Abstract
tRNA (tRNA) is a key molecule used for protein synthesis, with multiple points of stress-induced regulation that can include transcription, transcript processing, localization and ribonucleoside base modification. Enzyme-catalyzed modification of tRNA occurs at a number of base and sugar positions and has the potential to influence specific anticodon-codon interactions and regulate translation. Notably, altered tRNA modification has been linked to mitochondrial diseases and cancer progression. In this review, specific to Eukaryotic systems, we discuss how recent systems-level analyses using a bioanalytical platform have revealed that there is extensive reprogramming of tRNA modifications in response to cellular stress and during cell cycle progression. Combined with genome-wide codon bias analytics and gene expression studies, a model emerges in which stress-induced reprogramming of tRNA drives the translational regulation of critical response proteins whose transcripts display a distinct codon bias. Termed Modification Tunable Transcripts (MoTTs),Citation1 we define them as (1) transcripts that use specific degenerate codons and codon biases to encode critical stress response proteins, and (2) transcripts whose translation is influenced by changes in wobble base tRNA modification. In this review we note that the MoTTs translational model is also applicable to the process of stop-codon recoding for selenocysteine incorporation, as stop-codon recoding involves a selective codon bias and modified tRNA to decode selenocysteine during the translation of a key subset of oxidative stress response proteins. Further, we discuss how in addition to RNA modification analytics, the comprehensive characterization of translational regulation of specific transcripts requires a variety of tools, including high coverage codon-reporters, ribosome profiling and linked genomic and proteomic approaches. Together these tools will yield important new insights into the role of translational elongation in cell stress response.
Introduction
The central dogma of molecular biology highlights a unique role for transfer RNA (tRNA), one that bridges nucleobase sequence with amino acid sequence during protein synthesis. tRNA molecules can range in length from 73 to 94 nucleotides, and there are over 25 different enzymatically modified tRNA bases per organism.Citation2 tRNA plays a vital role in translation, highlighted by the fact that sequence variants, specific modifications and defects in modification systems can be linked to human diseases.Citation3-6 Recent evidence provides strong support for the idea that cells use tRNA to dynamically regulate gene expression in response to stress.Citation1,7-12 In this review, we highlight the roles that tRNA modification systems play in disease pathologies and in response to environmental insults. Further, we describe how specific transcripts with a distinct codon bias encode critical stress response proteins that can be translationally regulated by dynamic changes in tRNA wobble base modifications. We also describe how mass spectrometry and computational approaches, combined with molecular analyses, yield data that support the idea that critical stress response transcripts have codon biases that can be translationally linked to specific tRNA modifications. Further, these studies lead us to propose that reprogrammed tRNAs are involved in the selective translation of proteins from families of genes in which there is a second genetic code, in the form of a biased use of degenerate codons.
Our model finds strong parallels in the link between wobble base modifications and the use of the non-standard amino acid selenocysteine. Selenocysteine decoding is unconventional because it does not have a dedicated triplet codon, and instead “recodes” internal UGA stop codons in mRNA. Stop codon recoding depends upon the presence of a selenocysteine insertion sequence (SECIS) in the 3’ untranslated region (UTR) of the mRNA transcript as well as accessory factors to promote positioning of tRNASEC into the ribosome.Citation13-15 Importantly tRNASEC must contain modified uridine wobble bases at position 34 that include 5-methoxycarbonylmethyluridine (mcm5U) and 5-methoxycarbonylmethyl-2′-O-methyluridine (mcm5Um), which promote optimal anticodon-codon interactions needed to decode selenocysteine-containing proteins.Citation16-18
Stop-codon recoding illustrates our model of codon bias regulating translation, as the transcripts that encode the 25 selenoproteins in humans have at least twice as many stop codons as the other ∼25,000 open reading frames in the genome. Selenoproteins also encode critical stress response proteins, namely glutathione peroxidases (GPXs) and thioredoxin reductases (TrxRs),Citation15 with GPXs and TrxRs being vital to the detoxification of reactive oxygen species (ROS), and TrxRs being further implicated in regulating DNA damage responses. Lastly, efficient stop-codon recoding requires specifically modified tRNA wobble bases. Thus, there are established and emerging links between tRNA modifications, codon bias, and translational regulation in stress responses. As we continue to characterize dynamic tRNA modification signatures and classify gene subsets with an inherent codon bias, our future challenge will be to further develop these observations into models of translational regulation that will likely be disease-, stress-, toxicant- and cell-specific. This will require large-scale genomics, proteomics and computational approaches, as discussed in the final section of this review.
tRNA
tRNA structure and modification
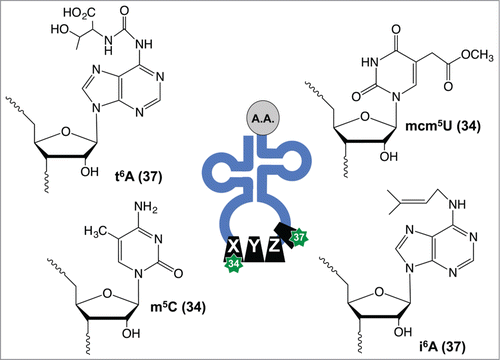
The regulatory potential for tRNA modifications can be understood in relation to tRNA structure. tRNA is initially transcribed by RNA polymerase III (RNA Pol III) using the canonical adenine (A), uracil (U), cytosine (C) and guanine (G) nucleobases in RNA. tRNA then folds into a 4 stem clover leaf secondary structure as defined by a D-stem and loop (DSL), T-stem and loop (TSL), anticodon stem and loop (ASL) and aminoacylation stem, and in some cases a fifth stem in the form of a variable loop.Citation19,20 The DSL and TSL contain the modified nucleotides dihydrouridine (D) and pseudouridine (ψ), respectively, but highlight the fact that many different tRNA nucleotides can be enzymatically modified post-transcriptionally. There are over 120 known post-transcriptional tRNA modifications that provide structural stability and help in decoding, among many other known and potential functions.Citation19,20 The aminoacylation stem contains a single-stranded 5′-CAA-3′ tail that is enzymatically charged with specific amino acids, with the associated aminoacyl tRNA synthetase (aaRS) enzymes well described in another review.Citation21 The ASL contains the 3-nucleotide anticodon sequence at positions 34 to 36, which will base pair with mRNA codons during translation. Positions 34 and 37 on tRNA can be enzymatically modified to influence binding of the tRNA molecule to the mRNA codon, with modifications including mcm5U34, 5-methylcytidine (m5C34), N6-threonylcarbamoyladenosine (t6A37) and N6-isopentenyladenosine (i6A37), (), to name just a few.Citation2 Notably, other modification chemistries within the anti-codon stem loop structure and throughout tRNA can also influence anticodon-codon interactions and alter the decoding capacity of the tRNA molecule.Citation22
tRNA function, codons and mistranslation
To better understand the role of tRNA modifications and mRNA codon bias in translational regulation, it is important to review aspects of protein synthesis and mistranslation relative to tRNA and codons. The recognized genetic code has 64 different 3-base combinations that comprise codons. The 61 codons that specify the 20 amino acids are considered to be degenerate, with significant regulatory potential in the multiplicity of codons for each amino acid. For example, there are 6 degenerate codons for arginine and 2 for glutamic acid; therefore, gene transcripts have multiple codon options for the decoding of these 2 particular amino acids.Citation11,24,25 The 6 codons for arginine are CGU, CGC, CGA, CGG, AGA and AGG, with the last 2 residing in a split codon box with the AGU and AGC codons for serine. The arginine and serine codons found in the split codon box only differ by their wobble bases, and paired with malfunctions in protein synthesis machinery highlight a potential for arginine to be misincorporated for serine and vice versa. Notably, deficiencies in specific wobble base tRNA modifications (e.g., mcm5U34) can lead to amino acid incorporation errors due to improper pairing of arginine tRNAs with non-cognate serine codons in the ribosome.Citation10 In addition anti-microbial aminoglycoside antibiotics can also promote protein errors by disrupting the protein synthesis machinery.Citation26-28 Translational errors have the potential to lead to mis-folded or aggregated proteins. The resulting faulty proteins can activate cellular heat shock (HSR) and unfolded protein (UPR) responses, both of which have been implicated in disease processes.Citation30-34
Human Diseases Linked to tRNA and Modification Systems
Mitochondrial diseases
In addition to protein errors, slowed translation can lead to pathological outcomes, as demonstrated by the strong links between tRNA hypomodification and human disease. For example, myoclonus epilepsy associated with ragged-red fibers (MERRF) and the condition of mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) are both mitochondrial encephalomyopathies that are caused by mutations in tRNA genes. A common feature of these mutant tRNAs is their hypomodification due to sequence changes that prevent enzyme-catalyzed modification of wobble bases. Both MERRF and MELAS are examples of diseases in which the pathology can be attributed to defective codon-anticodon interactions and decreased translation.Citation3,4 Both MERRF and MELAS also share common symptoms reflecting similar pathophysiological mechanisms, including lactic acidosis, muscle weakness, seizures and other neurological pathologies, such as a lack of physical coordination.Citation4,35 MERRF can occur due to a mutation that prevents wobble base thiolation (s2) in the tRNA-Lys that decodes AAG and AAA codons. This leads to a general decrease in translation of lysine in the mitochondria and an overall decrease in protein translation that corrupts mitochondrial function.
There are 2 classes of gene defects known to be etiologic for MELAS: 1) mutations in mitochondrial-specific genes that encode the complex I enzymes that operate the electron transport chain, and 2) mutations in mitochondrial-specific tRNA genes encoding leucine, histidine and valine isoacceptors; all cause a decrease in the levels of essential mitochondrial enzymes used to synthesize ATP.Citation36 For example, the wobble uridine of mitochondrial tRNALeu decodes UUG codons and is modified to 5-taurinomethyluridine (tm5U) using the β-amino acid taurine as a cofactor. MELAS arises when a mutation in tRNALeu prevents modification to tm5U34.Citation37 Furthermore, taurine deficiency can lead to hypomodified tRNAs, and promote a decrease in the translation of the UUG-rich transcript encoding the mitochondrial complex I protein, ND6, and promotes the onset of MELAS. Thus, taurine deficiency provides a clear mechanistic link between modified wobble uridines, codon-specific translation of a key protein, and the pathophysiology of a disease.Citation36,38
tRNA modification and cancer
tRNA hypomodification is associated with some cancers, with a decrease in the levels of the modified nucleoside queuosine (Q) identified in ovarian and blood cancers.Citation39,40 Decreased Q is further associated with higher tumor grades and a poor differentiation status,Citation41 which implicates a growth inhibitory role for this wobble base modification. Tumor growth suppressive roles have been reported for the confirmed and potential tRNA modification enzymes tRNA-isopentenyltransferase (tRNA-IPT) and the human tRNA methyltrasferase 9-like protein (hTRM9L), respectively. tRNA-IPT catalyzes the addition of i6A on position 37 in tRNA () and decreased levels have been reported in lung adenocarcinomas.Citation5 Increased expression of full-length tRNA-IPT in A549 lung cancer cells leads to decreased proliferation in colony formation assays.Citation5,6 Similarly, human TRM9L (hTRM9L), which is a homolog of the wobble base mcm5U-forming tRNA methyltransferase 9 from budding yeast (), has been demonstrated to be significantly decreased in colorectal, breast and bladder cancers.Citation8 Re-expression of hTRM9L in SW620 and HCT116 colorectal lines decreased tumor sizes in xenograft models and assessment of hTRM9L levels could be a useful cancer diagnostic.Citation8
Increased protein translation is linked to cell proliferation in many forms of cancer, with cancer-causing mutations affecting translation at several levels. For example, approximately half of all human cancers contain mutations of the p53 gene, most notably those of the breast, colon, brain, blood, lung and liver.Citation42 p53 is a negative regulator of RNA Pol III transcripts and thus tRNA gene transcription. In one of the most common p53 mutations, R175H, RNA Pol III is activated by the mutated protein, which has the effect of increasing the levels of tRNA, sRNA and rRNA,Citation43 and, in theory, enhancing protein synthesis in the cell.
At the level of translation, ALKBH8, the other human homolog of S. cerevisiae Trm9, and the confirmed wobble base mcm5U tRNA methyltransferase from humans,Citation18 has been demonstrated to be required for the survival of some bladder cancer cell lines. As evidence, knockdown of ALKBH8 in bladder cancer cell models increases apoptosis, and severely limits metastatic potential.Citation44 Given that ALKBH8-catalyzed mcm5U-based modifications are important for ROS detoxification, these finding suggest that some cancer cells may become “addicted” to specific tRNA modifications as a means of adapting to the inherently ROS-rich environment of cancer cells.Citation44-45 In theory, this could be a hypermodification of tRNA promoting a disease state, but that is speculative at this point and requires analytical studies in bladder cancer systems. Overall, the regulatory control, tumor growth suppression and oncogenic like connections between p53, protein synthesis and tRNA modification systems is further evidence of the important yet complex roles for tRNA and its modifications in cancer pathophysiology.
Considered together, the above described mitochondrial diseases and different cancers demonstrate that defects in tRNA regulation, the integrity of tRNA and modification status are linked to pathological outcomes. We and others have observed that controlled alteration of tRNA modifications in the anticodon can influence codon interactions in ways that affect positive outcomes in terms of normal cell response to environmental changes.Citation11,25,46 We have proposed the idea that stress-induced modifications are partnered with specific degenerate codons in certain transcripts, which appears as a codon bias, with both being used to regulate translation. These concepts will be discussed in further detail in following sections. Importantly, deciphering some of the rules behind this second genetic code is described further below and has lead to insights into regulatory aspects of gene expression. As many diseases are connected to stress-induced changes in signaling, understanding the regulation of tRNA as well as the regulatory potential of tRNA modifications could lead to diagnostic and therapeutic breakthroughs.
Stress-Induced Regulation of tRNA
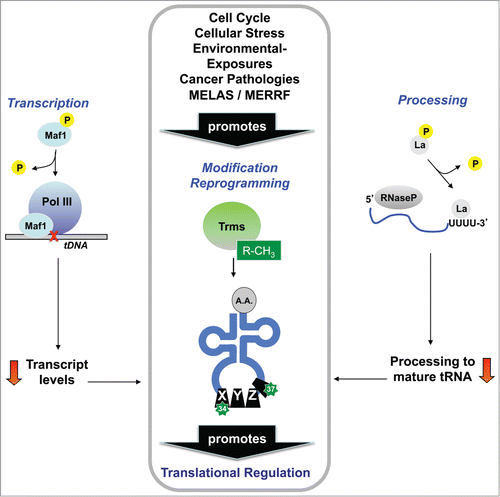
The pathway of tRNA biogenesis from gene to precursor tRNA (pre-tRNA) and on to a mature tRNA molecule for use in translation is a complex process that begins with RNA Pol III directed transcription, followed by 5′ and 3′ primary transcript processing, intron splicing, and, in the case of eukaryotes, export to the cytoplasm for aminoacylation.Citation47,48 The ribonucleosides that comprise the mature tRNA molecule are also extensively modified by enzymatic processes to form 20 - 25 different chemically altered ribonucleosides in every organism. The biosynthesis of many tRNA modifications has been defined for S. cerevisiae, with homologs of the corresponding enzyme systems present in humans.Citation49 Biological pathways as complex as those associated with tRNA metabolism allow for the regulation of tRNA and modifications at many discrete levels in order to fine tune translation, particularly under conditions of stress. Indeed, there are documented cases of stress-induced regulation of tRNA from the initial transcript to translation-associated forms (), and in this section we will address them and pay special attention to the re-programming of modified ribonucleosides in tRNA that occurs during replicative and oxidative stresses, as this represents a new and burgeoning area of RNA research.
Figure 2. Alteration of tRNA structure promotes functional regulation. Regulation of tRNA transcription, processing and modification has been reported in response to many stresses, with each regulatory change having the potential to contribute to changes in global tRNA modification levels. Reprogramming of tRNA modifications has been linked to tumor suppression, mitochondrial diseases, cell cycle progression and DNA damage- and ROS-responses, among other stress response pathways.
tRNA transcription and transcript processing can be regulated by stress
tRNA transcription is performed by RNA Pol III in coordination with Maf1, a key regulatory protein whose activity toward RNA Pol III is heavily influenced by cellular stress factors.Citation50 Several studies in S. cerevisiae have shown that under favorable cell growth conditions, Maf1 is phosphorylated by nutrient sensing and growth promoting kinases, and this phosphorylation reverses the normal inhibitory interaction of Maf1 with RNA Pol III.Citation51-55 Conversely, under conditions of stress such as DNA damage and nutrient deprivation, Maf1 remains in a non-phosphorylated form that inhibits RNA Pol III-dependent transcription.Citation56-60 Additionally, in human glioblastoma cells, Maf1 can negatively regulate Pol I- (rRNA) and Pol II- (mRNA) dependent transcription, a function that is linked to the transformed state of the cell, and likely evolved to coordinate all aspects of protein expression with the cellular milieu.Citation61
In almost all domains of life, initial pre-tRNA transcripts undergo processing at their 5′ and 3′ ends before adopting the canonical cloverleaf secondary structure. Processing at the 5′ end involves the removal of a 5′ “leader” sequence by an RNase P endonuclease complex, while mature 3′ ends are generated by RNase Z-mediated endonuclease activity, combined with other exonuclease activities that remove precursor “trailer” sequences until the proper 3′ end is generated; in some cases, the invariant C and/or A terminal nucleotides are added by a tRNA nucleotidyl transferase.Citation63,64 For most pre-tRNA molecules, 5′ end processing precedes 3′ end processing, but the temporal nature of this process is not completely understood.Citation63 In eukaryotes, it may be facilitated by the molecular chaperone protein La, which binds to 3′ oligo-U tracks, providing stability to the pre-tRNA molecule, and offering protection to the 3′ ends from exonuclease activity.Citation63,64 Human La is regulated in a manner similar to that of Maf1 in S. cerevisiae: under nutrient-rich conditions, La is phosphorylated by a nutrient-sensing kinase, and this phosphorylated form (pLa) binds and stimulates RNase P activity for tRNA biogenesis.Citation65,66 Conversely, in various mammalian thymocyte and cancer-derived cells, pLa is dephosphorylated by a protein phosphatase 2A-like activity in cells exposed to various forms of DNA damage or the death receptor activator, Fas,Citation67 representing a mechanism for the suppression of tRNA biogenesis under conditions of stress leading to apoptosis.
Certain subsets of pre-tRNA species also contain introns that typically disrupt the anticodon loop, and these intron sequences are removed by an endonuclease-mediated splicing process.Citation68 In Eukarya, the splicing endonuclease is comprised of Sen proteins and, although there is no evidence to suggest that these proteins are directly regulated by stress per se, point mutations in several human Sen family members are associated with the neurodegenerative disease, pontocerebellar hypoplasia (e.g., Sen2, Sen34 and Sen54).Citation69 Considering that neuronal dysfunction is tightly linked to free radical damage,Citation70,71 this raises the intriguing possibility that tRNA intron splicing may be influenced by oxidative stress in the brain, as is observed with tRNA modifications in response to oxidative stress in other eukaryotic and vertebrate cell systems.Citation11,12,72,73
tRNA aminoacylation and localization are regulated by reactive oxygen species
During tRNA aminoacylation, individual amino acids are attached to their associated tRNAs in a process that involves aminoacyl-tRNA synthetase (aaRS)-catalysis together with activation of the amino acid carboxyl group for peptide bond formation. It is important to note that translational fidelity is determined not only by tRNA anticodon recognition of the correct cognate codon, but also by attachment of the correct amino acid to its corresponding tRNA species. This specificity is achieved by the catalytic active site of the aaRS that typically accommodates only one amino acid for each of the appropriate codons,Citation74 and mischarging events are circumvented by the editing function of the aaRS. However, there are low-fidelity strains of S. cerevisiae that exhibit high levels of mischarged tRNAs, and this mutant-protein phenotype has been suggested to increase survival after stress as a form of “adaptive translation.”Citation75 Furthermore, it was observed that reactive oxygen species (ROS) stress increases the overall abundance of non-methionyl-tRNAs charged with methionine, leading to an overall increase in Met-misincorporated proteins, and serving to increase the ROS detoxification capacity of the cell as a mechanism to protect against oxidative stress-induced damage. Citation76,77
tRNA biogenic processing events are highly compartmentalized within the cell, and this compartmentalization can also be affected by cellular stress. After 5′-3′ processing in the nucleus, tRNA molecules are transported to the cytoplasm by export proteins belonging to the importin/karyopherin β superfamily (S. cerevisiae Los1, and vertebrate Xpo-t) where they undergo splicing and aminoacylation in the cytoplasm. At least 2 DNA damage response (DDR) proteins are linked to the regulation of tRNA molecules within this cellular landscape in S. cerevisiae. The kinases Mec1 and Rad53 can transmit a cytoplasmic localization signal to Los1 leading to the accumulation of unspliced intron-containing tRNAs in the nucleus, effectively coupling the G1 checkpoint response to a decrease in protein synthesis after exposures that cause DNA damage.Citation78
Modifications limit tRNA turnover
Overall tRNA levels are determined both by rates of RNA Pol III-dependent transcription and by tRNA turnover pathways, with these turnover pathways themselves regulated by mechanisms linked to the modification status of individual ribonucleosides. The nuclear pre-tRNA turnover pathway acts on diverse pre-tRNA transcripts; however, for S. cerevisiae it is thought to be particularly important for maintaining the level of initiator methionine tRNAs (tRNAMet).Citation79 This maintenance mechanism involves the Trm 6/61-mediated 1-methyladenosine (m1A) modification at position 58 of tRNAMet, which acts to protect the tRNA from polyadenylation by the TRAMP complex and subsequent degradation by Rrp6 and the 3′-nuclear exosome. In this way, the levels of m1A58-modified tRNAMet can conceivably control the overall rate of AUG-initiated mRNA translation.
In contrast to the pre-tRNA degradation pathway, the rapid tRNA decay (RTD) pathway acts on mature transcripts and involves degradation from the 5′ end that is not protected by aminoacylation. The RTD pathway primarily targets hypomodified tRNAs, which are tRNAs lacking key modifications to protect them from degradation by 5′-exonucleases, identified as Rat1 and Xrn1 in S. cerevisiae.Citation80 These protective modifications have been identified as 7-methylguanosine (m7G) and 5-methylcytidine (m5C), as catalyzed by Trm8 and Trm4, respectively, and these modification systems have been implicated in oxidative stress responses, which will be discussed in greater detail in the following sections.Citation81-83 Thus, methylation-based tRNA modifications are known to protect against stress-induced tRNA cleavage, possibly playing a role in stress signaling or allowing certain tRNA species to translate stress-response proteins despite a global decrease in translation.Citation84,85,87
Stress-Induced Reprogramming of Modifications in Global and Specific tRNAs
The link between hypomodified tRNA and tRNA degradation exemplifies the regulatory potential of tRNA modifications. Further, the editable chemical nature of tRNA modifications and their ability to regulate codon-anticodon interactions also makes them potential regulators of gene expression at the level of translation elongation. For enzyme-catalyzed tRNA modifications to be used in regulating translation, tRNA modifications must have the ability to be “reprogrammed” in response to different stimuli and this reprogramming needs to be linked to the decoding capabilities of the tRNA. The following sections describe recent advances in tRNA modification analysis, codon usage analytics, and gene expression studies that demonstrate the regulatory nature of wobble base tRNA modifications and the biased use of degenerate codons in stress response genes.
Technology for systems-level quantitation of tRNA modifications
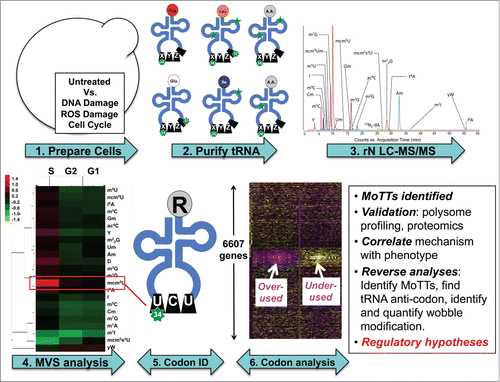
For tRNA modifications to be truly regulatory in nature, there must be coordinated changes in their levels in response to a stimulus. This was shown to be the case for the tRNA wobble mcm5U and m5C in response to stress.Citation7,12 (Chan et al., in press) To assess the generality and scope of stress-induced tRNA modification changes, we developed a chromatography-coupled mass spectrometry (LC-MS) platform (),Citation7 that involves (1) RNA isolation and HPLC purification of tRNA, (2) enzymatic hydrolysis to ribonucleosides for resolution by reversed-phase HPLC, (3) mass spectrometric identification and quantification of each ribonucleoside, and (4) multivariate statistical analysis of fold-change data obtained from control and stressed cells.Citation7,12 With 3 biological replicates, this approach detects relative changes in tRNA modification levels as small as 10% with statistical significance.Citation7,12 This platform was then used to analyze changes in the levels of tRNA modifications after arrest of yeast cells in distinct phases of the cell cycle and after yeast cells were exposed to equally toxic doses of 4 different toxicants: H2O2, methyl methanesulfonate (MMS), sodium arsenite (NaAsO2), and sodium hypochlorite (NaOCl).Citation9,12 Quantitative analysis of 23 of the 25 tRNA modifications in budding yeast revealed unique changes in response to each toxicant and dose. Further analysis using a hierarchical clustering programCitation88 to group data demonstrated that specific patterns modification patterns are present depending on agent and to some extent toxicant dose.Citation12 While the patterns of stress-induced changes in tRNA modification levels provided significant predictive power, the observation of changes in specific wobble modifications provided important clues about the link between tRNA reprogramming and selective translation of codon-biased genes in the cell response to each different stress.
Figure 3. A platform for systems-level quantification of stress-induced changes in tRNA modifications links them to regulation of specific genes based on codon usage. The pipeline for identifying connections between specific tRNA modifications and codon-biased genes begins with (1) exposing cells to different stresses, (2) isolation and hydrolysis of tRNA, (3) HPLC resolution of individual ribonucleosides, followed by quantification of each ribonucleoside by mass spectrometry, (4) analysis of stress-induced changes by multivariate statistical analysis, (5) assignment of significantly altered ribonucleosides to specific tRNAs, and (6) analysis of the cognate codons in genome-wide, gene-specific methods to identify codon trends in stress-response transcripts.
Stress-induced reprogramming of tRNA modifications
ROS stress in yeast
Intracellular ROS come in many forms and can act as important redox-altering molecules that signal to pathways regulating growth, hypoxia, immune cell recruitment, and cell mobility.Citation89-91 However, any failure of antioxidant enzyme systems to keep ROS levels in balance within the boundaries of normal cell survival and growth signaling (i.e., after exposure to environmental ROS-inducing toxicants) can lead to oxidative stress. In addition, exposure to agents that cause ROS can promote redox imbalances. tRNA modification systems have been linked to cellular ROS stress responses on several levels. First, 2′-O-methylcytidine (Cm), 5-methylcytidine (m5C) and N2,N2-dimethylguanosine (m22G) tRNA modifications have been shown to increase in S. cerevisiae after exposure to H2O2, but not in response to other stress-inducing agents.Citation12 Importantly, unique patterns of tRNA modification reprograming can distinguish mechanistically distinct damaging agents,Citation12 which supports the idea the tRNA modification patterns can be used as biomarkers of exposure. Secondly, strains lacking the enzymes responsible for catalyzing specific H2O2-induced modifications (i.e., Trm4 → m5C and Trm1 → m22G) display a cytotoxic hypersensitivity to H2O2, implying that these modification systems are important for cell survival after ROS stress.Citation12 The connection between tRNA modification systems and surviving cellular stress suggested that Trm1 and Trm4 could regulate critical DNA repair or ROS mitigation systems.
tRNA modifications in the yeast cell cycle and S-phase stress
Changes in tRNA modification patterns have also been noted for physiological programs and after DNA replication stress. Yeast cells cycle through G1, S and G2 phases during normal growth and distinct transcriptional reprogramming has been reported for these phases.Citation92 However, both transcriptional and post-translational regulation of gene expression are characteristic of cell cycle progression, suggesting that translational regulation can also be keyed to specific parts of the cell cycle. The levels of 16 tRNA modifications [ pseudouridine (Y), inosine (I), 2′-O-methylguanosine (Gm), 2′-O-methyluridine (Um), 2′-O-methylcytidine (Cm), 5-methyluridine (m5U), m5C, 3-methylcytidine (m3C), N7-methylguanosine (m7G), N4-acetylcytidine (ac4C), m22G, wybutosine (yW), N1-methylinosine (m1I), N1-methyladenosine (m1A), mcm5U, 5-methoxycarbonylmethyl-2-thiouridine (mcm5s2U)] have been demonstrated to oscillate throughout the cell cycle in S. cerevisiae, demonstrating that distinct regulation of tRNA is programmed into cell growth.Citation9 In fact, significant decreases in I, m5U, m5C, Cm, m7G, m1I and m1A modifications in G1 and G2 phases suggests that certain tRNA species become hypomodified as cells transit these phases of the cell cycle, and further suggests that they are being turned over since decreases in m1A, m7G and m5C are linked to tRNA degradation.Citation48,79-81 A significant 2-fold increase (P < 0.003) in mcm5U modifications was observed during S-phase under DNA-damaging conditions (i.e., treatment with hydroxyurea, HU),Citation9 which aligns with phenotypic data demonstrating that the corresponding tRNA methyltransferase (Trm9) is required to survive DNA-damaging agents hydroxyura (HU), MMS and ionizing radiation,Citation9,12,25 linking translation to DNA damage response.Citation46
tRNA modification reprogramming in human cells
The connection between global tRNA reprogramming and stress has also been reported in human cell lines. Several lines of tumorigenic colorectal cancer cells, including SW620 and HCT116, do not express the yeast Trm9 homolog hTRM9L, most likely due to epigenetic gene silencing.Citation8,93 The latter is consistent with the observation that treatment of SW620 cells with 5-deazacytidine promoted expression of hTRM9L and led to a significant decrease in tumor size in xenograft models. Similarly, SW620 and HCT116 cells reengineered to express hTRM9L also had a significant decrease in tumor growth as well as resistance to the aminoglycoside antibiotic paromomycin, which kills in part by inducing translational errors.Citation8 Expression of hTRM9L in yeast cells lacking native trm9 also had the effect of reducing sensitivity to killing by paromomycin.Citation10,94 To assess the effect of TRM9L expression on tRNA modifications, LC-MS analysis was performed on SW620 cells reengineered to express hTRM9L, with significant changes observed in the levels of 10 tRNA modifications [ac4C, Cm, Gm, I, i6A, m1A, N2-methylguanosine (m2G), 5,2′-O-dimethyluridine (m5Um), hydroxywybutosine (OHyW) and t6A].Citation8 Further, when hTRM9L-expressing SW620 cells were treated with the translational stressing agent, paromomycin, the levels of 4 tRNA modifications (ac4C, m1A, mcm5U and t6A) were significantly increased relative to untreated cells.Citation8 Taken together, these results support the idea that stress can promote reprogramming of tRNA modifications in human cancer models.
Trm4 and Trm9 drive codon-biased translation of critical stress response proteins
Two sets of coupled observations point to a mechanistic link between tRNA modification reprogramming and stress response in yeast. The observation that oxidative stress caused an elevation in the Trm4-dependent m5C was coupled with the observation that trm4Δ cells were sensitive to H2O2-induced cytotoxicity, while the S-phase damaging agents MMS and HU led to elevations in Trm9-dependent mcm5U and cells lacking Trm9 were similarly sensitive to killing by MMS and HUCitation12,24,25 (Chen et al., in press). Together these results suggested that specific modifications are vital to the cell response to specific stressors by regulating gene expression.Citation9,12
The Trm4/m5C connection was specifically linked to an H2O2-stimulated increase in the abundance of tRNALEU(CAA) isoacceptors with m5C at the wobble position. Further, protein expression studies linked Trm4, m5C and tRNALEU(CAA) isoacceptors to increased translation of transcripts that use UUG to decode leucine.Citation95 Interestingly, one such UUG-enriched transcript encoded the ribosomal protein RPL22A, which was identified as a key protein that prevents ROS sensitivity. The codon-specific relationship between UUG and RPL22A also supports the notion that cells may use an alternate ribosome to selectively translate proteins required for survival and DNA repair after oxidative stress exposure.Citation96,97
The Trm9/mcm5U connection to S-phase damage was also shown to be due to the increased translation of stress response transcripts that possess a specific codon bias. DNA replication in S-phase is limited by the available pool of intracellular 2-deoxyribonucleotides (dNTPs), which are formed from their ribonucleotide precursors by the ribonucleotide reductase (RNR) complex, composed of subunits 1 through 4.Citation98 The activity of the RNR complex is tightly linked to the cell cycle, having a high level of activity during the transition from G1 to S phase when the demand for dNTPs is accordingly high.Citation99-102 The Trm9-catalyzed mcm5U tRNA modification is required for the efficient translation of at least 2 subunits of the RNR complex (i.e., RNR1 and RNR3) after exposure to DNA damaging agents, when there is a similarly high demand for dNTPs in order to facilitate DNA repair.Citation9,103 RNR1 and RNR3 transcripts significantly over-use one codon from many split codon boxes, with mcm5U specifically linked to the AGA codon for arginine. It should be emphasized that the translational control by Trm9-catalyzed tRNA modifications is more complicated than simply linking mcm5U to AGA codons, as the mcm5U and its thiolated form (mcm5s2U) have been linked to the regulation of AGA, AAG, GAA, TTG an GAG codons, with last 2 being negatively regulated by Trm9.Citation9 The broader implication of these Trm9 studies is that tRNA modification-based translational control mechanisms can regulate RNR activity during S-phase damage and that they can be linked to specific codon usage patterns. In addition, they suggest that some tRNA modification and codon combinations can restrict translation. Apart from mcm5U, the biological significance of the 14 other tRNA modifications that change when cells enter G1 of G2 is yet to be determined,Citation9 but some will likely play regulatory roles similar to modified wobble bases.
Modification Tunable Transcripts (MoTTs)
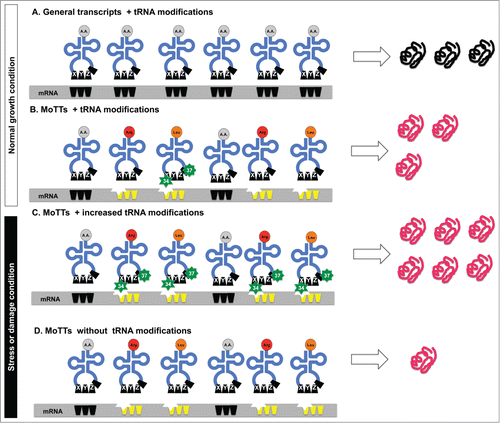
Although no codon in the genetic code can specify more than one amino acid, the degeneracy of the genetic code means that several codons can specify the same amino acid. Some synonymous codons are used more frequently than others (i.e., optimal codons), a fact that argues for an optimal code for translational efficiency and accuracy.Citation104 In support of this argument, the biased used of codons has been positively correlated with the number of copies of the associated tRNA gene, the number of copies of the expressed tRNA isoacceptor and gene expression levels.Citation105-110 It is now apparent that biased codon usage occurs at another level and represents a second genetic code. Here we discuss the idea of Modification Tunable Transcripts (MoTTs), which are distinct types of transcripts that have significantly different codon usage patterns when compared to the average transcript ( - bottom right panel and ), with changes in tRNA modification regulating MoTT translation. To understand the idea behind MoTTs, it is important to realize that gene codon usage for amino acid decoding is not random; certain gene subsets are enriched for one particular degenerate codon over another.Citation1,11,25 In tables that specify the genetic code, fold2- degenerate codons for one amino acid (i.e., arginine AGA & AGG) are found in a split codon box next to other 2-fold degenerate codons that specify a different amino acid (i.e., serine AGC & AGU). The cognate tRNAs for 2-fold degenerate codons require modification at the wobble position 34 and position 37 in order to discriminate the correct amino acid,Citation19,111 with hypomodification promoting amino acid misincorporation errors (i.e., arginine misincorporation for serine).Citation10 Computational studies and gene expression studies mechanistically demonstrated that Trm4- and Trm9-catalyzed tRNA modifications increase in response to specific stressors in order to stimulate the translation of transcripts that are enriched for specific degenerate codons (),Citation12,25,95 (Chan et al, submitted). In essence, MoTTs have identified one codon from a 2-fold degenerate box as the preferred codon, and are predicted to use tRNA modifications to translationally regulate the use of one degenerate codon from each of the 12 split boxes specific to 10 amino acids. The ability of cells to increase the levels of a specific modification in tRNA in response to stress (i.e., m5C after H2O2) allows for the increased efficiency of translation of a specific codon linked to the MoTTs code. The increased translation of specific codons should not only increase translational fidelity but also promote the faster translation of specific codons. In either case, be it fidelity or speed, increased tRNA modification should increase active protein levels, which in the case of stress response proteins allows for a rapid response to damage. In our model () of translational regulation of MoTTs by tRNA modifications, increased levels of specific modifications in the ASL stimulate the translation of codon-biased transcripts.
The translation of MoTTs is analogous to lock and key models of enzyme catalysis, in which specific structural fits are needed to meld the codon with the anticodon. Average transcripts that do not contain specific codon biases do not need the extra-modified tRNA, as they are efficiently translated under all conditions. In contrast, the translation of MoTTs in cells lacking tRNA modifications is severely reduced. In essence, increased tRNA modification in response to stress is proposed to drive increased translation of stress response proteins. MoTTs have their translation tightly linked to tRNA modification systems,Citation1 and are analogous to promoters that have been epigenetically regulated by methylation signals, in that only some but not all transcripts are regulated in this way.
Figure 4. Model for the regulation of MoTTs and ordinary transcripts under normal and stress conditions. Different stress conditions promote reprogramming of tRNA modifications that affects regular transcripts and MoTTs differentially. Under normal conditions, both (A) regular transcripts that are not codon biased and (B) MoTTs are efficiently translated. However, in response to stress-induced reprogramming of tRNA modifications, (C) MoTTs have their translation stimulated because the codon-bias (represented as indented stars) can be decoded by the stress-specific ASL modifications (green stars). In the absence of modified tRNA (D), MoTTs are poorly translated and this leads to decreased levels of stress response proteins and sensitivity to the stress.
MoTTs can be identified by their selective use of specific degenerate codons, and it should be noted that selective codon usage is a theme that carries over to another stress-related translational control mechanism, namely the recoding for selenocysteine incorporation into ROS detoxifying proteins. Selenocysteine is considered the 21st amino acid for which there is no dedicated codon. Rather, cells have expanded the genetic code by recoding internal UGA stop codons for the insertion of selenocysteine in a process that requires multiple factors, including specific SECIS signals in the 3′ UTR, accessory proteins, and specifically modified selenocystyl-tRNAs.Citation13-15 Selenocysteine-containing transcripts typically have at least 2 stop codons per transcript, which represents a significant codon bias that meets one of the MoTTs criteria. As a second criterion, the specific selenocystyl-tRNA used to decode the UGA stop codon requires anticodon loop modifications (mcm5U34, mcm5Um34 and i6A37),Citation13,17,18 further qualifying these stop codon enriched transcripts as MoTTs. As the third and last criterion, many of the 25 identified selenoproteins in humans are predicted to function as stress response proteins, with GPXs and TrxRs well represented.Citation15 With their biased codon use and requirement for tRNA modifications, in addition to encoding stress response proteins, the classification of selenocysteine transcripts as MoTTs offers another critical connection between translational regulation and the stress response. Furthermore, it suggests that, similar to yeast systems, the corresponding tRNA modifications will be reprogrammed in response to stress.
Future Outlook
The identification of stress-induced changes in tRNA modification, codon-biased translation, and MoTTs supports the idea that cells use distinct translational programs during stress responses. In addition to our described LC-MS-based platform for detecting changes in stress-induced tRNA modifications that predict translation regulation, other technologies offer insight into regulation of translation elongation. Specifically, codon-biased translation can be quantified by codon-specific reporter systems, ribosome profiling, and proteomic analysis, with the last 2 dependent on corresponding bioinformatic analysis. Codon-specific reporters use Green Fluorescent Protein (GFP) or Luciferase readouts to link specific modification systems or stresses with the translation of codon runs. While codon-specific reporters are typically used to analyze a subset of codons, codon reporter sets that address all 61 individual codons or the 3,904 combinations of 2 codons could be used to build a detailed picture of translation under specific stress-inducing conditions. Similarly, the use of modification-deficient cells in ribosome profiling provides a means to test the hypothesis that specific codons will be over- or under-represented in actively translated transcripts in tRNA modification mutants relative to wild-type cells. Proteomics offers another path to quantifying changes in codon usage since a computational analysis of codon use in proteins up-regulated in stressed cells, after accounting for proteins whose levels correlate with transcriptional changes, reveals trends related to tRNA modifications reprogramming and translational regulation. The combined use of ribosome profiling and proteomic analysis has the potential to identify significant codon bias in translated transcripts and upregulated proteins. As a corollary to translational up-regulation of stress response proteins, it is likely that stress-induced changes in tRNA modification will cause significant down-regulation in the translation groups of codon-biased transcripts, as an efficient means to shut down specific activities as the cell alters phenotype to survive the stress. Ongoing studies will test these models and other features of the translational control of cell stress response.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
NIH F32ES020670 (LE), NIH ES017010 (TJB), National Science Foundation CHE-1308839 (PCD), National Research Foundation of Singapore through the Singapore-MIT Alliance for Research and Technology Infectious Disease research program (PCD).
References
- Dedon PC, Begley TJ. A System of RNA Modifications and Biased Codon Use Controls Cellular Stress Response at the Level of Translation. Chem Res Toxicol (2014) 17:7
- Cantara WA, Crain PF, Rozenski J, McCloskey JA, Harris KA, Zhang X, Vendeix FA, Fabris D, Agris PF. The RNA Modification Database, RNAMDB: 2011 update. Nucleic Acids Res (2011) 39:D195-201; PMID:21071406; http://dx.doi.org/10.1093/nar/gkq1028
- Kirino Y, Yasukawa T, Ohta S, Akira S, Ishihara K, Watanabe K, Suzuki T. Codon-specific translational defect caused by a wobble modification deficiency in mutant tRNA from a human mitochondrial disease. Proc Natl Acad Sci U S A (2004) 101:15070-5; PMID:15477592; http://dx.doi.org/10.1073/pnas.0405173101
- Yasukawa T, Suzuki T, Ishii N, Ohta S, & Watanabe K. Wobble modification defect in tRNA disturbs codon-anticodon interaction in a mitochondrial disease. EMBO J 20:4794-802; PMID:11532943; http://dx.doi.org/10.1093/emboj/20.17.4794
- Spinola M, Galvan A, Pignatiello C, Conti B, Pastorino U, Nicander B, Paroni R, Dragani TA. Identification and functional characterization of the candidate tumor suppressor gene TRIT1 in human lung cancer. Oncogene (2005) 24:5502-09; PMID:15870694; http://dx.doi.org/10.1038/sj.onc.1208687
- Spinola M, Colombo F, Falvella FS, Dragani TA. N6-isopentenyladenosine: a potential therapeutic agent for a variety of epithelial cancers. Int J Cancer (2007) 120:2744-8; PMID:17304507; http://dx.doi.org/10.1002/ijc.22601
- Su D, Chan CT, Gu C, Lim KS, Chionh YH, McBee ME, Russell BS, Babu IR, Begley TJ, Dedon PC. Quantitative analysis of ribonucleoside modifications in tRNA by HPLC-coupled mass spectrometry. Nat Protoc (2014) 9:828-41; PMID:24625781; http://dx.doi.org/10.1038/nprot.2014.047
- Begley U, Sosa MS, Avivar-Valderas A, Patil A, Endres L, Estrada Y, Chan CT, Su D, Dedon PC, Aguirre-Ghiso JA. et al. A human tRNA methyltransferase 9-like protein prevents tumour growth by regulating LIN9 and HIF1-alpha. EMBO Mol Med (2013) 5:1-18; PMID:23283747; http://dx.doi.org/10.1002/emmm.201201161
- Patil A, Dyavaiah M, Joseph F, Rooney JP, Chan CT, Dedon PC, Begley TJ. Increased tRNA modification and gene-specific codon usage regulate cell cycle progression during the DNA damage response. Cell Cycle (2012) 11:3656-65; PMID:22935709; http://dx.doi.org/10.4161/cc.21919
- Patil A, Chan CT, Dyavaiah M, Rooney JP, Dedon PC, Begley TJ. Translational infidelity-induced protein stress results from a deficiency in Trm9-catalyzed tRNA modifications. RNA Biology (2012) 9:990-1001; PMID:22832247; http://dx.doi.org/10.4161/rna.20531
- Chan CTY, Pang YL, Deng W, Babu IR, Dyavaiah M, Begley TJ, Dedon PC. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat Commun (2012) 3:937; PMID:22760636; http://dx.doi.org/10.1038/ncomms1938
- Chan CT, Dyavaiah M, DeMott MS, Taghizadeh K, Dedon PC, Begley TJ. A quantitative systems approach reveals dynamic control of tRNA modifications during cellular stress. PLoS Genet (2010) 6:e1001247; PMID:21187895; http://dx.doi.org/10.1371/journal.pgen.1001247
- Carlson BA, Xu XM, Gladyshev VN, Hatfield DL. Selective rescue of selenoprotein expression in mice lacking a highly specialized methyl group in selenocysteine tRNA. J Biol Chem (2005) 280:5542-8; PMID:15611090; http://dx.doi.org/10.1074/jbc.M411725200
- Moustafa ME, Kumaraswamy E, Zhong N, Rao M, Carlson BA, Hatfield DL. Models for assessing the role of selenoproteins in health. J Nutr (2003) 133:2494S-6S; PMID:12840229
- Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, Gladyshev VN. Characterization of mammalian selenoproteomes. Science (2003) 300:1439-43; PMID:12775843; http://dx.doi.org/10.1126/science.1083516
- Novoselov SV, Calvisi DF, Labunskyy VM, Factor VM, Carlson BA, Fomenko DE, Moustafa ME, Hatfield DL, Gladyshev VN. Selenoprotein deficiency and high levels of selenium compounds can effectively inhibit hepatocarcinogenesis in transgenic mice. Oncogene (2005) 24:8003-11; PMID:16170372; http://dx.doi.org/10.1038/sj.onc.1208940
- Moustafa ME, Carlson BA, El-Saadani MA, Kryukov GV, Sun QA, Harney JW, Hill KE, Combs GF, Feigenbaum L, Mansur DB. et al. Selective inhibition of selenocysteine tRNA maturation and selenoprotein synthesis in transgenic mice expressing isopentenyladenosine-deficient selenocysteine tRNA. Mol Cell Biol (2001) 21:3840-52; PMID:11340175; http://dx.doi.org/10.1128/MCB.21.11.3840-3852.2001
- Songe-Moller L van den Born E, Leihne V, Vågbø CB, Kristoffersen T, Krokan HE, Kirpekar F, Falnes PØ, Klungland A. Mammalian ALKBH8 possesses tRNA methyltransferase activity required for the biogenesis of multiple wobble uridine modifications implicated in translational decoding. Mol Cell Biol (2010) 30:1814-27; PMID:20123966; http://dx.doi.org/10.1128/MCB.01602-09
- Agris PF, Decoding the genome: a modified view. Nucleic Acids Res (2004) 32:223-38; PMID:14715921; http://dx.doi.org/10.1093/nar/gkh185
- Durant PC, Bajji AC, Sundaram M, Kumar RK, Davis DR. Structural effects of hypermodified nucleosides in the Escherichia coli and human tRNALys anticodon loop: the effect of nucleosides s2U, mcm5U, mcm5s2U, mnm5s2U, t6A, and ms2t6A. Biochemistry (2005) 44:8078-89; PMID:15924427; http://dx.doi.org/10.1021/bi050343f
- Park SG, Schimmel P, Kim S. Aminoacyl tRNA synthetases and their connections to disease. Proc Natl Acad Sci U S A (2008) 105:11043-9; PMID:18682559; http://dx.doi.org/10.1073/pnas.0802862105
- Agris PF, Vendeix FA, Graham WD. tRNA's wobble decoding of the genome: 40 years of modification. J Mol Biol (2007) 366:1-13; PMID:17187822; http://dx.doi.org/10.1016/j.jmb.2006.11.046
- Vila-Sanjurjo A, Ridgeway WK, Seymaner V, Zhang W, Santoso S, Yu K, Cate JH. X-ray crystal structures of the WT and a hyper-accurate ribosome from Escherichia coli. Proc Natl Acad Sci U S A (2003) 100:8682-7; PMID:12853578; http://dx.doi.org/10.1073/pnas.1133380100
- Tumu S, Patil A, Towns WL, Dyavaiah M, Begley TJ. The gene-specific codon counting database: a genome-based catalog of one-, two-, three-, four- and five-codon combinations present in Saccharomyces cerevisiae genes. Database (2012) Feb 8; 2012:bas002; PMID:22323063
- Begley U, Dyavaiah M, Patil A, Rooney JP, DiRenzo D, Young CM, Conklin DS, Zitomer RS, Begley TJ. Trm9-Catalyzed tRNA Modifications Link Translation to the DNA Damage Response. Mol Cell (2007) 28:860-70; PMID:18082610; http://dx.doi.org/10.1016/j.molcel.2007.09.021
- Howard MT, Anderson CB, Fass U, Khatri S, Gesteland RF, Atkins JF, Flanigan KM. Readthrough of dystrophin stop codon mutations induced by aminoglycosides. Ann Neurol (2004) 55:422-6; PMID:14991821; http://dx.doi.org/10.1002/ana.20052
- Lai CH, Chun HH, Nahas SA, Mitui M, Gamo KM, Du L, Gatti RA. Correction of ATM gene function by aminoglycoside-induced read-through of premature termination codons. Proc Natl Acad Sci U S A (2004) 101:15676-81; PMID:15498871; http://dx.doi.org/10.1073/pnas.0405155101
- Lukacs GL, Durie PR. Pharmacologic approaches to correcting the basic defect in cystic fibrosis. The New England journal of medicine (2003) 349:1401-4; PMID:14534332; http://dx.doi.org/10.1056/NEJMp038113
- Strachan T, Read A. Human Molecular Genetics. Taylor & Francis, (1999) Chapter 7
- Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov (2008) 7:1013-30; PMID:19043451; http://dx.doi.org/10.1038/nrd2755
- Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol (2008) 3:399-425; PMID:18039139; http://dx.doi.org/10.1146/annurev.pathmechdis.3.121806.151434
- Turturici G, Sconzo G, Geraci F. Hsp70 and its molecular role in nervous system diseases. Biochem Res Int (2011) 2011:618127; PMID:21403864; http://dx.doi.org/10.1155/2011/618127
- Hoshino T, Murao N, Namba T, Takehara M, Adachi H, Katsuno M, Sobue G, Matsushima T, Suzuki T, Mizushima T. Suppression of Alzheimer's disease-related phenotypes by expression of heat shock protein 70 in mice. J Neurosci (2011) 31:5225-34; PMID:21471357; http://dx.doi.org/10.1523/JNEUROSCI.5478-10.2011
- Novo G, Cappello F, Rizzo M, Fazio G, Zambuto S, Tortorici E, Marino Gammazza A, Corrao S, Zummo G, De Macario EC. et al. Hsp60 and heme oxygenase-1 (Hsp32) in acute myocardial infarction. Translational research : the journal of laboratory and clinical medicine (2011) 157:285-92; PMID:21497776; http://dx.doi.org/10.1016/j.trsl.2011.01.003
- Shoffner JM, Lott MT, Lezza AM, Seibel P, Ballinger SW, Wallace DC. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell (1990) 61:931-7; PMID:2112427; http://dx.doi.org/10.1016/0092-8674(90)90059-N
- Schaffer S, Jong C. in Genes and Cardiovascular Function. (eds. B Ostadal, M Nagano & NS Dhalla) (Taylor & Francis US, 2011; 93-100)
- Yasukawa T, Suzuki T, Ueda T, Ohta S, Watanabe K. Modification defect at anticodon wobble nucleotide of mitochondrial tRNAs(Leu)(UUR) with pathogenic mutations of mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes. J Biol Chem (2000) 275:4251-7; PMID:10660592; http://dx.doi.org/10.1074/jbc.275.6.4251
- Kirino Y, Goto Y, Campos Y, Arenas J, Suzuki T. Specific correlation between the wobble modification deficiency in mutant tRNAs and the clinical features of a human mitochondrial disease. Proc Natl Acad Sci U S A (2005) 102:7127-32; PMID:15870203; http://dx.doi.org/10.1073/pnas.0500563102
- Baranowski W, Dirheimer G, Jakowicki JA, Keith G. Deficiency of queuine, a highly modified purine base, in transfer RNAs from primary and metastatic ovarian malignant tumors in women. Cancer Res (1994) 54:4468-71; PMID:8044797
- Huang BS, Wu RT, Chien KY. Relationship of the queuine content of transfer ribonucleic acids to histopathological grading and survival in human lung cancer. Cancer Res (1992) 52:4696-700; PMID:1511436
- Dirheimer G, Baranowski W, Keith G. Variations in tRNA modifications, particularly of their queuine content in higher eukaryotes. Its relation to malignancy grading. Biochimie (1995) 77:99-103; PMID:7599283; http://dx.doi.org/10.1016/0300-9084(96)88111-9
- Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science (1991) 253:49-53; PMID:1905840; http://dx.doi.org/10.1126/science.1905840
- Stein T, Crighton D, Boyle JM, Varley JM, White RJ. RNA polymerase III transcription can be derepressed by oncogenes or mutations that compromise p53 function in tumours and Li-Fraumeni syndrome. Oncogene (2002) 21:2961-70; PMID:12082526; http://dx.doi.org/10.1038/sj.onc.1205372
- Shimada K, Nakamura M, Anai S, De Velasco M, Tanaka M, Tsujikawa K, Ouji Y, Konishi N. A novel human AlkB homologue, ALKBH8, contributes to human bladder cancer progression. Cancer Res (2009) 69:3157-64; PMID:19293182; http://dx.doi.org/10.1158/0008-5472.CAN-08-3530
- Toyokuni S, Okamoto K, Yodoi J, Hiai H. Persistent oxidative stress in cancer. FEBS Lett (1995) 358:1-3; PMID:7821417; http://dx.doi.org/10.1016/0014-5793(94)01368-B
- Klassen R, Wemhoff S, Krause J, Meinhardt F. DNA repair defects sensitize cells to anticodon nuclease yeast killer toxins. Mol Genet Genomics (2011) 285:185-95; PMID:21188417; http://dx.doi.org/10.1007/s00438-010-0597-5
- Hopper AK, Phizicky EM. tRNA transfers to the limelight. Genes Dev (2003) 17:162-80; PMID:12533506; http://dx.doi.org/10.1101/gad.1049103
- Phizicky EM, Hopper AK. tRNA biology charges to the front. Genes Dev (2010) 24:1832-60; PMID:20810645; http://dx.doi.org/10.1101/gad.1956510
- Towns WL, Begley TJ. Transfer RNA methytransferases and their corresponding modifications in budding yeast and humans: activities, predications, and potential roles in human health. DNA Cell Biol (2012) 31:434-54; PMID:22191691; http://dx.doi.org/10.1089/dna.2011.1437
- Pluta K, Lefebvre O, Martin NC, Smagowicz WJ, Stanford DR, Ellis SR, Hopper AK, Sentenac A, Boguta M. Maf1p, a negative effector of RNA polymerase III in Saccharomyces cerevisiae. Mol Cell Biol (2001) 21:5031-40; PMID:11438659; http://dx.doi.org/10.1128/MCB.21.15.5031-5040.2001
- Graczyk D, Debski J, Muszyńska G, Bretner M, Lefebvre O, Boguta M. Casein kinase II-mediated phosphorylation of general repressor Maf1 triggers RNA polymerase III activation. Proc Natl Acad Sci U S A (2011) 108:4926-31; PMID:21383183; http://dx.doi.org/10.1073/pnas.1010010108
- Moir RD, Lee J, Haeusler RA, Desai N, Engelke DR, Willis IM. Protein kinase A regulates RNA polymerase III transcription through the nuclear localization of Maf1. Proc Natl Acad Sci U S A (2006) 103:15044-9; PMID:17005718; http://dx.doi.org/10.1073/pnas.0607129103
- Huber A Bodenmiller B, Uotila A, Stahl M, Wanka S, Gerrits B, Aebersold R, Loewith R. Characterization of the rapamycin-sensitive phosphoproteome reveals that Sch9 is a central coordinator of protein synthesis. Genes Dev (2009) 23:1929-43; PMID:19684113; http://dx.doi.org/10.1101/gad.532109
- Lee J, Moir RD, Willis IM. Regulation of RNA polymerase III transcription involves SCH9-dependent and SCH9-independent branches of the target of rapamycin (TOR) pathway. J Biol Chem (2009) 284:12604-8; PMID:19299514; http://dx.doi.org/10.1074/jbc.C900020200
- Wei Y, Tsang CK, Zheng XF. Mechanisms of regulation of RNA polymerase III-dependent transcription by TORC1. Embo J (2009) 28:2220-30; PMID:19574957; http://dx.doi.org/10.1038/emboj.2009.179
- Oler AJ, Cairns BR. PP4 dephosphorylates Maf1 to couple multiple stress conditions to RNA polymerase III repression. Embo J (2012) 31:1440-52; PMID:22333918; http://dx.doi.org/10.1038/emboj.2011.501
- Vannini A, Ringel R, Kusser AG, Berninghausen O, Kassavetis GA, Cramer P. Molecular basis of RNA polymerase III transcription repression by Maf1. Cell (2010) 143:59-70; PMID:20887893; http://dx.doi.org/10.1016/j.cell.2010.09.002
- Oficjalska-Pham D, Harismendy O, Smagowicz WJ, Gonzalez de Peredo A, Boguta M, Sentenac A, Lefebvre O. General repression of RNA polymerase III transcription is triggered by protein phosphatase type 2A-mediated dephosphorylation of Maf1. Mol Cell (2006) 22:623-32; PMID:16762835; http://dx.doi.org/10.1016/j.molcel.2006.04.008
- Roberts DN, Wilson B, Huff JT, Stewart AJ, Cairns BR. Dephosphorylation and genome-wide association of Maf1 with Pol III-transcribed genes during repression. Mol Cell (2006) 22:633-44; PMID:16762836; http://dx.doi.org/10.1016/j.molcel.2006.04.009
- Upadhya R, Lee J, Willis IM. Maf1 is an essential mediator of diverse signals that repress RNA polymerase III transcription. Mol Cell (2002) 10:1489-94; PMID:12504022; http://dx.doi.org/10.1016/S1097-2765(02)00787-6
- Johnson SS, Zhang C, Fromm J, Willis IM, Johnson DL. Mammalian Maf1 is a negative regulator of transcription by all three nuclear RNA polymerases. Mol Cell (2007) 26:367-79; PMID:17499043; http://dx.doi.org/10.1016/j.molcel.2007.03.021
- Singer M, Berg P. Genes and Genomes: A Changing Perspective. (Taylor & Francis, Mill Valley, CA; 1991)
- Wolin SL, Matera AG. The trials and travels of tRNA. Genes Dev (1999) 13:1-10; PMID:9887094; http://dx.doi.org/10.1101/gad.13.1.1
- Maraia RJ, Lamichhane TN. 3′ processing of eukaryotic precursor tRNAs. Wiley Interdiscip Rev RNA (2012) 2:362-75; http://dx.doi.org/10.1002/wrna.64
- Intine RV, Sakulich AL, Koduru SB, Huang Y, Pierstorff E, Goodier JL, Phan L, Maraia RJ. Control of transfer RNA maturation by phosphorylation of the human La antigen on serine 366. Mol Cell (2000) 6:339-48; PMID:10983981; http://dx.doi.org/10.1016/S1097-2765(00)00034-4
- Intine RV, Tenenbaum SA, Sakulich AL, Keene JD, Maraia RJ. Differential phosphorylation and subcellular localization of La RNPs associated with precursor tRNAs and translation-related mRNAs. Mol Cell (2003) 12:1301-7; PMID:14636586; http://dx.doi.org/10.1016/S1097-2765(03)00429-5
- Rutjes SA, Utz PJ, van der Heijden A, Broekhuis C, van Venrooij WJ, Pruijn GJ. The La (SS-B) autoantigen, a key protein in RNA biogenesis, is dephosphorylated and cleaved early during apoptosis. Cell Death Differ (1999) 6:976-86; PMID:10556975; http://dx.doi.org/10.1038/sj.cdd.4400571
- Abelson J, Trotta CR, Li H. tRNA splicing. J Biol Chem (1998) 273:12685-8; PMID:9582290; http://dx.doi.org/10.1074/jbc.273.21.12685
- Budde BS, Namavar Y, Barth PG, Poll-The BT, Nürnberg G, Becker C, van Ruissen F, Weterman MA, Fluiter K, te Beek ET. et al. tRNA splicing endonuclease mutations cause pontocerebellar hypoplasia. Nat Genet (2008) 40:1113-8; PMID:18711368; http://dx.doi.org/10.1038/ng.204
- Chan PH, Role of oxidants in ischemic brain damage. Stroke (1996) 27:1124-1129; PMID:8650725; http://dx.doi.org/10.1161/01.STR.27.6.1124
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci (1999) 22:391-7; PMID:10441299; http://dx.doi.org/10.1016/S0166-2236(99)01401-0
- Marston AL, Tham WH, Shah H, Amon A. A genome-wide screen identifies genes required for centromeric cohesion. Science (2004) 303:1367-70; PMID:14752166; http://dx.doi.org/10.1126/science.1094220
- Ivanov P, Emara MM, Villen J, Gygi SP, Anderson P. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol Cell (2011) 43:613-23; PMID:21855800; http://dx.doi.org/10.1016/j.molcel.2011.06.022
- Ling J, Reynolds N, Ibba M. Aminoacyl-tRNA synthesis and translational quality control. Annu Rev Microbiol (2009) 63:61-78; PMID:19379069; http://dx.doi.org/10.1146/annurev.micro.091208.073210
- Pan T, Adaptive translation as a mechanism of stress response and adaptation. Annu Rev Genet (2013) 47:121-37; PMID:23988117; http://dx.doi.org/10.1146/annurev-genet-111212-133522
- Netzer N, Goodenbour JM, David A, Dittmar KA, Jones RB, Schneider JR, Boone D, Eves EM, Rosner MR, Gibbs JS. et al. Innate immune and chemically triggered oxidative stress modifies translational fidelity. Nature (2009) 462:522-6; PMID:19940929; http://dx.doi.org/10.1038/nature08576
- Wiltrout E, Goodenbour JM, Frechin M, Pan T. Misacylation of tRNA with methionine in Saccharomyces cerevisiae. Nucleic Acids Res (2012) 40:10494-506; PMID:22941646; http://dx.doi.org/10.1093/nar/gks805
- Ghavidel A, Kislinger T, Pogoutse O, Sopko R, Jurisica I, Emili A. Impaired tRNA nuclear export links DNA damage and cell-cycle checkpoint. Cell (2007) 131:915-26; PMID:18045534; http://dx.doi.org/10.1016/j.cell.2007.09.042
- Kadaba S, Wang X, Anderson JT. Nuclear RNA surveillance in Saccharomyces cerevisiae: Trf4p-dependent polyadenylation of nascent hypomethylated tRNA and an aberrant form of 5S rRNA. RNA (2006) 12:508-21; PMID:16431988; http://dx.doi.org/10.1261/rna.2305406
- Chernyakov I, Whipple JM, Kotelawala L, Grayhack EJ, Phizicky EM. Degradation of several hypomodified mature tRNA species in Saccharomyces cerevisiae is mediated by Met22 and the 5′-3′ exonucleases Rat1 and Xrn1. Genes Dev (2008) 22:1369-80; PMID:18443146; http://dx.doi.org/10.1101/gad.1654308
- Alexandrov A, Chernyakov I, Gu W, Hiley SL, Hughes TR, Grayhack EJ, Phizicky EM. Rapid tRNA decay can result from lack of nonessential modifications. Mol Cell (2006) 21:87-96; PMID:16387656; http://dx.doi.org/10.1016/j.molcel.2005.10.036
- Alexandrov A, Grayhack EJ, Phizicky EM. tRNA m7G methyltransferase Trm8p/Trm82p: evidence linking activity to a growth phenotype and implicating Trm82p in maintaining levels of active Trm8p. Rna (2005) 11:821-30; PMID:15811913; http://dx.doi.org/10.1261/rna.2030705
- Alexandrov A, Martzen MR, Phizicky EM. Two proteins that form a complex are required for 7-methylguanosine modification of yeast tRNA. RNA (2002) 8:1253-66; PMID:12403464; http://dx.doi.org/10.1017/S1355838202024019
- Thompson DM, Parker R. The RNase Rny1p cleaves tRNAs and promotes cell death during oxidative stress in Saccharomyces cerevisiae. J Cell Biol (2009) 185:43-50; PMID:19332891; http://dx.doi.org/10.1083/jcb.200811119
- Thompson DM, Lu C, Green PJ, Parker R. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA (2008) 14:2095-103; PMID:18719243; http://dx.doi.org/10.1261/rna.1232808
- Yamasaki S, Ivanov P, Hu GF, Anderson P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol (2009) 185:35-42; PMID:19332886; http://dx.doi.org/10.1083/jcb.200811106
- Fu H, Feng J, Liu Q, Sun F, Tie Y, Zhu J, Xing R, Sun Z, Zheng X. Stress induces tRNA cleavage by angiogenin in mammalian cells. FEBS Lett (2009) 583:437-442; PMID:19114040; http://dx.doi.org/10.1016/j.febslet.2008.12.043
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. PNAS (1998) 95:14863-8; PMID:9843981; http://dx.doi.org/10.1073/pnas.95.25.14863
- Sies H, Role of metabolic H2O2 generation: redox signaling and oxidative stress. J Biol Chem (2014) 289:8735-41; PMID:24515117; http://dx.doi.org/10.1074/jbc.R113.544635
- Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal (2012) 24:981-90; PMID:22286106; http://dx.doi.org/10.1016/j.cellsig.2012.01.008
- Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol (2000) 279, L1005-1028; PMID:11076791
- Spellman PT, Sherlock G, Zhang MQ, Iyer VR, Anders K, Eisen MB, Brown PO, Botstein D, Futcher B. Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol Biol Cell (1998) 9:3273-97; PMID:9843569; http://dx.doi.org/10.1091/mbc.9.12.3273
- Flanagan JM, Healey S, Young J, Whitehall V, Trott DA, Newbold RF, Chenevix-Trench G. Mapping of a candidate colorectal cancer tumor-suppressor gene to a 900-kilobase region on the short arm of chromosome 8. Genes Chromosomes Cancer (2004) 40:247-60; PMID:15139003; http://dx.doi.org/10.1002/gcc.20039
- Kalhor HR, Clarke S. Novel methyltransferase for modified uridine residues at the wobble position of tRNA. Mol Cell Biol (2003) 23:9283-92; PMID:14645538; http://dx.doi.org/10.1128/MCB.23.24.9283-9292.2003
- Maraia RJ, Blewett NH, Bayfield MA. It's a mod mod tRNA world. Nat Chem Biol (2008) 3:162-4; http://dx.doi.org/10.1038/nchembio0308-162
- Dlakic M, The ribosomal subunit assembly line. Genome Biol (2005) 6:234; PMID:16207363; http://dx.doi.org/10.1186/gb-2005-6-10-234
- Mauro VP, Edelman G.M. The ribosome filter redux. Cell Cycle (2007) 6:2246-51; PMID:17890902; http://dx.doi.org/10.4161/cc.6.18.4739
- Yao R, Zhang Z, An X, Bucci B, Perlstein DL, Stubbe J, Huang M. Subcellular localization of yeast ribonucleotide reductase regulated by the DNA replication and damage checkpoint pathways. Proc Natl Acad Sci U S A (2003) 100:6628-33; PMID:12732713; http://dx.doi.org/10.1073/pnas.1131932100
- Turner MK, Abrams R, Lieberman I. Levels of ribonucleotide reductase activity during the division cycle of the L cell, J Biol Chem (1968) 243:3725-8; PMID:5658547
- Nordenskjold BA, Skoog L, Brown NC, Reichard P. Deoxyribonucleotide pools and deoxyribonucleic acid synthesis in cultured mouse embryo cells. J Biol Chem (1970) 245:5360-8; PMID:4319240
- Elledge SJ, Zhou Z, Allen JB. Ribonucleotide reductase: regulation, regulation, regulation. Trends Biochem Sci (1992) 17:119-23; PMID:1412696; http://dx.doi.org/10.1016/0968-0004(92)90249-9
- Elledge SJ, Zhou Z, Allen JB, Navas TA. DNA damage and cell cycle regulation of ribonucleotide reductase. Bioessays (1993) 15:333-9; PMID:8343143; http://dx.doi.org/10.1002/bies.950150507
- Chabes A, Georgieva B, Domkin V, Zhao X, Rothstein R, Thelander L. Survival of DNA damage in yeast directly depends on increased dNTP levels allowed by relaxed feedback inhibition of ribonucleotide reductase. Cell (2003) 112:391-401; PMID:12581528; http://dx.doi.org/10.1016/S0092-8674(03)00075-8
- Shields DC, Sharp PM. Synonymous codon usage in Bacillus subtilis reflects both translational selection and mutational biases. Nucleic acids Res (1987) 15:8023-40; PMID:3118331; http://dx.doi.org/10.1093/nar/15.19.8023
- Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O'Shea EK, Weissman JS. Global analysis of protein expression in yeast. Nature (2003) 425:737-41; PMID:14562106; http://dx.doi.org/10.1038/nature02046
- Ikemura T. Codon usage and tRNA content in unicellular and multicellular organisms. Mol Biol Evol (1985) 2:13-4; PMID:3916708
- Plotkin JB, Kudla G. Synonymous but not the same: the causes and consequences of codon bias. Nat Rev Genet (2011) 12:32-42; PMID:21102527; http://dx.doi.org/10.1038/nrg2899
- Novoa EM, Pavon-Eternod, M., Pan, T, Ribas de Pouplana, L. A role for tRNA modifications in genome structure and codon usage. Cell (2012) 149:202-13; PMID:22464330; http://dx.doi.org/10.1016/j.cell.2012.01.050
- Percudani R, Pavesi A, Ottonello S. Transfer RNA gene redundancy and translational selection in Saccharomyces cerevisiae. J Mol Biol (1997) 268:322-30; PMID:9159473; http://dx.doi.org/10.1006/jmbi.1997.0942
- Tuller T, Carmi A, Vestsigian K, Navon S, Dorfan Y, Zaborske J, Pan T, Dahan O, Furman I, Pilpel Y. An evolutionarily conserved mechanism for controlling the efficiency of protein translation. Cell (2010) 141:344-54; PMID:20403328; http://dx.doi.org/10.1016/j.cell.2010.03.031
- Yarian C, Townsend H, Czestkowski W, Sochacka E, Malkiewicz AJ, Guenther R, Miskiewicz A, Agris PF. Accurate translation of the genetic code depends on tRNA modified nucleosides. J Biol Chem (2002) 277:16391-5; PMID:11861649; http://dx.doi.org/10.1074/jbc.M200253200