Abstract
Bystander effects can be induced through cellular communication between irradiated cells and non-irradiated cells. The signals that mediate this cellular communication, such as cytokines, reactive oxygen species, nitric oxide and even microRNAs, can be transferred between cells via gap junctions or extracellular medium. We have previously reported that miR-21, a well described DDR (DNA damage response) microRNA, is involved in radiation-induced bystander effects through a medium-mediated way. However, the mechanisms of the microRNA transfer have not been elucidated in details. In the present study, it was found that exosomes isolated from irradiated conditioned medium could induce bystander effects. Furthermore, we demonstrated plenty of evidences that miR-21, which is up-regulated as a result of mimic transfection or irradiation, can be transferred from donor or irradiated cells into extracellular medium and subsequently get access to the recipient or bystander cells through exosomes to induce bystander effects. Inhibiting the miR-21 expression in advance can offset the bystander effects to some extent. From all of these results, it can be concluded that the exosome-mediated microRNA transfer plays an important role in the radiation-induced bystander effects. These findings provide new insights into the functions of microRNAs and the cellular communication between the directly irradiated cells and the non-irradiated cells.
Introduction
Radiation-induced bystander effects (RIBE) are well-defined phenomena, in which DNA damage responses can be induced in the non-irradiated cells either by gap junctional intercellular communication or various soluble extracellular factors released from irradiated cells.Citation1-8 Soluble signaling factors such as reactive oxygen species (ROS), secondary messengers like nitric oxide (NO), and cytokines like TGF-β and TNF-α, are relevant to the medium-mediated RIBE.Citation9-15 Recently, studies indicated that microRNAs (miRNAs) might play important roles in radiation responses and intercellular signaling pathway between irradiated cells and bystander cells.Citation16-19 In addition, our previous study shown that miR-21 is involved in radiation-induced bystander effects through a medium-mediated way.Citation20 However, more details of the miR-21 mediated RIBE need to be elucidated, especially the mechanisms of miRNA shuttle from irradiated cells to bystander cells.
In the last 2 decades, a new mechanism for intercellular communication has emerged which involves intercellular transfer of exosomes. Exosomes are small (40–100 nm in diameter) endocytic-original membrane vesicles that are released into the extracellular environment.Citation21 It was first demonstrated by tracking the fate of recycling transferrin receptor during the maturation of sheep reticulocytes.Citation22 Exosomes are formed intracellularly via endocytic invagination and are generated by the outward budding at the limiting endosomal membrane of the multivesicular bodies (MVBs), sharing the biochemical characteristics with the internal vesicles of MVBs.Citation23 Exosomes are released into the extracellular environment from many kinds of cells, such as, but not limited to, tumor cells, dendritic cells, lymphoid cells, epithelial cells, and cells from different tissues or organs.Citation24 Thus, cells may communicate through membrane transfer by the secretion of exosomes. In addition, exosomes have been detected in various body fluids such as urine, serum, saliva and breast milk, and function in intercellular communication, immune system modulation and tumor progression.Citation25-27 In most recent years, the relevance of exosomes and miRNAs in many fields has been recognized. For instance, Ratajczak et al. detected mRNA and miRNAs in extracellular vesicles in 2006. Valadi et al. demonstrated a exosome-mediated transfer of mRNA and miRNA between cells in 2007. In 2010, let-7 miRNA family was found to be selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line, implying the involvement of exosomes in miRNA-regulated tumorigenesis.
In this study, we identified that miRNA participated in radiation-induced bystander effects through exosomes. To test this, we isolated the exosome fraction in irradiated conditioned medium and tested the ability of miRNA-containing exosomes to induce bystander effect in non-irradiated cells. Our results show that miRNAs gain new properties to shuttle between irradiated cells and non-irradiated bystander cells through exosomes and miRNA-containing exosomes are able to induce the RIBE. Interpretation of such an exosome-mediated microRNA transfer will help us to understand the RIBE both in vitro and in vivo.
Results
Exosomes from irradiated cells induce bystander effects
We have previously reported that miR-21 was involved in the radiation-induced bystander effects through a medium-mediated way.Citation20 Recently, significant amounts of miRNAs were found in extracellular human body fluids including plasma/serum, saliva and urine.Citation28-32 Cultured mammalian cells can also export miRNAs into the extracellular environment.Citation32-34 These miRNAs are packaged into extracellular vesicles such as exosomes and microvesicles, and gain new properties such as resistant to RNase digestion, prolonged room temperature and multiple freeze-thawing.Citation35,36 Discoveries of the new miRNA properties inspire us to comprehend the miRNA mediated bystander effects.
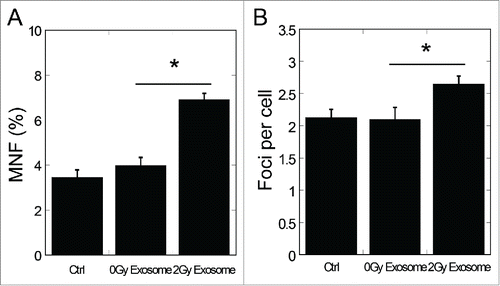
To investigate whether miR-21 participated in the RIBE through exosomes, we first detected the damage of non-irradiated cells treated with exosomes isolated from conditioned medium of irradiated cells. As shown in , the frequency of micronuclei in bystander cells was increased significantly by exosomes isolated from 2 Gy conditioned medium (P < 0.05), and the number of 53BP1 foci in bystander cells increased significantly (P < 0.05) (), too. These data suggest that exosomes from irradiated conditioned medium can induce bystander effects, which are consistent with earlier studies.Citation37,38
Figure 1. Bystander effects induced by exosomes isolated from conditioned medium of irradiated cells. (A) Frequency of micronuclei (MNF) in bystander cells treated for 48 h with exosomes isolated from conditioned medium harvested from directly irradiated cells. Cells were exposed to 0 Gy and 2 Gy of X-rays. Conditioned medium was harvested 4 h after exposure. (B) Yields of 53BP1 foci in bystander cells treated for 2 h with exosomes isolated from conditioned medium harvested from irradiated cells 4 h after exposure to 0 Gy and 2 Gy of X-rays. Error bars represent means ± standard error of 3 biological replicates and the superscript (*) denote a significant difference between groups (P < 0.05) as determined by Student's t test.
MicroRNAs shuttle from cells to cells by exosomes
To find out the specific role of miRNA-containing exosomes in the RIBE, we detected the shuttle of miRNA-containing exosomes between donor or irradiated cells and the recipient or bystander cells.
We first transfected MRC5 cells with Cel-miR-39, a miRNA from Caenorhabditis elegans which has no existence in human cells, and then refreshed the medium of transfection. Five hours later, the exosomes existing in the extracellular medium at different time points were isolated. Using qRT-PCR methods, we detected the expression of Cel-miR-39 in the isolated exosomes. The levels of Cel-miR-39 were represented by Ct values (lower Ct value, higher Cel-miR-39 expression). It was observed that the Ct values of Cel-miR-39 in exosomes from the refreshed medium isolated at 2,4 and 12 h were significantly lower than that at 0 h (P < 0.01), which means high expression of Cel-miR-39 in these exosomes (). After treatment of MRC5 cells with exosomes isolated from the refreshed medium and equal volumes of PBS, the expression of Cel-miR-39 in recipient MRC5 cells was detected. As can be seen in , the Ct value of exosome treated cells was significantly lower than that of PBS treated (P < 0.01), suggesting that Cel-miR-39 was receipted by MRC5 cells in the form of exosomes secreted from Cel-miR-39 transfected cells.
Figure 2. Exosome-mediated miRNA shuttle between cells. (A) Cel-miR-39 expression of exosomes isolated from the refreshed medium of Cel-miR-39 transfected cells at different time points. (B) The expression of Cel-miR-39 in bystander cells treated with PBS or exosomes from Cel-miR-39 transfected cells. (C) Fluorescent visuals of MRC5 cells transfected with NC (negative control) or Cy3-miR-21. (D) Fluorescent visuals of MRC5 cells that were co-cultured with conditioned medium harvested from NC or Cy3-miR-21 transfected cells or exosomes isolated from conditioned medium of Cy3-miR-21 transfected cells. Nuclei were stained blue (DAPI) while F actin was stained green by fluorescein isothiocyanate labeled phalloidin (Phall) and Cy3-miR-21 displayed red fluorescence (Cy3). Arrows indicated the Cy3-miR-21. Error bars represent means ± standard error of 3 biological replicates and the superscript (**) denote a highly significant difference between groups (P < 0.01) as determined by Student's t test.
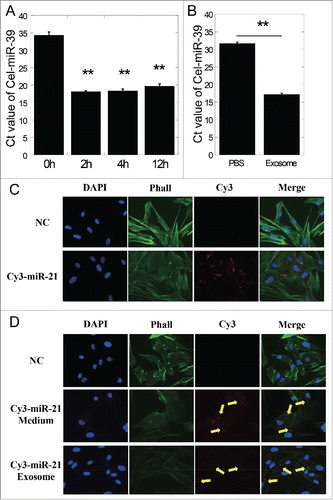
For visualized validation of exosome-mediated miRNA transfer between cells, we transiently transfected a fluorescent labeled miR-21 mimics (Cy3-miR-21) into MRC5 cells and then refreshed the medium. As shown by the apparent red fluorescence in , Cy3-miR-21 was successfully transfected into the cytoplasm. Then we harvested the conditioned medium from Cy3-miR-21 transfected cells, or further isolated the exosomes in the conditioned medium, and added them into untransfected MRC5 cells. Unambiguous red fluorescent points standing for Cy3-miR-21 could be seen in both conditioned medium and exosomes treated cells (), which indicated that Cy3-miR-21 had been successfully shuttled into the recipient cells through exosomes. These data suggest that miRNAs (including has-miR-21 and Cel-miR-39) can be packaged into exosomes and secreted into extracellular medium, and subsequently got accesses into recipient cells.
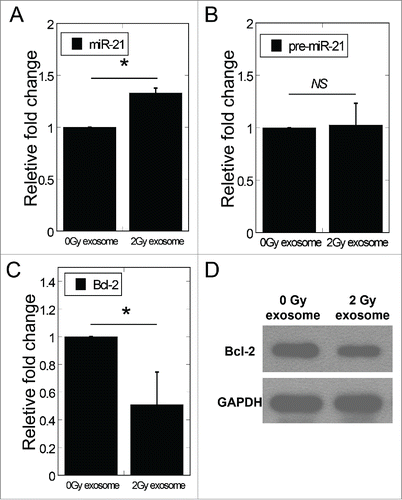
Our previous study demonstrated that miR-21 was upregulated in conditioned medium harvested from irradiated cells, and bystander cells treated with 2 Gy conditioned medium showed relatively increased miR-21 expression. Thus, we further checked the miR-21 expression in bystander cells treated with exosomes isolated from 0 Gy and 2 Gy conditioned medium. Compared to cells treated with exosomes from 0 Gy conditioned medium, cells treated with exosomes from 2 Gy conditioned medium showed relatively high expression of miR-21 and reduced expression of Bcl-2 (a approved target gene of miR-21) in bystander cells (). Consistent with these results, the protein level of Bcl-2 in bystander cells was also remarkably suppressed by exosomes from 2 Gy conditioned medium (). To identify the origin of the increased miR-21 expression in bystander cells, we further measured the expression of miR-21 precursor (pre-miR-21) in bystander cells and found no significant change, implying that the increased mature miR-21 levels in bystander cells were resulted from exosomes isolated from conditioned medium ().
Figure 3. Exosomes mediated the change of miR-21 expression levels in bystander cells. (A) MiR-21 expression in bystander MRC-5 cells after co-cultured with exosomes isolated from 0 and 2 Gy conditioned medium. (B) The expression of pre-miR-21 in bystander cells treated with exosomes isolated from 0 Gy or 2 Gy conditioned medium. (C) The mRNA expression of Bcl−2 in bystander cells 24 h after co-cultured with exosomes isolated from 0 Gy or 2 Gy conditioned medium. (D) Bcl-2 protein expression by Western blotting assay in bystander cells 24 h after co-cultured with exosomes isolated from 0 Gy or 2 Gy conditioned medium. β-actin, loading control. Error bars represent means ± standard error of 3 biological replicates and the superscript (*) denote a significant difference between groups (P < 0.05) as determined by Student's t test.
Taken these results together, it is concluded that miRNA can shuttle from cells to cells through exosomes and induce bystander effects.
Exosomal miR-21 transfer induces bystander-like effects
To investigate the role of exosomal miR-21 in the RIBE, we isolated the medium exosomes from MRC5 cells transfected with miR-21 mimics. Using qRT-PCR method, we found that the miR-21 expression in exosomes isolated from the conditioned medium of miR-21 mimic transfected cells was significantly higher than that of NC (negative control) transfected cells (43.2 fold, P < 0.05) (). Such exosomes with distinctly high levels of miR-21 mimics were referred as exosomal miR-21. After co-cultured with the exosomal miR-21, the expression levels of miR-21 in recipient cells increased, as shown in . In the meantime, the expression of pre-miR-21 showed no significant change in recipient cells, suggesting that increased mature miR-21 expression in recipient cells was due to the exosomal miR-21 taken up by recipient cells (). It was also found that the exosomal miR-21 led to significantly reduced mRNA level of Bcl-2 (P < 0.05, ). Consistently, western blotting results indicated that the protein level of Bcl-2 was also remarkably suppressed 24 h after exosomal miR-21 treatment ().
Figure 4. Exosomal miR-21 transfer induced bystander-like effects. (A) MiR-21 expression of exosomes isolated from medium of NC or miR-21 mimic transfected cells. (B) The expression of miR-21 in recipient cells treated with exosomes isolated from medium of NC or miR-21 mimic transfected cells. (C) The expression of pre-miR-21 in recipient cells treated with exosomes isolated from medium of NC or miR-21 mimic transfected cells. (D) The mRNA expression of Bcl−2 in bystander cells 24 h after co-cultured with NC or miR-21 mimic exosomes. (E) Bcl-2 protein expression by protein gel blotting assay in recipient cells 24 h after co-cultured with NC or miR-21 mimic exosomes. (F) Frequency of micronuclei (MNF) in recipient cells treated for 48 h with NC or miR-21 mimic exosomes. (G) Yields of 53BP1 foci in recipient cells treated for 2 h with NC or miR-21 mimic exosomes. Error bars represent means ± standard error of 3 biological replicates and the superscript (*) denote a significant difference between groups (P < 0.05) as determined by Student's t test.
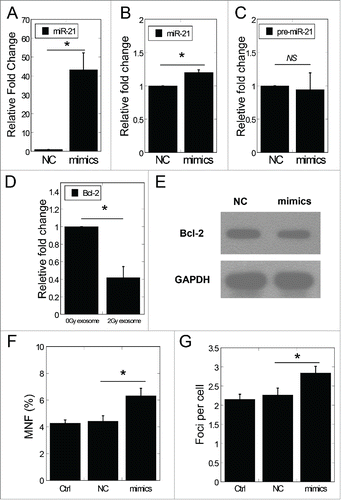
We further inspected the frequency of micronuclei and the 53BP1 foci in MRC-5 cells treated with exosomal miR-21. Remarkable increase of the frequency of micronuclei was found (P < 0.05) after exosomal miR-21 treatment (). Likewise, the number of 53BP1 foci increased after exosomal miR-21 treatment (). These increases are consistent with the results of the RIBE observed by conditioned medium transfer.
The above data indicate that the over-expressed miR-21 in donor cells can be secreted out into medium and taken up by the recipient cells to induce bystander-like effects through exosomal miR-21.
miR-21 inhibitor suppresses the RIBE mediated by exosomal miR-21
To verify that exosome-mediated miR-21 transfer plays a role in the RIBE, we analyzed the influence of miR-21 suppression in irradiated cells.
We transfected MRC5 cells with miR-21 inhibitor, and found significantly reduced miR-21 expression at different time points (). Next, 24 hours after transfected with NC or miR-21 inhibitor, we treated the cells with 0 Gy or 2 Gy of X-rays. The expression levels of miR-21 in irradiated cells and exosomes isolated from irradiated conditioned medium were measured. As is shown in , the expression level of miR-21 in 2 Gy irradiated cells was remarkably raised, but this up-regulation was suppressed by the inhibitor treatment and the expression level was even lower than non-irradiated cells. As for the exosomes, although the expression levels of miR-21 showed similar pattern with irradiated cells, the expression of exosomal miR-21 remained a relatively high level following miR-21 inhibitor treatment.
Figure 5. Inhibiting the miR-21 expression in directly irradiated cells suppressed the exosomal miR-21 induced RIBE. (A) The expression of miR-21 in cells at different time points after transfected with miR-21 inhibitor. (B) The expression of miR-21 in irradiated cells previously transfected with NC or miR-21 inhibitor and in exosomes 4 hours after irradiation. (C) MiR-21 expression of bystander cells co-cultured with exosomes in (B). (D) The expression of pre-miR-21 of bystander cells co-cultured with exosomes in (B). (E) The mRNA expression of Bcl−2 in bystander cells 24 h after co-cultured with exosomes in (B). (F) Bcl-2 expression in bystander cells 24 h after co-cultured with exosomes in (B) by western blotting assay. (G) Frequency of micronuclei (MNF) in bystander cells treated for 48 h with exosomes in (B). (H) Yields of 53BP1 foci in bystander cells treated for 2 h with exosomes in (B). Error bars represent means ± standard error of 3 biological replicates and the superscript (*) denote a significant difference between groups (P < 0.05) as determined by Student's t test.
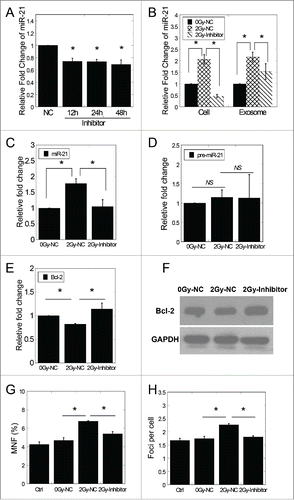
In addition, the bystander MRC-5 cells were co-cultured with exosomes isolated from conditioned medium of different treatments. It was observed that bystander cells treated with 2 Gy-NC exosomes showed significantly high miR-21 levels and reduced mRNA levels of Bcl-2, while bystander cells treated with 2 Gy-Inhibitor exosomes did not display significant changes (). As expected, the expression of pre-miR-21 showed no significant change in recipient cells after treatments of 2 Gy-NC and 2 Gy-Inhibitor exosomes (). Consistent with these results, Western blotting results indicated that the protein level of Bcl-2 in bystander cells was also remarkably suppressed by 2 Gy-NC exosomes and reverted to background level with 2 Gy-Inhibitor exosome treatment ().
The frequency of micronuclei and the number of 53BP1 foci were measured in bystander cells after co-cultured with exosomes isolated from conditioned medium of different treatments. Remarkable increase of the frequency of micronuclei and the number of 53BP1 foci was found (P < 0.05) after 2 Gy-NC exosomes treatment (). Similar with the miR-21 expression, the increase of the frequency of micronuclei and the number of 53BP1 was depressed by 2 Gy-Inhibitor exosomes ().
Discussion
Our previous study has reported firstly that a specific miRNA, miR-21, plays a definite role in the RIBE through a medium-mediated way.Citation20 In this continuous work, we first validated that exosomes secreted from irradiated cells induced the RIBE using the same cell model. Then we examined the shuttle of exosomal miRNAs between MRC5 cells quantitatively and qualitatively. In the quantitative analysis, we transfected an exogenous miRNA (Cel-miR-39) into MRC5 cells and found a rapid and abundant expression of Cel-miR-39 in extracellular exosomes 2 h after transfection. The high expression of Cel-miR-39 in exosomes could extend for a long period up to 12 hours, which indicated that the secreting of exosomes was spontaneous and stable. In the qualitative analysis, a fluorescent labeled miRNA mimics (Cy3-miR-21) was transfected into MRC5 cells and non-uniform distribution of fluorescence was observed in the cytoplasm. Partial of the mimics located in the MVBs where exosomes were generated and sorted. Cy3-miR-21 in the cytoplasm of non-transfected cells was visually detected by the red fluorescent points, indicating that exosomal miR-21 derived from transfected cells had been successfully transferred into non-transfected cells.
Although some researchers report that endocytosed exosomes can be targeted to the lysosome by entering the endosomal pathway and the cargo will be degraded along their pathway, much more studies reveal that exosomal miRNAs can be discharged to the cell cytosol upon fusion of the exosome membrane with its endosome, so that miRNAs can functionally modulate the gene expression of recipient cells.Citation39-41 Consistently, we found that exosomes isolated from irradiated conditioned medium caused the upregulation of miR-21 and corresponding suppression of the target gene in recipient cells. Supported by the pre-miR-21 results, it can be concluded that the up-regulation of miR-21 is due to the exosomal miR-21 transfer.
Varieties of molecules are contained in exosomes, including DNAs, lipids, proteins, mRNAs and miRNAs. Some profiling studies have shown that miRNAs are not randomly incorporated into exosomes.Citation42,43 Skog et al. reported that the level of miR-21 was lower in exosomes from the serum of healthy donors than those glioblastoma patients.Citation44 The levels of 8 specific exosomal miRNAs, including miR-21, were also found to be different between benign tumors and ovarian cancers.Citation45 These studies show that donor cells possess a sorting mechanism that guides specific intracellular miRNAs enter exosomes. Thus, one question emerges that whether the higher expression of miR-21 in exosomes is a response to radiation itself or the radiation induced miR-21 increment. We transfected MRC5 cells with miR-21 mimics to simulate the miR-21 upregulation induced by radiation, and found significant up-regulated miR-21 expression in exosomes secreted into the medium, indicating that the increased miR-21 expression in cells was sufficient to promote the sorting of miR-21 into exosomes. Adding exosomes derived from the medium of transfected cells to non-irradiated cells caused upregulated miR-21 expression, depressed target gene expression, increased chromosomal aberration and DNA damage, demonstrating that exosomal miR-21 could induce bystander effects, which was very similar to the bystander effects induced by exosomes isolated from irradiated conditioned medium. This finding was further verified by the experiment with miR-21 inhibitor.
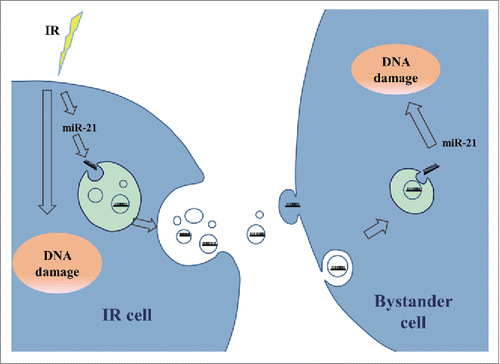
Based on these results, a model of the exosome-mediated miR-21 transfer in the radiation-induced bystander effects was proposed (). In irradiated cells, the expression level of miR-21 was increased along with the DNA damage response, resulted in motivated sorting of miR-21 to enter exosomes. With the diffusion of exosomes secreted from irradiated cells into extracellular medium, exosomes can be taken up by non-irradiated cells partially. Once the exosome cargo including miR-21 was released into the cytoplasm of recipient or bystander cells, increased miR-21 level regulated the relevant target gene expression and induced chromosome aberration and DNA damage.
Figure 6. A proposed model of the exosome-mediated miR-21 transfer in the RIBE. In irradiated cells, the expression of miR-21 is up-regulated and as a response, miR-21 sorting to exosomes is motivated. The exosomes are secreted out from the irradiated cells, diffused into extracellular medium, taken up by non-irradiated cells, and the miR-21 inside the exosomes are released into bystander cells to induce bystander effects.
Taken together, our results provide strong evidence that miR-21 acts as a bystander signal molecular shuttling from irradiated cells to bystander cells through exosomes, which support the hypothesis that the bystander effects are not nonspecific or nonselective. These findings might attract further research on exosomes-mediated communication and miRNA functions in the RIBE, and provide new insights into the development of radioprotection and radiotherapy.
Materials and Methods
Cell culture
Human normal embryonic lung fibroblast cell line MRC-5,Citation46 were purchased from the American Type Culture Collection (ATCC® CCL−171™). MRC-5 cells was cultured in minimal essential medium (Sigma, USA) supplemented with 10% fetal bovine serum (Hyclone, USA), 100 units/mL penicillin and 100 mg/mL streptomycin and maintained in a 5% CO2 humidified incubator (Thermo Scientific, NC, USA) at 37°C. For the preparation of conditioned medium, the culture medium was replaced with fresh medium supplemented with 10% FBS (depleted of bovine exosomes by overnight centrifugation at 100,000 × g) immediately before treatment with radiation.
Irradiation
X-rays were generated by Faxitron RX-650 (Faxitron Bioptics, Lincolnshire, IL, USA), which was operated with 100 kVp and 5 mA at a dose rate of 1.33 Gy/min. Exposure of all samples was performed at room temperature as previously described.Citation47
Conditioned medium (CM) transfer
For CM transfer experiments, MRC-5 cells (2 × 105) were plated in φ35 mm dishes for direct irradiation, while bystander MRC-5 cells (1×105) were plated in 12-well plates. After 18 h cultivation, MRC-5 cells were irradiated and immediately substituted with fresh medium. CM was harvested 4 h after irradiation, centrifuged at 1000 x g for 5 min to exclude cells and cellular fragments. The supernatant was used or exosome isolation or directly added to non-irradiated bystander cells. Control sample was treated in the same way, except for irradiation.
Exosomes transfer and exosomal RNA isolation
Exoquick exosome precipitation kit (SBI System Biosciences, Inc..) was used for exosome isolation from conditioned medium according to manufacturer's instruction. Briefly, the medium were collected and centrifuged at 1000 x g for 10 min to remove cells. Exoquick reagent (0.4 ml) was added to 2 ml of the supernatant, incubated 12 h at 4°C, and centrifuged at 1500 × g for 30 min to obtain pelleted exosomes. Resuspended the exosome pellet in 0.2 ml PBS or extracted the exosomal RNA with TRIzol Reagent (Invitrogen, USA) according to the manufacturer's instructions. The resuspended exosomes were transferred to non-irradiated cells. Control sample was treated in the same way, except for irradiation.
qRT-PCR of miRNA and Mrna
After irradiation or other treatment, cells were harvested at the indicated time-points. Then, RNA was isolated using TRIzol reagent (Invitrogen, USA). The quantity of isolated RNA was tested using a spectrophotometer (Eppendorf, Germany). For miRNA expression, total RNA reverse transcription and quantitative RT-PCR were preformed according to the protocol of All-in-One™ miRNA qRT-PCR Detection Kit (Genecopoeia, USA). Cel-miR-39 and RNU6 were used as an invariant control for Conditioned medium and cells, respectively. Primers for miR-21 were designed by Genecopoeia Co. (Guangzhou, China). Primer for pre-miR-21 was: GTACCACCTTGTCGGGTAGC. For mRNA expression, reverse transcription was performed using a First-Strand cDNA Synthesis Kit (GeneCopoeia, USA). The qRT-PCR reaction was performed using SYBR Green PCR master mix (GeneCopoeia, USA). Primers for GAPDH and Bcl-2 were designed by Genecopoeia Co. (Guangzhou, China). The PCR program was carried out with a Chromo4 system (Bio-Rad, CA, USA) under the following conditions: initiation for 10 min at 95°C, followed by 50 thermal cycles each at 95°C for 10 s and at 60°C for 20 s and at 70°C for at last 10 s. Data were analyzed with the C(t) value comparison method and normalized to RNU6, Cel-miR-39 or GAPDH expression for different sample.
Western blotting
Cells were lysed in RIPA buffer (Beyotime, China). Proteins were separated by 10% SDS-PAGE and transferred to a methanol-activated PVDF membrane (GE Healthcare, USA). The membrane was blocked for 1 h in PBST containing 5% milk and subsequently probed with anti-Bcl−2 antibody (ImmunoWay, USA), and anti-β-actin antibody (Santa Cruz) for 2 h. After 1 h incubation with goat-anti-mouse HRP-conjugated secondary antibody (Santa Cruz), the protein bands were detected with luminal reagent (GE Healthcare) and their relative intensities were quantified using Adobe Photoshop software (Adobe Systems Inc., San Jose, CA, USA).
MiRNA transfection assay
MiRNA (Cel-miR-39, Cy3-miR-21, miR-21 mimics or Inhibitor) were synthesized and purified by RiboBio Co. (Guangzhou, China). A miRNA with no sequence similarities to any reported human gene sequence was used as negative control (NC). Twenty-four hours prior to transfection, cells were plated at 40-60% confluence. Transfection was performed with Lipofectamine 2000 (Invitrogen) according to the manufacture's protocol. The medium was replaced with fresh medium 5 h after transfection.
Fluorescence tracking assay
At the time of analysis, the medium was aspirated and the cells were washed 3 times with PBS, fixed with 4% paraformaldehyde for 15 min, washed with PBS, and permeabilized in 0.1% Triton-X 100 for 10 min. Then, cell nuclei were labeled with DAPI (blue), actin were stained by phalloidin-FITC (green).
Micronucleus assay
Cells were treated with 15 μL 0.25 mg/mL cytochalasin B, cultured for 48 h, fixed with Carnoy's fluid for 15 min at room temperature, stained with acridine orange (30 μg/mL), and then observed under fluorescence microscope. At least 500 binucleate cells were scored for each sample.
Immunofluorescence staining
Immunofluorescent microscopy was conducted as described with minor modifications.Citation48 Briefly, cells were fixed in 4% paraformaldehyde for 15 min, washed with PBS, and permeabilized in 0.5% Triton-X 100. After blocked with 5% non-fat milk for 1 h, samples were incubated with a mouse monoclonal anti-53BP1 antibody (Upstate Biotechnology, USA) for 2 h at room temperature and washed. Followed by incubation with Alexa Fluor® 594 anti-mouse antibody (Molecular Probes, USA) for 1 h at room temperature, cells were counter-stained with DAPI and observed with a fluorescent microscope (Nikon, Tokyo, Japan). At least 100 cells were scored for each sample.
Statistical Analysis
All experiments were independently repeated at least 3 times and all data were presented as the means ± standard error. Student's t-tests were used for the statistical analysis. Probability (P) values less than 0.05 were considered to be statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 31270895, 31400723 and 11405235).
References
- Banaz-Yasar F, Lennartz K, Winterhager E, Gellhaus A. Radiation-induced bystander effects in malignant trophoblast cells are independent from gap junctional communication. J Cell Biochem 2008; 103:149-61; PMID:17516549
- Ryan LA, Smith RW, Seymour CB, Mothersill CE. Dilution of irradiated cell conditioned medium and the bystander effect. Radiation Res 2008; 169:188-96; PMID:18220470
- Belyakov OV, Mitchell SA, Parikh D, Randers-Pehrson G, Marino SA, Amundson SA, Geard CR, Brenner DJ. Biological effects in unirradiated human tissue induced by radiation damage up to 1 mm away. Proc Natl Acad Sci USA 2005; 102:14203-8; PMID:16162670
- Mancuso M, Pasquali E, Leonardi S, Tanori M, Rebessi S, Di Majo V, Pazzaglia S, Toni MP, Pimpinella M, Covelli V, et al. Oncogenic bystander radiation effects in Patched heterozygous mouse cerebellum. Proc Natl Acad Sci USA 2008; 105:12445-50; PMID:18711141
- Wang H, Yu KN, Hou J, Liu Q, Han W. Radiation-induced bystander effect: Early process and rapid assessment. Cancer Lett 2013; 356(1):137-44; PMID:24139967
- Azzam EI, Little JB. The radiation-induced bystander effect: evidence and significance. Human Exp Toxicol 2004; 23:61-5
- Mothersill C, Seymour CB. Radiation-induced bystander effects–implications for cancer. Nat Rev Cancer 2004; 4:158-64; PMID:14964312
- Widel M, Przybyszewski W, Rzeszowska-Wolny J. Radiation-induced bystander effect: the important part of ionizing radiation response Potential clinical implications. Postepy Hig I Med Dosw 2009; 63:377-88
- Shao C, Stewart V, Folkard M, Michael BD, Prise KM. Nitric oxide-mediated signaling in the bystander response of individually targeted glioma cells. Cancer Res 2003; 63:8437-42; PMID:14679007
- Narayanan PK, Goodwin EH, Lehnert BE. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res 1997; 57:3963-71; PMID:9307280
- Zhou H, Ivanov VN, Lien YC, Davidson M, Hei TK. Mitochondrial function and nuclear factor-kappaB-mediated signaling in radiation-induced bystander effects. Cancer Res 2008; 68:2233-40; PMID:18381429
- Shao C, Furusawa Y, Aoki M, Matsumoto H, Ando K. Nitric oxide-mediated bystander effect induced by heavy-ions in human salivary gland tumour cells. Int J Radiat Biol 2002; 78:837-44; PMID:12428924
- Hu W, Xu S, Yao B, Hong M, Wu X, Pei H, Chang L, Ding N, Gao X, Ye C, et al. MiR-663 inhibits radiation-induced bystander effects by targeting TGFB1 in a feedback mode. RNA Biol 2014; 11:1189-98; PMID:25483041
- Lyng FM, Maguire P, McClean B, Seymour C, Mothersill C. The involvement of calcium and MAP kinase signaling pathways in the production of radiation-induced bystander effects. Radiation Res 2006; 165:400-9; PMID:16579652
- Osterreicher J, Skopek J, Jahns J, Hildebrandt G, Psutka J, Vilasova Z, Tanner JM, Vogt J, Butz T. Beta1-integrin and IL-1alpha expression as bystander effect of medium from irradiated cells: the pilot study. Acta Histochemica 2003; 105:223-30; PMID:13677615
- Koturbash I, Zemp FJ, Kutanzi K, Luzhna L, Loree J, Kolb B, Kovalchuk O. Sex-specific microRNAome deregulation in the shielded bystander spleen of cranially exposed mice. Cell Cycle 2008; 7:1658-67; PMID:18560276
- Koturbash I, Boyko A, Rodriguez-Juarez R, McDonald RJ, Tryndyak VP, Kovalchuk I, Pogribny IP, Kovalchuk O. Role of epigenetic effectors in maintenance of the long-term persistent bystander effect in spleen in vivo. Carcinogenesis 2007; 28:1831-8; PMID:17347136
- Dickey JS, Zemp FJ, Martin OA, Kovalchuk O. The role of miRNA in the direct and indirect effects of ionizing radiation. Radiat Environ Biophys 2011; 50(4):491-9; PMID:21928045
- Chaudhry MA, Omaruddin RA. Differential regulation of microRNA expression in irradiated and bystander cells. Molekuliarnaia Biologiia 2012; 46:634-43; PMID:23113353
- Xu S, Ding N, Pei H, Hu W, Wei W, Zhang X, Zhou G, Wang J. MiR-21 is involved in radiation-induced bystander effects. RNA Biol 2014; 11:1161-70; PMID:25483031
- van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem 2006; 140:13-21; PMID:16877764
- Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nature Rev Immunol 2002; 2:569-79
- Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics 2010; 73:1907-20; PMID:20601276
- Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 2006; 20:1487-95; PMID:16791265
- Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic 2008; 9:871-81; PMID:18331451
- van der Grein SG, Nolte-'t Hoen EN. “Small Talk” in the Innate Immune System via RNA-Containing Extracellular Vesicles. Front Immunol 2014; 5:542; PMID:25400635
- Bang C, Thum T. Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol 2012; 44:2060-4
- Hanson EK, Lubenow H, Ballantyne J. Identification of forensically relevant body fluids using a panel of differentially expressed microRNAs. Anal Biochem 2009; 387:303-14; PMID:19454234
- Ai J, Zhang R, Li Y, Pu JL, Lu YJ, Jiao JD, Li K, Yu B, Li ZQ, Wang RR, et al. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochemical Biophys Res Commun 2010; 391:73-7
- Michael A, Bajracharya SD, Yuen PST, Zhou H, Star RA, Illei GG, Alevizos I. Exosomes from human saliva as a source of microRNA biomarkers. Oral Diseases 2010; 16:34-8; PMID:19627513
- Hanke M, Hoefig K, Merz H, Feller AC, Kausch I, Jocham D, Warnecke JM, Sczakiel G. A robust methodology to study urine microRNA as tumor marker: microRNA-126 and microRNA-182 are related to urinary bladder cancer. Urol Oncol-Semin Ori 2010; 28:655-61; http://dx.doi.org/10.1016/j.urolonc.2009.01.027
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biol 2007; 9:654-U72; PMID:17486113; http://dx.doi.org/10.1038/ncb1596
- Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res 2010; 38:7248-59; PMID:20615901; http://dx.doi.org/10.1093/nar/gkq601
- Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 2010; 285:17442-52; PMID:20353945; http://dx.doi.org/10.1074/jbc.M110.107821
- Chen X, Ba Y, Ma LJ, Cai X, Yin Y, Wang KH, Guo JG, Zhang YJ, Chen JN, Guo X, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 2008; 18:997-1006; PMID:18766170; http://dx.doi.org/10.1038/cr.2008.282
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 2008; 105:10513-8; PMID:18663219; http://dx.doi.org/10.1073/pnas.0804549105
- Al-Mayah AH, Irons SL, Pink RC, Carter DR, Kadhim MA. Possible role of exosomes containing RNA in mediating nontargeted effect of ionizing radiation. Radiation Res 2012; 177:539-45; PMID:22612287; http://dx.doi.org/10.1667/RR2868.1
- Jella KK, Rani S, O'Driscoll L, McClean B, Byrne HJ, Lyng FM. Exosomes are involved in mediating radiation induced bystander signaling in human keratinocyte cells. Radiation Res 2014; 181:138-45; PMID:24502353; http://dx.doi.org/10.1667/RR13337.1
- Lim PK, Bliss SA, Patel SA, Taborga M, Dave MA, Gregory LA, Greco SJ, Bryan M, Patel PS, Rameshwar P. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res 2011; 71:1550-60; PMID:21343399; http://dx.doi.org/10.1158/0008-5472.CAN-10-2372
- Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatol 2011; 54:1237-48; http://dx.doi.org/10.1002/hep.24504
- Viaud S, Thery C, Ploix S, Tursz T, Lapierre V, Lantz O, Zitvogel L, Chaput N. Dendritic cell-derived exosomes for cancer immunotherapy: what's next? Cancer Res 2010; 70:1281-5; PMID:20145139; http://dx.doi.org/10.1158/0008-5472.CAN-09-3276
- Guduric-Fuchs J, O'Connor A, Camp B, O'Neill CL, Medina RJ, Simpson DA. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genomics 2012; 13:357; PMID:22849433; http://dx.doi.org/10.1186/1471-2164-13-357
- Ohshima K, Inoue K, Fujiwara A, Hatakeyama K, Kanto K, Watanabe Y, Muramatsu K, Fukuda Y, Ogura S, Yamaguchi K, et al. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PloS one 2010; 5:e13247; PMID:20949044; http://dx.doi.org/10.1371/journal.pone.0013247
- Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr., Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nature Cell Biol 2008; 10:1470-6; PMID:19011622; http://dx.doi.org/10.1038/ncb1800
- Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecologic Oncol 2008; 110:13-21; http://dx.doi.org/10.1016/j.ygyno.2008.04.033
- Jacobs J, Jones C, Baille J. Characteristics of a human diploid cell designated MRC-5. Nature 1970; 227(5254):168-70; PMID:4316953
- Zhu J, Hu W, Ding N, Ye C, Usikalu M, Li S, Hu B, Sutherland BM, Zhou G. An optimized colony forming assay for low-dose-radiation cell survival measurement. Int Res J Biotechnol 2011; 2:164–72.
- Cowell IG, Sunter NJ, Singh PB, Austin CA, Durkacz BW, Tilby MJ. γH2AX foci form preferentially in euchromatin after ionising-radiation. PloS one 2007; 2:e1057; PMID:17957241
