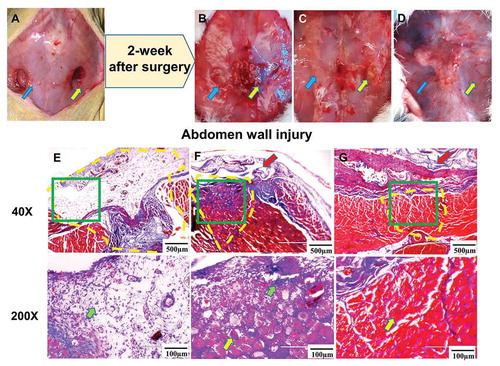
There was no tissue adhesion between the skin and the abdomen wall, nor was there intra-cavity adhesion, in both the DAM group and the DAM-POC group. Partial muscle defects could be grossly observed in the DAM group (, yellow arrow) but near-to-complete wound healing and a smooth abdominal surface were found in the DAM-POC group ( arrows). From the Masson’s Trichrome staining, the size of the muscle defect in the DAM group was much smaller than the defect in the wound group, but there were signs of fibrous tissue and inflammatory cell (, green arrow) deposition at the wound site. Newborn myofibers (, yellow arrow), with nuclei in the center of the cytoplasm, were also found at the periphery of the defect and sparsely towards the center of the defect (, inside yellow dotted line). In the DAM-POC group, many centro-nucleated myofibers (, yellow arrow) were observed in the wound region and the amount of fibrosis and inflammatory cells were decreased, which suggested that the DAM-POC dressing played a positive role in abdominal muscle repair (, inside yellow dotted line).
Wound healing in rats with back muscle defects
Two weeks after surgery, the muscle defect in the wound group narrowed without any obvious tissue adhesions from the gross view (, square). Based on the histology staining, we noticed that the muscle defect was filled with fibrous tissues in the wound group. Inflammatory cells (, green arrow) were also found in the defect area, invading the healthy muscles at the edges of the injury. Vascular ingrowth was also evident (, inside yellow dotted line).
Figure 3. (a) Gross view of the back muscle defect setting up in the rat; (b) gross view and (e) Masson’s trichrome staining of the back muscle defect after 2 weeks without any dressing material covering (back muscle wound group); (c) gross view and (f) Masson’s trichrome staining of the back muscle defect after 2-week repairing with DAM dressing (DAM group); (d) gross view and (g) Masson’s trichrome staining of the back muscle defect after 2-week repairing with DAM-POC dressing (DAM-POC group). Back muscle defects were indicated with yellow rectangles in A–D and yellow dotted lines in E–G. Lower magnification (top) and higher magnification (bottom) of area in green rectangle were indicated in E–G. DAM and DAM-POC were indicated by red arrows in F and G. In E–G, inflammatory cell was indicated by the green arrow and the newborn myofiber was indicated by the yellow arrow
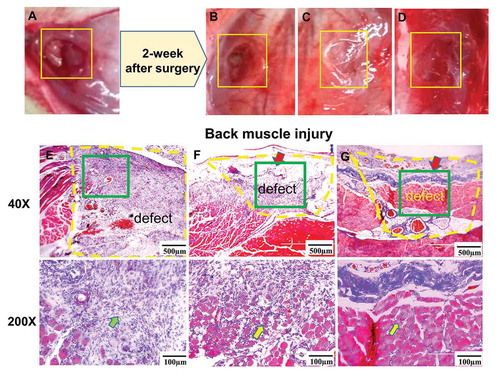
Both the DAM (, square) and DAM-POC dressings (, square) gradually degraded, but they could still be detected 2 weeks after surgery from the gross view. From histology staining, collagen fibers and fibrosis were observed inside the muscle defect region, pointing to the formation of new connective tissue and tissue remodeling in the DAM group. There were a few skeletal muscle fibers (, yellow arrow) in the muscle defect area, but the size of defect was much smaller than the defect in the wound group (, inside yellow dotted line). There was a reduction of fibrosis tissues in the DAM-POC group, but numerus of irregular, centro-nucleated myofibers (, yellow arrow) were found in the defect area suggesting the regeneration of muscle tissue. A limited inflammatory response was also found in the DAM-POC group (, inside yellow dotted line).
Anti-peritoneal adhesion in rats with liver injuries
In the wound group, tissue adhesion was extensive and dense around the liver, peritoneum, and intestines from the gross view 2 weeks after surgery, and sharp dissection was unable to detach the fibrous tissues from the organs ( right, arrow). Histological analysis showed that the liver was fused with the abdominal wall. Adhesions were thick, and deposits of a large number of fibroblasts were clearly observed. Furthermore, there was evidence of an infiltration of lymphocytes and macrophages, and several poorly formed granular tissues (, green arrow) composed of new, small blood vessels, as well as proliferating fibroblasts, could also be seen ().
Figure 4. (a) Gross view of the liver injuries after setting up (left) and after 2-week repairing without any dressing covering (right) in the liver wound group; (b) gross view of liver injuries that were covered with DAM dressing (left) and after 2-week repairing (right) in the DAM group; (c) gross view of liver injuries that were covered with DAM-POC dressing (left) and after 2-week repairing (right) in the DAM-POC group. Masson’s trichrome staining of liver injuries after 2-week repairing in the (d) liver wound group, (e) DAM group, and (f) DAM-POC group. Liver injuries were indicated with arrows in A–C. Lower magnification (left) and higher magnification (right) of area in green rectangle were indicated in D–F. DAM and DAM-POC were indicated by red arrows in E and F. In E–G, inflammatory cell was indicated by the green arrow and the newborn hepatocyte was indicated by the yellow arrow
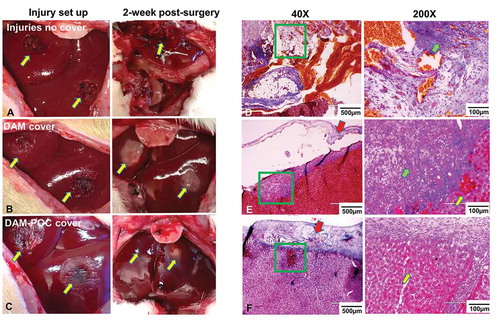
In contrast, liver injuries covered with DAM ( right, arrows) or DAM-POC dressing ( right, arrows) did not exhibit tissue adhesion or inflammation, and the surface of the injury site was smooth and soft from the gross view. Moreover, even as the DAM and DAM-POC dressings degraded, they still maintained adhesion to the injury site. Histological analysis verified that minor lymphocytic infiltration (, green arrow) was found in the injury area in the DAM group (). In the DAM-POC group, inflammatory response was absent, the injured region was filled with newly formed hepatocytes (, yellow arrow) and near-to-complete liver regeneration was observed ().
In all the above three animal models (abdominal wall, back muscle, and liver), both the DAM and DAM-POC dressings could be detected on the surface of the injury sites (indicated by the red arrows).
Bone defect healing in rats
Eight weeks after surgery, all the rats with tibia defects were euthanized. CT images showed that the tibia defect covered with the DAM-POC dressing () was near-to-completely healed and the density in the defect area was very close to the normal tibia bone in the control group (, normal tibia without surgery). In contrast, the wounds in the tibia wound group () or the DAM group () both showed incomplete bone healing as evidenced by lower bone density in the defect area compared with the control group ().
Figure 5. Cross (top) and longitudinal (bottom) views of CT scanning for the (a) control group (normal rat tibia with no defect), (b) tibia wound group (no dressing covering), (c) DAM group, and (d) DAM-POC group after 8-week repairing; Masson’s trichrome staining of the (e) control group, (F) tibia wound group, (g) DAM group, and (h) DAM-POC group after 8-week repairing. Bone defects were indicated with arrows in B–D. Lower magnification (top) and higher magnification (bottom) of area in green rectangle were indicated in E–H
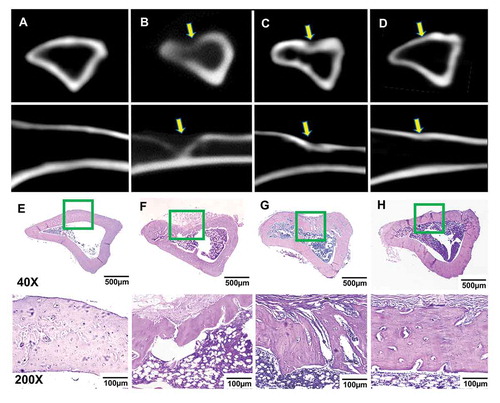
Histology staining showed that the cortical bone in the wound group () was not completely healed, but the defect area was filled with fibrous tissues, partial new bone formation was observed starting from the marrow cavity and progressing towards the edge of the cortical bone. In the DAM group (), an increased bone formation and thicker trabecular bone were found in the defect region compared to the wound group (). However, the outer contour of the tibia in the defect area was not as plump as the DAM-POC group. In contrast, the tibia contour and bone density of the DAM-POC group () were very similar to the control group ().
Discussion
Recently, various biomaterials have been developed into the advanced wound-care dressings, which can provide a well-maintained environment during wound healing by preventing inflammation and other subsequent tissue damages and stimulating a faster recovery and regeneration of the injured tissues.Citation21 This study investigated whether the DAM-POC graft could be used as a film dressing for wound covering and healing in a variety of tissue types, including abdominal wall, back muscle, tibia bone, and liver. This is an early-stage study in the development of the wound dressing materials, which can be assigned to stage 2a based on the IDEAL (The Idea, Development, Exploration, Assessment, and Long-term Follow-up) Recommendations of surgical innovation.Citation21
Our work presented a great promise of both the DAM and DAM-POC dressings in accelerating wound healing in both soft and hard tissues. The injuries in muscle, liver, and bone without a dressing covering all showed unsatisfied wound healing as evidenced by inflammation, scar formation, and limited tissue regeneration in the injured tissues. In contrast, the injuries that were covered by the DAM dressing showed an improved wound healing, and the best wound healing was found in all the DAM-POC dressing covered wounds, which could be indicated by the reduced amount of fibrosis and scar formation in the injured area, decreased volume of wound, and enhanced tissue regeneration and reorganization.
Besides the ability to accelerate the wound healing process, we also noticed that both the DAM and DAM-POC dressings were tissue adhesive when applied for soft and hard tissue covering. In this study, both the DAM and DAM-POC dressings were used to cover the wounds directly without an additional fixation method. Both the DAM and DAM-POC dressings could still be detected on the wound surface after two-week repairing in abdominal wall, back muscle, and liver, without a dressing moving or falling, which demonstrated a satisfied tissue adhesive ability. However, both the DAM and DAM-POC dressings were undetectable after 8-week covering on the tibia bone defects. Although the exact in vivo digestion rate of the DAM and DAM-POC dressings was not investigated in this study, we estimate that the degradation rate of the DAM dressing is about 3–4 week and the DAM-POC dressing has an extended degradation rate which is about 5–7 week based on our previous findings.,Citation17,Citation20 This might explain that the absence of the DAM and DAM-POC dressings in tibia defect is due to the complete material degradation. The resorbable and adhesive properties of both DAM and DAM-POC dressings allow the material to be firmly opposed to the wound surface without using an additive fixation method, such as sutures, staples, tape, or glue. Additional advantages of the tissue-adhesive dressing include ease of use, less pain, limited tissue damage at the covering area, and no need of an additional dressing change or removal after their resorption in the body.
Besides the above findings, we also noticed that in the liver injuries without a dressing covering, severe intra-abdominal tissue adhesion and inflammation were found after 2-week of surgery. When the DAM or DAM-POC dressing was applied to cover the liver injury, both dressings remained adhesion to the liver surface throughout the 2-week process with no post-surgery tissue adhesion and a reduced inflammatory response in the wound area. Post-operative tissue adhesion is a consequence of tissue response to incision, cauterization, suturing, traumatic injury, and inflammation.Citation22 The occurrence of post-operative adhesion can reach to 80%, causing serious complications, such as chronic pelvic pain, intestinal obstructions, and infertility, and imposing heavy financial burdens on the affected patients.Citation23–25 Post-operative tissue adhesion occurs for a number of reasons, including inflammation, ischemia, oxidation, and the over-generation of fibroblasts.Citation26 We speculate that the prevention of post-surgery tissue adhesion by the DAM or DAM-POC dressing is because both dressings can maintain an isolated environment during wound healing that may prevent fibrosis invasion into the injured region.
AM is an immune-privileged tissue that produces very slight immunologic and inflammatory responses and has been increasingly used in regenerative medicine and clinical treatment, especially in wound care.Citation9,Citation16, Citation27–30 Our results demonstrated that both the DAM and DAM-POC dressings were safe, well tolerated, easily handled, biocompatible, and anti-adhesive without causing severe foreign body reactions when they were applied as a film dressing covering multiple types of tissue wounds. In addition, the DAM-POC dressing showed an improved function in preventing adhesion between the wound and surrounding tissues, reducing fibroblast invasion and inflammation, and stimulating the wound healing and tissue regeneration process. Such an improved wound-healing effect of the DAM-POC dressing may be attributed to its prolonged in vivo resorption rate that helped constantly protect the injured tissues.
The DAM-POC presents suitable characteristics to be applied as film dressing for wound covering, such as 1) its ease of preparation and handling, 2) its adjustable shape and thickness to fit different types of clinical applications, 3) its tissue adhesive property is suitable for variety of tissue surface attachment without suturing, 4) the carboxyl and hydroxyl groups in POC provide opportunities to additionally modify the polymer with drugs and biomolecules, such as growth factors, angiogenic factors, and antibiotic and antiviral drugs, as well as allow the controlled releasing of drugs during wound healing. Thus, the DAM-POC dressing presents suitable characteristics for potential application as a wound dressing to promote wound healing and prevent the post-operative tissue adhesion.
The major limitation of this study is that the findings and conclusion from this study were based on gross observation and histological analysis, with insufficient analysis performed, especially, cells involved in inflammation, scar formation, or tissue regeneration were not specially stained, quantified, and statistically analyzed. Another limitation is that the time interval for wound healing was relatively short and the endpoints of the animal experiments were inadequate. In addition, the animal immune responses, wound healing rate, tissue regeneration, and the scaffold in vivo degradation were not assessed in this study. Future studies will add more time points (at weeks 1, 2, 4, 8, and 12, post-surgery) for the animal study to assess wound healing, soft and hard tissue regeneration, and scaffold resorption via histology and immunobiological analysis and quantification. Our future studies will also focus on developing a new generation of physical wound dressing based on the DAM-POC dressing, that will be able to carry and release drugs and/or functional molecules specifically selected to enhance the quality and rate of wound healing. In this way, we hope to alleviate much of the pain during treatment.
Materials and methods
DAM-POC dressing preparation
AM samples were collected from healthy mothers and approved by the IRB offices of Alabama State University, Medical College of Wisconsin, and Dalian Medical University. As described beforeCitation17, the AM was dissected from the center of the placenta, trimmed into square pieces (~1.5 cm ×1.5 cm), decellularized with 1% Triton X-100 and 0.1% Sodium dodecyl sulfate (SDS), and completely washed with water to prepare the DAM. As shown in , a two-layer DAM dressing was prepared by stacking two pieces of the DAM scaffold on a flat plastic frame and removing air bubbles between the layers.
POC was synthesized by dissolving equimolar ratios of citric acid and 1,8-octanediol in absolute ethanol to make a 1% (w/v) POC solution.Citation31 The two-layer DAM dressing was totally immersed in the 1% POC solution for 4 days at 45°C and thoroughly washed with PBS solution for 3 days to remove unbound POC pre-polymers following with lyophilization overnight at −80°C (Cole-Parmer, Vernon Hills, IL) to prepare the two-layer DAM-POC dressing ().
Animal experiments
The animal experiments about muscle and liver injuries were approved by the Animal Welfare Committee (IACUC) of Alabama State University and experiments about bone defect were approved by Dalian Medical University. Sprague-Dawley (SD) rats were kept in standard housing conditions with water and food ad libitum. At the age of 8–10-week-old, the rats were randomly chosen for one of the following surgeries. Before surgery, all the rats were anesthetized with an abdominal injection of a ketamine/xylazine solution (100 mg ketamine/10 mg xylazine/kg of body weight).
Abdominal wall wound
After shaving the fur on the belly and sterilization with iodophor, a median skin incision was made in the abdomen along the midline to expose the abdominal wall. Two full-thickness muscle defects (8-mm diameter round shape) were made with a sterile biopsy punch (MedexSupply, Passaic, NJ) on either side of the abdomen midline and the muscles were surgically removed with a scalpel ().
Back muscle defect
After shaving the fur on the back and sterilization with iodophor, the rats were placed in a prone position and a midline vertical skin incision was made to expose paraspinal muscles. One muscle defect (8 mm diameter × 3 mm deep) was created with a sterile biopsy punch on the right side of the spine and the muscles in the defects were surgically removed with a scalpel ().
Liver injury
A median incision was made in the abdominal wall to expose the liver. The middle lobe of the liver was exposed gently with wet sterile cotton swabs and two liver injuries (8 mm diameter × 3 mm deep) were made with a sterile biopsy punch (MedexSupply, Passaic, NJ, , left). The injured liver tissue was surgically removed with a scalpel. Active bleeding was stopped by direct pressure and electrocoagulation.
Tibia defect
After shaving the fur on the right leg and skin sterilization with iodophor, an incision was made to expose the tibia. A cubic bone defect (3.5 mm wide × 3.5 mm long × 2.5 mm depth) was made in the proximal portion of the tibia using a surgical micromotor (1,000 rpm) while irrigating with cold 0.9% sterile saline solution.
Wound covering and healing
All the rats with each type of the above tissue wounds (abdominal wall, back muscle, liver, and tibia wounds) were randomly divided into three groups and repaired with one of the following methods:
Group I – Wound group: The wounds in each rat were not repaired or covered with any dressing materials. The skin was directly closed with 4–0 silk sutures after the injury created (n = 4 for the wound group with each type of the tissue wounds).
Group II – DAM group: After the wounds were created, each wound was covered with the round-shape, 2-layer DAM dressing (12 mm diameter), making sure that the edge of the dressing was at least 2 mm beyond the border of the injury site. The skin was then closed with 4–0 silk sutures (n = 4 for the wound group with each type of the tissue wounds).
Group III – DAM-POC group: After the wounds were created, each wound was covered with the round-shape, 2-layer DAM-POC dressing (12 mm diameter), making sure that the edge of the dressing was at least 2 mm beyond the border of the injury site. The skin was then closed with 4–0 silk sutures (n = 4 for the wound group with each type of the tissue wounds).
All the rats in groups I, II, and III with the abdominal wall, back muscle, and liver surgeries were euthanized 2 weeks post-surgery, and all the rats in groups I, II, and III with tibia defects were euthanized 8 weeks post-surgery. The tibia was harvested for computed tomography (CT) scanning and histology analysis. The other types of the injured tissues and adjacent tissues were surgically isolated for histological analysis.
Histological and CT analyses
Samples that were harvested from abdominal wall, back muscle, and liver were fixed with a 4% paraformaldehyde solution for 24 hours, dehydrated with graduated concentrations of ethanol, cleared with xylene to remove the ethanol, and then embedded in paraffin. 5 µm thick, cross-sections of the samples were stained with Masson’s trichrome staining, and then imaged with bright-field light microscopy (Nikon EC600).
For all the rats with tibia defects, the tibias were scanned with a dental cone beam computed tomography (CBCT) system (90 kV, 8 mA, Cranex D, Germany) first with 0.3-mm voxel sizes, 14-bit grayscale, and 6-second integration time. After CT scanning, tibias were fixed with 10% formalin for 48 hours, decalcified with hydrochloric acid (10% HCl) for 3 days, dehydrated with graduated concentrations of ethanol, cleared with xylene to remove the ethanol, embedded in paraffin, and stained with hematoxylin and eosin (H&E) kit (Sigma, St. Louis, Missouri).
Conclusion
In accordance with the findings in this study, we conclude that both the DAM and DAM-POC dressings are easy-to-use, well tolerated by animals, tissue adhesive, and able to prevent tissue adhesion and inflammation and assist undisturbed wound healing. In addition, the DAM-POC dressing is superior to the DAM dressing in reducing inflammation, preventing fibrosis, healing wounds, and regenerating tissues. Thus, the DAM-POC may potentially be used as a film dressing in a wide range of therapeutic applications to protect the injured tissues from the external environment and prevent infections.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
Bo Wang: experiment design, animal surgery, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; Wuwei Li: animal surgery and tissue histology, data analysis, and manuscript writing; Justin Harrison: animal surgery.
References
- Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173:370–378.
- Chigurupati S, Mughal MR, Okun E, Das S, Kumar A, McCaffery M, Seal S, Mattson MP. Effects of cerium oxide nanoparticles on the growth of keratinocytes, fibroblasts and vascular endothelial cells in cutaneous wound healing. Biomaterials. 2013;34(9):2194–2201. doi:10.1016/j.biomaterials.2012.11.061.
- Tran NQ, Joung YK, Lih E, Park KD. In situ forming and rutin-releasing chitosan hydrogels as injectable dressings for dermal wound healing. Biomacromolecules. 2011;12:2872–2880. doi:10.1021/bm200326g.
- Gopinath D, Ahmed MR, Gomathi K, Chitra K, Sehgal PK, Jayakumar R. Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials. 2004;25:1911–1917. doi:10.1016/S0142-9612(03)00625-2.
- Bel A, Kachatryan L, Bruneval P, Peyrard S, Gagnieu C, Fabiani JN, Menasché P. A new absorbable collagen membrane to reduce adhesions in cardiac surgery. Interact Cardiovasc Thorac Surg. 2010;10:213–216. doi:10.1510/icvts.2009.215251.
- Iliopoulos J, Cornwall GB, Evans RO, Manganas C, Thomas KA, Newman DC, Walsh WR. Evaluation of a bioabsorable polylactide film in a large animal model for the reduction of retrosternal adhesions. J Surg Res. 2004;118(2):144–153. doi:10.1016/j.jss.2003.10.023.
- Lee MW, Tsai HF, Wen SM, Huang CH. Photocrosslinkable gellan gum film as an anti-adhesion barrier. Carbohydr Polym. 2012;90:1132–1138. doi:10.1016/j.carbpol.2012.06.064.
- Zhou Y, Zhang L, Zhao W, Wu Y, Zhu C, Yang Y. Nanoparticle-mediated delivery of TGF-beta1 miRNA plasmid for preventing flexor tendon adhesion formation. Biomaterials. 2013;34:8269–8278. doi:10.1016/j.biomaterials.2013.07.072.
- Toda A, Okabe M, Yoshida T, Nikaido T. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci. 2007;105:215–228. doi:10.1254/jphs.CR0070034.
- Koizumi N, Fullwood NJ, Bairaktaris G, Inatomi T, Kinoshita S, Quantock AJ. Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Invest Ophthalmol Vis Sci. 2000;41:2506–2513.
- Nakamura T, Endo K, Cooper LJ, Fullwood NJ, Tanifuji N, Tsuzuki M, Koizumi N, Inatomi T, Sano Y, Kinoshita S, et al. The successful culture and autologous transplantation of rabbit oral mucosal epithelial cells on amniotic membrane. Invest Ophthalmol Vis Sci. 2003;44:106–116. doi:10.1167/iovs.02-0195.
- Sakamoto T, Hirano K, Morishima Y, Masuyama K, Ishii Y, Nomura A, Uchida Y, Ohtsuka M, Sekizawa K. Maintenance of the differentiated type II cell characteristics by culture on an acellular human amnion membrane. In Vitro Cell Dev Biol Anim. 2001;37(8):471–479. doi:10.1290/1071-2690(2001)037<0471:MOTDTI>2.0.CO;2.
- Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88–99. doi:10.22203/eCM.v015a07.
- Prabhasawat P, Tseng SC. Impression cytology study of epithelial phenotype of ocular surface reconstructed by preserved human amniotic membrane. Arch Ophthalmol. 1997;115:1360–1367.
- Tseng SC. Amniotic membrane transplantation for ocular surface reconstruction. Biosci Rep. 2001;21:481–89. doi:10.1023/A:1017995810755.
- Kesting MR, Loeffelbein DJ, Steinstraesser L, Muecke T, Demtroeder C, Sommerer F, Hoelzle F, Wolff K-D. Cryopreserved human amniotic membrane for soft tissue repair in rats. Ann Plast Surg. 2008;60:684–691. doi:10.1097/SAP.0b013e31814fb9d2.
- Li W, Ma G, Brazile B, Li N, Dai W, Butler JR, Claude AA, Wertheim JA, Liao J, Wang B, et al. Investigating the potential of amnion-based scaffolds as a barrier membrane for guided bone regeneration. Langmuir. 2015;31:8642–8653. doi:10.1021/acs.langmuir.5b02362.
- Watanabe Y, Ajioka I, Akaike T. Tissue engineering: cooperation of molecular biology and polymer science. Tanpakushitsu Kakusan Koso. 2000;45:1376–1382.
- Roda A, Matias AA, Paiva A, Duarte ARC. Polymer science and engineering using deep eutectic solvents. Polymers (Basel). 2019;11. doi:10.3390/polym11050912.
- Li W, Fu Y, Jiang B, Lo AY, Ameer GA, Barnett C, Wang, B. Polymer-integrated amnion scaffold significantly improves cleft palate repair. Acta Biomater. 2019Jul 1;92:104–114.
- McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC, Nicholl J. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374(9695):1105–1112. doi:10.1016/S0140-6736(09)61116-8.
- Robertson D, Lefebvre G, Leyland N, Wolfman W, Allaire C, Awadalla A, Best C, Contestabile E, Dunn S, Heywood M. Clinical practice gynaecology C. Adhesion prevention in gynaecological surgery. J Obstet Gynaecol Can. 2010;32(6):598–602. doi:10.1016/S1701-2163(16)34530-3.
- Hammoud A, Gago LA, Diamond MP. Adhesions in patients with chronic pelvic pain: a role for adhesiolysis? Fertil Steril. 2004;82:1483–1491. doi:10.1016/j.fertnstert.2004.07.948.
- Kumar S, Wong PF, Leaper DJ. Intra-peritoneal prophylactic agents for preventing adhesions and adhesive intestinal obstruction after non-gynaecological abdominal surgery. Cochrane Database Syst Rev. 2009Jan 21;(1):CD005080.
- Vrijland WW, Jeekel J, van Geldorp HJ, Swank DJ, Bonjer HJ. Abdominal adhesions: intestinal obstruction, pain, and infertility. Surg Endosc. 2003;17:1017–1022. doi:10.1007/s00464-002-9208-9.
- Arung W, Meurisse M, Detry O. Pathophysiology and prevention of postoperative peritoneal adhesions. World J Gastroenterol. 2011;17:4545–4553. doi:10.3748/wjg.v17.i41.4545.
- Adamowicz J, Pokrywczynska M, Tworkiewicz J, Kowalczyk T, van Breda SV, Tyloch D, Kloskowski T, Bodnar M, Skopinska-Wisniewska J, Marszałek A. New amniotic membrane based biocomposite for future application in reconstructive urology. PLoS One. 2016;11:e0146012.
- Barski D, Gerullis H, Ecke T, Varga G, Boros M, Pintelon I, Timmermans J-P, Otto T. Human amniotic membrane is not suitable for the grafting of colon lesions and prevention of adhesions in a xenograft rat model. Surg Innov. 2017;24:313–320. doi:10.1177/1553350617709828.
- Barski D, Gerullis H, Ecke T, Varga G, Boros M, Pintelon I, Timmermans J-P, Otto T. Human amniotic membrane dressing for the treatment of an infected wound due to an entero-cutaneous fistula: case report. Int J Surg Case Rep. 2018;51:11–13. doi:10.1016/j.ijscr.2018.08.015.
- Barski D, Gerullis H, Ecke T, Yang J, Varga G, Boros M, Pintelon I, Timmermans J-P, Otto T. Bladder reconstruction with human amniotic membrane in a xenograft rat model: a preclinical study. Int J Sci Med. 2017;14:310–318. doi:10.7150/ijms.18127.
- Yang J, Motlagh D, Webb AR, Ameer GA. Novel biphasic elastomeric scaffold for small-diameter blood vessel tissue engineering. Tissue Eng. 2005;11:1876–1886. doi:10.1089/ten.2005.11.1876.