ABSTRACT
Hippo pathway is a cellular regulatory pathway composed of core molecules such as MST1/2, LATS1/2, SAV1, MOB1A/B and downstream YAP/TAZ. Fully involved in regulating cell proliferation, differentiation, migration and apoptosis, the Hippo pathway is critical in regulating stem cells of oral origin, for instance, DPSCs and PDLSCs, enamel formation and periodontium regeneration. Here, we summarized the Hippo pathway involved in these progresses and concluded crosstalks of the Hippo pathway with BCL-2, ERK1/2, ROCK, TGF-β/BMP and Wnt/β-catenin pathways, hoping to provide foundation for further clinical therapy.
Introduction
Since first discovered in Drosophila, Hippo pathway has been drawing interest in regulation on cell proliferation, migration and apoptosis.Citation1,Citation2 The Hippo pathway contains highly conserved kinases, involving mammalian sterile 20-like kinase 1/2 (MST1/2), large tumor suppressor kinase 1/2 (LATS1/2) and mps one binder 1 (MOB1) A/B, to regulate downstream Yes-associated protein 1 (YAP)/transcriptional coactivator with the PDZ-binding motif, WWTR1 (TAZ). YAP/TAZ could regulate target DNA via directly binding with DNA-binding transcription factors like TEA domain transcription factors (TEAD) and further formation of a complex. When the Hippo pathway is activated, LATS1/2 could phosphorylate YAP/TAZ, hence increasing its degradation and nuclear exclusion by 14-3-3 protein.Citation2,Citation3
Involvement of the Hippo pathway in tooth and periodontium is increasingly being studied, especially in enamel formation, cellular progresses of periodontal ligament cells (PDLCs) and cementoblasts.Citation4–8 Given that the essential role that the Hippo pathway plays in various stem cells,Citation9 the Hippo pathway is also noted to regulate stem cells of oral origin, for instance, dental pulp stem cells (DPSCs) and periodontal ligament stem cells (PDLSCs),Citation10,Citation11 which has shed light on stem cell therapy applied in dental tissue regeneration.Citation12 Therefore, we summarized the function of the Hippo pathway during cellular progresses of DPSCs and PDLSCs, enamel formation and periodontium regeneration, providing foundation for further clinical therapy.
Brief introduction of Hippo pathway
The earliest research on the Hippo pathway was carried out in Drosophila. The name Hippo was derived from the Ste20-like kinase Hippo (Hpo) molecule in this pathway, which was a drosophila kinase gene that had been identified closely related to regulating tissue growth and preventing excessive cell proliferation,Citation13 while Hpo mutants showed a broken balance of apoptosis and proliferation in the tissue.Citation14
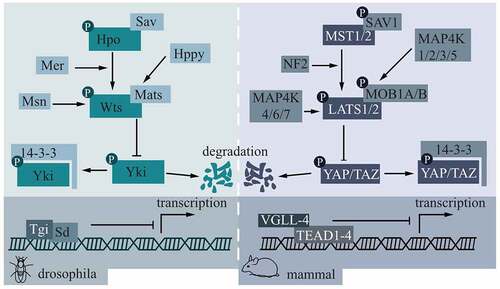
As shown in , the left part is a schematic diagram of the Hippo pathway in Drosophila, while the right part is a corresponding diagram of the Hippo pathway in mammals. The molecule that regulates the Hippo pathway from the start is TAO kinase (TAOK)1/2/3, which phosphorylates MST1 at Thr183 and phosphorylates MST2 at Thr180, thereby activating MST1/2.Citation15,Citation16 In addition to activating downstream LATS1/2 by the C-terminal hydrophobic motif,Citation17 MST1/2 can also phosphorylate Salvador family WW domain‑containing protein 1 (SAV1) and MOB1,Citation18,Citation19 SAV1 can assist the phosphorylation of LATS1/2 by MST1/2 in turn, and phosphorylated MOB1 can combine LATS1/2 as shown in .Citation20 Mer in Drosophila has similar functions to mammalian neurofibromatosis type 2 gene (NF2) and can directly bind to LATS1/2.Citation17 Two groups of mitogen-activated protein kinase kinase kinase kinase (MAP4K) can activate LAST1/2 in parallel with MST1/2.Citation21,Citation22 LATS1/2 directly phosphorylates downstream YAP/TAZ by combining with target consensus motifs (HXRXXS).Citation23 Phosphorylation activities inhibit YAP/TAZ from performing its normal function and cannot regulate downstream TEAD1-4.Citation24
Figure 1. Core molecules in the Hippo pathway and their mode of action.
YAP/TAZ is involved in the regulation of the growth and development of a variety of tissues and organs, including epidermis,Citation25 liverCitation26 and heart.Citation27 In controlling epidermal growth, YAP/TAZ could stabilize layers of cells of skin, where overexpressed activated YAP can induce excessive proliferation of keratinocytes with defective differentiation and reduced terminal differentiation.Citation25,Citation28 When YAP/TAZ overactivation was induced, excessive cell proliferation would occur in organs, such as the expansion of pancreatic duct cellsCitation29 and the change of the acinar structure.Citation30
Effects of the Hippo pathway on DPSCs and PDLSCs
Stem cells are featured by capacity to proliferate and differentiate into multiple cellular lineages and are the origin for multicellular organisms and organs, playing key roles during development and dysregulation of human diseases.Citation9 The Hippo pathway is found to play essential roles in various types of stem cells.Citation9 In recent years, new ideas for tooth and tissue regeneration were proposed with the foundation of theories and technologies of tissue engineering.Citation12 Therefore, it is of significance to summarize current understanding of the Hippo pathway involved in stem cells of oral origin, and the most typical among them are DPSCs and PDLSCs.
Hippo pathway controlling DPSCs
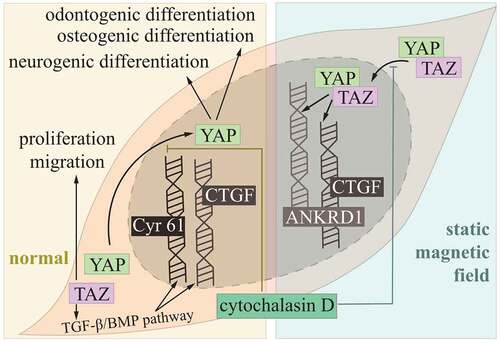
As multipotent stem cells, DPSCs had the potential of differentiating into osteoblast, chondroblasts and adipose and neuronal tissue, acting as promising generators of dentine, dental pulp, bone and muscle.Citation31 The Hippo pathway was proved to involve in these progresses under different conditions. YAP/TAZ had increased nuclear transportation and decreased phosphorylation in the static magnetic field, and then upregulating connecting tissue growth factor (CTGF) and ankyrin repeat domain 1 (ANKRD1), the nuclear localization was reversed by cytoskeleton inhibitor, cytochalasin D. The absence of YAP/TAZ contributed to diminished static magnetic field‐induced mineralization of DPSCs.Citation32
For osteogenic/odontogenic differentiation of DSPCs, upon F-actin alignment treatment, YAP was active in nuclear transportation, while treatment of cytochalasin D, inhibitor of F-actin, leads to reduced YAP nuclear/cytosolic ratios.Citation33 During neurogenic differentiation of DPSCs, YAP played a regulatory role in prominent activity of YAP in nucleus, and YAP expression was decreased after neurogenic differentiation.Citation34 Apart from YAP, TAZ was also expressed in DPSCs, where TAZ silencing inhibited proliferation and migration. Moreover, these effects were associated with downregulation of CTGF and cysteine-rich angiogenic inducer (Cyr) 61 via interfering with transforming growth factor beta (TGF-β)/bone morphogenetic proteins (BMP) pathway ().Citation10
Figure 2. Hippo pathway controlling DPSCs.
Hippo pathway controlling PDLSCs
During regeneration and reconstruction of periodontal tissues involving alveolar bone, cementum and periodontal ligament, PDLSCs play an essential role, which represent one of the mesenchymal stem cells that own the ability of self-renewal and multidifferentiation.Citation35 Studies have revealed that the Hippo pathway was fully involved in proliferation, differentiation, apoptosis and senescence of PDLSCs ().Citation11,Citation36–38
Figure 3. Hippo pathway controlling PDLSCs.
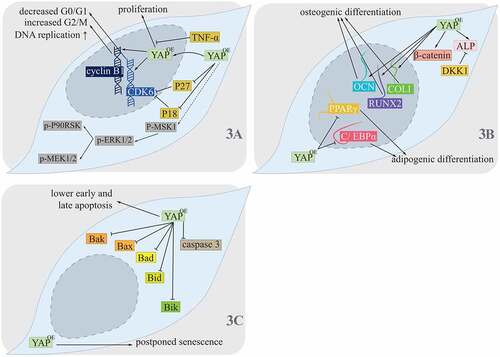
For YAP, it was observed to locate in both the nuclei and cytoplasm of PDLSCs, its location in nuclei increased under the condition of overexpression of YAP (OE YAP), and hence, it was proposed that OE YAP contributed to more activated and nucleus-translocated YAP in PDLSCs.Citation36 Higher DNA replication activities in PDLSCs in the OE YAP group were seen, which combined with increased expression of p-mitogen- and stress-activated protein kinase 1 (MSK1).Citation36 For acceleration of the PDLSC cell cycle, contribution of phases of cell cycle were also changed with OE YAP, where the proportion of G0/G1 decreased and that of G2/M increased.Citation36 Cyclin-dependent kinase 6 (CDK6), which was related to G1/S phase transition, and cyclin B1, which was responsible for G2/M phase transition, were both upregulated, while P18 and P27 that inhibited CDK were both downregulated. P-extracellular signal-regulated kinase (ERK) 1/2, which could be phosphorylated by p-MAPK kinase (MEK) 1/2, was also increased under the OE YAP condition, together with its downstream targets p-90 kD ribosomal S6 kinase (P90RSK) and p-MSK1, suggesting that the ERK1/2 pathway was upregulated with OE YAP and might thus form a crosstalk.Citation36 Similarly, under the condition of knocked down YAP, protein levels of p-ERK1/2, p-P90RSK and p-MSK1 were all reduced ().Citation37 Another study performed by Dong et al. revealed that the effect of OE YAP contributing to promotion of PDLSC proliferation was inhibited by TNF-α, giving us more insight into Hippo pathway controlling PDLSCs proliferation ().Citation38
Osteoblast lineage and adipocyte lineage, two essential directions of PDLSCs differentiation, were also shown to be finely balanced by the Hippo pathway, which might further contribute to tissue engineering, especially that of alveolar bone regeneration.Citation39 OE YAP was revealed for higher osteogenic potential by detection of stronger activity of alkaline phosphatase (ALP) and upregulation of both levels of mRNAs and proteins of collagen type I (COLI), runt-related transcription factor 2 (RUNX2) and osteocalcin (OCN). YAP knocked-down (SHYAP) induced less activated ALP, COLI, RUNX2 and OCN.Citation11 For YAP inhibiting adipogenesis of PDLSCs, mRNA and protein levels of peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer binding protein alpha (C/EBPα) were lower under the OE YAP condition and were higher under the SHYAP condition, which also resulted in more lipid droplets.Citation11 Thus, high expression of YAP could contribute to higher osteogenic differentiation and lower adipogenic differentiation. Moreover, the Wingless/Integrated (Wnt)/β-catenin pathway was also affected by the Hippo pathway in these progresses.Citation11 Higher nonphospho β-catenin (active form) was observed under more YAP conditions, whose effect was reversed by adding dickkopf1 (DKK1). DKK1 could further inhibit ALP activity, extracellular matrix mineralization and expression of osteogenic markers. Nevertheless, under the SHYAP condition, Wnt3a, another participant in the Wnt/β-catenin pathway, could promote osteogenic differentiation of PDLSCs.Citation11 Thus, the effects of YAP balancing PDLSCs osteogenesis and adipogenesis could be affected by the Wnt/β-catenin pathway ().
For cellular senescence of PDLSCs, it could be postponed by OE YAP,Citation36 while higher senescence PDLSCs was shown in the SHYAP group ().Citation37
In the progress of cellular apoptosis of PDLSCs, OE YAP contributed to lower early and late apoptosis, where the content of apoptosis-related proteins caspase 3 (C3) and B cell lymphoma 2 (BCL-2) family members, involving Bak, Bax, Bad, Bid and Bik, decreased.Citation36 Accordingly, SHYAP contributed to earlier and later apoptosis, and its related protein increased regarding expression levels.Citation37 These findings indicated that YAP involved in PDLSCs apoptosis, where crosstalk between the Hippo pathway and the BCL-2 pathway existed ().
In conclusion, the Hippo pathway wasshown to participate in various kinds of cellular activities of PDLSCs, involving proliferation, differentiation, apoptosis and senescence. To note, several crosstalks between the Hippo pathway and ERK1/2, Wnt/β-catenin and BCL-2 pathways in these progresses might of great significance for further reference of fully understanding the role the Hippo pathway plays in PDLSCs.
Hippo pathway involved in the development of enamel
Brief introduction of enamel organ
As diphyodonts, humans have a primary dentition of 20 teeth and a permanent dentition of 32 teeth. Initial development of tooth starts from thickened oral epithelium, which is dental lamina-oriented.Citation40
Tooth morphogenesis relies on reciprocal interactions between ectoderm and mesenchyme that is neural-crest-derived.Citation41 At the site of tooth eruption, thickened primary dental lamina could form dental placode, which invaginates into mesenchyme to form a tooth bud. It can further develop into the cap stage with differentiated inner and outer dental epithelium. To note, the transition from bud to cap gives the foundation of different types of teeth. During the cap stage, a condensed group of cells is named enamel organ for its potential of enamel formation, where enamel knot exists to regulate enamel formation.Citation41
Continuous growth of cap stage will transfer into the bell stage, where several cell groups could be seen, involving inner enamel epithelium, outer enamel epithelium, stratum intermedium and stellate reticulum. During this stage, ameloblasts and odontoblasts formed, making the hard tissues of the crown ().Citation41
Figure 4. Development of enamel organ.
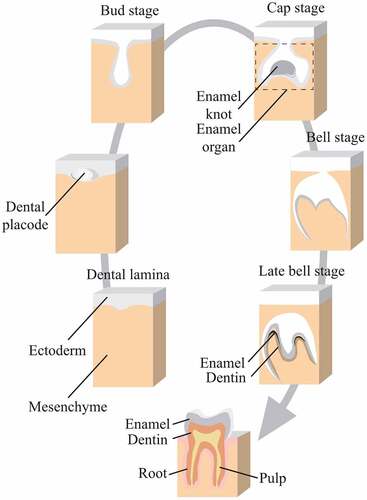
For human tooth development, dental lamina formation occurs during 42–48 days and the bud stage for deciduous incisors, canines and molars occurs in 55–56 days. Bell stages for deciduous teeth and bud stage for permanent teeth happen at 14 weeks. In 18 weeks, dentin and functional ameloblasts start formation in deciduous teeth.Citation41
Hippo pathway regulates the development of enamel organ
During different stages of enamel organ, the Hippo pathway was proved to participate, where YAP/TAZ had everchanging expression in various cells.Citation5,Citation42 The in vivo model of incisor showed that in the lamina stage (embryonic day 11, E11), few cells of dental epithelium displayed strong YAP expression, while in the late bud stage (E13), high YAP expression was observed in the basal cell’s nucleus and that of dental lamina and dental mesenchyme was weak.Citation42 During the cap stage (E14), YAP was still strongly positive in nucleus of a majority of basal cells and weak in cells of the stellate reticulum and dental papilla.Citation42 In E14.5, both YAP and TAZ had expression patterns in enamel organ and dental mesenchyme.Citation43 In the early bell stage (E16), high YAP expression mainly occurred in transit amplifying cells.Citation42
Nevertheless, conflicts were found regarding YAP expression of late enamel development. Li et al. proposed that from the middle bell stage (E18) to the eruption stage (postnatal day 20, PN20), YAP expression was quite similar, which remained strong in the transit amplifying cells and weak in both the apical bud and ameloblasts.Citation42 However, Zhang et al. proposed that from PN0 to PN14, YAP expression was high in ameloblasts, odontoblasts and stratum intermedium. TAZ expression was also high in ameloblasts and odontoblasts during PN0 to PN7, and in PN14, only odontoblasts and dentin tubules showed high expression of TAZ.Citation43 These conflicts might own to different anatomical structures of molars used in their study and incisor in study performed by Li et al.Citation43 Further research studies were expected.
OE YAP was described as a contributor to deformed tooth morphogenesis with widened dental lamina and a “mislocated enamel knot,” whose abnormal effects were observed from E13.5 to E16.5.Citation4 In E13.5, normal expression of YAP was detected in both epithelial and mesenchymal cells with a slightly intense expression in dental epithelia, while OE YAP was seen to increase greatly throughout dental epithelia. In E14.5, widened dental lamina was observed under the OE YAP condition.Citation4 In E16.5, OE YAP brought distinct morphological alterations of enamel organ, involving arrested cap stage, deformed enamel organ with folds of inner dental epithelium and a widened dental lamina, which proved essential roles the Hippo pathway displayed in enamel formation and tooth shape formation.Citation4
Hippo pathway involved in periodontium regeneration
Hippo pathway controlling periodontal ligament remodeling
PDLCs, the connective tissue localized between alveolar bone and tooth cementum, could mediate bone regeneration after sense of mechanical signals of tension and compression during orthodontic tooth movement (OTM) as bone formation at the tension side and bone resorption at the compressive side.Citation44 The Hippo pathway was proved to involve in these progresses.Citation6–8,Citation45
In vivo studies showed that, under cyclic tensile stress (CTS), both mRNA and protein expression of TAZ in PDLCs were increased, together with its interaction with RUNX2, one of the osteogenic proteins, and nucleus aggregation of TAZ was detected.Citation45 Treatment of Y27632, an inhibitor of ras homologue-associated coiled-coil protein kinase (ROCK), could inhibit CTS-induced TAZ expression, together with expressions of several osteogenic proteins like RUNX2, COLI, osterix (OSX) and OCN. Further studies revealed that Y27632-induced inhibition of the ROCK pathway impeded TAZ’s translocation from cytoplasm to nucleus by blocking its combination with RUNX2.Citation45 These findings implicated that nucleus accumulation and activation of TAZ was required in CTS-induced osteogenic differentiation of PDLCs, and crosstalk between the Hippo pathway and the ROCK pathway might assist a fine regulation of this progress ().Citation45
Figure 5. Hippo controlling PDLCs under the cyclic tensile stress condition.
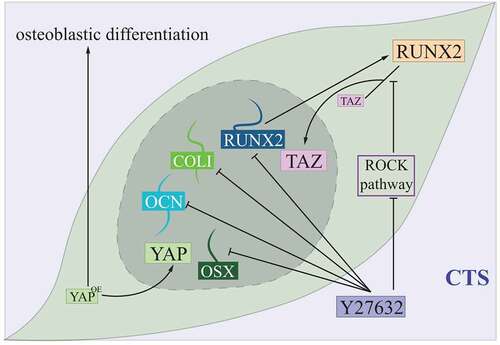
Similar functions were observed in YAP affecting CTS-induced osteogenic differentiation, as it depended on increased nuclear localization and activity of YAP, and depletion of YAP reduced this differentiation of PDLCs.Citation6
Apart from PDLCs, alveolar bone and cementum also involved in periodontal tissue remodeling under OTM and were related to the Hippo pathway. Without force of orthodontic appliance, both TAZ and RUNX2 were situated in the periodontal ligament, alveolar bone and cementum, while under force application, colocalization of TAZ and RUNX2 was observed in both nuclei of PDLCs and osteoblasts. YAP expression was also increased under force treatment, but showed no colocalization with RUNX2.Citation7 It was proposed that the force-Hippo-TAZ-RUNX2 pathway might mainly contribute to bone remodeling, while the force-Hippo-YAP pathway was mainly associated with cell proliferation and differentiation.Citation7
As a result, similar to Hippo pathway’s contribution to PDLSCs, it fully involved in periodontal tissue remodeling of OTM, and crosstalk between pathways was also observed. Nevertheless, different functional molecules of the Hippo pathway and its interaction with downstream targets might enjoy various specific effects on periodontal tissue remodeling, but studies revealing more underlying signaling mechanisms remained to be further elucidated.
Hippo pathway plays an emerging role in differentiation and mineralization of cementoblast
Cementoblasts are cells forming the cementum, which provides attachment of Sharpey’s fiber of the periodontal ligament.Citation46 OE YAP displayed upregulated mRNA levels of cementogenesis phenotypic markers like ALP, RUNX2, dentin matrix acidic phosphoprotein 1 (DMP1) and OCN, while knockdown of YAP showed lower levels of these markers, suggesting that YAP promoted the differentiation and mineralization of cementoblasts.Citation8
Dentin sialophosphoprotein (DSPP), which is expressed in both odontoblasts and cementoblasts, enhanced nuclear translocation under the OE YAP condition, whereas its transcriptional potential decreased by nearly 30% in the knockdown YAP group.Citation8
Crosstalks were also seen in cementoblast, as OE YAP increased downstream target p-mothers against DPP homolog 2 (SMAD)1/5/9 of the TGF-β/BMP pathway and decreased p-ERK1/2 of the ERK1/2 pathway. YAP-induced activation of the SMAD-dependent BMP pathway contributed to cementoblast differentiation, while YAP-induced inhibition of the ERK1/2 pathway contributed to cementoblast mineralization.Citation8
Interactions between the Hippo pathway and other signaling in tooth and periodontium
Interactions of the Hippo pathway with other signaling have been seen in cellular progresses of DPSCs and PDLSCs, enamel formation and periodontium tissue formation, including PDLCs and cementoblasts.Citation6,Citation8,Citation10,Citation36,Citation37,Citation45
Crosstalk between the BCL-2 pathway and the Hippo pathway mainly participates in apoptosis of PDLSCs.Citation36,Citation37 The BCL-2 gene family encodes various proteins that regulate apoptosis, balancing between cellular survival and death.Citation47 Research studies regarding BCL-2 revealed that mechanisms for cell death were highly conserved, and genes that activate the BCL-2 gene were associated with malignant disease in humans.Citation47 Bak, Bax, Bad, Bid and Bik, which were all apoptosis-related BCL-2 family members, were found to be decreased under the OE YAP condition, hence inducing lower proportion of early and late apoptosis of PDLSCs,Citation36 while YAP silencing was related to upregulation of these proteins. That is, in the process of decreasing PDLSCs apoptosis, the Hippo pathway could inhibit the BCL-2 pathway through targets Bak, Bax, Bad, Bid and Bik.Citation37 However, the mechanism by which the Hippo pathway regulated the BCL-2 pathway required further study.
Crosstalk between the ERK1/2 pathway and the Hippo pathway contributes to proliferation of PDLSCs, together with differentiation and mineralization of cementoblast.Citation36,Citation37 The ERK1/2 pathway is subordinate to the mitogen-activated protein kinase (MAPK) pathway.Citation48 The ERK1/2 pathway regulates various cellular progresses, for instance, proliferation, differentiation and transformation.Citation48 During progress of DNA replication of PDLSCs, YAP was tested to assist MEK1 phosphorylation, then triggering downstream phosphorylation of ERK1/2, P90RSK and MSK1.Citation36 During differentiation and mineralization of cementoblast, a lower level of p-ERK1/2 was observed under OE YAP.Citation8 These conclusions indicated that the ERK1/2 pathway might receive contradicting regulation by the Hippo pathway in periodontal tissues repair and regeneration.
Crosstalk between the ROCK pathway and the Hippo pathway regulates CTS-induced osteogenic differentiation of PDLCs.Citation45 ROCK is a downstream target of RhoA, which exists in two forms, namely ROCK1 and ROCK2.Citation49 This pathway mainly regulates cell growth, differentiation, migration and development.Citation49,Citation50 Inhibition of the ROCK pathway could inhibit TAZ expression and translocation from cytoplasm to nucleus under CTS induction and then inhibits osteogenic differentiation of PDLCs.Citation45 In these progresses, the ROCK pathway might act as an upstream regulator of the Hippo pathway.
Crosstalk between the TGF-β/BMP pathway and the Hippo pathway participates in DPSCs proliferation and differentiation, migration and cementoblast differentiation.Citation8,Citation10 BMPs are the largest subdivisions of the TGF-β family, which complete intracellular signal transmission by binding to receptors on the cell membrane. In the canonical BMP pathway, the activation of type 1 receptors leads to the formation of the SMAD1/5/8/SMAD4 complex, which is transported in the nucleus to complete the transcriptional regulation of downstream genes.Citation51 The TGF-β/BMP pathway is interfered by the Hippo pathway for further regulation of CTGF and Cyr 61 in DPSCs,Citation10 while in cementoblast, this pathway is also upregulated by the Hippo pathway. Further studies regarding the complete mechanism are still expected.Citation8
Crosstalk between the Wnt/β-catenin pathway and the Hippo pathway are mainly focused on periodontal tissues regeneration, which PDLSCs osteogenic differentiation represented.Citation11 The core molecules in the Wnt/β-catenin pathway are Wnt protein and β-catenin. It is generally believed that the Wnt protein family is the start of activating the Wnt/β-catenin pathway, which helps complete the transmission of extracellular signals into cell and nucleus.Citation52 Silencing the Hippo pathway could activate the Wnt/β-catenin pathway via Wnt3a, hence promoting osteogenic differentiation of PDLSCs.Citation53
Conclusion and Perspectives
Development and regeneration of enamel and periodontium involves several pathways; in this review, we focused on the Hippo pathway during these progresses.
As is known, YAP/TAZ acts as the most critical molecules in the Hippo pathway, and this point has been reflected in various research studies focusing on its role in enamel and periodontium formation. Nevertheless, how do other molecules like MST1/2 and LATS1/2 affect these progresses? Further studies are expected.
Studies focusing on different types of cells mentioned in this review are not all in vivo, but it is believed that in vivo studies are closer to the real function and state of the corresponding pathways in human tissues. Compared with other pathways involved in oral tissue development and regeneration, the Hippo pathway is expecting more in vitro studies for further clinical potentials of therapy.
Moreover, as crosstalks of the Hippo pathway with various pathways are demonstrated in intestinal regeneration and disease, further studies are encouraged to perform in-depth discovery of more complete crosstalks of the Hippo pathway with other pathways in dental regeneration and disease.
As the Hippo pathway has been proved to involve in enamel formation, what role does it play in other tissues of tooth, for instance, dentin and root formation? Does the Hippo pathway regulate other stem cells of oral origin? Are the Hippo pathway and its crosstalks ready for clinical therapy of tooth regeneration? We hope that our opinion could inspire further in-depth studies.
In conclusion, emerging roles of the Hippo pathway in enamel formation and periodontium regeneration have been discovered. Nevertheless, it remains far from clinical treatment of Hippo targeting when it comes to tooth development dysplasia and regeneration of periodontium. Much more related studies are highly encouraged and expected.
Abbreviation
Author contributions
T. W: drafting of the article and drawing schematic figures; T. W, K. L and H. L: critical revision of the article; E. L: guidance of this project and preformation of the final approval of the version to be submitted.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Maugeri-Sacca M, De Maria R. The Hippo pathway in normal development and cancer. Pharmacol Ther. 2018;186:60–12. doi:10.1016/j.pharmthera.2017.12.011.
- Ma S, Meng Z, Chen R, Guan KL. The Hippo pathway: biology and pathophysiology. Annu Rev Biochem. 2019;88(1):577–604. doi:10.1146/annurev-biochem-013118-111829.
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21(21):2747–61. doi:10.1101/gad.1602907.
- Liu M, Zhao S, Wang XP. YAP overexpression affects tooth morphogenesis and enamel knot patterning. J Dent Res. 2014;93(5):469–74. doi:10.1177/0022034514525784.
- Kwon HJ, Li L, Jung HS. Hippo pathway/Yap regulates primary enamel knot and dental cusp patterning in tooth morphogenesis. Cell Tissue Res. 2015;362:447–51. doi:10.1007/s00441-015-2267-8.
- Yang Y, Wang BK, Chang ML, Wan ZQ, Han GL. Cyclic stretch enhances osteogenic differentiation of human periodontal ligament cells via YAP activation. Biomed Res Int. 2018;2018:2174824. doi:10.1155/2018/2174824.
- Sun B, Wen Y, Wu X, Zhang Y, Qiao X, Xu X. Expression pattern of YAP and TAZ during orthodontic tooth movement in rats. J Mol Histol. 2018;49:123–31. doi:10.1007/s10735-017-9752-1.
- Yang B, Sun H, Song F, Wu Y, Wang J. Yes-associated protein 1 promotes the differentiation and mineralization of cementoblast. J Cell Physiol. 2018;233:2213–24. doi:10.1002/jcp.26089.
- Liu H, Jiang D, Chi F, Zhao B. The Hippo pathway regulates stem cell proliferation, self-renewal, and differentiation. Protein Cell. 2012;3:291–304. doi:10.1007/s13238-012-2919-3.
- Tian S, Tian X, Liu Y, Dong F, Wang J, Liu X, Zhang Z, Chen H. Effects of TAZ on human dental pulp stem cell proliferation and migration. Mol Med Rep. 2017;15(6):4326–32. doi:10.3892/mmr.2017.6550.
- Jia L, Zhang Y, Ji Y, Xiong Y, Zhang W, Wen Y, Xu X. YAP balances the osteogenic and adipogenic differentiation of hPDLSCs in vitro partly through the Wnt/beta-catenin signaling pathway. Biochem Biophys Res Commun. 2019;518(1):154–60. doi:10.1016/j.bbrc.2019.08.024.
- Zhai Q, Dong Z, Wang W, Li B, Jin Y. Dental stem cell and dental tissue regeneration. Front Med. 2019;13(2):152–59. doi:10.1007/s11684-018-0628-x.
- Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114(4):445–56. doi:10.1016/S0092-8674(03)00549-X.
- DeRan M, Yang J, Shen CH, Peters EC, Fitamant J, Chan P, Hsieh M, Zhu S, Asara J, Zheng B, et al. Energy stress regulates hippo-YAP signaling involving AMPK-mediated regulation of angiomotin-like 1 protein. Cell Rep. 2014;9(2):495–503. doi:10.1016/j.celrep.2014.09.036.
- Boggiano JC, Vanderzalm PJ, Fehon RG. Tao-1 phosphorylates Hippo/MST kinases to regulate the Hippo-Salvador-Warts tumor suppressor pathway. Dev Cell. 2011;21(5):888–95. doi:10.1016/j.devcel.2011.08.028.
- Poon CL, Lin JI, Zhang X, Harvey KF. The sterile 20-like kinase Tao-1 controls tissue growth by regulating the Salvador-Warts-Hippo pathway. Dev Cell. 2011;21(5):896–906. doi:10.1016/j.devcel.2011.09.012.
- Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154(6):1342–55. doi:10.1016/j.cell.2013.08.025.
- Praskova M, Xia F, Avruch J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol. 2008;18(5):311–21. doi:10.1016/j.cub.2008.02.006.
- Callus BA, Verhagen AM, Vaux DL. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J. 2006;273(18):4264–76. doi:10.1111/j.1742-4658.2006.05427.x.
- Hergovich A, Schmitz D, Hemmings BA. The human tumour suppressor LATS1 is activated by human MOB1 at the membrane. Biochem Biophys Res Commun. 2006;345(1):50–58. doi:10.1016/j.bbrc.2006.03.244.
- Zheng Y, Wang W, Liu B, Deng H, Uster E, Pan D. Identification of Happyhour/MAP4K as alternative Hpo/Mst-like kinases in the Hippo kinase cascade. Dev Cell. 2015;34(6):642–55. doi:10.1016/j.devcel.2015.08.014.
- Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW, Park HW, Mo J-S, Lu W, Lu S, et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun. 2015;6(1):8357. doi:10.1038/ncomms9357.
- Chan SW, Lim CJ, Guo F, Tan I, Leung T, Hong W. Actin-binding and cell proliferation activities of angiomotin family members are regulated by Hippo pathway-mediated phosphorylation. J Biol Chem. 2013;288(52):37296–307. doi:10.1074/jbc.M113.527598.
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang C-Y, Chinnaiyan AM, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22(14):1962–71. doi:10.1101/gad.1664408.
- Nishio M, Miyachi Y, Otani J, Tane S, Omori H, Ueda F, Togashi H, Sasaki T, Mak TW, Nakao K, et al. Hippo pathway controls cell adhesion and context-dependent cell competition to influence skin engraftment efficiency. FASEB J. 2019;33(4):5548–60. doi:10.1096/fj.201802005R.
- Koo JH, Plouffe SW, Meng Z, Lee DH, Yang D, Lim DS, Wang C-Y, Guan K-L. Induction of AP-1 by YAP/TAZ contributes to cell proliferation and organ growth. Genes Dev. 2020;34(1–2):72–86. doi:10.1101/gad.331546.119.
- Chen X, Yuan W, Li Y, Luo J, Hou N. Role of Hippo-YAP1/TAZ pathway and its crosstalk in cardiac biology. Int J Biol Sci. 2020;16(13):2454–63. doi:10.7150/ijbs.47142.
- Yuan Y, Park J, Feng A, Awasthi P, Wang Z, Chen Q, Iglesias-Bartolome R. YAP1/TAZ-TEAD transcriptional networks maintain skin homeostasis by regulating cell proliferation and limiting KLF4 activity. Nat Commun. 2020;11(1):1472. doi:10.1038/s41467-020-15301-0.
- Ansari D, Ohlsson H, Althini C, Bauden M, Zhou Q, Hu D, Andersson R. The Hippo signaling pathway in pancreatic cancer. Anticancer Res. 2019;39(7):3317–21. doi:10.21873/anticanres.13474.
- Swidnicka-Siergiejko AK, Gomez-Chou SB, Cruz-Monserrate Z, Deng D, Liu Y, Huang H, Ji B, Azizian N, Daniluk J, Lu W, et al. Chronic inflammation initiates multiple forms of K-Ras-independent mouse pancreatic cancer in the absence of TP53. Oncogene. 2017;36(22):3149–58. doi:10.1038/onc.2016.461.
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. PNAS. 2000;97(25):13625–30. doi:10.1073/pnas.240309797.
- Zheng L, Zhang L, Chen L, Jiang J, Zhou X, Wang M, Fan Y. Static magnetic field regulates proliferation, migration, differentiation, and YAP/TAZ activation of human dental pulp stem cells. J Tissue Eng Regen Med. 2018;12(10):2029–40. doi:10.1002/term.2737.
- Du Y, Montoya C, Orrego S, Wei X, Ling J, Lelkes PI, Yang M . Topographic cues of a novel bilayered scaffold modulate dental pulp stem cells differentiation by regulating YAP signalling through cytoskeleton adjustments. Cell Prolif. 2019;52(6):e12676. doi:10.1111/cpr.12676.
- Baysal E, Zirh EB, Buber E, Jakobsen TK, Zeybek ND. The effect of melatonin on Hippo signaling pathway in dental pulp stem cells. Neurochem Int. 2021;148:105079. doi:10.1016/j.neuint.2021.105079.
- Tomokiyo A, Wada N, Maeda H. Periodontal ligament stem cells: regenerative potency in periodontium. Stem Cells Dev. 2019;28(15):974–85. doi:10.1089/scd.2019.0031.
- Jia L, Gu W, Zhang Y, Jiang B, Qiao X, Wen Y. Activated yes-associated protein accelerates cell cycle, inhibits apoptosis, and delays senescence in human periodontal ligament stem cells. Int J Med Sci. 2018;15(11):1241–50. doi:10.7150/ijms.25115.
- Wen Y, Ji Y, Zhang Y, Jiang B, Tang C, Wang Q, Chen X, Jia L, Gu W, Xu X . Knockdown of yes-associated protein induces the apoptosis while inhibits the proliferation of human periodontal ligament stem cells through crosstalk between Erk and Bcl-2 signaling pathways. Int J Med Sci. 2017;14(12):1231–40. doi:10.7150/ijms.20504.
- Dong T, Sun X, Jin H. Role of YAP1 gene in proliferation, osteogenic differentiation, and apoptosis of human periodontal ligament stem cells induced by TNF-alpha. J Periodontol. 2021;92(8):1192–200. doi:10.1002/JPER.20-0176.
- Yang S, Guo L, Su Y, Wen J, Du J, Li X, Liu Y, Feng J, Xie Y, Bai Y, et al. Nitric oxide balances osteoblast and adipocyte lineage differentiation via the JNK/MAPK signaling pathway in periodontal ligament stem cells. Stem Cell Res Ther. 2018;9(1):118. doi:10.1186/s13287-018-0869-2.
- Lacruz RS, Habelitz S, Wright JT, Paine ML. Dental enamel formation and implications for oral health and disease. Physiol Rev. 2017;97(3):939–93. doi:10.1152/physrev.00030.2016.
- Nanci A. 2018. Ten Cate’s oral histology development, structure, and function. Ninth (Elsevier), 400:9780323485241.
- Li L, Kwon HJ, Harada H, Ohshima H, Cho SW, Jung HS. Expression patterns of ABCG2, Bmi-1, Oct-3/4, and Yap in the developing mouse incisor. Gene Expr Patterns. 2011;11(3–4):163–70. doi:10.1016/j.gep.2010.11.001.
- Zhang B, Sun BY, Ji YW, Zhang YP, Wang XX, Xu X, Wen Y . Expression and localization of Yap and Taz during development of the mandibular first molar in rats. Biotech Histochem. 2017;92(3):212–21. doi:10.1080/10520295.2016.1267799.
- Deng L, Chen Y, Guo J, Han X, Guo Y. Roles and mechanisms of YAP/TAZ in orthodontic tooth movement. J Cell Physiol. 2021;236(11):7792–800. doi:10.1002/jcp.30388.
- Wang Y, Hu B, Hu R, Tong X, Zhang M, Xu C, He Z, Zhao Y, Deng H . TAZ contributes to osteogenic differentiation of periodontal ligament cells under tensile stress. J Periodontal Res. 2020;55:152–60. doi:10.1111/jre.12698.
- MI C, PR G. Development and general structure of the periodontium. Periodontol. 2000;24:9–27. doi:10.1034/j.1600-0757.2000.2240102.x.
- Delbridge AR, Grabow S, Strasser A, Vaux DL. Thirty years of BCL-2: translating cell death discoveries into novel cancer therapies. Nat Rev Cancer. 2016;16:99–109. doi:10.1038/nrc.2015.17.
- Lake D, Correa SA, Muller J. Negative feedback regulation of the ERK1/2 MAPK pathway. Cell Mol Life Sci. 2016;73:4397–413. doi:10.1007/s00018-016-2297-8.
- Deng Z, Jia Y, Liu H, He M, Yang Y, Xiao W, Li Y. RhoA/ROCK pathway: implication in osteoarthritis and therapeutic targets. Am J Transl Res. 2019;11:5324–31.
- Chen K, Zhang W, Chen J, Li S, Guo G. Rho-associated protein kinase modulates neurite extension by regulating microtubule remodeling and vinculin distribution. Neural Regener Res. 2013;8:3027–35. doi:10.3969/j.1673-5374.2013.32.006.
- Katagiri T, Watabe T. Bone morphogenetic proteins. Cold Spring Harb Perspect Biol. 2016;8:a021899. doi:10.1101/cshperspect.a021899.
- Taciak B, Pruszynska I, Kiraga L, Bialasek M, Krol M. Wnt signaling pathway in development and cancer. J Physiol Pharmacol. 2018;69. doi:10.26402/jpp.2018.2.07.
- Wang Y, Zhang X, Shao J, Liu H, Liu X, Luo E. Adiponectin regulates BMSC osteogenic differentiation and osteogenesis through the Wnt/βcatenin pathway. Sci Rep. 2017;7:3652–3665. doi:10.1038/s41598-017-03899-z. .
