Abstract
Alternative Lengthening of Telomeres (ALT) mechanisms allow telomerase-negative immortal cells to buffer replicative telomere shortening. ALT is naturally active in a number of human cancers and might be selected upon telomerase inactivation. ALT is thought to operate through homologous recombination (HR) occurring between telomeric repeats from independent chromosome ends. Indeed, suppression of a number of HR factors impairs ALT cell proliferation. Yet, how HR is initiated at ALT telomeres remains elusive. Mounting evidence suggests that the long noncoding telomeric RNA TERRA renders ALT telomeres recombinogenic by forming RNA:DNA hybrids with the telomeric C-rich strand. TERRA and telomeric hybrids act in concert with a number of other factors, including the RNA endoribonuclease RNaseH1 and the single stranded DNA binding protein RPA. The functional interaction network built upon these different players seems indispensable for ALT telomere maintenance, and digging into the molecular details of this previously unappreciated network might open the way to novel avenues for cancer treatments.
Abbreviations
| ALT | = | alternative lengthening of telomeres |
| APBs | = | ALT-associated PML bodies |
| ATRX | = | α thalassemia/mental retardation syndrome X-linked |
| FEN1 | = | flap endonuclease 1 |
| HR | = | homologous recombination |
| MMS4 | = | methyl methane sulfonate sensitivity |
| MRN | = | MRE11, RAD50 and NBS1 |
| PML | = | promyelocytic leukemia |
| RPA | = | replication protein A |
| TERRA | = | telomeric repeat-containing RNA |
| T-SCE | = | telomeric sister chromatid exchange |
| XRCC3 | = | X-ray repair complementing defective repair in chinese hamster cells 3 |
De novo telomere synthesis is a pre-requisite for cell population immortality. Telomerase activity is the most common tool utilized by cells to replenish telomeric sequences, which are naturally lost during successive replication cycles. However, in a more limited number of cases, telomere maintenance is achieved through mechanisms collectively known as ALT.Citation1-3 ALT has been documented in several species including humans, mice, chickens, C. elegans, and yeasts.Citation4-8 Approximately 15% of human cancers such as sarcomas, gastric carcinomas, central nervous system malignancies, and bladder carcinomas have activated ALT.Citation9 ALT tumors will be resilient to anti-telomerase therapy and treatment of telomerase-positive tumors with anti-telomerase drugs could eventually select for drug-resistant ALT cells. Indeed genetic or chemical inhibition of telomerase led to insurgence of ALT in cultured human cancer cells and mice.Citation10,11 Hence it is necessary to gain a thorough understanding of the molecular basis of the ALT pathway and to develop targeted anti-ALT therapies to be used alone or together with telomerase inhibitors.
Telomeres in ALT cells retain numerous canonical features including the presence of a duplex (TTAGGG)n sequence bound by the specialized shelterin complex. Yet, ALT telomeres can be recognized based on unique markers. These distinct traits include an extremely heterogeneous telomere length, the association of multiple telomeres in nuclear bodies containing promyelocytic leukemia (PML) to form the so-called ALT-associated PML bodies (APBs), elevated rates of exchange of telomeric sequences between newly replicated sister telomeres (telomeric sister chromatid exchange; T-SCE), abundant extrachromosomal telomeric DNA in the form of double-stranded (ds) telomeric circles (t-circles), partly single-stranded (ss) circles (C-circles and G-circles) and linear double-stranded DNA.Citation1-3 Moreover, ALT cell lines are often characterized by the loss of expression of ATRX, which is an ATP-dependent helicase involved in chromatin remodeling, an unstable karyotype and impaired DNA damage detection and repair.Citation12
It is commonly accepted that in the large majority of ALT tumors telomere maintenance occurs via homologous recombination (HR) between telomeric sequences.Citation1-3 In fact, several HR proteins have been found to localize to APBs and their functional inactivation leads to loss of telomeric sequences and eventually cell growth arrest or death.Citation1-3 Among these factors are the MRN complex components MRE11, RAD50 and NBS1,Citation13,14 which are essential for the early steps of HR, the 2 interacting proteins MUS81 and MMS4,Citation15 and the 2 RAD51 paralogs XRCC3 and RAD51D, which promote resolution of Holliday junctions in HR intermediates.Citation16,17 Different HR-based models for telomere maintenance have been suggested. Unequal T-SCEs could sustain elongation of one sister telomere at the expense of shortening of the other one, a model supported by the elevated rates of T-SCEs in ALT cells. Telomere synthesis could also involve break-induced replication (BIR), an HR-based repair mechanism that uses a homologous donor template to synthesize up to several kilobases of new DNA starting from a break site. Finally, ALT telomeres could engage in HR with extra-chromosomal telomeric DNA.Citation1-3 Several factors either not directly involved in HR or having multiple functions are also essential for ALT telomere maintenance, for example the flap endonuclease FEN1Citation18 and the single ss DNA binding protein RPA.Citation19
The long noncoding RNA ‘telomeric repeat-containing RNA’ (TERRA), a DNA-dependent RNA polymerase II transcript produced from chromosome ends,Citation20,21 is emerging as a novel feature associated to ALT. Already in the very first reports on TERRA discovery it was shown that osteosarcoma-derived U2OS cells, ALT cells commonly used in laboratories, contained elevated levels of TERRA as compared to telomerase-positive cancer cells or primary human fibroblasts.Citation22,23 Successive work employing additional ALT cells has indeed confirmed that TERRA is generally up-regulated in ALT.Citation12,24,25 Moreover, TERRA largely localizes to APBs.Citation25 Thus TERRA levels and localization can now be used as additional markers for ALT tumor screening. TERRA increase in ALT is mainly due to augmented telomere transcription, as TERRA half-life in ALT cells appears not to be markedly different than in telomerase-positive cells.Citation22,24 Consistently, in ALT cells, subtelomeric TERRA CpG island promoters are hypomethylated and more avidly bound by RNAPII while the density of the transcriptionally repressive histone H3 trimethylated at lysine 9 (H3K9me3) mark is decreased.Citation24-26 Moreover, contrary to what is observed in telomerase positive cancer cells, TERRA levels do not decline during progression from S-phase to G2/M, possibly due to a lack of ATRX activity.Citation27,28 Thus increased transcription and loss of its cell cycle regulation account for the overall increase of TERRA cellular levels characteristic of ALT lines.
Having established that elevated TERRA levels are a hallmark of ALT, a major question arises: is TERRA increase necessary for ALT telomeres or is it a mere consequence of reduced telomeric chromatin compaction and therefore functionally irrelevant? Growing evidence supports a scenario where TERRA promotes telomere length maintenance in ALT cells by converting telomeres into efficient substrates for HR. Several groups have shown that TERRA from different organisms can form recombinogenic RNA:DNA hybrid structures by base pairing with the C-rich strand of telomeres.Citation25,29–31 Although it has been previously proposed that TERRA retention at telomeres is mediated by physical interactions with the telomeric factors TRF1 and TRF2,Citation32 formation of RNA:DNA hybrids could additionally participate in this process () How telomeric hybrids are formed needs to be carefully tested. In vitro transcription of telomeric DNA repeats leads to generation of abundant RNA:DNA hybrids, in particular when the telomeric tract is transcribed in the direction producing TERRA-like molecules.Citation25 Given the constant proximity of TERRA to its template DNA, telomeric hybrids could also originate through invasion of TERRA into ds telomeric DNA or when ss C-rich DNA is exposed for example during telomere replication. Telomeric hybrids might therefore form both co- and post-transcriptionally and investigating this aspect should help understand not only the biology of ALT but also how TERRA is regulated in different cellular settings.
Figure 1. Hypothetical model for TERRA-mediated telomere elongation in ALT cells. TERRA associates with ALT telomeres possibly through 2 independent mechanisms: formation of co-transcriptional and perhaps post-transcriptional RNA:DNA hybrids with the C-rich telomeric strand and physical interaction with TRF1 (in blue) and TRF2 (in green) within the shelterin multiprotein complex. Stripping off TERRA from telomeric hybrids would generate ss C-rich DNA that could be immediately bound by RPA. Although not indicated, RPA can also associate with G-rich ss DNA. In addition, TERRA molecules, which are elevated in ALT cells, would further prevent RPA dissociation from telomeres in G2/M. A switch between RPA and RAD51 could mediate homology searches and annealing between C-rich ss DNA previously engaged in a hybrid and the G-rich ss overhangs from heterologous telomeres. The C-rich strand of telomeric hybrid-containing telomeres could therefore serve as a template for de novo synthesis of telomeric repeats, which are directly added to the overhangs of the invading telomeres.
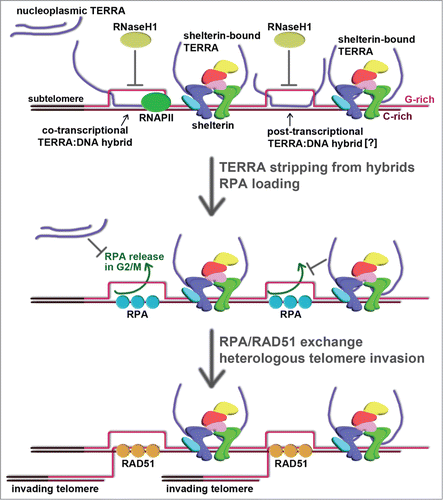
Regardless of the way telomeric hybrids originate, they are controlled in unique ways in ALT cells. The endoribonuclease RNaseH1, which specifically degrades the RNA moiety within a hybrid, preferentially accumulates at ALT telomeres over other repetitive genomic sequences and telomeres of telomerase-positive cancer cells.Citation25 Moreover, perturbing RNaseH1 levels in ALT cells proportionally alters the levels of hybrids at telomeres, while the ones formed at the highly transcribed Actin gene remain unaffected.Citation25 Depletion of RNaseH1 in U2OS cells induces rapid telomere loss due to exaggerated telomeric circle excision, which is accompanied by exposure of ss C-rich DNA and accumulation of RPA32 phosphorylated at Serine 33 (pSer33) at telomeres. RPA is most likely bound to C-rich ss telomeric DNA as the levels of ss G-rich DNA are not increased upon RNaseH1 depletion.Citation25 On the other hand, over-expression of RNaseH1 limits the potential of telomeres to be maintained as they become shorter upon prolonged culturing of cells. Again, telomeric defects are not evident upon manipulation of RNaseH1 levels in telomerase-positive cancer cells.Citation25
A connection between RPA and telomere maintenance in ALT has also been underscored in independent studies. As mentioned above, TERRA is not down-regulated in ALT cells during S to G2/M phase progression and, similarly, its gradual decline in S to G2/M is prevented in telomerase-positive cells depleted for ATRX.Citation27,28 These data suggest that the lack of ATRX in ALT cells might be responsible for the loss of regulation of TERRA levels during the cell cycle and re-expressing functional ATRX in ALT cells should uncover whether this hypothesis is correct. Moreover, while RPA is normally released from telomeres in G2/M, depletion of ATRX in telomerase-positive cells averts this event and, consistently, RPA foci can readily be detected in ALT cells during G2/M.27 In vitro data have shown that RPA binding to telomeres is facilitated by TERRACitation33 suggesting that, in telomerase-positive but not in ALT cells, TERRA down-regulation in G2/M could promote displacement of RPA.Citation27,33
In a general model integrating the ensemble of the above-described data, in ALT cells, TERRA would favor stable association of RPA with telomeres through at least 2 independent mechanisms () On one side, telomeric hybrids, once stripped off of their RNA component, possibly in a cell cycle dependent manner, would expose ss C-rich DNA as a binding substrate for RPA. On the other hand, elevated TERRA levels would prevent RPA dissociation from telomeres during G2/M () RPA constantly present at telomeres is the probable trigger of HR between telomeric sequences, likely through recruitment of and exchange with RAD51 molecules () an obligatory step for homology searches occurring during HR.Citation34 Consistently, RAD51 has recently been shown to be necessary for long range telomere movement and clustering of independent chromosome ends in ALT cells.Citation35 RAD51-coated ss C-rich telomeric DNA would constitute a recombinogenic nucleoprotein complex promoting base pairing with the G-rich overhangs of independent telomeres, an event that could be followed by polymerase-mediated elongation of the invading G-overhangs in a process resembling BIR ().
A straightforward assessment of this model requires depleting TERRA in cells, a goal that has not been reached yet because robust and reproducible protocols for TERRA downregulation are still missing. Development of such protocols will once and for all establish whether and to what extent TERRA promotes telomere maintenance in ALT cells. Moreover they will also clarify whether TERRA can be envisaged as a druggable target for curing ALT. Data from budding yeast suggest that TERRA might assist telomere lengthening in telomerase-positive cells by recruiting telomerase to the shortest telomeres in cells.Citation36 In this light, depleting TERRA levels in cancers could simultaneously suppress telomerase-mediated and ALT-mediated telomere elongation and therefore progression of different cancer types.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank members of our laboratory for discussions.
Funding
The Azzalin laboratory is funded by the European Research Council, the Swiss National Science Foundation, the Promedica Stiftung and ETH Zürich.
References
- Cesare AJ, Reddel RR. Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet 2010; 11:319-30; PMID:20351727; http://dx.doi.org/10.1038/nrg2763
- Conomos D, Pickett HA, Reddel RR. Alternative lengthening of telomeres: remodeling the telomere architecture. Front Oncol 2013; 3:27; PMID:23429284; http://dx.doi.org/10.3389/fonc.2013.00027
- Draskovic I, Londono-Vallejo A. Telomere recombination and alternative telomere lengthening mechanisms. Front Biosci 2013; 18:1-20; PMID:23276906; http://dx.doi.org/10.2741/4084
- Bryan TM, Englezou A, Dalla-Pozza L, Dunham MA, Reddel RR. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat Med 1997; 3:1271-4; PMID:9359704; http://dx.doi.org/10.1038/nm1197-1271
- Lackner DH, Raices M, Maruyama H, Haggblom C, Karlseder J. Organismal propagation in the absence of a functional telomerase pathway in Caenorhabditis elegans. EMBO J 2012; 31:2024-33; PMID:22425786; http://dx.doi.org/10.1038/emboj.2012.61
- Hande MP, Samper E, Lansdorp P, Blasco MA. Telomere length dynamics and chromosomal instability in cells derived from telomerase null mice. J Cell Biol 1999; 144:589-601; PMID:10037783; http://dx.doi.org/10.1083/jcb.144.4.589
- Le S, Moore JK, Haber JE, Greider CW. RAD50 and RAD51 define two pathways that collaborate to maintain telomeres in the absence of telomerase. Genetics 1999; 152:143-52; PMID:10224249
- O'Hare TH, Delany ME. Molecular and cellular evidence for the alternative lengthening of telomeres (ALT) mechanism in chicken. Cytogenet Genome Res 2011; 135:65-78; PMID:21822009; http://dx.doi.org/10.1159/000330125
- Henson JD, Reddel RR. Assaying and investigating Alternative Lengthening of Telomeres activity in human cells and cancers. FEBS Lett 2010; 584:3800-11; PMID:20542034; http://dx.doi.org/10.1016/j.febslet.2010.06.009
- Hu J, Hwang SS, Liesa M, Gan B, Sahin E, Jaskelioff M, Ding Z, Ying H, Boutin AT, Zhang H, et al. Antitelomerase therapy provokes ALT and mitochondrial adaptive mechanisms in cancer. Cell 2012; 148:651-63; PMID:22341440; http://dx.doi.org/10.1016/j.cell.2011.12.028
- Chen W, Xiao BK, Liu JP, Chen SM, Tao ZZ. Alternative lengthening of telomeres in hTERT-inhibited laryngeal cancer cells. Cancer Sci 2010; 101:1769-76; PMID:20545697; http://dx.doi.org/10.1111/j.1349-7006.2010.01611.x
- Lovejoy CA, Li W, Reisenweber S, Thongthip S, Bruno J, de Lange T, De S, Petrini JH, Sung PA, Jasin M, et al. Loss of ATRX, genome instability, and an altered DNA damage response are hallmarks of the alternative lengthening of telomeres pathway. PLoS Genet 2012; 8:e1002772; PMID:22829774; http://dx.doi.org/10.1371/journal.pgen.1002772
- Jiang WQ, Zhong ZH, Henson JD, Neumann AA, Chang AC, Reddel RR. Suppression of alternative lengthening of telomeres by Sp100-mediated sequestration of the MRE11/RAD50/NBS1 complex. Mol Cell Biol 2005; 25:2708-21; PMID:15767676; http://dx.doi.org/10.1128/MCB.25.7.2708-2721.2005
- Zhong ZH, Jiang WQ, Cesare AJ, Neumann AA, Wadhwa R, Reddel RR. Disruption of telomere maintenance by depletion of the MRE11/RAD50/NBS1 complex in cells that use alternative lengthening of telomeres. J Biol Chem 2007; 282:29314-22; PMID:17693401; http://dx.doi.org/10.1074/jbc.M701413200
- Zeng S, Xiang T, Pandita TK, Gonzalez-Suarez I, Gonzalo S, Harris CC, Yang Q. Telomere recombination requires the MUS81 endonuclease. Nat Cell Biol 2009; 11:616-23; PMID:19363487; http://dx.doi.org/10.1038/ncb1867
- Tarsounas M, Muñoz P, Claas A, Smiraldo PG, Pittman DL, Blasco MA, West SC. Telomere maintenance requires the RAD51D recombination/repair protein. Cell 2004; 117:337-47; PMID:15109494; http://dx.doi.org/10.1016/S0092-8674(04)00337-X
- Compton SA, Choi JH, Cesare AJ, Ozgur S, Griffith JD. Xrcc3 and Nbs1 are required for the production of extrachromosomal telomeric circles in human alternative lengthening of telomere cells. Cancer Res 2007; 67:1513-9; PMID:17308089; http://dx.doi.org/10.1158/0008-5472.CAN-06-3672
- Saharia A, Stewart SA. FEN1 contributes to telomere stability in ALT-positive tumor cells. Oncogene 2008; 28:1162-7; PMID:19137021; http://dx.doi.org/10.1038/onc.2008.458
- Grudic A, Jul-Larsen A, Haring SJ, Wold MS, Lønning PE, Bjerkvig R, Bøe SO. Replication protein A prevents accumulation of single-stranded telomeric DNA in cells that use alternative lengthening of telomeres. Nucleic Acids Res 2007; 35:7267-78; PMID:17959650; http://dx.doi.org/10.1093/nar/gkm738
- Arora R, Brun CM, Azzalin CM. TERRA: long noncoding RNA at eukaryotic telomeres. Prog Mol Subcell Biol 2011; 51:65-94; PMID:21287134; http://dx.doi.org/10.1007/978-3-642-16502-3_4
- Azzalin CM, Lingner J. Telomere functions grounding on TERRA firma. Trends Cell Biol 2015; 25:29-36; PMID:25257515; http://dx.doi.org/10.1016/j.tcb.2014.08.007
- Azzalin CM, Reichenbach P, Khoriauli L, Giulotto E, Lingner J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 2007; 318:798-801; PMID:17916692; http://dx.doi.org/10.1126/science.1147182
- Schoeftner S, Blasco MA. Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II. Nat Cell Biol 2008; 10:228-36; PMID:18157120; http://dx.doi.org/10.1038/ncb1685
- Episkopou H, Draskovic I, Van Beneden A, Tilman G, Mattiussi M, Gobin M, Arnoult N, Londoño-Vallejo A, Decottignies A. Alternative Lengthening of Telomeres is characterized by reduced compaction of telomeric chromatin. Nucleic Acids Res 2014; 42:4391-05; PMID:24500201; http://dx.doi.org/10.1093/nar/gku114
- Arora R, Lee Y, Wischnewski H, Brun CM, Schwarz T, Azzalin CM. RNaseH1 regulates TERRA-telomeric DNA hybrids and telomere maintenance in ALT tumour cells. Nat Commun 2014; 5:5220; PMID:25330849; http://dx.doi.org/10.1038/ncomms6220
- Nergadze SG, Farnung BO, Wischnewski H, Khoriauli L, Vitelli V, Chawla R, Giulotto E, Azzalin CM. CpG-island promoters drive transcription of human telomeres. RNA 2009; 15:2186-94; PMID:19850908; http://dx.doi.org/10.1261/rna.1748309
- Flynn RL, Cox KE, Jeitany M, Wakimoto H, Bryll AR, Ganem NJ, Bersani F, Pineda JR, Suvà ML, Benes CH, et al. Alternative lengthening of telomeres renders cancer cells hypersensitive to ATR inhibitors. Science 2015; 347:273-7; PMID:25593184; http://dx.doi.org/10.1126/science.1257216
- Porro A, Feuerhahn S, Reichenbach P, Lingner J. Molecular dissection of telomeric repeat-containing RNA biogenesis unveils the presence of distinct and multiple regulatory pathways. Mol Cell Biol 2010; 30:4808-17; PMID:20713443; http://dx.doi.org/10.1128/MCB.00460-10
- Balk B, Maicher A, Dees M, Klermund J, Luke-Glaser S, Bender K, Luke B. Telomeric RNA-DNA hybrids affect telomere-length dynamics and senescence. Nat Struct Mol Biol 2013; 20:1199-205; PMID:24013207; http://dx.doi.org/10.1038/nsmb.2662
- Pfeiffer V, Crittin J, Grolimund L, Lingner J. The THO complex component Thp2 counteracts telomeric R-loops and telomere shortening. EMBO J 2013; 32:2861-71; PMID:24084588; http://dx.doi.org/10.1038/emboj.2013.217
- Yu TY, Kao YW, Lin JJ. Telomeric transcripts stimulate telomere recombination to suppress senescence in cells lacking telomerase. Proc Natl Acad Sci U S A 2014; 111:3377-82; PMID:24550456; http://dx.doi.org/10.1073/pnas.1307415111
- Deng Z, Norseen J, Wiedmer A, Riethman H, Lieberman PM. TERRA RNA binding to TRF2 facilitates heterochromatin formation and ORC recruitment at telomeres. Mol Cell 2009; 35:403-13; PMID:19716786; http://dx.doi.org/10.1016/j.molcel.2009.06.025
- Flynn RL, Centore RC, O'Sullivan RJ, Rai R, Tse A, Songyang Z, Chang S, Karlseder J, Zou L. TERRA and hnRNPA1 orchestrate an RPA-to-POT1 switch on telomeric single-stranded DNA. Nature 2011; 471:532-6; PMID:21399625; http://dx.doi.org/10.1038/nature09772
- Renkawitz J, Lademann CA, Kalocsay M, Jentsch S. Monitoring homology search during DNA double-strand break repair in vivo. Mol Cell 2013; 50:261-72; PMID:23523370; http://dx.doi.org/10.1016/j.molcel.2013.02.020
- Cho NW, Dilley RL, Lampson MA, Greenberg RA. Interchromosomal homology searches drive directional ALT telomere movement and synapsis. Cell 2014; 159:108-21; PMID:25259924; http://dx.doi.org/10.1016/j.cell.2014.08.030
- Cusanelli E, Romero CA, Chartrand P. Telomeric noncoding RNA TERRA is induced by telomere shortening to nucleate telomerase molecules at short telomeres. Mol Cell 2013; 51:780-91; PMID:24074956; http://dx.doi.org/10.1016/j.molcel.2013.08.029
