ABSTRACT
The use of primary human hepatocytes has been hampered by limited availability of adequate numbers of fresh and viable cells due to the ongoing shortage of liver donors. Thus, there is no surplus of healthy organs from which freshly isolated cells can be prepared when needed. However, primary hepatocytes can be successfully isolated from explanted liver specimens obtained from patients receiving orthotopic liver transplantation for decompensated liver cirrhosis or for metabolic liver disease without end-stage liver disease and are a valuable resource for the pharmaceutical industry research. This review focuses on the isolation, characterization and cryopreservation of hepatocytes derived from therapeutically resected livers with various hepatic diseases.
Introduction
Primary human hepatocytes are still considered the “gold standard” for evaluating in vitro liver disease models.Citation1 The presence of complete, undisrupted metabolic pathways and cofactors in these cells allows for the generation of more relevant data and analysis of interactions that reflect many of the features of the liver in vivo. However, the use of human hepatocytes is limited by the availability of unused donor liver tissues and difficulties in isolating and preserving isolated cells.Citation2 Application of primary human hepatocytes allows for the development of data more relevant to human liver in vivo than liver hepatoma cell lines such as HepG2 or HepaRG cells. Hepatic lineage cells derived from human hepatocarcinoma are less suitable cell systems for assessing drug metabolism and induction in vitro and some hepatic functions of these cells are limited.Citation3 Since its initial establishment, the HepG2 cell line has lost a substantial and variable set of liver-specific functions, especially the functions of major cytochromes P450 (CYPs) involved in xenobiotic metabolism.Citation4 Another platform, the HepaRG cell line expresses high levels of the fetal enzyme CYP3A7, but low levels of the adult enzymes CYP1A2 and CYP2D6.Citation5
One potential solution to the lack of available primary human hepatocytes is the use of iPSC-derived hepatocyte-like cells (iPSC-Heps). Such cells provide a limitless supply of cells with hepatocyte characteristics that can mimic the pathophysiology of liver disease.Citation5 Recently, several protocols of iPSC-Heps have been developed. According to Takeishi et al., iPSC-Heps expressed similar expression of hepatocyte-specific nuclear factors (HNF4alpha and FOXA2) as normal hepatocytes, but the hepatocyte-specific cytochrome P450 activity (CYP3A4) was inferior to that observed in adult human hepatocytes.Citation6 Other recently published data show similar results: iPSC-Heps retain a hepatoblast phenotype rather than undergoing full maturation and although they reveal similar morphological profiles, these cells have limited functionality when compared with primary hepatocytes.Citation7,Citation8
Overall, current alternative liver cell models are mostly based on immortalized cell lines that are limited by poor, or incomplete function, which elucidates why there is a huge unmet need for primary hepatic cell systems that can be efficiently used in modeling liver disease in vitro. Nevertheless, primary human hepatocytes offer immediate resources for studying liver diseases and cell transplantation, and thus represent the most powerful experimental model for drug development, allowing early evaluation of human drug properties and selection of drug candidates with a high probability of clinical success.Citation9 Properly isolated and viable hepatocytes can be used in various formats, either fresh or following cryopreservation, as an experimental model for liver diseases, drug screening and in predictive drug toxicology ().Citation10–12
Figure 1. Isolated cells obtained from diseased explanted livers can be used to mimic the in vivo environment and become an effective experimental model to study a number of liver diseases including ALD, NAFLD, NASH, and metabolic diseases.
To date, hepatocytes have been isolated from a variety of liver samples, including whole livers, wedge biopsy samples, and small biopsy samples.Citation13 The major limitation is that primary hepatocytes isolated from the livers that had been deemed unsuitable for transplantation are scarce and their availability is unpredictable.Citation14 Since obtaining human non-diseased livers for the traditional isolation of viable hepatocytes is more difficult, planning of any kind of experiments with their use becomes more complicated. However, primary hepatocytes can be successfully isolated from explanted diseased liver specimens of patients undergoing liver transplantation. In the process of an industrial novel drug discovery, many liver diseases including ALD (alcoholic liver disease), DILI (drug-induced liver injury), NAFLD (nonalcoholic fatty liver disease), NASH (nonalcoholic steatohepatitis), inborn errors of metabolism (e.g., alpha-1 antitrypsin deficiency, cystic fibrosis, urea cycle disorders), abnormalities of the immune system (e.g., autoimmune hepatitis), or viral infections, are being extensively studied by using a variety of liver models.Citation15–17
Reported studies indicate that properly prepared hepatocytes derived from the diseased liver tissue retain their liver-specific characteristics and can provide an extremely valuable in vitro hepatic model for both research and clinical applications.Citation18–22 Therefore, we have developed a very efficient program which allows for acquisition of the therapeutically resected livers and fresh human liver tissue specimens from patients (IRB: STUDY20090069 – designated as an exempt study) undergoing liver transplantation in the pediatric and adult liver transplant programs at the University of Pittsburgh Medical Center (UPMC). In most cases, these are elective procedures, allowing for the proper scheduling and planning in advance of the highly complicated cell isolation procedures.
As an exceptional organization linking together liver research professionals at the University of Pittsburgh, we are in a unique position to provide highly viable and functional human hepatocytes obtained directly from explanted diseased livers. We believe that these cells can be efficiently used in vitro to study fundamental aspects of the disease processes in various hepatic disorders. This concise review focuses on the evaluation of the potential use of primary hepatocytes isolated from patient-derived diseased livers based on our unpublished raw data, in comparison with published up to date reports.
Isolation of human hepatocytes from diseased livers
As the liver is the major site for drug metabolism, there is a continuous increase in the demand for isolated primary human hepatocytes for drug screening and in predictive drug toxicology. An important application of primary hepatocytes is modeling of liver disease, which significantly facilitates the development of high throughput platforms for investigation of hepatic injury and rare genetic disorders.Citation9,Citation23,Citation24 Although the interest in developing liver-based in vitro models is very high, one of the major problems limiting the application of human hepatocytes is the chronic shortage of liver donors. Primary human hepatocytes are usually isolated from non-diseased livers that had been deemed unsuitable for orthotopic transplantation, or from resected liver tissue. However, published reports indicate that isolation of hepatocytes from explanted diseased livers would substantially increase availability of these valuable cells for any type of research applications.Citation16,Citation17 The utility of diseased liver specimens obtained during liver transplantation has been demonstrated by several groups. According to these reports, even substantial histopathological alterations of the liver tissue such as fibrosis, cirrhosis, steatosis, and cholestasis still allow for the isolation of metabolically competent hepatocytes and/or NPCs (non-parenchymal cells). Nevertheless, the overall published results are variable in terms of cell quality (cell yields and viability might be significantly reduced) that using one highly optimized protocol for successful hepatocyte isolation from such livers is questionable.Citation9,Citation25,Citation26
Since the introduction of the classic two-step methodology, human hepatocyte isolation protocols have been significantly modified mostly by applying various methods of tissue perfusion and optimization of reagents that are used for media supplementation.Citation2,Citation25,Citation27,Citation28 Hepatocyte isolation from human liver tissue is a very demanding and logistically complicated procedure that requires an extensive experience, establishment of the efficient network between the cell isolation team members and transplant surgeons, as well as compliance with regulatory standards. The cell isolation outcome depends on many factors including donor characteristics, condition of the liver tissue, cold/warm ischemic time, as well as a complex conditions of cell isolation and processing. The isolation of primary hepatocytes from diseased liver tissue is an even more complicated and challenging process that involves instant analysis and judgment throughout each stage of the procedure. Only a few studies have assessed modified protocols for the isolation of primary human hepatocytes with improved viability and function from diseased livers (e.g. NASH and ALD). According to some reports, introducing a specific cannulation technique using commercially available Foley catheters might enable efficient hepatocyte isolation from explanted diseased livers with technically difficult vascular access.Citation29 Another study reported some additional modifications compared to previous reports. One such modification was the use of N-acetyl cysteine in isolations involving steatotic livers.Citation30 Some authors have demonstrated the potential of obtaining human hepatocytes from both discarded steatotic and cirrhotic livers by using two-step portal vein perfusion. The presence of cirrhosis significantly impaired the cell yield, but not the metabolic function of the isolated cells.Citation31
Although published reports demonstrate that explanted diseased livers can be used as a possible source of active human hepatocytes, there are currently no systematic studies in the literature that have investigated methods of a large-scale hepatocyte isolation from end-stage cirrhotic livers. Therefore, it is important to standardize the protocols that can be used to maximize the efficient, and reproducible isolation of highly viable cells from such compromised livers.Citation25,Citation28 This applies mostly to highly fibrotic/scarred tissue specimens dissected from livers of patients with decompensated liver cirrhosis. Other studies have used different protocols of cell isolation from explanted diseased livers, however the procedures applied resulted in poor isolation outcome which most likely was related to a median perfusion/digestion time and type of enzymes used.Citation9 In order to improve the outcome of cell isolation procedure in these cases, implementations of instant modifications to already optimized protocols are often required.
Thus far, in our laboratory, hepatocytes have been isolated from 30 diseased liver specimens (unpublished data). Resected livers are always protected from ischemic injury by flushing with ice-cold University of Wisconsin (UW-Belzer) solution immediately after vascular clamping and resection in the Operating Room, placing on ice and transporting immediately to the cell isolation laboratory. Hepatocytes are usually isolated from encapsulated liver segments (preferably the left lateral segment) by a modified three-step perfusion technique. Briefly, the specimen is placed in the custom-made perfusion apparatus and 2–3 hepatic vessels are cannulated with tubing attached to a multi-channel manifold. For a given vessel, tubing that most closely matches the size of the vessel is inserted into the exposed vessel opening. It may be necessary to use two catheters in large vessels, or just one catheter for a small fragment of the tissue. Each catheter is secured in place by suturing or clamping adjacent tissue with a hemostat. In most of the cases, the cell isolation is initiated immediately by perfusion (recirculation technique) with pre-warmed to 37°C calcium-free HBSS (Sigma Aldrich) supplemented with 0.5 mM EGTA (Bio World), and then with collagenase/protease (VitaCyte, LLC) solution, until the tissue is fully digested. Since the resected tissue is initially flushed with UW solution, it can be left on wet ice for up to 6 hours prior to initiation of the cell isolation processing. However, our long-term observations revealed that cell isolation procedures performed immediately after tissue resection resulted in significantly better yields and viability of isolated hepatocytes. The digestion time for each preparation ranges from 30 to 60 min, depending mostly on the overall quality of the tissue.
Specimens with a higher score of fibrosis, or steatosis, dissected from adult livers with ALD, NAFLD, or NASH are difficult to process and require more sophisticated techniques and prolonged times of the enzymatic digestion. Determination of the optimal digestion time and the perfusion rate may vary significantly, which depends on the overall condition of the processed liver specimen. It is critical that the perfusion rate is sufficient to cause visible distention of the tissue specimen. For pediatric livers with metabolic diseases including: Methylmalonic Acidemia (MMA), Propionic Acidemia (PA), Ornithine Transcarbamylase Deficiency (OTC), Maple Syrup Urine Disease (MSUD) and Carbamoyl Phosphate Synthetase-1 deficiency (CPS1), the digestion time is more predictable and usually does not exceed 35 minutes. In our protocol, the digested liver is removed, immediately cooled with ice-cold Leibovitz’s L-15 medium (GIBCOTM), and strained through serial progressively smaller stainless-steel sieves, with the final filtration through a 100 µm mesh. The final crude cell suspensions are centrifuged twice, and the post-digest medium is aspirated. Sporadically, when the initial cell viability is lower than 80%, we further purify our crude cell preparations by using a standard Percoll (GE Healthcare) treatment. The yield and viability of freshly isolated hepatocytes is usually estimated by trypan blue or live/dead fluorescent staining. Patient’s demographic data and viability of freshly isolated hepatocytes are presented in (unpublished data). Considering the initial quality of most liver tissue specimens that we have processed, our success rate of cell isolation outcome is significant.
Table 1. Patient’s demographic data, yield and viability of isolated hepatocytes
Only a few studies have dealt with human hepatocytes isolated from explanted diseased livers. Analysis of cells harvested from discarded livers revealed no differences in viability, cell integrity and metabolic activity in cell culture in comparison to hepatocytes isolated from the normal liver tissue.Citation2 Other studies described a modified method, combining the use of Liberase and N-acetylcysteine for the isolation of primary human hepatocytes with improved viability and function from normal and diseased human livers.Citation26 According to other reports, hepatocytes isolated from end-stage biliary cirrhosis (Primary Biliary Cirrhosis and Primary Sclerosing Cholangitis) show the typical features of cells in culture with a cubic and binucleate morphology. Furthermore, in the same study, cells isolated from ALD show similar morphological features to human hepatocytes isolated from normal non-steatotic livers.Citation9 Moreover, the same authors reported that the time delay between hepatectomy and the beginning of liver perfusion is the most important factor in determining the likelihood of success of this procedure. They suggest that the shortest possible digestion time is desirable for the successful outcome of the isolation procedure, which confirms our observations.
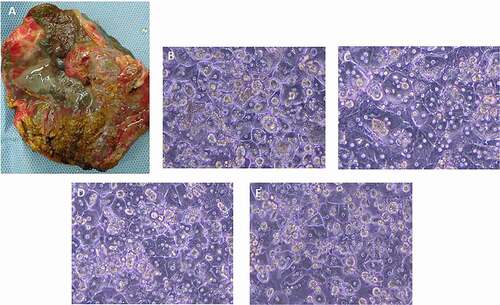
Our unpublished data clearly demonstrate that isolation of hepatocytes from highly cirrhotic and fibrotic livers with alcoholic disease and NASH () has a significant success rate (over 80%), higher than that reported by other authors.Citation9 This might be explained by the different strategies and implementation of particular modifications in our system, as well as techniques that we have approached, mostly relating to the supplementation of the liver perfusion solutions including the type of used enzymes. In our protocols, we use a powerful VitaCyte collagenase/protease kit which has a superior lot-to-lot enzyme consistency that substantially improves the productivity of our cell isolation. Furthermore, a novel system developed in our laboratory enables a very efficient isolation of primary hepatocytes from livers explanted from pediatric patients with metabolic diseases (). The final cell yield and average viability in these cases are always high without Percoll purification (). Viable hepatocytes isolated from metabolic diseased livers show typical and intact morphology as demonstrated by light microscopy, and the cells attach to collagen-coated dishes within 24 hours after seeding, forming confluent cultures ().
Figure 2. (a) Example of NASH liver explant, (partial segments 2,3, Child-Pugh score C) before cell isolation; ongoing liver damage causing significant disruption of the normal hepatic architecture; surface of affected liver becomes irregular and nodular, with a progression of fibrosis. Representative phase-contrast images of cultured hepatocytes from NASH (b), MSUD (c), and ALD (d) livers, 24 hr culture. (e) Phase-contrast image of hepatocytes isolated from an ALD liver, recovered from cryopreservation, 24 h culture.
In the case of successful cell isolation, the yield of primary hepatocytes from a dissected liver segment is often greater than one billion cells, well in excess of the capacity of large-scale in vitro assays. A practical solution to save these valuable cells for on-demand utilization includes hepatocyte cryopreservation and banking. However, an efficient and reproducible hepatocyte preservation method has been difficult to establish due to the fact that these cells are extremely fragile and require very specific microenvironmental conditions to maintain the cell phenotype in vitro. We have previously shown that properly prepared human hepatocytes can be successfully cryopreserved in a mixture in which one of the major components is the potassium-rich UW solution, and that prolonged storage in ultra-low temperatures does not lead to significant loss of cell viability and function.Citation22,Citation32 We have recently modified this protocol by using much more effective cryopreservation media including Cryostor CS10 (Biolife Solutions), and applying a controlled rate freezing method, that substantially improves the overall cell survival rates ().
Characterization of hepatocytes isolated from diseased livers
As important as the maintenance of hepatocyte viability is, it is even more critical to maintain the activities of certain liver-specific functions at a high level. Mature, primary hepatocytes have a variety of differentiated functions, including drug metabolism, detoxification, and protein synthesis.Citation33 Applications of human hepatocytes in novel drug development include the evaluation of metabolic stability, metabolite profiling and identification, drug–drug interaction potential, and hepatotoxic potential.Citation34 Isolated cells must display robust biotransformation activity, which is critical for further in vitro applications of these cells. The data indicate that hepatocytes isolated from some livers with metabolic disease (MMA, MSUD) performed as well or better than those isolated from organ donors with respect to viability, cell yield, plating efficiency and drug metabolism.Citation28 However, the critical liver functions can be compromised by several liver diseases such as NAFLD, NASH, or ALD, predicted to be the major cause of liver failure as well as a leading indication for liver transplantation once progressed into severe form of liver fibrosis and cirrhosis. Despite the gravity of the situation, there are currently no drugs approved by the US FDA to treat those specific diseases and conditions. Additionally, given significant species-specific differences in diseases profiles, current animal models do not reflect key features and progression of liver diseases.Citation24 Therefore, primary human hepatocytes isolated from explanted livers obtained from patients receiving orthotopic liver transplantation for decompensated liver cirrhosis or for metabolic liver disease are physiologically relevant and extremely valuable cell source to construct in vitro models of the human liver disease.Citation24
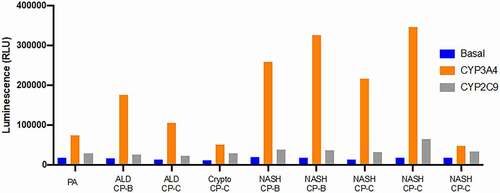
The successful use of primary human hepatocytes in research depends on a reliable demonstration of the functional integrity of isolated cells.Citation35,Citation36 It is essential that hepatocytes isolated from diseased human livers express different CYP450 functions. To address this issue, it is desirable to examine several functional properties of isolated cells including analysis of major Phase I and Phase II drug-metabolizing enzymes. In , we show analysis of activities of CYP3A4 and CYP2C9 (the two, major Phase I drug-metabolizing enzymes) of hepatocytes isolated from nine different diseased or cirrhotic explanted livers (PA, ALD, Crypto and NASH). Our unpublished data indicates that the cells isolated from diseased livers express substantial activities of both CYP3A4 and CYP2C9 and also demonstrates that their functional activities are highly variable after the isolation process. Nevertheless, human hepatocytes of patients with either metabolic diseases of the liver or cirrhosis due to NASH or alcohol consumption can be isolated. These hepatocytes show measurable functional activities, as demonstrated previously using ischemic donor livers that were not used for organ transplantation.Citation36 In the future, it will be interesting and informative to compare functional abilities of cirrhotic hepatocytes vs normal donor hepatocytes. Here, we only show a limited number of liver specimens. It would be necessary to compare a greater number of specimens in order to understand their functional differences. Overall, we observed considerable sample-to-sample variation in the levels of CYP3A4 and we are not sure to what extent (if at all) the ongoing liver disease contributes to the measured enzyme activity. We can conclude that hepatocytes isolated from diseased livers have the potential to provide a large cell source for studies of human CYP450 function and regulation, although these in vitro studies have yet to be extended to all major Phase I and Phase II drug-metabolizing enzymes.
Conclusions
The importance of embracing new technologies to model human liver diseases in vitro is critical for development of effective treatments. Considering the need for preclinical liver models to recapitulate the features of chronic liver diseases, primary human hepatocytes isolated from discarded livers are of great importance. However, implementing a sustainable, continuous improvement of standard methods for hepatocyte isolation from such livers as well as improvements of cell characterization and cryopreservation procedures are essential for efficacy and viability of the final cell product.
Disclosure statement
Potential conflict of interest: A.O. is inventor on a provisional patent application related to methods to enhance hepatic functions in human failing livers (PCT/US2020/055500). A.O. is a co-founder and has a financial interest in Pittsburgh ReLiver Inc., a company focused on programming liver failure and her interests are managed by the Conflict of Interest Office at the University of Pittsburgh in accordance with their policies.
Additional information
Funding
References
- Green CJ, Charlton CA, Wang LM, Silva M, Morten KJ, Hodson L. The isolation of primary hepatocytes from human tissue: optimising the use of small non-encapsulated liver resection surplus. Cell Tissue Bank. 2017;18:597–604. doi:https://doi.org/10.1007/s10561-017-9641-6.
- Belaschk E, Rohn S, Mukiibi R, Reutzel-Selke A, Tang P, Sawitzki B, Pratschke J, Sauer IM, Mogl MT. Isolation, characterization and cold Storage of cells isolated from diseased explanted livers. Int J Artif Organs. 2017;40(6):294–306. doi:https://doi.org/10.5301/ijao.5000594.
- Yamaguchi T, Matsuzaki J, Katsuda T, Saito Y, Saito H, Ochiya T. Generation of functional human hepatocytes in vitro: current status and future prospects. Inflamm Regen. 2019;39:13. doi:https://doi.org/10.1186/s41232-019-0102-4.
- Guguen-Guillouzo C, Guillouzo A. General review on in vitro hepatocyte models and their applications in hepatocytes. Meth Mol Biol. 2010;640:1–40. doi:https://doi.org/10.1007/978-1-60761-688-7_1.
- Corbett JL, Duncan SA. iPSC-derived hepatocytes as a platform for disease modeling and drug discovery. Front Med. 2019;6:265. doi:https://doi.org/10.3389/fmed.2019.00265.
- Takeishi K, Collin de I’Hortet A, Wang Y, Handa K, Guzman-Lepe J, Matsubara K, Morita K, Jang S, Haep N, Florentino RM, et al. Assembly and function of a bioengineered human liver for transplantation generated solely from induced pluripotent stem cells. Cell Rep. 2020;2(9):31. doi:https://doi.org/10.1016/j.celrep.2020.107711.
- Mawer CM, Ene D, and Watt FM. Functional comparison of adult and pluripotent stem cell-derived hepatocytes. Matters 3 9 . 2020.
- Orge ID, Gadd VL, Barouh JL, Rossi EA, Carvalho RH, Smith I, Allahdadi KJ, Paredes BD, Silva DN, Damasceno PKF, et al. Phenotype instability of hepatocyte-like cells produced by direct reprogramming of mesenchymal stromal cells. Stem Cell Res Ther. 2020;11(1):154. doi:https://doi.org/10.1186/s13287-020-01665-z.
- Bhogal RH, Hodson J, Bartlett DC, Weston CJ, Curbishley SM, Haughton E, Williams KT, Reynolds GM, Newsome PN, and Adams DH, et al. Isolation of primary human hepatocytes from normal and diseased liver tissue: a one hundred liver experience. PLoS One. 2011;6(3):e18222. doi:https://doi.org/10.1371/journal.pone.0018222.
- Li Y, Gao M, Wu D, Bao J. Isolations and cultures of primary hepatocytes. J Clin Exp Pathol. 2017;7:322. doi:https://doi.org/10.4172/2161-0681.1000322.
- Zhou Y, Shen JX, Lauschke VM. Comprehensive evaluation of organotypic and microphysiological liver models for prediction of drug-induced liver injury. Front Pharmacol. 2019;10:1093. doi:https://doi.org/10.3389/fphar.2019.01093.
- Lee JH, Ho KL, Fan SK. Liver microsystems in vitro for drug response. J Biomed Sci. 2019;26:88. doi:https://doi.org/10.1186/s12929-019-0575-0.
- Stock P, Christ B. Hepatocyte transplantation methods and protocols. New York (NY): Humana Press; 2017. doi:https://doi.org/10.1007/978-1-4939-6506-9.
- Iansante V, Mitry RR, Filippi C, Fitzpatrick E, Dhawan A. Human hepatocyte transplantation for liver disease: current status and future perspectives. Pediatr Res. 2018;83:232–40. doi:https://doi.org/10.1038/pr.2017.284.
- Oseini AM, Cole BK, Issa D, Feaver RE, Sanyal AJ. Translating scientific discovery: the need for preclinical models of nonalcoholic steatohepatitis. Hepatol Int. 2018;12(1):6–16. doi:https://doi.org/10.1007/s12072-017-9838-6.
- McCarron S, Bathon B, Abbey D, Conlon DM, Rader DJ, Olthoff K, Shaked A, and Raabe TD. NASH patient liver derived organoids exhibit patient specific NASH phenotypes and drug responses bioRxiv preprint . 2019. doi:https://doi.org/10.1101/791467.
- Lasli S, Kim HJ, Lee K, Suurmond CE, Goudie M, Bandaru P, Sun W, Zhang S, Zhang N, Ahadian S, et al. A human liver-on-a-chip platform for modeling non-alcoholic fatty liver disease. Adv Biosyst. 2019;3(8):e1900104. doi:https://doi.org/10.1002/adbi.201900104.
- Hewitt NJ, Lechon MJ, Houston JB, Hallifax D, Brown HS, Maurel P, Kenna JG, Gustavsson L, Skonberg C, Guillouzo A, et al. Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab Rev. 2007;39:159–234. doi:https://doi.org/10.1080/03602530601093489.
- LeCluyse EL, Alexandre E, Hamilton GA, Viollon-Abadie C, Coon DJ, Jolley S, Richert L. Isolation and culture of primary human hepatocytes. Methods Mol Biol. 2005;290:207–29. doi:https://doi.org/10.1385/1-59259-838-2:207.
- Tanaka K, Soto-Gutierrez A, Navarro-Alvarez N, Carrillo JD, Kobayashi N. Functional hepatocyte culture and its application to cell therapies. Cell Transplant. 2006;15:855–64. doi:https://doi.org/10.3727/000000006783981332.
- Ullrich A, Berg C, Hengstler JG, Runge D. Use of standardised and validated long-term human hepatocyte culture system for repetitive analyses of drugs: repeated administrations of Acetaminophen reduces albumin and urea secretion. ALTEX. 2007;24:35–40. doi:https://doi.org/10.14573/altex.2007.1.35.
- Ostrowska A, Gu K, Bode DC, Van Buskirk RG. Hypothermic storage of isolated human hepatocytes: a comparison between University of Wisconsin solution and a hypothermosol platform. Arch Toxicol. 2009;83:493–502. doi:https://doi.org/10.1007/s00204-009-0419-x.
- Kermanizadeh A, Moritz W. Next generation in vitro primary hepatic cell test systems—their suitability as an alternative to in vivo testing? Hepatobiliary Surg Nutr. 2020 Feb;9(1):103–05. doi:https://doi.org/10.21037/hbsn.2019.09.09.
- Underhill GH, Khetani SR. Emerging trends in modeling human liver disease in vitro. L Bioeng. 2019;3(4):040902. doi:https://doi.org/10.1063/1.5119090.
- Kleine M, Riemer M, Krech T, DeTemple D, Jäger MD, Lehner F, Manns MP, Klempnauer J, Borlak J, Bektas H, et al. Explanted diseased livers - a possible source of metabolic competent primary human hepatocytes. PLoS One. 2014;9(7):e101386. doi:https://doi.org/10.1371/journal.pone.0101386.
- Bartlett DC, Newsome PN, Modified A. Protocol for the isolation of primary human hepatocytes with improved viability and function from normal and diseased human liver. Methods Mol Biol. 2017;1506:61–73. doi:https://doi.org/10.1007/978-1-4939-6506-9_4.
- Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969;43:506–20. doi:https://doi.org/10.1083/jcb.43.3.506.
- Gramignoli R, Tahan V, Dorko K, Skvorak KJ, Hansel MC, Zhao W, Venkataramanan R, Ellis EC, Jorns C, Ericzon BG, et al. New potential cell source for hepatocyte transplantation: discarded livers from metabolic disease liver transplants. Stem Cell Res. 2013;11(1):563–73. doi:https://doi.org/10.1016/j.scr.2013.03.002.
- Kehr DC, Raschzok N, Sauer IM. A novel cannulation technique for isolation of human hepatocytes from explanted diseased whole livers. Transplant Proc. 2012 May;44(4):999–1001. PMID: 22564608. doi:https://doi.org/10.1016/j.transproceed.2012.03.006.
- Hossein Aghdaie M, Geramizadeh B, Azarpira N, Esfandiari E, Darai M, Rahsaz M, Nikeghbalian S, Malekhosseini SA. Hepatocyte isolation from unused/rejected livers for transplantation: initial step toward hepatocyte transplantation, the first experience from Iran. Hepat Mon. 2013 Aug 3;13(8):e10397. doi:https://doi.org/10.5812/hepatmon.10397.
- Baccarani U, Donini A, Risaliti A, Piccolo G, Dialti V, Cautero N, Degrassi A, Sirchia G, Bresadola F. Steatotic versus cirrhotic livers as a source for human hepatocyte isolation. Transplant Proc. 2001 Feb-Mar;33(1–2):664–65. doi:https://doi.org/10.1016/s0041-1345(00)02191-6.
- Ostrowska A, Bode DC, Pruss J, Bilir B, Smith GD, and Zeisloft S. Investigation of functional and morphological integrity of freshly isolated and cryopreserved human hepatocytes Cell Tissue Bank . 2000;1(1):55–68. doi:https://doi.org/10.1023/A:1010175906791.
- Li AP. In vitro human hepatocyte-based experimental systems for the evaluation of human drug metabolism, drug-drug interactions, and drug toxicity in drug development. Curr Top Med Chem. 2014;14(11):1325–38. doi:https://doi.org/10.2174/1568026614666140506114411.
- Beckwitt CH, Clark AM, Wheeler S, Taylor DL, Stolz DB, Griffith L, Wells A. Liver ‘organ on a chip.’ Exp Cell Res. 2018;363(1):15–25. doi:https://doi.org/10.1016/j.yexcr.2017.12.023.
- Gramignoli R, Dorko K, Tahan V, Skvorak KJ, Ellis E, Jorns C, Ericzon BG, Fox IJ, Strom SC. Hypothermic storage of human hepatocytes for transplantation. Cell Transplant. 2014;23(9):1143–51. doi:https://doi.org/10.3727/096368913X668627.
- Gramignoli R, Tahan V, Dorko K, Venkataramanan R, Fox IJ, Ellis E, Vosough M, Strom SC. Rapid and sensitive assessment of human hepatocyte functions. Cell Transplant. 2014;23(12):1545–56. doi:https://doi.org/10.3727/096368914X680064.