ABSTRACT
Antibody-mediated rejection (ABMR) is the major cause of chronic allograft dysfunction and loss in kidney transplantation. The immunological mechanisms of ABMR that have been featured in the latest studies indicate a highly complex interplay between various immune and nonimmune cell types. Clinical diagnostic standards have long been criticized for being arbitrary and the lack of accuracy. Transcriptomic approaches, including microarray and RNA sequencing of allograft biopsies, enable the identification of differential gene expression and the continuous improvement of diagnostics. Given that conventional bulk transcriptomic approaches only reflect the average gene expression but not the status at the single-cell level, thereby ignoring the heterogeneity of the transcriptome across individual cells, single-cell RNA sequencing is rising as a powerful tool to provide a high-resolution transcriptome map of immune cells, which allows the elucidation of the pathogenesis and may facilitate the development of novel strategies for clinical treatment of ABMR.
Introduction
Allograft kidney transplantation has become a treatment of choice for patients with end-stage renal disease (ESRD) which has less impact on patients’ quality of life as compared with dialysis.Citation1 Following the introduction of modern immunosuppressive regimens, the outcomes of kidney transplant recipients have been improving. However, chronic allograft rejection remains a knotty clinical issue despite the advances in immunosuppressants.Citation2 Antibody-mediated rejection (ABMR) is reported to be the leading cause of chronic allograft rejection.Citation3,Citation4 Over the years, the weakness of diagnostic standards and the complexity of the immunological pathogenesis of ABMR have affected patient care and hindered the successful development of novel therapeutic strategies.
The major focus of this article is to review the advances in transcriptomic approaches including microarray, RNA sequencing (RNA-seq), and the rapidly emerging single-cell transcriptome analysis, with emphasis on their applications to the ABMR study. I attempt to provide a comprehensive overview of the insights and the opportunities that these techniques provide to improve the diagnosis and treatment of ABMR.
Immunological mechanisms of ABMR
The allograft rejection starts from the recognition of alloantigens by recipient T cells. Allorecognition can be divided into direct, indirect, and semi-direct types.Citation5 In direct allorecognition, the recipient T cells recognize the alloantigens presented by donor antigen-presenting cells (APCs). In indirect allorecognition, the alloantigens are processed into peptides by the recipient APCs and presented to the recipient T cells. The activated CD4 T cells help activate CD8 T cells to differentiate into cytotoxic effectors.Citation6 In semi-direct allorecognition, recipient APCs acquire donor anti-human leukocyte antigen (HLA) molecules that present peptides directly to recipient T cells.Citation7 In indirect allorecognition, T cells differentiate into T follicular helper T (Tfh) cells.Citation8 The interaction between Tfh cells and B cells requires the signals of co-stimulatory and co-inhibitory molecules, and cytokines.Citation9
After being activated by antigens, some of the B cells differentiate into short-lived plasma cells (SLPCs) that secrete antibodies, while some other B cells migrate to germinal centers and become long-lived plasma cells (LLPCs) or memory B cells with the aid of Tfh cells.Citation10,Citation11 Both LLPCs and memory B cells account for the donor-specific antibody (DSA) production; the quiescent memory B cells rapidly differentiate into SLPCs upon alloantigen re-exposure and account for the generation of de novo DSA, while the LLPCs constitutively secrete antibodies and produce long-term circulating DSA but do not react upon alloantigen re-exposure.Citation12 The affinity maturation of the memory B cells in the germinal centers during the primary response involves mutations in the antigen combining site. This somatic hypermutation-based mechanism may account for the unpredictable nature of DSA in terms of antigen specificity and the frequent failure of optimal HLA matching by using serum alone.Citation12
The formation of DSA results in three following consequences: complement-dependent cytotoxicity, antibody-dependent cellular toxicity, and direct endothelial injury.Citation13 Upon the binding of DSA to alloantigen, the classical complement pathway is activated, which produces anaphylatoxins including C3a and C5a, recruits inflammatory cells, and ultimately leads to tissue injury.Citation14 During this process, C4d is produced as a degradation product that binds to the endothelial basement membrane and appears as an in situ marker of complement activation in renal allografts.Citation15 The Fc receptor binding to the Fc of innate immune cells including macrophages and natural killer (NK) cells is responsible for antibody-dependent cellular toxicity and leads to degranulation, cell lysis, and phagocytosis.Citation14 The direct binding of the antigens on allograft endothelial cells also causes endothelial activation and proliferation. Instead of being a passive victim, the endothelial cells also participate in the pathogenesis of rejection including leukocyte adhesion and recruitment, lymphocyte activation and differentiation, as well as the secretion of cytokines and chemokines after activation.Citation16
Current diagnosis, treatment, and clinical challenges of ABMR
ABMR was first identified based on a very high correlation found between the occurrence of antibodies reactive to graft antigens and histologic evidence of microvascular lesions.Citation17 Later on, it was found to be associated with classical complement activation based on the intense deposition of complement fragments C4d and C3d in peritubular capillaries shown by immunostaining.Citation18
The diagnosis of ABMR in the current clinical practice primarily depends on the Banff classification. The Banff Classification of Kidney Allograft Pathology was initially established in August 1991 by a group of pathologists and clinicians in Banff, Alberta, Canada. Diagnostic criteria for acute/active ABMR were first added to the Banff classification after the 2001 conference.Citation19 Since then, Banff conferences have been held every 2 years worldwide, and several major revisions and modifications have been made to improve the diagnostic and prognostic values. According to the most recent Banff 2019 Kidney Meeting Report,Citation20 all three of the criteria listed below must be met for diagnosis: (1) histological evidence of allograft injury via microvascular inflammation (MVI), intimal or transmural arteritis, acute thrombotic microangiopathy, or acute tubular injury in the absence of any other apparent cause; (2) histological evidence of antibody-endothelial interactions either by C4d deposition, at least moderate MVI, or increased expression of gene transcripts/classifiers in the biopsy tissue that have been validated to be strongly associated with ABMR; and (3) the presence of circulating DSA, predominantly HLA antibody. C4d staining or expression of validated transcripts/classifiers as noted in criterion (2) may substitute for DSA, and biopsies meeting criterion 1 but not criterion 2 with current or prior evidence, but not remote DSA, may be stated as showing chronic ABMR.
In retrospect, the Banff classification was once arbitrated by the recognition of C4d deposition in peritubular capillaries. C4d positive staining in peritubular capillaries was the only indicator of antibody interaction with vascular endothelium in criterion (2) and must be met to make the diagnosis of ABMR according to the 2007 Banff classification.Citation21 This has been shown to be problematic since many cases with features of chronic antibody-mediated rejection were observed to be C4d-negative, which suggests that C4d may be specific but not sensitiveCitation22; on the other hand, C4d staining may be positive in the absence of clinically significant ABMR.Citation23 As a result, one major revision made in the 2013 Banff classification was to include C4d-negative ABMR, embracing other features including MVI and validated molecular markers as an alternative assessment for criterion (2).Citation24 Further, the Banff working group specified the molecular markers as the expression of gene transcripts/classifiers associated with ABMR as a major update from the 2015 to 2017 Banff classification.Citation25,Citation26 The revised Banff classification was found to outperform previous versions in terms of ABMR recognition and prediction of graft loss,Citation27 and such advances are largely aided by the results yielded with some of the techniques listed in the present article which will be described in detail in the next section.
Despite these major advances, the current Banff classification still carries several pitfalls, including the subjective quantitative scoring of histological lesions, uncertain pathogenesis for similar morphologies, and difficulty in determining the activity of inflammatory cells.Citation23 In addition, the complexity of the participating cells and the immune crosstalk among them have created an arduous challenge in the development of effective treatments for ABMR. Common therapeutic strategies are based on the reduction of antibody titers using plasmapheresis, along with intravenous immune globulins (IVIG) plus immunosuppressants such as tacrolimus or mycophenolic acid.Citation6 Novel agents including antibodies targeting B cells, plasma cells, and the complement system have featured in recent studies of ABMR. However, an updated systemic review and meta-analysis done by Wan et al.Citation28 reported that rituximab showed little or no benefit when used in addition to the standard-of-care combination of plasmapheresis plus IVIG, and the efficacy of bortezomib and complement inhibitors for the treatment of ABMR remained unclear. Likewise, studies evaluating the beneficial effect of eculizumab, a humanized monoclonal antibody that binds to the human C5 complement protein, have reported mixed results in treating ABMR.Citation29–31 Therefore, there is a constant unmet need for increasing the diagnostic precision in ABMR and in-depth knowledge of the interplay between the immune and nonimmune cells in the onset and progression of ABMR.
Improving the diagnosis of ABMR: transcriptome analysis
The unsolved inconsistency in the conventional diagnostic system has called for the discovery and validation of molecular diagnostic tools in ABMR assessment. In transplantation, an allograft meets the graft-infiltrating cells containing genomes from both the donor and the recipient, which not only contributes to the variability of outcomes but also confers a unique genetic perspective on the approach to the illness.Citation32 This section will cover the transcriptomic techniques that have been utilized to improve the diagnostic accuracy of ABMR including reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR), microarray, and RNA-seq, and summarize major research corresponding to each technique.
Since the late 1990s, RT-qPCR has shown promise to explore differential gene expression (DGE) in various diseases. The principal steps of RT-qPCR include RNA isolation, generating complementary DNA (cDNA) with reverse transcriptase, and PCR amplification of cDNA with primers for specific genes of interest, in which DNA molecules labeled with fluorescent probes can be quantified by monitoring the signals.Citation33 In renal allograft rejection, RT-qPCR has revealed elevation of multiple immune activation transcripts in allografts, including expression of interleukin (IL)-2, IL-7, IL-10, IL-15, Fas ligand, perforin, and granzyme B in acute renal allograft rejection,Citation34,Citation35 as well as transforming growth factor beta 1 (TGF-β1) in chronic rejection.Citation35
First introduced by Brown et al.Citation36 at Stanford University in 1995, microarray emerged as another powerful tool in molecular studies of transplant biopsies. In a typical microarray experiment, the mRNA extracted from tissues of interest is first converted into cDNA and tagged with fluorescent probes. The cDNA samples are then hybridized with numerous transcript sequences printed at a high density on a microscope slide. Following the hybridization, the microarray slide is then scanned to measure the fluorescence, which reveals the expression of each gene printed on the slide. Thus, microarray has the distinguished capability of monitoring the expression of many genes in parallel, and it has also been widely applied to reveal DGE in transplant rejection and, more specifically, ABMR.Citation37–44
Some studies have compared the quantification of mRNA gene expression in renal allograft biopsies by RT-qPCR with the results yielded by microarray. One study reported that RT-qPCR and microarray gave similar results in abnormal kidneys and were both competent to detect the relevant changes in rejection.Citation45 In contrast, a meta-analysis of the kidney microarray dataset investigating cytokine gene detection and the correlation with RT-qPCR results showed that microarray failed to detect a majority of cytokine-related genes, which are generally expressed at low abundance.Citation46 Hence, it is worth attention that microarray, despite being high throughput, is limited by the range of detection owing to both background and saturation of signals, and could lead to false-negative results especially in detecting changes in the expression of genes of low abundance. Moreover, one major drawback shared by RT-qPCR and microarray is that both methods rely upon existing knowledge about genome sequence. RNA-Seq, emerging on the advent of next-generation sequencing (NGS) technology, can address these limitations.
RNA-Seq has clear advantages over the existing approaches and revolutionized the manner of transcriptome analysis.Citation47 In a typical RNA-seq experiment, a population of RNA is extracted from samples, converted into cDNA, made into an adaptor-ligated sequencing library, and then sequenced to a depth of 10–30 million reads per sample.Citation48 Exploiting the advances in computational methods, the resulting sequencing reads are either aligned to a reference genome or assembled de novo without the genomic sequence to produce a genome-scale transcription map for analyzing DGE.Citation47,Citation48 RNA-seq has been increasingly utilized in the investigation of different types of renal allograft rejection, including ABMR, albeit currently available studies are relatively limited compared to microarray method. One study performing RNA-seq on biopsy-paired peripheral blood samples from patient cohorts with different types of rejection identified 102 genes with enrichment in the regulation of endoplasmic reticulum stress, adaptive immunity, and Ig class-switching to be associated with ABMR, including the SIGLEC17P pseudogene and the related coding genes,Citation49 of which the expression is almost exclusively in NK cells.Citation50 Dooley et al.Citation51 performed RNA-seq with human urine samples matched to TCMR, ABMR, as well as non-rejection biopsies and identified three novel mRNAs (ITM2A, SLAMF6, and IKZF3) of which the expressions were significantly higher in urine matched to TCMR or ABMR than in the non-rejection biopsies.
In terms of the translation of these transcriptomic techniques into clinical diagnostics, one known example is the work done by the Edmonton group. To facilitate the determination of the molecular phenotype of renal allograft biopsies using microarray, Mueller et al.Citation39 in Edmonton, Alberta, first created pathogenesis-based transcript sets (PBTs) to reflect the biological processes in the alloimmune response. The study showed no absolute specificity of individual molecules or PBTs for rejection but revealed quantitative specificity, including interferon-gamma (IFN-γ) for rejection, T cell and macrophage transcripts for T cell-mediated rejection (TCMR), as well as endothelial and NK cell transcripts for ABMR.Citation39 Based on the hypothesis that alloantibody acting on the microcirculation could be a sensitive indicator of ABMR, Sis et al.Citation40 identified 119 endothelial-associated transcripts (ENDATs) from literature and studied their expression by microarray in 173 renal allograft biopsies; ENDAT expression was correlated with histopathologic lesions of ABMR but not TCMR, and high ENDAT expression in patients with alloantibody could serve as an indicator of active ABMR and poor graft outcome. To distinguish between ABMR and TCMR with molecular tests, the Edmonton group performed microarray on renal allograft biopsies to develop classifiers that assigned ABMR scores to each biopsy that had been assigned diagnoses including C4d-negative ABMR based on histology and donor-specific HLA antibody.Citation41 The author group then conducted the prospective INTERCOM study (NCT01299168) in biopsies obtained from six centers: Baltimore, Barcelona, Edmonton, Hannover, Manchester, and Minneapolis to validate the ABMR score, which showed that the score was more strongly associated with allograft failure than conventional assessments and could predict early progression to failure.Citation42 Further, the author group developed the Molecular Microscope Diagnostic System (MMDx) using machine learning-derived classifier algorithms,Citation43 which showed better agreement with clinical judgment than histology did in an extended survey of the INTERCOM study (INTERCOMEX).Citation44 The serial studies demonstrated the feasibility of molecular assessment in biopsy interpretation.
The measurement of ENDAT and other immune activation transcripts in allograft has also been used to evaluate the therapeutic effects of novel immunosuppressive regimens on ABMR. Kulkarni et al.Citation31 assessed the efficacy of eculizumab therapy for chronic ABMR with ENDAT confirmed by RT-qPCR in a pilot randomized controlled trial. Apart from this study, most previous animal model studies and clinical trials of immunosuppressive treatments for ABMR used renal function, serum C4d, or DSA levels for outcome monitoring instead of DGE. A possible explanation is that the patients who met the diagnostic criteria with positive results for C4d and DSA might not be tested for ENDAT and would not be monitored with ENDAT as parameters in the follow-up studies. Nevertheless, since gene classifiers/transcripts have already been included in the Banff classification and their significance in differential diagnosis has been widely recognized, the measurement of ENDAT and other immune activation transcripts in allograft still has great potential to increasingly serve as biological markers for treatment response.
The advances in transcriptome profiling techniques have facilitated the refinement of the diagnostic Banff Classification and have shown their great potential to become useful adjuncts to histopathology-based diagnostics. However, there are still unaddressed issues related to improving clinical care and patient outcomes. Regarding improving diagnosis, the microarray-based MMDx, the most established model of applications of transcriptomics in ABMR, has received some critiques from pathologists specifying its questionable statistical measures of variability for classifier scores used for MMDx assignment, the inability to differentiate overlapping phenotypes such as non-rejection inflammation, thresholding and sampling problems arising from the various degrees of inflammation within a tissue sample, and the interlaboratory variability of microarray-based assays.Citation52 Regarding progress in therapeutic strategies, the treatment responses to conventional immunosuppressive therapies vary widely and the outcomes are generally suboptimal. Such treatment hurdles may arise from the complex immune mechanisms and the heterogeneity of the cell types that are involved in the development of ABMR, including not only the immune cells but also the resident cells in the allograft.Citation15,Citation53 Moreover, the extremely dynamic nature of the immunological response, as well as the ever-changing surface expression of surface markers of immune cells, have also been reported as causes of resistance to treatments.Citation13,Citation54,Citation55
The transcriptomic approaches described in this section analyze bulk samples, which only reflect the average gene expression but not the status of individual cells and are unable to distinguish the gene expression profile of donors from recipients in a mixed cell population.Citation56,Citation57 To make a breakthrough in either improving diagnosis or treatment, it is important to address the cellular heterogeneity and to dissect the behaviors of individual cells, so as to identify the cell populations that play a decisive role in the response or lack of response to certain treatments and to determine whether patients, of which clinical and molecular phenotypes, at which time point of the disease course, may benefit from any given treatments. The advent of single-cell RNA sequencing (scRNA-seq) could address cellular heterogeneity and has largely improved our understanding of transcriptomics during cell–cell interaction.Citation58 In the following two sections, I will touch on the basic principles of scRNA-seq, describe how it has been utilized to decipher the transcriptomes of various cell subtypes in studies of ABMR, and catalog the published data yielded with this technique.
Single-cell transcriptome analysis in ABMR
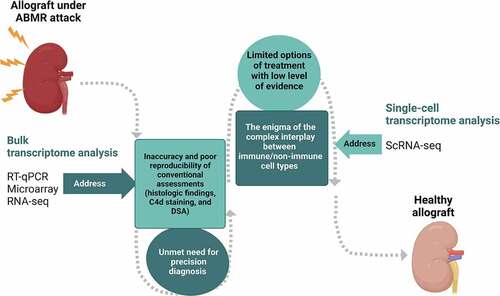
ScRNA-seq is a promising research tool that provides unprecedented resolution to examine cellular heterogeneity in tissues and organs in a high-throughput fashion,Citation59 and it exhibits unique values in providing new insights into renal pathophysiology. There are more than 20 cell types with distinct spatial organization in kidneys,Citation60 and the behaviors and gene expression in response to certain insults or pathological conditions, including ABMR, may differ between different compartments of the organ.Citation61 ScRNA-seq not only detects specific changes of a known cell type but also redefines kidney cell types based on the transcriptome patterns.Citation53 As a result, it can provide a new entry point for enhancing the knowledge of the immunological response behind the rejection and for developing the therapeutic strategy that has been limited by the complexity of the disease (). In addition, its distinguished ability to present a dynamic and transient profile of the cell–cell interaction makes it a powerful tool to aid our understanding of immunologic mechanisms in renal allograft rejection.Citation57
Figure 1. The potential clinical applications of transcriptomic approaches to improve graft outcomes in patients with antibody-mediated rejection in kidney transplantation. The figure was created with BioRender.com.
The technique encompasses the following principal steps. Briefly, single cells are isolated from a tissue sample by various methods including serial dilution, micromanipulation, microdissection, flow cytometry and cell sorting (FACS), magnetic-activated cell sorting (MACS), microfluidic platform, and droplet-associated methods.Citation62 Next, isolated cells are lysed individually to allow the capture of RNA molecules. Poly T primers that bind to the mRNA poly A tails are used to capture the mRNA. The mRNA is then converted to cDNA and amplified by reverse transcription. The primers used in this step contain adaptor sequences and unique molecular identifiers (UMIs) to label the cellular origin. The cDNA is then amplified by polymerase chain reaction, pooled, and analyzed using library preparation techniques, sequencing platforms, and genomic alignment. Data analysis after sequencing usually comprises quality control, batch effect correction, normalization, data imputation, dimensionality adjustment, expression analysis, and cell subpopulation identification.Citation63
There are some major challenges in sample preparation and cell isolation for biopsies obtained from patients. In certain areas, the distance between the clinics and laboratories may hamper the timely delivery of samples.Citation64 As a result, tissues often but not always require special processing after collection in clinical scenarios. Cryopreservation and methanol fixation are the most used methods for tissue preservation, and currently available evidence shows that both methods do not significantly alter the integrity of nucleic acids or the transcriptome profiles.Citation65–67 However, according to the observation and the comments by Wu and Humphrey,Citation68 adult kidneys contain a relatively dense matrix and are difficult to be enzymatically digested, and there is indeed a chance that proteolytic dissociation becomes a stressor to transcriptomes and RNA integrity, although that has not been evidenced by comparative studies.
Single-cell isolation is the most critical step for obtaining transcriptome profiles from an individual cell. Each of the aforementioned methods that are commonly used to isolate single cells from tissues has its own strengths and flaws.Citation64 Of them, droplet-associated methods and FACS are mostly used in currently available scRNA-seq studies in kidneys ().Citation53,Citation57,Citation76 FACS has the strength of being able to sort cell populations with high specificity by evaluating multiple parameters at once but has downsides including being operator-dependent, need for specialized equipment, and more cell damageCitation64; it also requires large starting volumes of cells (>10,000 cells) in suspension, which hinders its application to analyze limited tissue samples.Citation77 In terms of droplet-based methods, the three most commercially available systems are Drop-Seq, InDrop, and 10X Genomics Chromium. Drop-Seq has been extensively used but has a low cell capture rate (<5%) and is poorly suitable for cell isolation from a small starting volume of renal biopsy,Citation68 while the latter two systems have remarkably higher capture efficiency (>50%) and thus are much more capable of the analysis of human biopsies.Citation68,Citation78,Citation79 10X Genomics Chromium has become the most prevalent droplet-associated system in ABMR scRNA-seq studies based on the present review ().
Table 1. Summary of single-cell RNA sequencing studies on antibody-mediated rejection/chronic allograft rejection in kidney transplantation.
To date, published studies that utilize scRNA-seq to analyze ABMR, including both animal and human-based data, are relatively limited (). The cells of interest in most studies are isolated from the renal allograft biopsies or the peripheral blood, but the feasibility of scRNA-seq analysis of individual cells in urine samples matched to human kidney allograft biopsies has also been reported.Citation80 The cell clusters that are identified to take part in ABMR can be generally classified into three types: cells in the innate immune system, cells in the adaptive immune system, as well as nonimmune populations including renal epithelial cells, endothelial cells, and stromal cells.
In 2018, Wu et al.Citation71 published the first scRNA-seq analysis of a single human renal allograft biopsy. By generating data from single-cell transcriptomes from a healthy adult kidney and a single renal allograft diagnosed as chronic ABMR, they identified two subclusters of monocytes, a nonclassical FCGR3A(CD16)+ group and a classic CD16− group expressing dendritic cell (DC) maturation markers. CD16+ cells were strongly associated with allograft rejection and were found to highly express two receptors (SDC3 and ABCA1) and a panel of DC maturation markers, suggesting differentiation into DC in situ. Of the six distinct epithelial cell clusters identified, including podocytes, proximal tubules, the loop of Henle, distal tubules, principal cells, and intercalated cells, the proximal tubule particularly expressed proinflammatory cytokine genes, such as CXCL14 and IL32. They also identified three clusters of donor endothelial cells and three clusters of stromal cells that were involved in the ABMR to various degrees. In 2020, Malone et al.Citation73 used scRNA-seq to analyze human allograft biopsies and found that the recipient macrophages and donor T cells displayed inflammatory activation, and that donor macrophages persisted for years post-transplantation. Liu et al.Citation72 collected scRNA-seq data from three healthy adult kidneys and two renal allograft biopsies of chronic renal transplant rejection; in addition to five subclasses of Natural killer T (NKT) cells, two subclasses of B cells, and two subclasses (classic and non-classical) of monocytes, they identified myofibroblasts, which expressed collagen-related genes, platelet-derived growth factor receptor (PDGFR)-related genes, and epithelial-to-mesenchymal transition (EMT)-related genes, as a novel subpopulation involved in the development of chronic renal allograft rejection. In another study, scRNA-seq of the peripheral blood from patients diagnosed with chronic ABMR and two healthy individuals identified four subtypes in T-cell subsets and two subtypes in B cell subsets; the expression of MTND6, CXCL8, NFKBIA, NFKBIZ, and other genes was up-regulated in both T and B cells and was associated with pro-inflammatory response, including the tumor necrosis factor (TNF), IL-17, and Toll-like receptor signaling pathways. Mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NF-kB) signaling pathways were also involved in the development of chronic ABMR.Citation74 Finally, Asano et al.Citation75 performed a scRNA-seq analysis focusing on the role of the B cell population in chronic ABMR. By comparing the Ig class-switch states and DGE between the human tonsillar and the intrarenal B cells, they reported that intrarenal B cells have a unique transcriptional state that resembles gene expression data of mouse B1 innate-like (Bin) cells acquired from The Immunological Genome Project (Immgen)Citation69; several migration- and adhesion-related genes were highly expressed in this population.
ScRNA-seq analysis on animal models also provides an abundance of insights into the disease mechanism of ABMR. One study performing scRNA-seq on a murine kidney transplant model, in which BALB/c kidneys were transplanted into fully MHC-mismatched bilaterally nephrectomized C57BL/6 J recipients, demonstrated that the allograft-infiltrating myeloid cells followed a trajectory of differentiation from monocytes to proinflammatory macrophages and exhibited distinct interactions with kidney allograft parenchymal cells. Axl, a gene of the receptor tyrosine kinase family Tyro3/Axl/Mertk (TAM), was correlated with the differentiation.Citation70 Another study performing scRNA-seq on CD45+ leukocytes in mouse renal allograft rejection model on days 7 and 15, respectively, showed that the proportion of proliferating and naïve CD8 + T cells, B cells, and neutrophils decreased, while the proportion of macrophages and DCs increased significantly, especially in Ly6cloMrc1+ and Ly6cloEar2+ macrophages during the progression from acute to chronic rejection.Citation81
As described above, the currently available studies vary widely in the target species, the organ origins, and the matched diagnoses/experimental designs of the specimens, which might have led to mixed results in terms of the cell subtypes that were identified to be involved in the ABMR. However, some of these studies were in accordance with each other. A few of the aforementioned studies showed that the non-classical, CD16+ macrophages, had a major role in ABMR.Citation71,Citation72 Likewise, there was consistency among different studies that included the ligand and receptor (LR) pair analysis, reporting increased chemokine ligands production, such as CXCL8, CXCL12, or CXCL14 on various cell populations.Citation71,Citation74 Interestingly, more than one study pointed out that DGE not only mediated activation of immune cells but was enriched in the mitochondria oxidative stress and the endoplasmic reticulum (ER) stress,Citation71,Citation73 while the LR analysis in tolerated allograft found an increased Hsp90b1–Trl7 interaction,Citation70 indicating that the cellular stress response was involved in the post-transplant ABMR, although its exact role and mechanism in the development of ABMR remains undetermined.
As with other techniques, scRNA-seq has its limitation. The basic steps of scRNA-seq require tissue samples to be dissociated into single-cell suspensions, which may change the transcriptome and proteome of cells. Tissue processing can also disrupt the spatial arrangement of cells in each organ, and the cell profile observed may not be expanded into other tissue environments.Citation82 At a higher level of view, the disparity of experimental protocols across studies has not been systematically examined and would thus hamper the correct interpretation and further applications of the results. The optimization and standardization of the workflow of this technique, from the yield and the storage of tissue to data analysis, may help overcome the disadvantage and improve the consistency across studies. Currently, the relatively small sample number in most scRNA-seq studies, primarily due to the high cost of this technology, has also caused the divergence of the study goal. Thus, it is necessary to carefully examine the biological plausibility when drawing new conclusions from scRNA-seq data.Citation83 Despite these limitations, scRNA-seq is developing rapidly and has shown great potential for discovering novel disease mechanisms and effective therapeutic strategies in clinical allograft rejection, including ABMR.
Conclusion
The clinical management of ABMR is largely limited by not only the lack of an ideal diagnostic tool but also the diverse phenotypes and the elusive immunological mechanisms behind the disease. The transcriptome analysis including the microarray technique and RNA-seq enabled the identification of protein-coding and noncoding genes and the segregation of different rejection phenotypes. Such molecular diagnostics could be used in parallel with or even replace the conventional diagnostics based on Banff Classification to increase diagnostic accuracy and have shown promise to serve as biological markers for treatment response. To popularize the application of transcriptome analyses in response evaluation, more well-designed prospective clinical studies in which the baseline transcriptome profiles of patients are clearly documented for future comparisons are needed. ScRNA-seq is rising as a powerful tool to dissect cell–cell interactions, which sheds light on the complicated immunological interplay among various cell types in the allograft during the ABMR and identifies new cell populations that are involved in disease development. This would help explain the limitations of current treatments, as well as provide evidence for novel therapeutic strategies (). Either animal or human-based scRNA-seq studies have been increasingly available. The shared results of scRNA-seq studies that have been conducted on ABMR reveal several panels of DGE associated with innate immunity, the chemotaxis of inflammatory cells, and the cellular stress response, which could be new targets for ABMR therapy. Although more studies under a uniform and well-established experimental protocol are awaited, it is hoped that the scRNA-seq technology can be widely translated into ABMR studies in humans, which will result in improved therapeutics for kidney transplant patients with chronic allograft dysfunction.
Acknowledgments
The author would like to thank Dr Alejandro Soto-Gutiérrez and Dr Diana Maria Metes for the insightful direction and guidance of the manuscript, and Dr Chun-Cheng Chiang for the manuscript review.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Tsai HI, Yu HP. A review of nationwide population study of organ transplantation in Taiwan. Acta Anaesthesiol Taiwan. 2016;54(70–74):70–13. doi:10.1016/j.aat.2016.05.003.
- Wekerle T, Segev D, Lechler R, Oberbauer R. Strategies for long-term preservation of kidney graft function. Lancet. 2017;389(2152–2162):2152–62. doi:10.1016/S0140-6736(17)31283-7.
- Hart A, Singh D, Brown SJ, Wang JH, Kasiske BL. Incidence, risk factors, treatment, and consequences of antibody-mediated kidney transplant rejection: a systematic review. Clin Transplant. 2021;35(e14320). doi:10.1111/ctr.14320.
- Gaston RS, Cecka JM, Kasiske BL, Fieberg AM, Leduc R, Cosio FC, Gourishankar S, Grande J, Halloran P, Hunsicker L, et al. Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation. 2010;90(68–74). doi:10.1097/TP.0b013e3181e065de
- DeWolf S, Alloimmune SM. T cells in transplantation. J Clin Invest. 2017;127(2473–2481):2473–81. doi:10.1172/JCI90595.
- Becker LE, Morath C, Suesal C. Immune mechanisms of acute and chronic rejection. Clin Biochem. 2016;49(320–323):320–23. doi:10.1016/j.clinbiochem.2016.02.001.
- Herrera OB, Golshayan D, Tibbott R, Ochoa FS, James MJ, Marelli-Berg FM, Lechler RI. A novel pathway of alloantigen presentation by dendritic cells. J Immunol. 2004;173(4828–4837). doi:10.4049/jimmunol.173.8.4828.
- Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular helper T cells. Annu Rev Immunol. 2016;34(335–368):335–68. doi:10.1146/annurev-immunol-041015-055605.
- Leibler C, et al. Control of humoral response in renal transplantation by belatacept depends on a direct effect on B cells and impaired T follicular helper-B cell crosstalk. J Am Soc Nephrol. 2018;29(1049–1062):1049–62. doi:10.1681/ASN.2017060679.
- Wallin EF. Follicular regulatory T. Cells and antibody responses in transplantation. Transplantation. 2018;102(1614–1623):1614–23. doi:10.1097/TP.0000000000002224.
- Louis K, Macedo C, Metes D. Targeting T follicular helper cells to control humoral allogeneic immunity. Transplantation. 2021;105(11):e168–e180. doi:10.1097/TP.0000000000003776.
- Chong AS, Sciammas R. Memory B cells in transplantation. Transplantation. 2015;99(21–28):21–28. doi:10.1097/TP.0000000000000545.
- Kim MY, Brennan DC. Therapies for chronic allograft rejection. Front Pharmacol. 2021;12(651222). doi:10.3389/fphar.2021.651222.
- Zhang R. Donor-Specific antibodies in kidney transplant recipients. Clin J Am Soc Nephrol. 2018;13(182–192):182–92. doi:10.2215/CJN.00700117.
- Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nat Rev Immunol. 2005;5(807–817):807–17. doi:10.1038/nri1702.
- Cross AR, Glotz D, Mooney N. The role of the endothelium during antibody-Mediated rejection: from victim to accomplice. Front Immunol. 2018;9(106). doi:10.3389/fimmu.2018.00106.
- Jeannet M, Pinn VW, Flax MH, Winn HJ, Russell PS. Humoral antibodies in renal allotransplantation in man. N Engl J Med. 1970;282(111–117):111–17. doi:10.1056/NEJM197001152820301.
- Feucht HE, et al. Vascular deposition of complement-split products in kidney allografts with cell-mediated rejection. Clin Exp Immunol. 1991;86(464–470). doi:10.1111/j.1365-2249.1991.tb02954.x
- Loupy A, Mengel M, Haas M. Thirty years of the international Banff classification for allograft pathology: the past, present, and future of kidney transplant diagnostics. Kidney Int. 2022;101(678–691). doi:10.1016/j.kint.2021.11.028.
- Loupy A, Haas M, Roufosse C, Naesens M, Adam B, Afrouzian M, Akalin E, Alachkar N, Bagnasco S, Becker JU, et al. The Banff 2019 kidney meeting report (I): updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant. 2020;20:2318–31. doi:10.1111/ajt.15898.
- Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8(753–760). doi:10.1111/j.1600-6143.2008.02159.x
- Correa RR, Machado JR, da Silva MV, Helmo FR, Guimarães CSO, Rocha LP, Faleiros ACG, Dos Reis MA. The importance of C4d in biopsies of kidney transplant recipients. Clin Dev Immunol. 2013;2013(678180). doi:10.1155/2013/678180.
- Jeong HJ. Diagnosis of renal transplant rejection: Banff classification and beyond. Kidney Res Clin Pract. 2020;39(17–31):17–31. doi:10.23876/j.krcp.20.003.
- Haas M. The revised (2013) Banff classification for antibody-Mediated rejection of renal allografts: update, difficulties, and future considerations. Am J Transplant. 2016;16(1352–1357):1352–57. doi:10.1111/ajt.13661.
- Loupy A, et al. The Banff 2015 kidney meeting report: current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant. 2017;17(28–41):28–41. doi:10.1111/ajt.14107.
- Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, Nankivell BJ, Halloran PF, Colvin RB, Akalin E, et al. The Banff 2017 kidney meeting report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018;18(293–307). doi:10.1111/ajt.14625
- Solar-Cafaggi D, Marino L, Uribe-Uribe N, Morales-Buenrostro LE. Antibody-mediated rejection in the Banff classifications of 2007 and 2017: a comparison of renal graft loss prediction capability. Transpl Immunol. 2018;51(40–44):40–44. doi:10.1016/j.trim.2018.08.008.
- Wan SS, Ying TD, Wyburn K, Roberts DM, Wyld M, Chadban SJ. The treatment of antibody-mediated rejection in kidney transplantation: an updated systematic review and meta-Analysis. Transplantation. 2018;102(557–568). doi:10.1097/TP.0000000000002049.
- Khan SA, Al-Riyami, D., Abed, Y. W. A. M., Mohammed, S., Al-Riyami, M., & Al-Lawati, N. M. Successful salvage treatment of resistant acute antibody-mediated kidney transplant rejection with eculizumab. Sultan Qaboos Univ Med J. 2016;16(e371–374):e371–37. doi:10.18295/squmj.2016.16.03.020.
- Tan EK, Bentall A, Dean PG, Shaheen MF, Stegall MD, Schinstock CA. Use of eculizumab for active antibody-mediated rejection that occurs early post-kidney transplantation: a consecutive series of 15 cases. Transplantation. 2019;103(2397–2404). doi:10.1097/TP.0000000000002639.
- Kulkarni S, Kirkiles-Smith NC, Deng YH, Formica RN, Moeckel G, Broecker V, Bow L, Tomlin R, Pober JS. Eculizumab therapy for chronic antibody-Mediated injury in kidney transplant recipients: a pilot randomized controlled trial. Am J Transplant. 2017;17(682–691). doi:10.1111/ajt.14001.
- Thareja G, Yang H, Hayat S, Mueller FB, Lee JR, Lubetzky M, Dadhania DM, Belkadi A, Seshan SV, Suhre K, et al. Single nucleotide variant counts computed from RNA sequencing and cellular traffic into human kidney allografts. Am J Transplant. 2018;18(2429–2442). doi:10.1111/ajt.14870
- Deepak S, Kottapalli KR, Rakwal R, Oros G, Rangappa KS, Iwahashi H, Masuo Y, Agrawal GK. Real-Time PCR: revolutionizing detection and expression analysis of genes. Curr Genomics. 2007;8(234–251). doi:10.2174/138920207781386960.
- Strehlau J, Pavlakis M, Lipman M, Shapiro M, Vasconcellos L, Harmon W, Strom T. Quantitative detection of immune activation transcripts as a diagnostic tool in kidney transplantation. Proc Natl Acad Sci U S A. 1997;94(695–700). doi:10.1073/pnas.94.2.695.
- Suthanthiran M. Clinical application of molecular biology: a study of allograft rejection with polymerase chain reaction. Am J Med Sci. 1997;313(264–267):264–67. doi:10.1097/00000441-199705000-00003.
- Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995;270(467–470):467–70. doi:10.1126/science.270.5235.467.
- Sarwal M, Chua M-S, Kambham N, Hsieh S-C, Satterwhite T, Masek M, Salvatierra O. Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N Engl J Med. 2003;349(125–138). doi:10.1056/NEJMoa035588.
- Flechner SM, Kurian SM, Head SR, Sharp SM, Whisenant TC, Zhang J, Chismar JD, Horvath S, Mondala T, Gilmartin T, et al. Kidney transplant rejection and tissue injury by gene profiling of biopsies and peripheral blood lymphocytes. Am J Transplant. 2004;4(1475–1489). doi:10.1111/j.1600-6143.2004.00526.x
- Halloran PF, De Freitas DG, Einecke G, Famulski KS, Hidalgo LG, Mengel M, Reeve J, Sellares J, Sis B. The molecular phenotype of kidney transplants. Am J Transplant. 2010;10(2215–2222). doi:10.1111/j.1600-6143.2010.03267.x.
- Sis B, et al. Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am J Transplant. 2009;9(2312–2323):2312–23. doi:10.1111/j.1600-6143.2009.02761.x.
- Sellares J, Reeve J, Loupy A, Mengel M, Sis B, Skene A, de Freitas DG, Kreepala C, Hidalgo LG, Famulski KS, et al. Molecular diagnosis of antibody-mediated rejection in human kidney transplants. Am J Transplant. 2013;13(971–983). doi:10.1111/ajt.12150
- Halloran PF, Pereira, A. B., Chang, J., Matas, A., Picton, M., De Freitas, D. & Reeve, J. Microarray diagnosis of antibody-mediated rejection in kidney transplant biopsies: an international prospective study (INTERCOM). Am J Transplant. 2013;13(2865–2874). doi:10.1111/ajt.12465
- Halloran PF, Famulski KS, Reeve J. Molecular assessment of disease states in kidney transplant biopsy samples. Nat Rev Nephrol. 2016;12(534–548):534–48. doi:10.1038/nrneph.2016.85.
- Halloran PF, Reeve J, Akalin E, Aubert O, Bohmig GA, Brennan D, Bromberg J, Einecke G, Eskandary F, Gosset C, et al. Real time central assessment of kidney transplant indication biopsies by microarrays: the INTERCOMEX study. Am J Transplant. 2017;17(2851–2862). doi:10.1111/ajt.14329
- Allanach K, Mengel, M., Einecke, G., Sis, B., Hidalgo, L. G., Mueller, T., & Halloran, P. F. Comparing microarray versus RT-PCR assessment of renal allograft biopsies: similar performance despite different dynamic ranges. Am J Transplant. 2008;8(1006–1015):1006–15. doi:10.1111/j.1600-6143.2008.02199.x.
- Park WD, Stegall MD. A meta-analysis of kidney microarray datasets: investigation of cytokine gene detection and correlation with rt-PCR and detection thresholds. BMC Genomics. 2007;8(88):88. doi:10.1186/1471-2164-8-88.
- Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10(57–63):57–63. doi:10.1038/nrg2484.
- Stark R, Grzelak M, Hadfield J. RNA sequencing: the teenage years. Nat Rev Genet. 2019;20(631–656):631–56. doi:10.1038/s41576-019-0150-2.
- Pineda S, Sur S, Sigdel T, Nguyen M, Crespo E, Torija A, Meneghini M, Gomà M, Sirota M, Bestard O, et al. Peripheral blood RNA sequencing unravels a differential signature of coding and noncoding genes by types of kidney allograft rejection. Kidney Int Rep. 2020;5(1706–1721). doi:10.1016/j.ekir.2020.07.023
- Wang X, Mitra, N., Secundino, I., Banda, K., Cruz, P., Padler-Karavani, V. & Young, A. Specific inactivation of two immunomodulatory SIGLEC genes during human evolution. Proc Natl Acad Sci U S A. 2012;109(9935–9940). doi:10.1073/pnas.1119459109
- Dooley BJ, Verma A, Ding R, Yang H, Muthukumar T, Lubetzky M, Shankaranarayanan D, Elemento O, Suthanthiran M. Urinary cell transcriptome profiling and identification of ITM2A, SLAMF6, and IKZF3 as biomarkers of acute rejection in human kidney allografts. Transplant Direct. 2020;6(e588). doi:10.1097/TXD.0000000000001035.
- Randhawa PS. The molecular microscope diagnostic system (MMDx) in transplantation: a pathologist’s perspective. Am J Transplant. 2020;20(1965–1966):1965–66. doi:10.1111/ajt.15887.
- Chen L, Lee JW, Chou C-L, Nair AV, Battistone MA, Păunescu TG, Merkulova M, Breton S, Verlander JW, Wall SM, et al. Transcriptomes of major renal collecting duct cell types in mouse identified by single-cell RNA-seq. Proc Natl Acad Sci U S A. 2017;114:E9989–E9998. doi:10.1073/pnas.1710964114.
- Choi J, Aubert O, Vo A, Loupy A, Haas M, Puliyanda D, Kim I, Louie S, Kang A, Peng A, et al. Assessment of Tocilizumab (Anti-Interleukin-6 receptor monoclonal) as a potential treatment for chronic antibody-Mediated rejection and transplant glomerulopathy in HLA-Sensitized renal allograft recipients. Am J Transplant. 2017;17(9):2381–89. doi:10.1111/ajt.14228.
- Mathews DV, Wakwe WC, Kim SC, Lowe MC, Breeden C, Roberts ME, Farris AB, Strobert EA, Jenkins JB, Larsen CP, et al. Belatacept-Resistant rejection is associated with CD28 + memory CD8 T cells. Am J Transplant. 2017;17(2285–2299). doi:10.1111/ajt.14349
- Liao J, Yu Z, Chen Y, Bao M, Zou C, Zhang H, Liu D, Li T, Zhang Q, Li J, et al. Single-cell RNA sequencing of human kidney. Sci Data. 2020;7(4). doi:10.1038/s41597-019-0351-8
- Park J, Shrestha R, Qiu C, Kondo A, Huang S, Werth M, Li M, Barasch J, Suszták K. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science. 2018;360(758–763). doi:10.1126/science.aar2131.
- Tang F, Barbacioru C, Wang Y, Nordman E, Lee C, Xu N, Wang X, Bodeau J, Tuch BB, Siddiqui A, et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nature Methods. 2009;6(377–382). doi:10.1038/nmeth.1315
- Denyer T, Timmermans MCP. High-throughput single-cell RNA sequencing. Trends Plant Sci. 2022;27(104–105). doi:10.1016/j.tplants.2021.09.003.
- Al-Awqati Q, Oliver JA. Stem cells in the kidney. Kidney Int. 2002;61(387–395):387–95. doi:10.1046/j.1523-1755.2002.00164.x.
- Trailin A, Mrazova P, Hruba P, Voska L, Sticova E, Slavcev A, Novotny M, Kocik M, Viklicky O. Chronic active antibody-mediated rejection is associated with the upregulation of interstitial but not glomerular transcripts. Front Immunol. 2021;12(729558). doi:10.3389/fimmu.2021.729558.
- Haque A, Engel J, Teichmann SA, Lonnberg T. A practical guide to single-cell RNA-sequencing for biomedical research and clinical applications. Genome Med. 2017;9(75). doi:10.1186/s13073-017-0467-4.
- Wang Y, Wang JY, Schnieke A, Fischer K. Advances in single-cell sequencing: insights from organ transplantation. Mil Med Res. 2021;8(45). doi:10.1186/s40779-021-00336-1.
- Tian B, Li LQ. Single-Cell sequencing and its applications in liver cancer. Front Oncol. 2022;12(857037). doi:10.3389/fonc.2022.857037.
- Srinivasan M, Sedmak D, Jewell S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am J Pathol. 2002;161(1961–1971):1961–71. doi:10.1016/S0002-9440(10)64472-0.
- Alles J, Karaiskos N, Praktiknjo SD, Grosswendt S, Wahle P, Ruffault P-L, Ayoub S, Schreyer L, Boltengagen A, Birchmeier C, et al. Cell fixation and preservation for droplet-based single-cell transcriptomics. BMC Biol. 2017;15(44). doi:10.1186/s12915-017-0383-5
- Rao DA, Arazi A, Wofsy D, Diamond B. Design and application of single-cell RNA sequencing to study kidney immune cells in lupus nephritis. Nat Rev Nephrol. 2020;16(238–250):238–50. doi:10.1038/s41581-019-0232-6.
- Wu H, Humphreys BD. The promise of single-cell RNA sequencing for kidney disease investigation. Kidney Int. 2017;92(1334–1342):1334–42. doi:10.1016/j.kint.2017.06.033.
- Heng TS, Painter MW, Elpek K, Lukacs-Kornek V, Mauermann N, Turley SJ, Koller D, Kim FS, Wagers AJ, Asinovski N; Immunological Genome Project, C. The immunological genome project: networks of gene expression in immune cells. Nat Immunol. 2008;9(1091–1094). doi:10.1038/ni1008-1091.
- Dangi A, Natesh NR, Husain I, Ji Z, Barisoni L, Kwun J, Shen X, Thorp EB, Luo X. Single cell transcriptomics of mouse kidney transplants reveals a myeloid cell pathway for transplant rejection. JCI Insight. 2020:5. doi:10.1172/jci.insight.141321.
- Wu H, Malone AF, Donnelly EL, Kirita Y, Uchimura K, Ramakrishnan SM, Gaut JP, Humphreys BD. Single-Cell transcriptomics of a human kidney allograft biopsy specimen defines a diverse inflammatory response. J Am Soc Nephrol. 2018;29(2069–2080). doi:10.1681/ASN.2018020125.
- Liu Y, Hu J, Liu D, Zhou S, Liao J, Liao G, Yang S, Guo Z, Li Y, Li S, et al. Single-cell analysis reveals immune landscape in kidneys of patients with chronic transplant rejection. Theranostics. 2020;10(8851–8862). doi:10.7150/thno.48201
- Malone AF, Wu H, Fronick C, Fulton R, Gaut JP, Humphreys BD. Harnessing expressed single nucleotide variation and single cell RNA sequencing to define immune cell chimerism in the rejecting kidney transplant. J Am Soc Nephrol. 2020;31(1977–1986). doi:10.1681/ASN.2020030326.
- Kong F, Ye, S., Zhong, Z., Zhou, X., Zhou, W., Liu, Z. & Ye, Q. Single-Cell transcriptome analysis of chronic antibody-mediated rejection after renal transplantation. Front Immunol. 2021;12(767618). doi:10.3389/fimmu.2021.767618
- Asano Y, Daccache J, Jain D, Ko K, Kinloch A, Veselits M, Wolfgeher D, Chang A, Josephson M, Cunningham P, et al. Innate-like self-reactive B cells infiltrate human renal allografts during transplant rejection. Nat Commun. 2021;12(4372). doi:10.1038/s41467-021-24615-6
- Zimmerman KA, Bentley, M. R., Lever, J. M., Li, Z., Crossman, D. K., Song, C. J. & Yoder, B. K. Single-Cell RNA sequencing identifies candidate renal resident macrophage gene expression signatures across species. J Am Soc Nephrol. 2019;30(767–781):767–81. doi:10.1681/ASN.2018090931.
- Chen G, Ning B, Single-Cell ST. RNA-Seq Technologies and Related Computational Data Analysis. Front Genet. 2019;10(317). doi:10.3389/fgene.2019.00317.
- Zheng GX, Terry JM, Belgrader P, Ryvkin P, Bent ZW, Wilson R, Ziraldo SB, Wheeler TD, McDermott GP, Zhu J, et al. Massively parallel digital transcriptional profiling of single cells. Nat Commun. 2017;8(14049). doi:10.1038/ncomms14049
- Zhang X, Li T, Liu F, Chen Y, Yao J, Li Z, Huang Y, Wang J. Comparative analysis of droplet-Based Ultra-High-Throughput single-Cell RNA-Seq systems. Mol Cell. 2019;73:130–42. doi:10.1016/j.molcel.2018.10.020.
- Muthukumar T,YH, Belkadi A, Thareja G, Li C, Snopkowski C, Chen K, Salinas T, Lubetzky M, Lee J, Dadhania D, et al. Single cell RNA-Sequencing of urinary cells and defining the immune landscape of rejection in human kidney allografts [abstract]. Am J Transplant. 2021;21(suppl 3):32–36. https://atcmeetingabstracts.com/abstract/single-cell-rna-sequencing-of-urinary-cells-and-defining-the-immune-landscape-of-rejection-in-human-kidney-allografts/.
- Shen Q, Wang Y, Chen J, Ma L, Huang X, Tang SCW, Lan H, Jiang H, Chen J. Single-Cell RNA sequencing reveals the immunological profiles of renal allograft rejection in mice. Front Immunol. 2021;12(693608). doi:10.3389/fimmu.2021.693608.
- Stewart BJ, Ferdinand JR, Clatworthy MR. Using single-cell technologies to map the human immune system - implications for nephrology. Nat Rev Nephrol. 2020;16(112–128):112–28. doi:10.1038/s41581-019-0227-3.
- Malone AF. Monocytes and macrophages in kidney transplantation and insights from single cell RNA-Seq studies. Kidney360. 2021;2(1654–1659):1654–59. doi:10.34067/KID.0003842021.
