ABSTRACT
Cells under stressful microenvironmental conditions initiate integrated molecular circuitries that aim at reducing general protein translation rates while redirecting protein synthesis toward a selective set of stress-response proteins. The consequence of the activation of this dynamic system is a reduction of the energy expenditure of the cell, and a metabolic rewiring that shapes adaptation under stress, which will, in fine, promote cell survival. In general, the translation initiation step is the prime target of translation reduction, with 2 molcular modules inhibiting translation initiation: the mechanistic target of Rapamycin complex 1, and the stress related kinases eIF2 kinases, which are all involved in the cellular responses to kidney injuries. tRNA (tRNA) dynamics and fragmentation have recently gained a considerable weight in the field of the non-coding RNA biology, and emerge as an important system for protein translation modulation under cellular stress. More precisely, stress-induced tRNA (tiRNA), the cleavage products of the ribonuclease angiogenin, are generated under various stress conditions, including oxidative stress and endoplasmic reticulum stress, and contribute to protein translation reprogramming in mammal cells. Current clinical and experimental evidence indicates that the angiogenin-tRNA fragmentation system is initiated under renal insults, and is involved in the tissue adaptation upon kidney injury. In addition, this system represents a potential source for minimally-invasive or non invasive biomarkers of early kidney injury. Besides RNA interference, tRNA fragments are likely involved in other fundamental cellular functions, including inflammation, and a better understanding of the molecular basis of tRNA functions will drive discoveries on the fundamental role of non coding RNA biology, as exemplified by microRNA, in the regulation of kidney homeostasis.
Introduction
Kidneys continuously have to cope with a wide array of insults that translate into elementary stressors at the cellular level (e.g., nutrient starvation, hypoxia, oxidative stress, and endoplasmic reticulum (ER) stress). Adaptive responses to stress are often evolutionarily conserved molecular systems that primarily aim to eradicate or reduce stress intensity and promote metabolic reprograming to maintain cellular homeostasis and other vital functions. Molecular modules sense microenvironmental fluctuations in nutrients, oxygen, and temperature, as well as disordered intracellular fluctuations, such as the accumulation of unfolded proteins, or energy (Adenosine tri phosphate, ATP) shortage, among others. These modules then transduce signals that will fuel metabolic reprogramming to maintain basal functions, while adapting the cell to the new environmental conditions. These integrative molecular circuitries include, but are not limited to, various molecules, such as the mechanistic Target of Rapamycin Complex 1 (mTORC1), which senses amino acids, growth factors, and glucose; hypoxia induced factors (HIFs), which sense oxygen; the unfolded protein response (UPR) that senses misfolded proteins; the stress related kinases eIF2 kinases (eIF2K) that sense misfolded proteins amino acid shortages, viruses and glucose; AMPK (5' AMP-activated protein kinase), which senses energy (ATP); and heat shock proteins, which sense physical stress. In general, programmed cell death pathways are activated in parallel to adaptive stress responses, and the net effect will dictate cell fate pending the intensity and the duration of the stress Citation[1]. In addition to cellular decisions of life and death, these adaptive responses also participate in building communication networks that shape the stressed cell microenvironment, generally in a paracrine manner, leading to the activation of preemptive responses in cells not yet subjected to the stress, and the production of alarm signals that will activate innate immune cells such as macrophages or dendritic cells Citation[1]. These processes are particularly relevant in renal pathophysiology given the strong interplay between injured resident renal cells such as tubular cells that communicate with fibroblasts and macrophages to promote fibrosis and inflammation, which are critical for kidney structural deterioration and loss of function Citation[2–5].
The demand for global protein synthesis decreases under stress conditions, where the translation machinery is diverted to preferentially promote synthesis of stress response genes Citation[6–8]. Various types of environmental stress, including nutrient stress, temperature shock, DNA damage, and hypoxia, require rapid and adequate reprogramming of gene expression at different regulatory levels and timescales Citation[7]. In general, these stresses lead to changes in gene expression patterns caused by a general shutdown and reprogramming of protein synthesis to elicit a response that is relevant to the type of stress induced and the targeted cell. Almost all steps of gene expression are altered to generate the cell stress responses, including chromatin remodeling, gene transcription regulation, pre-mRNA splicing, mRNA degradation, translation efficiency modulation, post-translational modifications, and protein degradation.
In addition to allowing for specific and directed reprogramming of protein synthesis, reducing global protein synthesis rates is critical for cell survival under stress, including nutrient and energy shortages. In line with the importance of translational repression in response to cellular stress, the artificial maintenance of a high protein synthesis level under ER stress leads to ATP depletion, oxidative stress and cell death Citation[9]. Protein translation is among the most energy-consuming cellular processes. Synthesising a 100 amino acid polypeptide chain requires 100 ATP molecules and 100 GTP (Guanosine Tri Phosphate) molecules, which roughly correspond to 1 Kcal for 1 g of new protein; thus, a global shutdown of translational might be an energy- and resource-saving program, and this process can be rapidly annulled upon stress relief Citation[10]. A fine-tuning of the energy expenditure for modulating protein synthesis, which heavily consumes ATP, appears to be of particular relevance for renal cell homeostasis maintenance under stress Citation[11]. The basal metabolism of renal epithelial cells requires a considerable amount of energy, under the form of ATP, because apico-basal polarization and molecular transport, which are fundamental processes for renal function, significantly rely on Na+/K+ ATPase co-transporters Citation[12]. Consistent with the notion that the kidney is a highly aerobic tissue with many mitochondria producing ATP, more than 50% of the transcriptional output of the whole organ occurs in the mitochondria Citation[13]. In addition, the deep regions of the kidneys have to cope with low oxygen and nutrient concentrations, which render them acutely sensitive to ischemia Citation[14]. As a consequence, the molecular circuitries that support the cell to adapt its metabolism to an energy shortage are critical for renal cell homeostasis and survival Citation[15]. Overall, the net effect of the activation of these pathways is a reduction of energy expense on a global level, accompanied by the selective activation of an array of genes encoding a stress response program, which will evade translational repression and provide a mechanism for adaptation Citation[7].
Several molecular pathways are involved in general protein reprograming under stress repression target translation initiation, the first step of protein translation. Among them, the processing and cleavage of tRNA (tRNA) emerge as important contributors of cell adaptation under stress Citation[16,17]. Indeed, tRNA fragments possess distinct expression patterns, abundance, cellular localizations, or biological roles compared with their parental tRNA molecules. Importantly, a growing body of evidence indicates that these tRNA fragments are involved in tubular adaptation under stress and could be used as potential biomarkers of early kidney injury.
Mechanisms of protein synthesis reprograming under cellular stress
Eukaryotic translation initiation control
A typical eukaryotic mRNA comprises a coding sequence that directs protein synthesis, flanked by 5' and 3' untranslated regions (UTRs). In general, translation begins with the recruitment of the smaller ribosome subunit to the 5' extremity of the mRNA (the cap), which is modified by methylation of a guanine, called the m7G cap Citation[18]. This process of cap-dependent translation initiation is coordinated by a multimeric complex of initiation factors (eIFs) called eIF4F, that include eIF4G (a scaffold protein), eIF4E, which recognizes the 5'm7G cap structure, and eIF4A, an RNA helicase. The mRNA is then circularized and stabilized by the interaction of the poly(A) binding protein (PABP) with eIF4G and eIF4B (). A ternary complex composed of eIF2, GTP and methionyl tRNAi is recruited and drives loading of the ribosome, which will scan along the 5' UTR until an AUG codon (the starting codon) is recognized. The recognition of the AUG codon by the ribosome loaded with these factors will prompts hydrolysis of eIF2-GTP, and uncoupling of tRNAi from eIF2. Recruitment of the large ribosomal subunit then occurs, and translation elongation begins. The complexity of translation initiation offers targets for regulation under stress, with 2 critical checkpoints, the phosphorylation statuses of eIF2 and 4EBPs (eIF4E Binding Proteins). The phosphorylation of the α subunit of eIF2 (eIF2α) on serine 51 inhibits eIF2 recycling and reloading with GTP. Indeed, phosphorylated eIF2 has an increased affinity for eiF2B, its Guanine nucleotide exchange factor, which, as a consequence, won't be able to exchange GDP for GTP. The reduction of the recycling of the eIF2-GTP complex will lower ternary complex formation and reduce translation rates Citation[19]. Conversely, the dephosphorylation of 4EBPs increases its affinity for eIF4E and therefore will impede its recruitment in formation of the eIF4F complex and reduce translation initiation Citation[20]. The phosphorylation of eIF2 and 4EBPs, and their activation, depends on the activity of the kinases that integrate extracellular and intracellular fluctuations to support protein translation reprogramming, namely, eIF2 kinases and mTOR, respectively.
Figure 1. Mechanisms of translation initiation repression. A wide array of cell stresses cause a repression of protein synthesis via molecular modules that modify the phosphorylation status of proteins involved in translation initiation. The phosphorylation of eIF2α by eIF2 kinases PKR, GCN2, PERK, or HRI inhibits ternary complex recycling, leading to a reduction in initiation codon recognition. Indeed, phosphor-eIF2α inhibits the guanine exchange factor (GEF) activity of eIF2B. In parallel, the inhibition of mTOR leads to a reduction in 4EBP phosphorylation and consequently inhibits eIF4E-eIF4G interaction. Moreover, the activities of eIF4B and eIF4A are reduced. MicroRNAs, generally through binding in the 3' UTRs of RNAs, reduce the expression levels of mRNA. In parallel to the global inhibition of protein synthesis, individual mRNA expression is specifically maintained, which supports the expression of stress-response genes. IRESs in the 5' UTRs of many stress-response mRNAs allow for the synthesis of proteins when cap-dependent translation is compromised. ATF4 mRNA contains 2 upstream open reading frames (uORFs) in the 5' UTR, which are recognized by preinitiation complexes. Because the second uORF overlaps with the authentic ATF4 coding sequence, but in a different frame, expression of functional ATF4 is repressed. However, under conditions in which eIF2α is phosphorylated, a reduction in ternary complex concentration occurs, and the second uORF is less likely to be recognized, allowing translation of functional ATF4 from the authentic open reading frame.
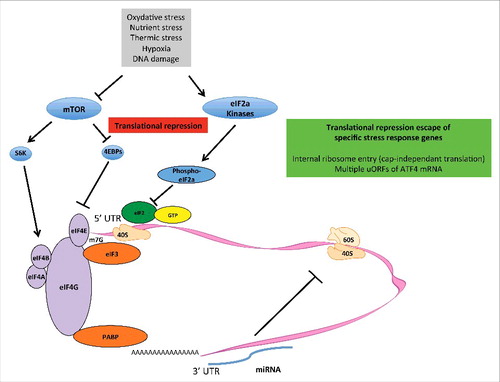
Eukaryotic translation initiation repression under stress
Translation initiation is the most important target repressed under cellular stress, and several factors modulate protein synthesis and translation rates, including the stress related kinases [GCN2 (General control non derepressible), PERK (protein kinase RNA-like endoplasmic reticulum kinase), HRI (Heme regulated eiF2α kinase), and PKR (Protein kinase R)], which, when activated, lead to eiF2α phosphorylation and translation initiation inhibition Citation[19], and the mTOR kinase, which, when inhibited, is associated with a reduced phosphorylation of 4EBPs, leading to the impairment of m7G cap-dependent translation of mRNA, including mRNAs that encode proteins involved in the translation machinery. mTOR inhibition is also associated with a reduced activation of S6K1 (S6 kinase 1), leading to translation initiation repression related with inactivated eIF4A and eIF3 Citation[20].
Under situations leading to global protein translation repression, that is, when mTOR is inhibited (by growth factors, amino-acids, ATP or glucose shortage) and/or eIF2 kinases are activated (GCN2 by amino-acid starvation, PERK by misfolded proteins, PKR by dsDNA and glucose, and HRI by low levels of heme in red blood cell precursors), a set of mRNAs encoding stress response proteins evade global repression of translation to preferentially shape a stress response program (). mRNAs that evade cap-dependent translation inhibition are thought to contain internal ribosome entry sites (IRESs). Although the existence of cellular IRESs is still debated, 10% of mRNA contain IRES in their 5' UTRs Citation[8], and therefore have the potential to be translated by internal ribosome entry, a process that allows recruitment of the translation machinery downstream and independently of the 5' m7G cap. IRESs containing mRNA encode, for example, the cat-1 Arg/Lys transporter (CAT-1), the sodium-coupled neutral amino acid transporter (SNAT2), the chaperone glucose related protein (GRP) 78, antiapoptotic proteins B-cell lymphoma 2 (BCL2), X-linked inhibitor of apoptosis protein (XIAP), the angiogenic factors HIF-1α, vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF) Citation[21].
Another mechanism by which stress response mRNAs evade translation repression relies on the presence of multiple upstream open reading frames (uORFs, in-frame start and stop codons in the 5' UTR) that can promote the preferential expression of certain stress-related mRNAs when the translation initiation complex is inhibited Citation[22]. The canonical example of such reprograming is the selective translation of the transcription factor ATF4 (Activating transcription factor 4) under amino-acid deprivation, which, despite a general repression of translation mediated by the activation of the eIF2 kinase-eIF2α axis, will transcribe an array of genes, including amino acid transporters, asparagine synthetase and antioxidants Citation[6,23,24].
Protein synthesis reprogramming upon cellular stress occurs during a wide range of kidney injuries, and many examples have been described, including ischemic, toxic, and immune-mediated kidney injuries that implicate either eIF2 kinase signaling pathways Citation[11,25–31], or mTOR Citation[32–36]. In aggregate, these studies demonstrate that the biological modules regulating stress responses and protein translation rates under kidney injury are critical for the maintenance of cellular homeostasis under kidney injury.
Targeting mRNA under stress
Coordination of the stress responses that impact mRNA translation rates is also mediated by processes that exquisitely regulate mRNA during its translation. These processes also regulate dissociation of endoplasmic reticulum (ER)-targeted mRNA from ribosomes Citation[37], and the degradation of ER-targeted mRNA mediated by the ribonuclease activity of Inositol requiring Enzyme 1α (IRE1α), in a process called RIDD, for regulated IRE1-dependent decay of mRNA Citation[38]. By removing mRNAs from the site of translocation and by degrading mRNA encoding proteins targeted for ER retrotranslocation, these mechanisms may serve as a potent means to transiently reduce ER protein folding load and restore proteostasis in affecting the dynamic subcellular localization of mRNAs and translation as a selective and rapid regulatory feature of the cellular response to protein folding stress. Of note, these 2 processes occur under ER stress and are mediated by the UPR.
Finally microRNA (miRNA)-mediated RNA interference provides a mechanism for regulation of genes expression, and miRNAs are involved in protein synthesis reprogramming under cellular stress Citation[39]. miRNAs are short (≈22 nucleotides) non-coding RNAs that imperfectly bind the 3' UTR to destabilize and inhibit mRNA translation. Notably, miRNA-mediated RNA interference is the best-described example of the critical roles that non-coding RNAs (ncRNAs) might play in gene expression regulation and likely represent the tip of the iceberg given that our functional understanding of ncRNA biology has undergone dramatic changes in the recent past. Numerous studies have provided insights into how miRNA participate in the stress responses under various kidney injuries Citation[40–42], and the detection of circulating miRNA may serve as predictive biomarkers of ongoing injury Citation[43,44].
Rewiring protein expression in targeting tRNA
Genomes of higher eukaryotes are almost entirely transcribed into RNA, while only a minor portion is subsequently translated into proteins Citation[45]. Therefore, the ncRNA transcriptome is the major output of genome expression, and recent evidence strongly suggests that ncRNAs promote post-transcriptional gene silencing by targeting mRNAs and also act on DNA by playing pivotal roles in regulating genome stability and guiding chromatin remodeling Citation[46]. More importantly, fragments of these ncRNA are increasingly recognized as regulated and conserved entities with specific biological functions Citation[47]. tRNA-derived RNA fragments belong to a family of short ncRNAs present in most organisms and that can be both constitutively generated and produced in the context of stress. Over the past few years, our understanding about tRNA fragmentation and the biological properties of tRNA fragments has dramatically changed Citation[16].
tRNA and tRNA fragments
tRNA biology
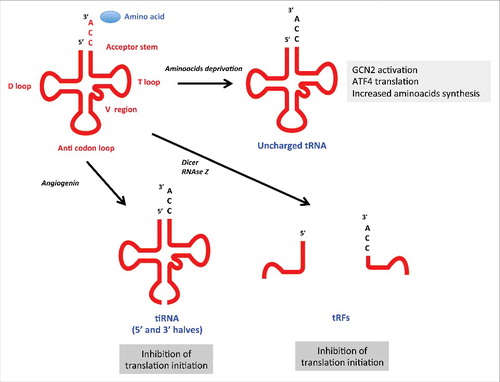
tRNAs represent one of the most abundant cellular transcripts (up to 15% of total cellular RNAs) and link the genetic code to the amino acid sequence of proteins. tRNAs are 73–90 nucleotides long and have a characteristic tertiary structure made of 4 base-paired stems and 5 unpaired regions, the D-loop, the anticodon loop, the variable loop, the T-loop, and the 3′ CCA tail (). tRNAs are charged with an amino acid linked to the adenosine of the 3′ CCA end by cognate aminoacyl-tRNA synthetases. By reading the mRNA, 3 nucleotides at a time by base-paring, the codon (mRNA)–anticodon (tRNA) interaction defines the amino acid position in the protein.
Figure 2. Processing and functions of tRNA fragments under cellular stress tRNAs have a length of 73–90 nucleotides (nt) and have a characteristic secondary and tertiary structure. The backbone of the so-called cloverleaf structure is made of 4 base-paired stems and 5 unpaired regions, the D-loop, anticodon loop, variable loop, T-loop, and in case of mature tRNAs, the 3′ CCA tail. Each tRNA is charged with an amino acid that is covalently linked to the adenosine of the 3′ CCA end, a reaction that is performed by cognate aminoacyl-tRNA synthetases. Oxidative stress-induced deactivation of the CCA tail shuts down global translation. Angiogenin-mediated tiRNA-derived fragments repress translation initiation through eIF4E and eIF4G displacement. tRFs produced under cellular stress also negatively impact gene expression by displacing transcripts from YBX1, leading to transcript destabilization. Under amino acid starvation, uncharged tRNA will directly activate the GCN2 kinase, leading to translation repression and increased translation of ATF4 mRNAs to enhance amino acid biosynthesis. In addition, nutrient starvation induced by glucose, nitrogen, amino acid or inorganic phosphate deprivation causes tRNA retrograde transport into the nucleus. Depleting translation competent tRNAs when nutrients are limited is a means to reduce protein synthesis (not shown).
In addition to their roles in polypeptide chain production, tRNAs have biological functions under nutritional stress. Under deprivation of either amino acids or other nutrients, a proportion of mature cytoplasmic tRNAs are rapidly re-imported into the nucleus as a mechanism for global translational repression Citation[48]. Moreover, in situations of amino acid shortages, tRNAs become uncharged in amino acids. Uncharged tRNAs have the potential to activate the eIF2 kinase GCN2, which will in turn phosphorylate eIF2α on serine 51 to reduce translation initiation Citation[49]. Consequently, uncharged tRNAs promote the expression of genes involved in amino acid transport and synthesis through selective translation of the GCN4/ATF4 transcription factors Citation[17].
tRNA fragments
Processed RNA fragments are tRNA-derived pieces represent a rapidly growing class of novel regulatory ncRNAs and are no longer considered random degradation products Citation[16]. Rather, their production is regulated, with identified ribonucleases, and they share a set of similar functional properties. In addition, their nomenclature has been edited to facilitate scientific exchanges Citation[50]. Two main species of tRNA fragments have been characterized Citation[16]. First, tRNA halves have been identified (also known as “tRNA-derived, stress-induced small RNAs” or tiRNAs), which are derived from both the 5′ and 3′ region of full-length tRNA and are produced by cleavage of the anticodon loop (and are consequently composed of 30–50 nucleotides). Second, shorter tRNA-derived fragments (tRFs) have been characterized, which are classified as 5′ tRFs or 3′ CCA tRFs, depending on the region from which the tRNA is derived ().
The production of tRNA-derived fragments has been observed in a number of human diseases, including acute tissue injury (toxic injury, irradiation and ischemia-reperfusion), cancer or neurodegenerative disease (including Amyotrophic lateral sclerosis) Citation[17,51]. These tRNA-derived fragments are likely involved in the pathophysiology of the disease, with ambivalent impact, since they are protect tissue upon acute injury, and may also promote tumor growth and angiogenesis. In some cases, these tRNA fragments may serve as useful biomarkers of ongoing tissue injury or cancer (see below).
tiRNA
A majority of cellular stresses promote tRNA cleavage into tiRNA, including physicochemical, nutritional, and oxidative stresses, but not γ irradiation or DNA damage agents such as etoposide Citation[52–55]. The only-known ribonuclease for tRNA cleavage into tiRNA is angiogenin Citation[53]. Angiogenin is a secreted ribonuclease that was first identified as an angiogenic factor found in tumor cell–conditioned medium Citation[56]. The secretion of angiogenin is enhanced by hypoxia, which indicates that it may be a component of a stress response program Citation[57,58]. Angiogenin binds to an uncharacterized receptor on the surface of endothelial cells that facilitate its internalization and transport to the nucleolus Citation[59,60]. Remarkably, the promotion of new blood vessel growth is dependent on its ribonuclease activity Citation[61]. Angiogenin promotes rRNA transcription and cellular proliferation Citation[62].
In fact, angiogenin has multiple cellular functions, pending the stress status of the cell. Angiogenin is a stress-activated ribonuclease that cleaves tRNA into tiRNAs that can directly inhibit protein synthesis. The primary target of angiogenin is tRNA with CA sequences in their anticodon loop. The 5' half of certain tRNA, such as the 5'-halves from tRNACys and tRNAAla, but not their 3' half, has a terminal oligo-G motif containing 4 to 5 consecutive guanosines, that displaces eIF4E and eIF4G and interferes with translation initiation leading to translation repression Citation[52]. Therefore, not every 5'-tiRNA but only selected, derived from Ala and Cys, inhibit translation initiation. In addition, tiRNA facilitates the expression of stress-response genes by interfering with siRNA-mediated silencing Citation[63]. Importantly, cleavage by angiogenin can be regulated by tRNA methylation mediated by the Drosophilia RNA methyltrasferases Nsun2 and Dnmt2 in Drosophilia Citation[64], or by the ribonuclease inhibitor RNH1 Citation[65].
Initially angiogenin was thought to mediate translational arrest as a consequence of depleted pools of tRNAs; however, steady-state levels of tRNAs do not actually change significantly upon tRNA production Citation[54]. Rather, tiRNAs contribute to the displacement of eIF4G/A from capped and uncapped mRNA and the displacement of eIF4E/G/A (eIF4F) from the m7G cap of mRNAs by cooperating with the translational silencer protein YB-1, a versatile RNA-binding protein, which stabilizes transcripts (including oncogenes) and mediates their enhanced expression Citation[52]. This may explain their ability to induce the assembly of stress granules (SG), which are dense cytoplasmic aggregates containing mRNA stalled in the initiation complex of translation, eventually promoting the adaptation and survival of stressed cells Citation[66]. In addition, angiogenin deactivates tRNAs by cleavage of the 3' CCA termini, adding a second layer of protein translation control upon cellular stress Citation[67]. Translation-independent cytoprotective actions of tiRNA have been described. For example, the interaction between tiRNA and cytochrome C will inhibit the formation of the apoptosome plateform, leading to an apoptosis-inhibiting effect Citation[68].
tRFs
tRFs are thought to arise from the ribonucleolytic processing of tRNAs by Dicer and RNase Z Citation[50], but there are likely multiple mechanisms by which they exert their inhibitory effects on translation and protein expression, which remain to be fully described. Nearly all functionally characterized 3′ CCA tRFs associate with components of the RNAi machinery and exert, at least in some cases, miRNA-like functions Citation[16]. Another mechanism has been proposed, where tRFs bind YB1. The interaction between tRFs and YB1 displaces several transcripts from YB1, thereby antagonizing YB1 activity. The displacement of these transcripts by tRFs reduces their stability and expression Citation[69].
Emerging roles of the tRNA ribonuclease angiogenin and tRNA catabolism in kidney diseases
The combination of high-throughput sequencing technology with new molecular, biochemical, and computational methods led to a significant improvement in our understanding of the fundamental biology of tRNA dynamics in response to cellular stress over the past few years. Therefore, it is not unexpected that the recent advances in this field have begun to reach the pathophysiology of acute kidney injuries. Thus, our understanding of how stress-induced tRNA fragments operate in adaptation to kidney injury is beginning to expand. Recently, experimental and clinical studies have provided insights into how angiogenin and tiRNA modulate the response to kidney injury, but surprisingly, the early literature also focused on the relationship between angiogenin and kidneys disease. These early studies should be reanalyzed and put in perspective in light of recent discoveries on the biological functions of angiogenin.
Angiogenin is an uremic toxin produced by the kidneys
A relationship between angiogenin and renal diseases was described decades ago, and in general, angiogenin is considered as a uremic toxin with immunomodulating properties Citation[70,71]. Indeed, plasma angiogenin concentrations increase in patients with chronic kidney disease (CKD) and patients under hemodialysis therapy, and angiogenin purified from uremic patients inhibits polymorphonuclear cell degranulation Citation[72–74]. Consistent with these results, a decrease in plasma concentrations of angiogenin during hemodialysis therapy is associated with a marked release of lactoferrin and metalloproteinase from polymorphonuclear leukocytes Citation[75]. Other than the immunomodulating properties of angiogenin, no clear biological or clinical consequences for increased concentrations of angiogenin during CKD have been determined, and whether plasma angiogenin accumulation participates in the complications of CKD is unknown.
Regarding the contribution of systemic angiogenin production, there is clear evidence for local renal production, at least under metabolic stress, as it can be observed during hypoxia or nutrient starvation Citation[76]. In addition, kidney cancers, including Wilm's tumors, produce angiogenin, along with other angiogenic factors such as vascular endothelial growth factor and basic Fibroblast growth factor 2 Citation[77,78], which likely contribute to tissue remodeling under ischemia to allow for adaptation to nutrient and oxygen shortages Citation[79]. These effects are consistent with the known angiogenic properties of angiogenin, which are at play in a wide array of cancers, and are mediated by its endothelial proliferative and antiapoptotic properties. Notably, the gene encoding angiogenin is regulated by HIF-1α Citation[58], but other stress response pathways, including the transcription factor sXBP1 (X box binding protein 1), also participate in angiogenin expression Citation[80].
tRNA fragmentation under ischemic kidney injury
tRNA cleavage by angiogenin under cellular stress is a regulated process that implies initiation of native tRNA conformational modifications, leading to the loss of the tertiary structure of the molecule, allowing the ribonuclease activity of angiogenin to access the anticodon loop of the tRNA and promote its cleavage into 5' and 3' halves (). Once produced, these tRNA fragments, the so-called tiRNA, will interfere with the initiation of protein translation and reduce translation rates. Unfolded tRNA are immunogenic, as they expose a specific nucleoside called 1-methyl adenosine, which is located in position 58 on the T loop (A58m) and can be recognized by artificial cognate antibodies.
To monitor tRNA conformational changes under cellular stress, as a marker of tRNA catabolism, antibodies have been raised against the methylated adenosine in position 58 that specifically recognize unfolded tRNA Citation[81]. Moreover, tRNA fragments that contain the methyl A58 can be detected by mass spectrometry and may serve to monitor tRNA fragmentation for diagnostic purposes. Use of these techniques has provided insights into the dynamics of tRNA catabolism in injured kidneys and systemic circulation during kidney disease. Unfolded (and therefore 'precleaved') tRNAs accumulate in the proximal tubules of rat kidneys soon after ischemia-reperfusion injury; interestingly, they also appear in the medullary regions even in sham animals, suggesting that tRNA fragmentation occurs physiologically, at least in tissues with a high basal level of oxidative stress. tRNA fragmentation is an early event that occurs after injury (i.e., after half an hour) but before apoptosis, which is reasonable if we consider that tRNA fragmentation is an early stress-response event that could insufficiently protect against programmed cell death if the intensity and duration of the stress are too high. In addition, kidney injury is associated with the detection and possible accumulation of tRNA fragments in the plasma of animals subjected to renal ischemia-reperfusion and individuals who underwent total aortic surgery (and consequently, acute ischemic kidney injury). These tRNA derivatives increase earlier in plasma than Kidney Injury Molecule (KIM)-1 in urine and precede plasma creatinine rise. The concentration of tRNA derivatives also increases in the blood of CKD patients and might be associated with an increased mortality Citation[81]. These latter findings can be reconciled with the earlier studies cited above, indicating that angiogenin concentrations also increase in the plasma of individuals with renal failure. It is tempting to speculate that accumulated angiogenin during CKD generates tiRNA, which promotes tissue adaptation against CKD-associated cellular stress, such as oxidative stress.
tiRNA protect against Endoplasmic reticulum stress-associated kidney injury
Recent experimental evidence indicates that tiRNA produced by angiogenin might promote cellular adaptation during acute kidney injury. In a mouse model of toxic acute tubular injury associated with ER stress and induced by the injection of tunicamycin, the genetic deletion of angiogenin is associated with more severe structural deterioration and altered renal function compared to control mice Citation[80]. In addition, apoptotic events occurred more frequently in the kidneys of angiogenin knockdown mice. The molecular bases of the cytoprotective effects of angiogenin regulation under ER stress have also been uncovered. Angiogenin expression is specifically induced during ER stress, under the control of the transcription factor sXBP1, which relies on the ribonuclease activity of IRE1α. Mechanistically, angiogenin is activated after dissociation from its inhibitor RNH1 (ribonuclease/angiogenin inhibitor) and promotes cellular adaptation to ER stress through the production of tiRNA, leading to the induction of SG assembly and translation inhibition. The cytoprotective properties of angiogenin likely extend the model of tunicamycin-induced kidney injury because angiogenin is expressed in the tubules of mouse kidneys with nephrotoxic serum-induced glomerulonephritis, ischemic injury, or cyclosporine nephrotoxicity. The model is clinically relevant because angiogenin is expressed in the tubules of kidneys transplant recipients with ischemia-reperfusion injury and cyclosporine nephrotoxicity.
Working model and future prospects
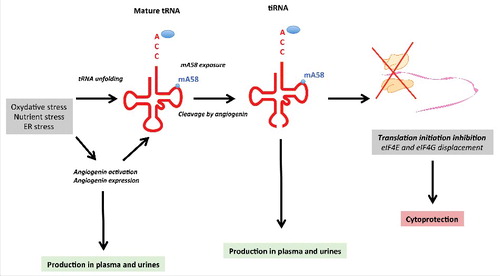
Collectively, these studies provide a rational for a model of the regulation and biological function of angiogenin and tRNA fragmentation during kidney injury, as well as the potential for biomarkers of kidney injury (). Soon after kidney injury as a result of, for example, oxidative stress under ischemia-reperfusion injury, tRNA unfolds. In parallel, angiogenin is activated (after the release from its inhibitor RNH1), and its expression is increased. Angiogenin cleaves tRNA at the anticodon loop, leading to the formation of 5' and 3' tRNA halves (i.e., tiRNA), which will interfere with the initiation of protein translation and reduce global protein synthesis. The reduction of protein synthesis rates appears to be cytoprotective under ER stress, but whether a set of stress response genes escape this inhibition to be selectively expressed remains to be demonstrated. If the intensity or duration of the stress is too high or prolonged, programmed cell death will occur. Because angiogenin and tRNA fragments are produced in the epithelium under stress, they can accumulate in the plasma of individuals with either acute or chronic kidney injury and may reflect the severity of the prognosis. In addition, they can be detected in urine and can be used as a non-invasive biomarker of ongoing kidney injury.
Figure 3. Model for angiogenin and tRNA fragmentation during kidney injury Cellular stress, such as oxidative stress, as it might occur during ischemia-reperfusion injury, promotes mature tRNA unfolding and exposition of a methylated adenosine at position 58 (mA58). Antibodies that recognize this epitope can be used to monitor tRNA unfolding under stress. In parallel, angiogenin is freed from its inhibitor, RNH1, and then becomes activated. Hypoxia and ER stress responses increase angiogenin expression. Angiogenin cleaves tRNA at the anticodon loop and fuel tiRNA formation, including 5' tRNA halves, which will interfere with the initiation of protein translation. Consequently, global protein synthesis will be reduced, which appears to be cytoprotective under cellular stress. In addition, mRNAs stalled in the initiation complex accumulate in the cytoplasm to produce stress granules. Because angiogenin and tRNA fragments are produced in the epithelium under stress, they can accumulate in the plasma of individuals with renal failure and may provide information on the prognosis.
The potential biological impact of tRNA fragmentation on renal cell homeostasis extends the roles of tiRNA and their cytoprotective effects on the stressed cell, and it opens the field of the biology of ncRNA and RNA fragmentation in renal pathophysiology with exciting perspectives. For example, an intriguing question would be to investigate whether these tRNA fragments are immunogenic and how are they sensed by primitive pattern recognition receptors. Indeed, the RNase activity of IRE1α cleaves mRNA, which produces endogenous RNA fragments that induce an inflammatory response. The mRNA fragments engage retinoic-acid inducible gene 1 (RIG-I), a cytosolic sensor of RNA viruses, to activate the NF-κB and interferons pathway Citation[82]. Whether tiRNAs mirror the effect of cleaved mRNA and are also immunogenic remain to be established. But such a process would contribute to the general immunogenicity of sterile stress responses, including ER stress, which are critical to mount an inflammatory response under a sterile milieu. Consistent with the possible immunogenicity of these cell-intrinsic responses, the accumulation of tRNA fragments observed during CKD, which is associated with a poorer prognosis, could contribute to the chronic inflammatory state associated with this condition.
Another research field in which tRNA biology impacts kidney pathophysiology is the tRNA-based pathologies. Several genetic disorders in which tRNA are thought to play a role have been described, and some may be associated with renal disease. These diseases include 2 types of pathogenic mutations, either within tRNA or in tRNA processing and modifying enzymes. For example, the 3243 A > G point mutation in the leucine tRNA gene causes MELAS syndrome in children (myopathy, encephalomyopathy, lactic acidosis, and stroke-like episodes) Citation[83]. Mutations in the leucine tRNA gene may be associated with diabetes and sensory deafness in adults, with a disproportional number of patients with end-stage renal failure secondary to a proteinuric renal disease consistent with glomerulosclerosis. Given that some organs seem to be affected more than others, this indicates that variations in tRNA expression and abundance may modulate the effect of tRNA mutations. This example paves the way for further genetics studies that attempt link mutations in either genes encoding tRNA or tRNA processing enzymes with kidney disease phenotypes. In addition, it would be of great interest to map the polymorphisms of the tRNA genomes to identify factors that would impact renal disease severity and/or progression.
Conclusion
In humans, there are 513 nuclear tRNA genes for 49 isoacceptors (different tRNA species carrying the same amino acids but with different anticodon sequences), which is metabolically wasteful for the cell and implies that several functions of tRNA are unrelated to the transmission of the message from the mRNA to the polypeptide chain. As part of the array of the known biological functions of tRNA, emerging evidence indicates that they are key regulators of stress responses by functioning directly as signaling molecules. tRNA dynamics provide the necessary plasticity to mount different regulatory layers for coping with stress and promoting multiple biological responses. tRNA fragments accumulate under stress after dedicated cleavage by ribonucleases, with the most important and best described being angiogenin. tRNA fragments are no longer considered to be simple debris but rather entities that perform critical and conserved functions to manage cellular adaptation under stress. tiRNAs promote RNA interference and silencing to repress protein translation and might support stress-response gene expression.
With advances in technologies and experimental approaches, we are only beginning to appreciate the contribution of the variety of programs that coordinate tRNA and tRNA fragment expression to tune translation and promote protein expression reprogramming upon cellular stress that may occur during kidney injury. With a deep quantitative and qualitative knowledge of tRNA biology in the kidney at a cell type-dependent level, we will gain a more accurate understanding of the specificity of the tRNA fragment dynamics that modulate renal disease severity and progression and define potential diagnostic and prognostic biomarkers of ongoing kidney injury.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Chovatiya R, Medzhitov R. Stress, inflammation, and defense of homeostasis. Mol Cell. 2014;54:281–8. doi:10.1016/j.molcel.2014.03.030
- Duffield JS. Macrophages and immunologic inflammation of the kidney. Semin Nephrol. 2010;30:234–54. doi:10.1016/j.semnephrol.2010.03.003
- Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11:264–76. doi:10.1038/nrneph.2015.3
- Nelson PJ, Rees AJ, Griffin MD, Hughes J, Kurts C, Duffield J. The renal mononuclear phagocytic system. J Am Soc Nephrol. 2012;23:194–203. doi:10.1681/ASN.2011070680
- Yang L, Humphreys BD, Bonventre JV. Pathophysiology of acute kidney injury to chronic kidney disease: maladaptive repair. Contrib Nephrol. 2011;174:149–55. doi:10.1159/000329385
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–108. doi:10.1016/S1097-2765(00)00108-8
- Spriggs KA, Bushell M, Willis AE. Translational regulation of gene expression during conditions of cell stress. Mol Cell. 2010;40:228–37. doi:10.1016/j.molcel.2010.09.028
- Spriggs KA, Bushell M, Mitchell SA, Willis AE. Internal ribosome entry segment-mediated translation during apoptosis: the role of IRES-trans-acting factors. Cell Death Differ. 2005;12:585–91. doi:10.1038/sj.cdd.4401642
- Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–90. doi:10.1038/ncb2738
- Hand SC, Hardewig I. Downregulation of cellular metabolism during environmental stress: mechanisms and implications. Annu Rev Physiol. 1996;58:539–63. doi:10.1146/annurev.ph.58.030196.002543
- Peng W, Robertson L, Gallinetti J, Mejia P, Vose S, Charlip A, Chu T, Mitchell JR. Surgical stress resistance induced by single amino acid deprivation requires Gcn2 in mice. Sci Transl Med. 2012;4:118ra11. doi:10.1126/scitranslmed.3002629
- Jones DP. Renal metabolism during normoxia, hypoxia, and ischemic injury. Annu Rev Physiol. 1986;48:33–50. doi:10.1146/annurev.ph.48.030186.000341
- Mele M, Ferreira PG, Reverter F, DeLuca DS, Monlong J, Sammeth M, Young TR, Goldmann JM, Pervouchine DD, Sullivan TJ, et al. Human genomics. The human transcriptome across tissues and individuals. Science. 2015;348:660–5. doi:10.1126/science.aaa0355
- Heyman SN, Rosen S, Rosenberger C. Renal parenchymal hypoxia, hypoxia adaptation, and the pathogenesis of radiocontrast nephropathy. Clin J Am Soc Nephrol. 2008;3:288–96. doi:10.2215/CJN.02600607
- Liu J, Krautzberger AM, Sui SH, Hofmann OM, Chen Y, Baetscher M, Grgic I, Kumar S, Humphreys BD, Hide WA, et al. Cell-specific translational profiling in acute kidney injury. J Clin Invest. 2014;124:1242–54. doi:10.1172/JCI72126
- Gebetsberger J, Polacek N. Slicing tRNAs to boost functional ncRNA diversity. RNA Biol. 2013;10:1798–806. doi:10.4161/rna.27177
- Kirchner S, Ignatova Z. Emerging roles of tRNA in adaptive translation, signalling dynamics and disease. Nat Rev Genet. 2015;16:98–112. doi:10.1038/nrg3861
- Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–45. doi:10.1016/j.cell.2009.01.042
- Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi:10.1042/BST0340007
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. doi:10.1016/j.cell.2012.03.017
- Stoneley M, Willis AE. Cellular internal ribosome entry segments: structures, trans-acting factors and regulation of gene expression. Oncogene. 2004;23:3200–7. doi:10.1038/sj.onc.1207551
- Somers J, Poyry T, Willis AE. A perspective on mammalian upstream open reading frame function. Int J Biochem Cell Biol. 2013;45:1690–700. doi:10.1016/j.biocel.2013.04.020
- Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, De Panis DN, Bobrovnikova-Marjon E, Diehl JA, Ron D, Koumenis C. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. Embo J. 2010;29:2082–96. doi:10.1038/emboj.2010.81
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi:10.1016/S1097-2765(00)80330-5
- Chaudhary K, Shinde R, Liu H, Gnana-Prakasam JP, Veeranan-Karmegam R, Huang L, Ravishankar B, Bradley J, Kvirkvelia N, McMenamin M, et al. Amino Acid Metabolism Inhibits Antibody-Driven Kidney Injury by Inducing Autophagy. J Immunol. 2015;194:5713–24. doi:10.4049/jimmunol.1500277
- Cai Q, Brooks HL. Phosphorylation of eIF2alpha via the general control kinase, GCN2, modulates the ability of renal medullary cells to survive high urea stress. Am J Physiol Renal Physiol. 2011;301:F1202–7. doi:10.1152/ajprenal.00272.2011
- Johno H, Nakajima S, Kato H, Yao J, Paton AW, Paton JC, Katoh R, Shimizu F, Kitamura M. Unfolded protein response causes a phenotypic shift of inflamed glomerular cells toward redifferentiation through dual blockade of Akt and Smad signaling pathways. Am J Pathol. 2012;181:1977–90. doi:10.1016/j.ajpath.2012.08.015
- Cybulsky AV, Takano T, Papillon J, Bijian K. Role of the endoplasmic reticulum unfolded protein response in glomerular epithelial cell injury. J Biol Chem. 2005;280:24396–403. doi:10.1074/jbc.M500729200
- Liang G, Yang J, Wang Z, Li Q, Tang Y, Chen XZ. Polycystin-2 down-regulates cell proliferation via promoting PERK-dependent phosphorylation of eIF2alpha. Hum Mol Genet. 2008;17:3254–62. doi:10.1093/hmg/ddn221
- Wu CT, Sheu ML, Tsai KS, Weng TI, Chiang CK, Liu SH. The role of endoplasmic reticulum stress-related unfolded protein response in the radiocontrast medium-induced renal tubular cell injury. Toxicol Sci. 2010;114:295–301. doi:10.1093/toxsci/kfq006
- Yokouchi M, Hiramatsu N, Hayakawa K, Kasai A, Takano Y, Yao J, Kitamura M. Atypical, bidirectional regulation of cadmium-induced apoptosis via distinct signaling of unfolded protein response. Cell Death Differ. 2007;14:1467–74. doi:10.1038/sj.cdd.4402154
- Grahammer F, Haenisch N, Steinhardt F, Sander L, Roerden M, Arnold F, Cordts T, Wanner N, Reichardt W, Kerjaschki D, et al. mTORC1 maintains renal tubular homeostasis and is essential in response to ischemic stress. Proc Natl Acad Sci U S A. 2014;111:E2817–26. doi:10.1073/pnas.1402352111
- Grahammer F, Wanner N, Huber TB. mTOR controls kidney epithelia in health and disease. Nephrol Dial Transplant. 2014;29 Suppl 1:i9-i18. doi:10.1093/ndt/gft491
- Mariappan MM, Prasad S, D'Silva K, Cedillo E, Sataranatarajan K, Barnes JL, Choudhury GG, Kasinath BS. Activation of glycogen synthase kinase 3beta ameliorates diabetes-induced kidney injury. J Biol Chem. 2014;289:35363–75. doi:10.1074/jbc.M114.587840
- Peruchetti DB, Cheng J, Caruso-Neves C, Guggino WB. Mis-regulation of mammalian target of rapamycin (mTOR) complexes induced by albuminuria in proximal tubules. J Biol Chem. 2014;289:16790–801. doi:10.1074/jbc.M114.549717
- Rai P, Plagov A, Lan X, Chandel N, Singh T, Lederman R, Ayasolla KR, Mathieson PW, Saleem MA, Husain M, et al. mTOR plays a critical role in p53-induced oxidative kidney cell injury in HIVAN. Am J Physiol Renal Physiol. 2013;305:F343–54. doi:10.1152/ajprenal.00135.2013
- Reid DW, Chen Q, Tay AS, Shenolikar S, Nicchitta CV. The unfolded protein response triggers selective mRNA release from the endoplasmic reticulum. Cell. 2014;158:1362–74. doi:10.1016/j.cell.2014.08.012
- Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186:323–31. doi:10.1083/jcb.200903014
- Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125:1111–24. doi:10.1016/j.cell.2006.04.031
- Anglicheau D, Muthukumar T, Suthanthiran M. MicroRNAs: small RNAs with big effects. Transplantation. 2010;90:105–12. doi:10.1097/TP.0b013e3181e913c2
- Bhatt K, Mi QS, Dong Z. microRNAs in kidneys: biogenesis, regulation, and pathophysiological roles. Am J Physiol Renal Physiol. 2011;300:F602–10. doi:10.1152/ajprenal.00727.2010
- Godwin JG, Ge X, Stephan K, Jurisch A, Tullius SG, Iacomini J. Identification of a microRNA signature of renal ischemia reperfusion injury. Proc Natl Acad Sci U S A. 2010;107:14339–44. doi:10.1073/pnas.0912701107
- Aguado-Fraile E, Ramos E, Conde E, Rodriguez M, Martin-Gomez L, Lietor A, Candela Á, Ponte B, Liaño F, García-Bermejo ML. A Pilot Study Identifying a Set of microRNAs As Precise Diagnostic Biomarkers of Acute Kidney Injury. PLoS One. 2015;10:e0127175. doi:10.1371/journal.pone.0127175
- Lorenzen JM, Kielstein JT, Hafer C, Gupta SK, Kumpers P, Faulhaber-Walter R, Haller H, Fliser D, Thum T. Circulating miR-210 predicts survival in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2011;6:1540–6. doi:10.2215/CJN.00430111
- Huttenhofer A, Brosius J, Bachellerie JP. RNomics: identification and function of small, non-messenger RNAs. Curr Opin Chem Biol. 2002;6:835–43. doi:10.1016/S1367-5931(02)00397-6
- Sabin LR, Delas MJ, Hannon GJ. Dogma derailed: the many influences of RNA on the genome. Mol Cell. 2013;49:783–94. doi:10.1016/j.molcel.2013.02.010
- Tuck AC, Tollervey D. RNA in pieces. Trends Genet. 2011;27:422–32. doi:10.1016/j.tig.2011.06.001
- Whitney ML, Hurto RL, Shaheen HH, Hopper AK. Rapid and reversible nuclear accumulation of cytoplasmic tRNA in response to nutrient availability. Mol Biol Cell. 2007;18:2678–86. doi:10.1091/mbc.E07-01-0006
- Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol. 2005;59:407–50. doi:10.1146/annurev.micro.59.031805.133833
- Sobala A, Hutvagner G. Transfer RNA-derived fragments: origins, processing, and functions. Wiley Interdiscip Rev RNA. 2011;2:853–62. doi:10.1002/wrna.96
- Anderson P, Ivanov P. tRNA fragments in human health and disease. FEBS Lett. 2014;588:4297–304. doi:10.1016/j.febslet.2014.09.001
- Ivanov P, Emara MM, Villen J, Gygi SP, Anderson P. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol Cell. 2011;43:613–23. doi:10.1016/j.molcel.2011.06.022
- Yamasaki S, Ivanov P, Hu GF, Anderson P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol. 2009;185:35–42. doi:10.1083/jcb.200811106
- Saikia M, Krokowski D, Guan BJ, Ivanov P, Parisien M, Hu GF, Anderson P, Pan T, Hatzoglou M. Genome-wide identification and quantitative analysis of cleaved tRNA fragments induced by cellular stress. J Biol Chem. 2012;287:42708–25. doi:10.1074/jbc.M112.371799
- Thompson DM, Lu C, Green PJ, Parker R. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA. 2008;14:2095–103. doi:10.1261/rna.1232808
- Fett JW, Strydom DJ, Lobb RR, Alderman EM, Bethune JL, Riordan JF, Vallee BL. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985;24:5480–6. doi:10.1021/bi00341a030
- Hartmann A, Kunz M, Kostlin S, Gillitzer R, Toksoy A, Brocker EB, Klein CE. Hypoxia-induced up-regulation of angiogenin in human malignant melanoma. Cancer Res. 1999;59:1578–83.
- Nakamura M, Yamabe H, Osawa H, Nakamura N, Shimada M, Kumasaka R, Murakami R, Fujita T, Osanai T, Okumura K. Hypoxic conditions stimulate the production of angiogenin and vascular endothelial growth factor by human renal proximal tubular epithelial cells in culture. Nephrol Dial Transplant. 2006;21:1489–95. doi:10.1093/ndt/gfl041
- Wiedlocha A. Following angiogenin during angiogenesis: a journey from the cell surface to the nucleolus. Arch Immunol Ther Exp (Warsz). 1999;47:299–305.
- Hu GF, Riordan JF, Vallee BL. A putative angiogenin receptor in angiogenin-responsive human endothelial cells. Proc Natl Acad Sci U S A. 1997;94:2204–9. doi:10.1073/pnas.94.6.2204
- Shapiro R, Vallee BL. Human placental ribonuclease inhibitor abolishes both angiogenic and ribonucleolytic activities of angiogenin. Proc Natl Acad Sci U S A. 1987;84:2238–41. doi:10.1073/pnas.84.8.2238
- Tsuji T, Sun Y, Kishimoto K, Olson KA, Liu S, Hirukawa S, Hu GF. Angiogenin is translocated to the nucleus of HeLa cells and is involved in ribosomal RNA transcription and cell proliferation. Cancer Res. 2005;65:1352–60. doi:10.1158/0008-5472.CAN-04-2058
- Durdevic Z, Mobin MB, Hanna K, Lyko F, Schaefer M. The RNA methyltransferase Dnmt2 is required for efficient Dicer-2-dependent siRNA pathway activity in Drosophila. Cell Rep. 2013;4:931–7. doi:10.1016/j.celrep.2013.07.046
- Schaefer M, Pollex T, Hanna K, Tuorto F, Meusburger M, Helm M, Lyko F. RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage. Genes Dev. 2010;24:1590–5. doi:10.1101/gad.586710
- Pizzo E, Sarcinelli C, Sheng J, Fusco S, Formiggini F, Netti P, Yu W, D'Alessio G, Hu GF. Ribonuclease/angiogenin inhibitor 1 regulates stress-induced subcellular localization of angiogenin to control growth and survival. J Cell Sci. 2013;126:4308–19. doi:10.1242/jcs.134551
- Anderson P, Kedersha N. Stress granules. Curr Biol. 2009;19:R397–8. doi:10.1016/j.cub.2009.03.013
- Czech A, Wende S, Morl M, Pan T, Ignatova Z. Reversible and rapid transfer-RNA deactivation as a mechanism of translational repression in stress. PLoS Genet. 2013;9:e1003767. doi:10.1371/journal.pgen.1003767
- Saikia M, Jobava R, Parisien M, Putnam A, Krokowski D, Gao XH, Guan BJ, Yuan Y, Jankowsky E, Feng Z, et al. Angiogenin-cleaved tRNA halves interact with cytochrome c, protecting cells from apoptosis during osmotic stress. Mol Cell Biol. 2014;34:2450–63. doi:10.1128/MCB.00136-14
- Goodarzi H, Liu X, Nguyen HC, Zhang S, Fish L, Tavazoie SF. Endogenous tRNA-Derived Fragments Suppress Breast Cancer Progression via YBX1 Displacement. Cell. 2015;161:790–802. doi:10.1016/j.cell.2015.02.053
- Ouseph R, Hutchison CA, Ward RA. Differences in solute removal by two high-flux membranes of nominally similar synthetic polymers. Nephrol Dial Transplant. 2008;23:1704–12. doi:10.1093/ndt/gfm916
- Vasandani VM, Wu YN, Mikulski SM, Youle RJ, Sung C. Molecular determinants in the plasma clearance and tissue distribution of ribonucleases of the ribonuclease A superfamily. Cancer Res. 1996;56:4180–6.
- Cohen G, Haag-Weber M, Horl WH. Immune dysfunction in uremia. Kidney Int Suppl. 1997;62:S79–82.
- Haag-Weber M, Horl WH. The immune system in uremia and during its treatment. New Horiz. 1995;3:669–79.
- Tschesche H, Kopp C, Horl WH, Hempelmann U. Inhibition of degranulation of polymorphonuclear leukocytes by angiogenin and its tryptic fragment. J Biol Chem. 1994;269:30274–80.
- Schmaldienst S, Oberpichler A, Tschesche H, Horl WH. Angiogenin: a novel inhibitor of neutrophil lactoferrin release during extracorporeal circulation. Kidney Blood Press Res. 2003;26:107–12. doi:10.1159/000070992
- Bouvier N, Fougeray S, Beaune P, Thervet E, Pallet N. The unfolded protein response regulates an angiogenic response by the kidney epithelium during ischemic stress. J Biol Chem; 287:14557–68. doi:10.1074/jbc.M112.340570
- Kluth B, Hess S, Engelmann H, Schafnitzel S, Riethmuller G, Feucht HE. Endothelial expression of CD40 in renal cell carcinoma. Cancer Res. 1997;57:891–9.
- Skoldenberg EG, Christiansson J, Sandstedt B, Larsson A, Lackgren G, Christofferson R. Angiogenesis and angiogenic growth factors in Wilms tumor. J Urol. 2001;165:2274–9. doi:10.1016/S0022-5347(05)66183-6
- Ramani P, Headford A, Sowa-Avugrah E, Hunt LP. Angiogenin expression in human kidneys and Wilms' tumours: relationship with hypoxia and angiogenic factors. Int J Exp Pathol. 2013;94:115–25. doi:10.1111/iep.12012
- Mami I, Bouvier N, El Karoui K, Gallazzini M, Rabant M, Laurent-Puig P, Li S, Tharaux PL, Beaune P, Thervet E, et al. Angiogenin Mediates Cell-Autonomous Translational Control under Endoplasmic Reticulum Stress and Attenuates Kidney Injury. J Am Soc Nephrol;.
- Mishima E, Inoue C, Saigusa D, Inoue R, Ito K, Suzuki Y, Jinno D, Tsukui Y, Akamatsu Y, Araki M, et al. Conformational change in transfer RNA is an early indicator of acute cellular damage. J Am Soc Nephrol. 2014;25:2316–26. doi:10.1681/ASN.2013091001
- Cho JA, Lee AH, Platzer B, Cross BC, Gardner BM, De Luca H, Luong P, Harding HP, Glimcher LH, Walter P, et al. The unfolded protein response element IRE1alpha senses bacterial proteins invading the ER to activate RIG-I and innate immune signaling. Cell Host Microbe. 2013;13:558–69. doi:10.1016/j.chom.2013.03.011
- Emma F, Montini G, Salviati L, Dionisi-Vici C. Renal mitochondrial cytopathies. Int J Nephrol. 2011;2011:609213.
