ABSTRACT
This study is to investigate the therapeutical effect and mechanisms of human-derived adipose mesenchymal stem cells (ADSC) in relieving adriamycin (ADR)-induced nephropathy (AN). SD rats were separated into normal group, ADR group, ADR+Losartan group (20 mg/kg), and ADR + ADSC group. AN rats were induced by intravenous injection with adriamycin (8 mg/kg), and 4 d later, ADSC (2 × 105 cells/mouse) were administrated twice with 2 weeks interval time (i.v.). The rats were euthanized after the 6 weeks’ treatment. Biochemical indicators reflecting renal injury, such as blood urea nitrogen (BUN), neutrophil gelatinase alpha (NGAL), serum creatinine (Scr), inflammation, oxidative stress, and pro-fibrosis molecules, were evaluated. Results demonstrated that we obtained high qualified ADSCs for treatment determined by flow cytometry, and ADSCs treatment significantly ameliorated renal injuries in DN rats by decreasing BUN, Scr and NGAL in peripheral blood, as well as renal histopathological injuries, especially protecting the integrity of podocytes by immunofluorescence. Furthermore, ADSCs treatment also remarkably reduced the renal inflammation, oxidative stress, and fibrosis in DN rats. Preliminary mechanism study suggested that the ADSCs treatment significantly increased renal neovascularization via enhancing proangiogenic VEGF production. Pharmacodynamics study using in vivo imaging confirmed that ADSCs via intravenous injection could accumulate into the kidneys and be alive at least 2 weeks. In a conclusion, ADSC can significantly alleviate ADR-induced nephropathy, and mainly through reducing oxidative stress, inflammation and fibrosis, as well as enhancing VEGF production.
Introduction
The adriamycin (ADR)-induced nephropathy model mimics human focal segmental glomerular sclerosisCitation1,Citation2 and is a valuable tool to evaluate renal protective effect of various treatment strategies, including stem cell therapies.Citation3–5 The pathogenesis of adriamycin nephropathy (AN) is complicated, mainly including complement activation, over-production of reactive oxygen species (ROS), dysregulation of the renin–angiotensin system and so on.Citation6,Citation7 Besides those above, the equilibrium between proangiogenic and antiangiogenic factors is crucial for maintaining vascular homeostasis in kidneys. However, in AN, this equilibrium is disrupted, leading to a microvessel loss due to an antiangiogenic environment.Citation8 Therefore, effectively alleviating renal inflammation, oxidative stress, fibrosis, or promoting angiogenesis may be the effective strategies for treating AN.
The mesenchymal stem cells (MSCs), including adipose-derived mesenchymal stem cells (ADSC), are multipotent adult stem cells derived from the mesoderm, capable of differentiating into various cell types and possessing the capacity for self-renewal and differentiation.Citation9,Citation10 Numerous clinical studies have shown that MSCs regulate inflammation and promote tissue healing.Citation11 The migration of MSCs to the site of tissue injury can induce the secretion of a diverse range of growth factors, including epidermal growth factor, angiopoietin-1, fibroblast growth factor, platelet-derived growth factor, transforming growth factor-β1 (TGF-β1), vascular endothelial growth factor (VEGF), hepatocyte growth factor, insulin growth factor-1 and so on, thereby facilitating tissue repair.Citation12–15 By activating paracrine trophic factors, endothelial cells can manufacture extracellular matrix, enhancing endothelial barrier function, and inhibiting leukocyte-endothelial cell interactions to facilitate tissue regeneration.Citation16,Citation17 Morigi et al. demonstrated that intravenous injection of MSCs significantly reversed the declined renal function in drug-induced acute injury mice, accompanied by a rapid improvement of pathological damage, although the mechanisms and signaling pathways involved in MSC-mediated renal repair are poorly understood.Citation18
Compared with other sources to get the MSCs, ADSCs have advantages of being abundant and easy to obtain.Citation19 ADSCs have been reported to have a satisfactory beneficial effect in reducing the cardiac fibrosis, which is one of serious diabetic complications.Citation20 Despite the well-established efficacy of ADSC in ameliorating various diseases, their reliable therapeutic efficiency on nephropathy still remains to be confirmed. Therefore, in the current study, we investigated the potential therapeutic effect of ADSC and its potential mechanisms with the help of rat AN model. Meanwhile, despite this potential, the distribution, survival time and destiny of the delivered cells in the circulation are limited, and in the present study, by in vivo imaging technology, we also try to clarify their in vivo pharmacodynamic characteristics of ADSCs via intravenous administration.
Materials and methods
Isolation, identification, and cultivation of ADSC
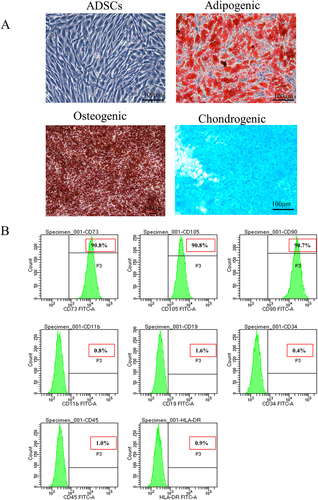
The protocol of ADSC isolation adheres to the previous publications.Citation21,Citation22 Briefly, human normal subcutaneous adipose tissue (obtained in healthy individuals undergoing selective liposuction or undergoing plastic surgery) was obtained from Peking Union Medical College Hospital (Beijing, China) and was approved by ethical committee of Peking Union Medical College Hospital (approval number: ZS-3186). The adipose tissue was digested with type I collagenase for 60 min and terminated with DME/F12 complete medium with 10% fetal bovine serum. The centrifuge was used to pellet cell debris at a speed of 1000 rpm for a duration of 5 min. Every 3 d, the medium was changed, and the cells were cultured in 5% CO2 at 37°C. When the confluence reaches 80%, the cells were defined as passage and replated until passage 4 for subsequent experiments. Fifth-generation cells were used in the animal experiments. Adipogenesis was confirmed employing Oil Red O staining, osteogenesis was verified with Alizarin Red staining, and chondrogenesis was examined using Alcian Blue staining (). By flow cytometry, the following cell surface markers were detected: CD73, CD105, CD90, HLA-DR, CD11b, CD19, CD34, and CD45 (Biolegend, USA) ().
Figure 1. Induced differentiation ability and characteristic surface markers of ADSC. (a) Differentiation abilities of cells were detected by cellular staining. The order from left to right and from top to bottom: uninduced ADSC, adipogenesis using Oil red O staining, osteogenesis using Alizarin red staining, and chondrogenesis using Alcian blue staining. (b) Specific surface markers of cells were examined by flow cytometry. The ADSC associated with markers were positive for CD73, CD105, and CD90 and were negative for CD11b, CD19, CD34, CD45, and HLA-DR.
Establishment of rat adriamycin-induced nephropathy and treatment
Male Sprague – Dawley rats (180–200 g) (SPF Biotechnology Co., Ltd., Beijing, China) were used. The animals were separated into normal control groups (Control), AN + Vehicle (Model), AN + Losartan (20 mg/kg), and AN+ADSC group,and N = 7 for each group. A single injection of 8 mg/kg ADR via the tail vein was used to induce nephropathy in this study. Two weeks after ADR injection, saline (0.5 mL) with or without 2 × 105 ADSC was injected into the model and ADSC groups via the tail vein. The losartan group was orally given 20 mg/kg of losartan daily as positive control to compare the ADSCs efficiency. The time interval for ADSC administration was 2 weeks. The rats were sacrificed for cervical translocation after a 4-week treatment, and before that, urine, serum and kidneys from each animal were collected for renal function test and histopathological analysis. BUN, Scr, serum triglyceride, serum low-density lipoprotein cholesterol (LDL-C), and urinary creatinine were measured by commercial kits (Nanjing Jiancheng Biotechnology, Nan Jing, China). The microalbumin in the urine was measured by ELISA kit (Abcam, CA, USA). The NGAL concentration in serum and urine were examined using ELISA kit (Abcam, USA). The experimental procedure was illustrated in . The optional dosage of ADSCs was determined by highest tolerance, and losartan dosage was selected based on previous publications.Citation7,Citation23
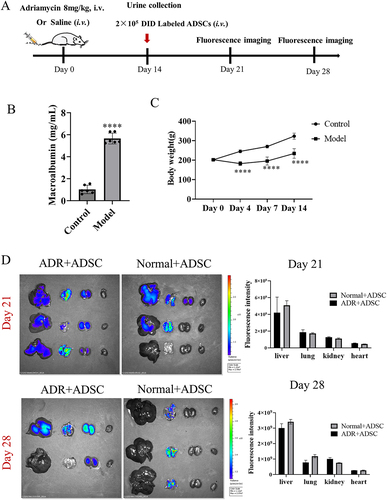
Figure 2. Dynamic ADSC distribution in vivo in an rats. (a) the timetable and flowchart of in vivo imaging (N = 3 for each time point). (b) Significantly elevated albuminuria in an model rats compared with normal rats after adriamycin challenge for 14 d (N = 6). (c) the growth curve of body weight. d) Fluorescence imaging of major organs from an model and normal rats after injection of DID-labeled ADSC through tail vein 7 and 14 d (N = 3 or 2). Data are presented as mean ± SEM.****p < 0.0001 vs normal control group.
Fluorescence imaging
ADSC distribution after transfusion was visualized using fluorescence imaging. Briefly, 2 × 105 cells were labeled with DiD (Thermo Fisher Scientific, USA) before intravenous transfusion into AN rats. Normal rats also received intravenous ADSC transfusion to assess whether their distribution in normal rats was different from AN animals. ADSC migration and accumulation were observed and analyzed on days 7 and 14 using an in vivo imaging system (IVIS Spectrum, PerkinElmer). The detailed procedure was shown in .
Microscopy, immunofluorescence and immunohistochemistry
The kidneys were fixed using 4% paraformaldehyde and embedded into the paraffin. Slices with 4 μm thick were cut from the tissue sections for hematoxylin and eosin (H&E) staining. The digital images were captured using a camera equipped with a charge-coupled device (Hamamatsu Photonics, Tokyo, Japan). Glomerular hypertrophy was determined by measuring the diameter of the glomeruli. Tubular injury was evaluated based on the proportions of tubular dilation and brush border loss. Scoring was conducted on five fields of view for each rat and calculated as follows: 0, none; 1, 0–10%; 2, 11–25%; 3, 26–45%; 4, 46–75%; and 5, >76%.
To perform immunofluorescence, 3 μm thick frozen slices were treated with a drop-wise addition of 5% BSA and incubated for half an hour. Subsequently, an overnight incubation at 4°C was performed using rabbit anti-nephrin antibody (Abcam, USA, 1:100). After PBS washing, the corresponding FITC-conjugated secondary antibody (SeviceBio, China) was added and incubated for 50 min in the darkness. Subsequently, the images were captured using a panoramic scanner (NanoZoomer, S210, Tokyo, Japan) following DAPI staining (Zsbio, Beijing, China). With the help of Image Pro-plus 5.0 (Media Cybernetics, USA), the intensity and percentage of positive nephrin staining were calculated.
Immunohistochemistry was performed as previous studies.Citation23,Citation24 Briefly, kidney sections embedded in paraffin were deparaffinized using water and subsequently subjected to antigen retrieval by utilizing sodium citrate buffer solution (pH 6.0). After washing three times, the kidney tissue was incubated with the antibody 1:100 anti-fibronectin (Abcam, USA), 1:500 TGF-β1 (Abcam, USA), 1:1000 CD31 (Abcam, USA) and 1:100 VEGF (Abcam, USA) overnight at 4°C. The sections were subsequently exposed to anti-rabbit antibody (ZSGB-Bio, Beijing, China) conjugated with peroxidase and stained with hematoxylin as a counterstain. Expression of fibronectin, CD31, TGF-β1 and VEGF was evaluated with Image-Pro Plus based on the intensity of staining.
Western blot analysis
Approximately 10 mg of kidney tissue was dissected using scissors and then lysed by 100 μL of RIPA lysis buffer (Solarbio, Beijing, China) containing 1 μL of protease inhibitors (Targetmol, Beijing, China) for a duration of 45 min on ice. Following centrifugation at 12,000 g for 15 min, the supernatant was collected for protein quantification using the bicinchoninic acid kit (Lablead, Beijing, China). About 40 μg protein samples were loaded in 12% sodium dodecyl sulfate-polyacrylamide gel (Dakewe, Beijing, China) and transferred to the 0.45 μm PVDF membrane (Millipore, USA). The membrane was then obstructed by 5% skim milk at room temperature for 1 h. Primary antibodies (TGF-β1, fibronectin, nephrin, VEGF and β-actin, 1:1000, Abcam, USA) were incubated overnight, followed by additional incubation with anti-mouse or anti-rabbit second antibodies at room temperature for 1 h. Protein bands were achieved by Enhanced chemiluminescence (Tanon, Shanghai, China). The quantitative analysis was conducted using Image J.Citation25,Citation26
Total RNA extraction and real-time PCR
The TRIzol RNA extraction reagent (Yeasen Technologies, Beijing, China) was employed for the isolation of total mRNA. Then cDNA reverse transcription kit (TransGen Biotech, Beijing, China) was used to reverse transcription. Real-time PCR was performed on an qTOWERCitation3 PCR system (Analytik Jena, Berlin, Germany) using specific primers shown in . The mRNA expression levels were comparatively quantified against β-actin using the 2ΔΔCT method.Citation27
Table 1. The primer sequence.
In vitro angiogenesis experiment
Aims to evaluate whether the bioactive growth factors secreted by ADSCs could promote angiogenesis, and in vitro endothelial cell tube formation assay was performed. Briefly, a 24-well plate was evenly coated with growth-factor-reduced Matrigel (356234, Corning, CA, USA) at 37°C for 30 min, then the rat vascular endothelial cells were seeded at a concentration of 1 × 104 cells/well. The vascular endothelial cells were treated with cell culture medium, 10 μg/mL adriamycin plus cell culture medium and 10 μg/mL adriamycin plus ADSC cell culture supernatant for 48 h, respectively. To observe the tube formation, a phase-contrast microscope was utilized, and photographs were captured. ImageJ software was employed to calculate the number of tubes after examining five fields for each test.
Statistical methods
All the data were presented as Mean ± standard error of mean (SEM) and visualized using GraphPad Prism 8.0.2 software, and when comparing two groups, unpaired t-tests were employed, and when comparing multiple groups, ANOVA followed by Tukey’s post hoc analysis was conducted. p < 0.05 was considered significantly different between groups.
Results
Biological properties of ADSC
In the present study, the current ADSC are derived from the human adipose mesenchymal tissue and exhibit various basic biological characteristics for identifying cells as ADSCs. The ADSC in the current research showed potential to differentiate into pre-adipocytes, chondrocytes, and osteoblasts (). Flow cytometry experiments revealed that the expression of CD73, CD105, and CD90 on ADSCs was highly positive (90.8%, 90.8%, and 90.7% respectively), while the expression of CD11b, CD19, CD45, CD34, and HLA-DR was significantly low (0.8%, 1.6%, 1.0%, 0.4%, and 0.9%, respectively) ().
Pharmacodynamics of intravenous administration of ADSC in An and normal rats by in vivo imaging
Before exploring the therapeutic effects of ADSC on AN, we tried to investigate the pharmacodynamics of ADSC in vivo through intravenous administration, and their distribution in different major organs, especially in kidneys. On day 0, by injecting ADR (8 mg/kg) into the tail veins, the experimental AN rats were induced. Two weeks later, after confirmation of albuminuria (), the 2 × 105 ADSC cells were intravenous administrated per rats. The same number of normal rats also received ADSC administration as control (). After undergoing ADR challenge, the AN rats exhibited a consistent and significant reduction in body weight throughout the days 1 to 14 (). The partial animals were sacrificed on 7th and 14th d after ADSC injection, respectively (N = 3 or 2), and the specimens were collected for fluorescence imaging. The fluorescence distribution in the main organs was detected, and ADSC were predominantly found in the liver, lungs, kidneys, and exhibited limited presence in other major organs such as heart (). Compared with normal rats, ADSC retained longer in ADR rats, and fluorescence was still observed in kidneys on 14th d. Based on these results, we confirmed that ADSC preferred to reach to the injured kidneys and also could keep alive at least 14 d. Therefore, we conducted biweekly administration of ADSC in the current study.
ADSC treatment improves the renal function of An rats
Compared with normal control group, the body weights of all the AN rats from the three groups significantly deceased, but both ADSC and losartan treatment did not change the body weight compared with model group (). However, compared to ADR rats, we observed that increased kidney index and spleen index of the Adriamycin-treated rats were significantly reduced by ADSC treatment, and the effect of ADSC on kidney index was better than Losartan (). At the same time, we detected the relevant biochemical indices in the serum and found that the extent of LDL, TG, BUN, and Scr in ADR rats were significantly increased, which were all reversed after treatment with ADSC or losartan, suggesting that renal function had improved (). For these biochemical parameters, ADSCs had the similar effect with losartan. NGAL, a sensitive index of renal injury, increased significantly in the AN model group, whereas its level decreased significantly after treatment both in serum and urine by ADSCs treatment, and its effect on reducing the NAGL was slightly better than losartan, which suggested that ADSCs might have the advantage than losartan on reducing inflammation (). These results show that ADSC can effectively improve the renal function of rats with adriamycin nephropathy.
Figure 3. Beneficial effects of ADSC treatment on biochemical indexes and histopathological injuries of an rats. (a) Timetable and flowchart of an establishment and ADSC therapy. (b) The growth curve of body weight in each group (N = 7). (c) kidney index (kidney weight/body weight) in each group (N = 7). (d) Spleen index (spleen weight/body weight) in each group (N = 7). (e-i): Biochemical indexes in each group (N = 7): (e) Serum low-density lipoprotein. (f) Serum triglycerides. (g) Serum urea nitrogen. h) Serum creatinine. (i) serum neutrophil gelatinase-associated lipocalin. (j) Urine neutrophil gelatinase-associated lipocalin/urine creatinine ratio. (k) Hematoxylin-eosin staining. (l) Statistical chart of glomerular diameter (N = 7). (m) Renal tubular injury score (N = 7). Data are presented as mean ± SEM. ****p < 0.05, *p < 0.01, **p < 0.001 vs Model group, ***p < 0.05 vs Control.
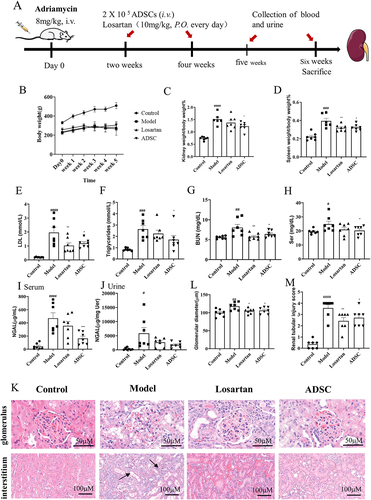
H&E pathological staining demonstrated the presence of segmental glomerulosclerosis and infiltration of inflammatory cells in the ADR group; however, ADSC significantly ameliorated these degenerative changes ().
ADSC treatment reduces oxidative stress and inflammation in An rats
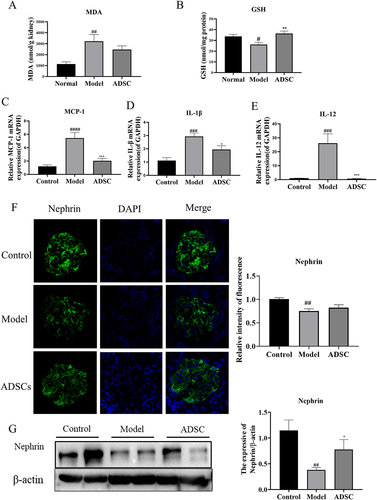
The results of the H&E showed that ADSC reduced lymphocyte infiltration and alleviated renal injury, suggesting the properties that combat oxidation and inflammation. Therefore, we measured the level of oxide MDA and the antioxidant GSH in the kidney tissue. It showed that the level of MDA increased while that of the GSH decreased in the kidneys from ADR group, whereas the content of MDA and GSH in the ADSC group reversed the change in the AN group (). Besides, we detected the kidney-related inflammatory factor mRNA using qRT-PCR and found that the mRNA expressions of MCP-1, IL-1β, and IL-12 were markedly elevated in the model group. In contrast, receiving ADSC treatment resulted in a decrease in the levels of mRNA for inflammatory cytokines compared to the model group (). This shows that ADSC treatment has antioxidant and anti-inflammatory effects in ADR model rats. Meanwhile, nephrin, a key barrier protein of the podocyte integrity, increased remarkably in the ADSC group by immunofluorescence examination (). We arrived at the same conclusion at protein levels by western blot ().
Figure 4. Effects of ADSC treatment on oxidative stress, inflammation of the kidney and nephrin expression. Determination of MDA (a) and GSH (b) in renal oxidative stress indexes. Relative mRNA expression of MCP-1(c), IL-1β(d), and IL-12(e) in kidney tissues (N = 7). (f) Representative images of nephrin expression in kidney site detected by immunofluorescence, and quantitative statistics of nephrin expression (N = 7). (g) Western blot analysis of nephrin in the kidney, and Bands were quantified and expressed in the graph. Data are presented as mean ± SEM. ****p < 0.05 vs Model group, *p < 0.05 vs Control, **p < 0.001 vs Control.
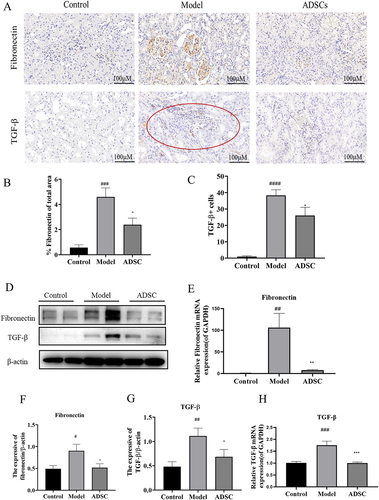
ADSC treatment ameliorates renal fibrosis in An rats
With the progress of AN rats, most will develop into irreversible glomerular and interstitial fibrosis. Further investigating ADSC’s potential role in anti-renal fibrosis, we examined renal fibrosis-associated molecules, such as fibronectin and TGF-β1. Immunohistochemical staining revealed that fibronectin was mainly expressed in the glomeruli in the ADR group and it was significantly reduced after ADSC treatment (). Meanwhile, TGF-β1 immunohistochemical results showed that the infiltrating inflammatory cells mainly secreted TGF-β1 in the ADR group, while the secretion of TGF-β1 decreased in the ADSC group (). RT-qPCR and western blot assays were also performed to further confirm that expression of fibronectin and TGF-β1 involved in renal fibrosis. The protein () and mRNA () levels of fibronectin and TGF-β1 were significantly upregulated in ADR group. However, this enhancement was significantly inhibited by ADSC treatment (). It suggests that inhibiting the expression of fibronectin and TGF-β1 was one of underlying mechanisms of ADSC reducing fibrosis.
Figure 5. ADSC treatment alleviated glomerular and tubulointerstitial fibrosis in an model rats. a) Immunohistochemistry staining of Fibronectin and TGF-β1 in kidney tissues (N = 7). b) the Fibronectin positive area was quantitatively analyzed (N = 7). c) the TGF-β positive cells were quantitatively analyzed (N = 7). d) Western blot bands of Fibronectin and TGF-β1 in kidney tissues. e) Western blot analysis of fibronectin in kidney tissues. f) Western blot analysis of TGF-β1 in kidney tissues. g) the mRNA expression of fibronectin in kidney tissues (N = 7). h) the mRNA expression of TGF-β1 in kidney tissues (N = 7). Data are presented as mean ± SEM. ****p < 0.05, *p < 0.01, **p < 0.01 vs model. ***p < 0.05, #P < 0.001, *p < 0.001 vs control group.
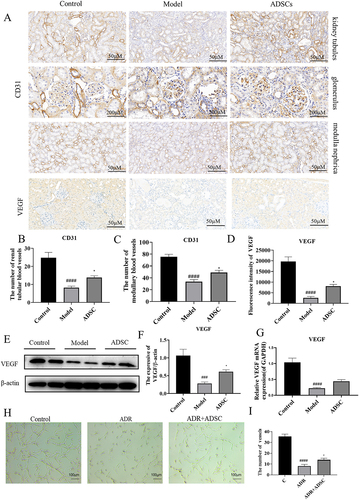
ADSC treatment promotes angiogenesis via enhancing secretion of VEGF
The advantage of ADSC over other mesenchymal stem cells is their strong ability to promote angiogenesis. As a result of the antiangiogenic environment, the equilibrium between pro- and anti-angiogenic factors is disrupted in the AN model. Therefore, we investigated whether ADSC alleviates AN by promoting angiogenesis. The CD31 was used as microvessel biomarker, and we observed the blood vessels are reduced in the model group, while neovascularization significantly increased following ADSC treatment (). Because ADSC can promote the secretion of pro-angiogenic molecules, such as VEGF, we detected the expression of VEGF and found that kidney VEGF secretion significantly increased in ADSC treatment compared with model group (). We also detected the mRNA and protein expression level of VEGF and found that they were remarkably increased after ADSC treatment (). An in vitro co-culture experiment of rat vascular endothelial cells and ADSC supernatant further confirmed that various growth factors in ADSC supernatant could promote vascular network formation (). All these results suggested that ADSC treatment could increase renal angiogenesis in AN group by enhancing VEGF secretion.
Figure 6. ADSC treatment promotes angiogenesis by increasing VEGF secretion in renal tissue. a) Immunohistochemistry staining of CD31 in kidney tissues, including kidney tubules, glomerulus, and medulla (N = 7). b–d) the number of vessels in renal tubule (b) and medulla (c) and the fluorescence intensity of VEGF expression (d) (N = 7). e) Western blot bands of VEGF in kidney tissues. f) Western blot analysis of TGF-β1 in kidney tissues. g) the mRNA expression of VEGF in kidney tissues (N = 7). h–i) ADSC treatment promotes rat vascular endothelial cell angiogenesis in vitro (N = 3). Data are presented as mean ± SEM. ****p < 0.05 vs. model. *p < 0.001, **p < 0.001, ***p < 0.0001 vs control group.
Discussion
The ADSC can differentiate into various cell lineages.Citation28 There have been several clinical trials using ADSC in human patients over the past few decades that have examined their safety, feasibility, and effectiveness.Citation19 Developing uniform protocols for preparing and characterizing ADSC, including standardized functional assays to evaluate their biological potential, is a critical factor contributing to their clinical utility.Citation29 We performed quality control on the liposuction patient-sourced ASDC, including differentiation potential and cell surface markers. These and other functionalities of ADSC can be used in clinical applications to achieve predictably favorable results for stem cell therapies with comprehensive and uniform guidelines and release criteria.
Labeling ADSC with DID, we studied ADSC’s distribution and metabolism in the AN model and normal rats’ kidneys. We found that ADSC had a longer retention time and slower metabolic clearance in AN model rats, which were possibly related to renal function impairment in AN model rats. Finally, we decided to administer ADSC injections every 2 weeks as a treatment option. In previous studies, the homing feature of MSCs has been observed to predominantly target the site of injury in cases of ischemia, hypoxia, and injury.Citation30 The results of our study indicatethat transplanted ADSC may be play a role in repairing kidney damage in situ after they have been injected into kidney tissues.
The adriamycin nephropathy mouse model closely resembles the human chronic proteinuric kidney disease.Citation2 An indicator of glomerular disease, proteinuria is a measure of glomerular injury and plays a pivotal role in triggering the subsequent oxidative stress response, inflammatory response, and fibrosis. Albuminuria increased significantly after adriamycin injection, confirming the successful AN modeling in rats (p < 0.0001). The protein synthesis and catabolism are dysregulated in the condition of proteinuria, leading to excessive production of lipoproteins in the liver and high levels of triglycerides and cholesterol in the blood.Citation31 ADSC treatment alleviated these symptoms. BUN, Scr, and urine NGAL-to-creatinine ratio (NGAL/URE) measure the glomerular function and extent of injury to the glomeruli. In our study, ADSC treatment reduced Scr, BUN, and NAGL/URE levels and improved kidney function. In addition, lymphocyte infiltration decreased, and renal tubule injury was alleviated.
A comprehensive evaluation of renal histology includes histological variants of glomerulosclerosis and the pathology of inflammatory cell infiltration.Citation32,Citation33 In this study, ADSC treatment reduced glomerular hypertrophy and alleviated renal tubular injury. Besides, podocytopathies result in a reduction in nephrin in the glomerulus.Citation34 ADSC treatment mitigated this reduction in nephrin levels. The presence of inflammatory cells and fibrosis between the tissues in AN is linked to various inflammatory cytokines, including IL-1β, IL-12 and MCP-1.Citation35,Citation36 IL-1β, IL-12, and MCP-1 expression increased in the AN rat kidneys. ADSC significantly down-regulated the mRNA expression of inflammatory cytokines. In addition, ADSC treatment inhibited the generation of MDA, increased GSH levels, and reduced the oxidative stress response in AN rats.
The extensive distribution and low immunogenicity of ADSC have rendered it a widely utilized therapeutic approach for fibrotic diseases.Citation37 It has been reported that ADSC could improve fibrosis in various organs, including cardiac, liver and pulmonary.Citation38 However, there is little research on its ability to alleviate kidney fibrosis. In this study, we confirmed the anti-fibrotic properties of ADSC. TGF-β1 is primarily produced by macrophages and actively participate in the progression of renal interstitial fibrosis.Citation39 Our results showed that ADSC reduces the secretion of TGF-β1 in infiltrating cells and reduces the production of fibronectin. In addition, the protein and mRNA expression levels of TGF-β1 and fibronectin were decreased in renal tissues. However, the mechanism of ADSC action in renal fibrosis requires further investigation.
Induced angiogenesis by MSCs is mainly regulated by proangiogenic paracrine activity.Citation40 VEGF can stimulate endothelial cell maturation, migration, and proliferation, thereby promoting angiogenesis. Our study found that ADSC treatment increases the number of new blood vessels in the kidneys. Experimental studies have shown that ADSC can stimulate angiogenesis in vitro. In addition, ADSC treatment increased the VEGF mRNA and protein levels. Therefore, ADSC treatment promotes the formation of new blood vessels by increasing VEGF secretion.
Conclusion
In this study, an animal model of AN was used to demonstrate the therapeutic effects of ADSC on kidney function. ADSC’s renoprotective effects are also discussed in relation to its underlying mechanisms. Our study has demonstrated that the therapeutic effects of ADSC on ADR were mediated through the following four mechanisms: anti-inflammation, antioxidative stress, anti-fibrosis, and induction of angiogenesis. ADSC treatment improved the activity of anti-oxidative enzymes (GSH), reduced MDA levels in kidney tissue, reduced inflammatory factors secreted by the kidney, and protected the cell membrane of glomerular podocytes. Additionally, ADSC treatment can reduce renal fibrosis and increase VEGF secretion to promote angiogenesis. Our study establishes a solid groundwork for the medical application of ADSC and presents them as a promising substitute for hormone therapy in treating clinical CKD.
Authors’ contributions
Xiaodi Zhao and Chengyan Ma are in charge of animal study and pathological study (Immunohistochemistry and immunofluorescence), Lijie Li is in charge of flow cytometry and data analysis; Yuemei Yang is in charge of data analysis; Sen Zhang and Xiaoli Wang are in charge of project design and manuscript writing.
Availability of data and materials
All the data can be accessed upon request.
Consent for publication
All of the authors have given their consent for the publication of this manuscript, including all associated data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Fogo AB. Causes and pathogenesis of focal segmental glomerulosclerosis. Nat Rev Nephrol. 2015;11:76–15. doi:10.1038/nrneph.2014.216.
- Lee VW, Harris DC. Adriamycin nephropathy: a model of focal segmental glomerulosclerosis. Nephrology (Carlton). 2011;16(1):30–38. doi:10.1111/j.1440-1797.2010.01383.x.
- Hori H, Sakai K, Ohashi A, Nakai S. Chitin powder enhances growth factor production and therapeutic effects of mesenchymal stem cells in a chronic kidney disease rat model. J Artif Organs. 2023;26(3):203–11. doi:10.1007/s10047-022-01346-z.
- Xia C, Shao L, Ma Y, Wang X, Zhang Y, Shi C, Li H, Wang J. Ultrasound-guided transplantation of mesenchymal stem cells improves adriamycin nephropathy in rats through the RIPK3/MLKL and TLR-4/NF-κB signaling. Stem Cells Dev. 2021;30(20):1003–16. doi:10.1089/scd.2021.0087.
- Chen F, Chen N, Xia C, Wang H, Shao L, Zhou C, Wang J. Mesenchymal stem cell therapy in kidney diseases: potential and challenges. Cell Transplant. 2023;32:9636897231164251. doi:10.1177/09636897231164251.
- Qiu Y, Lei C, Zeng J, Xie Y, Cao Y, Yuan Q, Su H, Zhang Z, Zhang C. Asparagine endopeptidase protects podocytes in adriamycin-induced nephropathy by regulating actin dynamics through cleaving transgelin. Mol Ther. 2023;31(11):3337–54. doi:10.1016/j.ymthe.2023.09.003.
- Li Z, Zhang X, Wu H, Ma Z, Liu X, Ma J, Zhang D, Sheng L, Chen X, Zhang S. et al. Hydrangea paniculata coumarins attenuate experimental membranous nephritis by bidirectional interactions with the gut microbiota. Commun Biol. 2023;6(1):1189. doi:10.1038/s42003-023-05581-9.
- Deluque AL, Oliveira BM, Souza CS, Maciel ALD, Francescato HDC, Giovanini C, de Almeida LF, de Paula FJA, Costa RS, Antunes-Rodrigues J., et al. Paricalcitol improves the Angiopoietin/Tie-2 and VEGF/VEGFR2 signaling pathways in adriamycin-induced nephropathy. Nutrients. 2022;14(24):5316. doi:10.3390/nu14245316.
- Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, Wang Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14(8):493–507. doi:10.1038/s41581-018-0023-5.
- Zheng J, Lu Y, Lin Y, Si S, Guo B, Zhao X, Cui L. Epitranscriptomic modifications in mesenchymal stem cell differentiation: advances, mechanistic insights, and beyond. Cell Death Differ. 2024;31(1):9–27. doi:10.1038/s41418-023-01238-6.
- Han L, Wu X, Wang O, Luan X, Velander WH, Aynardi M, Halstead ES, Bonavia AS, Jin R, Li G., et al. Mesenchymal stromal cells and alpha-1 antitrypsin have a strong synergy in modulating inflammation and its resolution. Theranostics. 2023;13(9):2843–62. doi:10.7150/thno.83942.
- Chen J, Fujita N, Takeda T, Hanyu W, Takatani H, Nakagawa T, Nishimura R. Canine bone marrow peri-adipocyte cells could therapeutically benefit acute spinal cord injury through migration and secretion of hepatocyte growth factor to inflammatory milieu. Exp Anim. 2023;72(1):19–29. doi:10.1538/expanim.22-0026.
- Lu J, Zhang Y, Wang D, Xu X, Xu J, Yang X, Qian H, Zhang H. Basic fibroblast growth factor promotes mesenchymal stem cell migration by regulating glycolysis-dependent β-catenin signaling. Stem Cells. 2023;41(6):628–42. doi:10.1093/stmcls/sxad024.
- Xu T, Chen T, Fang H, Shen X, Shen X, Tang Z, Zhao J. Human umbilical cord mesenchymal stem cells repair endothelial injury and dysfunction by regulating NLRP3 to inhibit endothelial cell pyroptosis in Kawasaki disease. Inflammation. 2023;47:483–502.
- Qi R, Hou J, Yang Y, Yang Z, Wu L, Qiao T, Wang X, Song D. Integrin beta1 mediates the effect of telocytes on mesenchymal stem cell proliferation and migration in the treatment of acute lung injury. J Cell Mol Med. 2023;27(24):3980–94. doi:10.1111/jcmm.17976.
- Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise review: mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells (Dayton, Ohio). 2011;29(6):913–19. doi:10.1002/stem.643.
- Giacca M, Zacchigna S. VEGF gene therapy: therapeutic angiogenesis in the clinic and beyond. Gene Ther. 2012;19(6):622–29. doi:10.1038/gt.2012.17.
- Herrera M, Mirotsou M. Stem cells: potential and challenges for kidney repair. Am J Physiol Renal Physiol. 2014;306:F12–23. doi:10.1152/ajprenal.00238.2013.
- Rochette L, Mazini L, Malka G, Zeller M, Cottin Y, Vergely C. The crosstalk of adipose-derived stem cells (ADSC), oxidative stress, and inflammation in protective and adaptive responses. Int J Mol Sci. 2020;21(23):21. doi:10.3390/ijms21239262.
- Yu S, Cheng Y, Zhang L, Yin Y, Xue J, Li B, Gong Z, Gao J, Mu Y. Treatment with adipose tissue-derived mesenchymal stem cells exerts anti-diabetic effects, improves long-term complications, and attenuates inflammation in type 2 diabetic rats. Stem Cell Res Ther. 2019;10(1):333. doi:10.1186/s13287-019-1474-8.
- Chen YJ, Liu HY, Chang YT, Cheng YH, Mersmann HJ, Kuo WH, Ding S-T. Isolation and differentiation of adipose-derived stem cells from porcine subcutaneous adipose tissues. J Vis Exp. 2016;(109):e53886. doi:10.3791/53886-v.
- Mailey B, Hosseini A, Baker J, Young A, Alfonso Z, Hicok K, Wallace AM, Cohen SR. Adipose-derived stem cells: methods for isolation and applications for clinical use. Methods Mol Biol. 2014;1210:161–81.
- Wang W, Sheng L, Chen Y, Li Z, Wu H, Ma J, Zhang D, Chen X, Zhang S. Total coumarin derivates from Hydrangea paniculata attenuate renal injuries in cationized-BSA induced membranous nephropathy by inhibiting complement activation and interleukin 10-mediated interstitial fibrosis. Phytomedicine. 2022;96:153886. doi:10.1016/j.phymed.2021.153886.
- Shi X, Li Z, Lin W, Shi W, Hu R, Chen G, Li X, Li X, Zhang S. Altered intestinal microbial flora and metabolism in patients with idiopathic membranous nephropathy. Am J Nephrol. 2023;54(11–12):451–70. doi:10.1159/000533537.
- Zhang S, Ma J, Sheng L, Zhang D, Chen X, Yang J, Wang D. Total coumarins from hydrangea paniculata show renal protective effects in lipopolysaccharide-induced acute kidney injury via anti-inflammatory and antioxidant activities. Front Pharmacol. 2017;8. doi:10.3389/fphar.2017.00872.
- Zhang Y, He W, Zhang S. Seeking for correlative genes and signaling pathways with bone metastasis from breast cancer by integrated analysis. Front Oncol. 2019;9. doi:10.3389/fonc.2019.00138.
- Sen Z, Weida W, Jie M, Li S, Dongming Z, Xiaoguang C. Coumarin glycosides from Hydrangea paniculata slow down the progression of diabetic nephropathy by targeting Nrf2 anti-oxidation and smad2/3-mediated profibrosis. Phytomedicine. 2019;57:385–95. doi:10.1016/j.phymed.2018.12.045.
- Zhu M, Heydarkhan-Hagvall S, Hedrick M, Benhaim P, Zuk P. Manual isolation of adipose-derived stem cells from human lipoaspirates. J Vis Exp. 2013;(79):e50585. doi:10.3791/50585-v.
- Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM. Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl Med. 2017;6(12):2173–85. doi:10.1002/sctm.17-0129.
- Lopes-Pacheco M, Rocco PRM. Functional enhancement strategies to potentiate the therapeutic properties of mesenchymal stromal cells for respiratory diseases. Front Pharmacol. 2023;14:1067422. doi:10.3389/fphar.2023.1067422.
- Shin JI, Kronbichler A, Oh J, Meijers B. Nephrotic syndrome: genetics, mechanism, and therapies. Biomed Res Int. 2018;2018:6215946. doi:10.1155/2018/6215946.
- Zhang Y, Li Z, Wu H, Wang J, Zhang S. Esculetin alleviates murine lupus nephritis by inhibiting complement activation and enhancing Nrf2 signaling pathway. J Ethnopharmacol. 2022;288:115004. doi:10.1016/j.jep.2022.115004.
- Shi W, Li Z, Wang W, Liu X, Wu H, Chen X, Zhou X, Zhang S. Dynamic gut microbiome-metabolome in c-BSA induced experimental immune-complex glomerulonephritis and effect of losartan and MMF on microbiota modulation. J Pharmaceut Anal. 2023;14(4):100931. doi:10.1016/j.jpha.2023.12.021.
- Yogavijayan Kandasamy RS, Lumbers ER, Rudd D. Nephrin – a biomarker of early glomerular injury. Biomarker Res. 2014;2(1):21. doi:10.1186/2050-7771-2-21.
- D’Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med. 2011;365(25):2398–411. doi:10.1056/NEJMra1106556.
- Angeletti A, Cantarelli C, Petrosyan A, Andrighetto S, Budge K, D’Agati VD, Hartzell S, Malvi D, Donadei C, Thurman JM., et al. Loss of decay-accelerating factor triggers podocyte injury and glomerulosclerosis. J Experiment Medi. 2020;217(9). doi:10.1084/jem.20191699.
- Zhou Y, Yuan J, Zhou B, Lee AJ, Lee AJ, Ghawji M Jr., Yoo TJ. The therapeutic efficacy of human adipose tissue-derived mesenchymal stem cells on experimental autoimmune hearing loss in mice. Immunology. 2011;133(1):133–40. doi:10.1111/j.1365-2567.2011.03421.x.
- Han HS, Lee H, You D, Nguyen VQ, Song DG, Oh BH, Shin S, Choi JS, Kim JD, Pan CH., et al. Human adipose stem cell-derived extracellular nanovesicles for treatment of chronic liver fibrosis. J Control Release. 2020;320:328–36. doi:10.1016/j.jconrel.2020.01.042.
- Li JH, Zhu HJ, Huang XR, Lai KN, Johnson RJ, Lan HY. Smad7 inhibits fibrotic effect of TGF-β on Renal tubular epithelial cells by blocking smad2 activation. J Am Soci Nephro. 2002;13(6):1464–72. doi:10.1097/01.ASN.0000014252.37680.E4.
- Koellensperger E, Gramley F, Preisner F, Leimer U, Germann G, Dexheimer V. Alterations of gene expression and protein synthesis in co-cultured adipose tissue-derived stem cells and squamous cell-carcinoma cells: consequences for clinical applications. Stem Cell Res Ther. 2014;5(3):65. doi:10.1186/scrt454.
