ABSTRACT
Over the past 2 decades, different types of circular RNAs have been discovered in all kingdoms of life, and apparently, those circular species are more abundant than previously thought. Apart from circRNAs in viroids and viruses, circular transcripts have been discovered in rodents more than 20 y ago and recently have been reported to be abundant in many organisms including humans. Their exact function remains still unknown, although one may expect extensive functional studies to follow the currently dominant research into identification and discovery of circRNA by sophisticated sequencing techniques and bioinformatics. Functional studies require models and as such methods for preparation of circRNA in vitro. Here, we will review current protocols for RNA circularization and discuss future prospects in the field.
Abbreviations
| AMP | = | adenosine monophosphate |
| AppRNA | = | 5′-adenylated RNA |
| ATP | = | adenosine triphosphate |
| bp | = | base pair |
| CEV-A | = | citrus exocortis viroid strain A |
| circRNA | = | circular RNA |
| ciRNA | = | circular intronic RNA |
| CuAAC | = | cupper catalyzed azide alkyne cycloaddition |
| EDC | = | ethyl-3-(3′-dimethylaminopropyl)-carbodiimide |
| HIV | = | human immunodeficiency virus |
| IEciRNA | = | intron-exon circular RNA |
| nt | = | nucleotide |
| PIE | = | permuted intron exon |
| T4 Dnl | = | T4 DNA Ligase |
| T4 Rnl | = | T4 RNA Ligase |
Introduction
In recent years, the interest in circular RNAs (circRNA) as a novel type of endogenous non-coding RNA has been strongly grown. CircRNAs are found in all kingdoms of life; thousands have been identified across species from Archaea to humans.Citation1-8 CircRNAs have been known for a long time in form of genomes of viroidal plant pathogenes or Hepatitis delta virus.Citation9 Furthermore, circRNAs can be formed from introns (hence termed circular intronic RNA (ciRNA),Citation6 or from exons with introns retained between the exons (termed exon-intron circRNA (IEciRNA).Citation7 However, the vast majority of circRNAs apparently is transcribed and spliced from exons of protein and non-coding genes.Citation1,10,Citation11 Initially discovered as ubiquitous molecules in mice and human cells, those circRNAs were subsequently found as abundant, conserved and stable species in all eukaryotes studied today.Citation3,4
Whereas a large number of data have allowed suggesting models for their formation,Citation10,11 the biological function of circRNAs has remained elusive. For a long time, circRNAs were seen just as side products of aberrant splicing reactions. This view has however dramatically changed due to the fact that with the development of deep sequencing and bioinformatic tools a large number of circRNAs have been discovered in mammalian and other cells.Citation1-5,Citation7 Strikingly, often circRNA is even the more abundant isoform than mRNA.Citation2,12,Citation13 Due to their covalently closed ring structure, circRNAs have a higher stability in the cellular environment as compared with their linear counterparts, implying that possible functions of circRNA may be associated with the longer life time of these molecules. In fact, based on recent research in the field, circRNAs have been suggested to act as miRNA and protein sponges, to be involved in the modulation of gene expression and splicing, and to be associated with diseases initiation and progression.Citation4,7,Citation14-18 Thus, circRNAs have also been considered as biomarkers for diseases prognostics and diagnostics and as targets or tools for disease treatment.Citation19-21
The field of investigations into circRNA and in particular into their putative function within the complex network of regulation of gene expression and splicing is currently very active. In addition to the technical and conceptual challenges, raised for example by sequence overlap with mRNA or linear non-coding RNA transcribed from the same locus, synthetic circRNAs are needed as models for structure and function studies. This has put demand on the side of chemistry and biochemistry to elucidate and optimize protocols for the generation of circRNA. Basically, procedures for intermolecular RNA-ligation may be adapted to proceed in intramolecular fashion. The key issue is the design of the ligation assays in favor of intramolecular end joining (circularization) versus intermolecular reaction (oligomerization). Circular RNAs can be made from their linear counterparts by enzymatically or chemically supported reactions and in addition by the help of specially designed ribozymes. RNA circularization by chemical means can produce a natural phosphodiester bond. In addition, a number of powerful chemistries may be used, which however, would lead to the formation of non-natural linkages. This is not necessarily a problem, because one modified linkage among the many natural phosphodiester bonds may be tolerated without significantly affecting the function. Here we will review the methods available for formation of nucleic acid phosphodiester bonds, highlight those protocols that have been used already for production of circRNAs, but also show potential strategies that might be used in the future.
RNA synthesis and functionalization
Chain assembly
At the beginning of all circRNA preparations is the synthesis of the linear RNA with suitable 5′- and 3′-terminal functionalities for end-joining. For enzymatic protocols these functionalities are usually 5′-phosphate and 3′-OH. However, for chemical ligation, the required terminal functionalities may be rather diverse (). Therefore, chemical RNA synthesis allowing the site-specific introduction of a desired functionality at the 5′- and/or 3′-end is a valuable option. Chemical RNA synthesis is limited to oligomers of about 50 to 70 nucleotides length. However, protocols for joining of RNA fragments are available,Citation22 which could be implemented into protocols for RNA circularization. For example, recently we have prepared a modified 129mer RNA for fluorescence studies by enzymatic and chemical ligation of 2 RNA fragments.Citation23 As an alternative to chemical synthesis, RNA can be produced by in vitro transcription, which allows preparation of RNAs of virtually unlimited length, however, requires post-synthetic functionalization strategies.
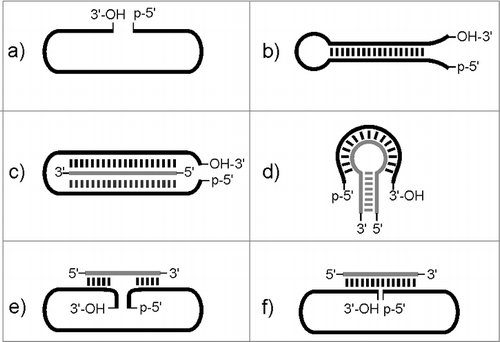
Figure 1. Oligonucleotide circularization strategies. (a) enzymatic ligation of a 5′-phosphate with a 3′-OH terminus; (b) chemical ligation of a phosphate with OH-terminus (the 5′- or the 3′-end can be phosphorylated); (c) chemical ligation of a 3′-thiophosphate with a tosylated 5′-end; (d) chemical ligation of a 3′- thiophosphate with a iodinated 5′-end; (e) chemical ligation of a 3′-aldehyde with a 5′oxoamine (oxime circularization); (f) chemical ligation of a 5′- or 3′-azide with a 3′- or 5′- alkyne (Click circularization); (g) circularization by metal chelation (M = Zn2+ or Fe2+, ( = terpyridine).
5′-terminal functionalization
In case of chemical RNA synthesis, 5′-terminal modifications such as phosphates, amines, thiols, azides or alkynes to be used directly for cyclization or, prior to cyclization, for further conjugation chemistry to introduce the required functionality, can be introduced by coupling the respective phosphoramidite as last building block in the chain assembly proceeding from the 3′- to the 5′-end.Citation23
A characteristic feature of in vitro transcribed RNA is the 5′-terminal triphosphate, because 5′-triphosphates of nucleosides are used as building blocks for enzymatic polymerization. As mentioned above, enzymatic end-joining for circularization mostly requires a 5′-monophosphate, and also chemical procedures rely on specific functionalities. Therefore, the 5′-terminal triphosphate needs to be removed prior to further functionalization and ligation. This can be easily achieved by treatment with alkaline phosphatase or any other dephosphorylation enzyme, and subsequent introduction of the required 5′-functionality. A phosphate group can be easily attached onto the 5′-OH by reaction with ATP in the presence of T4 Polynucleotide kinase. This protocol would also allow for further chemical modification, if instead of ATP γ-thio-ATP is used, such that the sulfur of the transferred thiophosphate can be used for further chemical conjugation of a desired functionality. Another option is the reaction of the unique primary alcohol at the 5′-end with triphenylphosphonium iodide to generate a good leaving group and thus making the 5′-terminus suitable for reaction with a nucleophile, as for example an azide.Citation23,24 The azide may be directly used for Click cyclization (see below) or, upon reduction to an amino group, for conjugation with a desired functionality via peptide chemistry.Citation25
Specific 5′-RNA functionalization can be also achieved by transcription priming, adding to the transcription mixture an initiator nucleotide that carries no triphosphate for polymerization and therefore can be used only for priming RNA synthesis at the 5′-end.Citation26,27 This protocol has been mostly used with T7-RNA-Polymerase and therefore requires guanosine conjugated via a 5′- phosphate with the desired functionality, as initiator nucleotide (the 5′-phosphate is necessary for recognition/acceptance of the modified building block by the polymerase). In our laboratory, we have made use of this strategy for enzymatic preparation of RNAs carrying a biotinylated nucleosideCitation28 or a monophosphateCitation29 at the 5′-end.
3′-terminal functionalization
As for 5′-terminal modification, 3′-end modification can be easily achieved by chemical synthesis provided that a suitable modified building block attached to a solid support is available or alternatively, can be made in the laboratory. Preparation of 3′-phosphorylated RNAs, as well as of RNAs with 3′-terminal amines, thiols, azides or alkynes can be easily performed and belongs to the standard repertoire of solid phase phosphoramidite chemistry. Moreover, protocols for post-synthetic modification of the 3′-terminus are available, therein using the characteristic features of the unique 3′-terminal cis-diol. The cis-diol, in the presence of periodate, is easily oxidized to a dialdehyde, which subsequently is reacted with N-nucleophiles, such as amines or hydrazines to introduce a desired functionality.Citation30 In our hands, this procedure works very well, and we have prepared a number of RNAs modified with fluorescence dyes or with flavine derivatives.Citation31
Circularization strategies by 5′-3′-end joining
In general, the preparation of circRNAs from linear precursors is very challenging, due to the negative entropy associated with the circularization step. The most significant side reaction is intramolecular bond forming leading to oligomerization of the linear precursor instead of circularization. This can be counteracted by a clever choice of reaction conditions, in particular by strict control of the concentration regime. Intramolecular reaction would be favored over intermolecular reaction at lower concentration of the linear precursor, which however, has the disadvantage that production of circRNAs at preparative scale becomes rather challenging. Therefore special protocols for pre-orientation of the 2 termini to be linked are of utmost importance. A most favorable scenario is a self-templating effect based on the intrinsic secondary structure of the linear precursorCitation32,33 (). Dependent on the specific ligation protocol, the ligation junction can be positioned in a double-stranded area of the folded nucleic acid structure (nick site) or in the single-stranded area with the 2 reacting ends positioned next to each other (e.g. in a hairpin loop). This strategy would allow also for a template-assisted convergent approach of circularization: 2 or even more fragments constituting the full-lengths linear/circular nucleic acid need to be designed to interact in the required way to achieve multi-site ligation and circularization in one.Citation34 Other ways of pre-orienting the 2 reacting ends may include a linear or hairpin helper oligonucleotide (), or a linear oligonucleotide (splint) that interacts with the 5′- and 3′-end of the linear precursor bringing them in a flanking alignment and thus serving as splint for template assisted ligation ().Citation35-37 Thus, the choice of the ligation site and of the explicit strategy depends on the RNA substrate to be circularized and in addition is affected by the decision of enzymatic vs. chemical ligation.
Enzymatic strategies
Several protocols for enzymatic ligation of RNA fragments are available,Citation38 most commonly employing 3 different polynucleotide ligases:
T4 DNA ligase (T4 Dnl). The enzyme requires ATP as cofactor and supports the formation of a phosphodiester bond between a 5′-phosphate and a 3′-hydroxyl group within double stranded regions of DNA or RNA or even hybrid structures. Very importantly, the enzyme catalyzes the covalent joining of the 2 ends only in case of full undistorted complementarity and if no nucleotides are missing at the junction.Citation39 A splint can be used to create the required double stranded region around the ligation site (as depicted in ).Citation40 T4 Dnl-assisted ligation of 2 RNA fragments was pioneered by Moore and Sharp,Citation41,42 and is also suitable for RNA circularization. Using a DNA splint is most favorable, because after reaction the splint can be digested with DNAse. The circularized RNA product can then easily be separated from the linear precursor by polyacryl amide gel electrophoresis. For intermolecular ligation, it has been shown that the length of the splint affects the efficiency of the ligation.Citation40 However, in general it is impossible to predict an optimal splint length of universal validity. Thus, for each individual system, reaction conditions with special focus on temperature, stoichiometry and splint length need to be defined in order to achieve efficient ligation.
The T4 DNA ligase directed closure of linear DNA is common in the literature,Citation43 whereas examples of RNA circularization with T4 DnI are rather rare. A representative example is the T4 Dnl assisted circularization of a 453nt linear RNA precursor that was shown to be translated into protein by the eukaryotic translational apparatus.Citation44 Furthermore, circular dumbbell RNA/DNA chimeric oligonucleotides were designed for the efficient inhibition of HIV-1 replication.Citation45 These circular chimeras consisted of a sense RNA and its complementary antisense DNA, connected via 2 ethylene glycol loop structures. Circularization was carried out by enzymatic ligation of the 5′-phosphorylated nicked 46-mer RNA/DNA oligonucleotides with T4 DNA ligase.
T4 RNA Ligase 1 (T4 Rnl1). The enzyme, in the presence of ATP as cofactor, supports the ligation of single stranded nucleic acids, and accepts DNA and RNA fragments as substrates.Citation46 T4 RNA ligase is useful for joining RNA to RNA, provided that the donor contains a 5´-phosphate and the acceptor a 3′-OH (). When designing the experiment and defining the ligation junction for circularization, it should be taken into account that the ligase has different preferences for the terminal nucleotides of the donor- and acceptor strand. This is A > G ≥ C > U for the 3′-terminal nucleotide of the acceptor strand, and pC > pU > pA > pG for the 5′-terminal nucleotide of the donor strand.Citation47-50 T4 Rnl1 assisted RNA ligation has been used to produce large amounts of homogeneous and pure circRNA.Citation51 A key element of these syntheses was the exploitation of the RNA intrinsic secondary structure for orientation of the 5′- and 3′-termini for efficient ligation. Furthermore, dumbbell-shaped circular RNAs for RNAi applications were designed and synthesized by ligation of 2 RNA strands that form a stem structure with overhanging single stranded regions and covalent joining of the thus pre-oriented 5′-and 3′-termini at both sites with T4 Rnl1.Citation52-54 Successful ligation in linear arrangements has been also achieved for tRNAs with the ligation junction positioned in the anticodon and the D loop pre-oriented by the intrinsic tRNA structure.Citation55,56 Further examples of RNA circularization by T4 Rnl1 include the synthesis of an infectious circRNA of the citrus exocortis viroid strain A (CEV-A),Citation57 circular versions of the hammerhead ribozyme,Citation58 and others more.Citation19 Apart from intrinsic secondary structure elements that help to orient the 2 termini to be ligated close to each other, proper orientation of the reactive ends can be achieved by using a splint that by hybridization with both the donor and the acceptor brings the substrates close together, however, leaves the terminal 2 to 3 nucleotides of both strands single stranded at the ligation junction ().Citation59 In our laboratory, best results were obtained with a DNA splint leaving 2 nucleotides of both donor and acceptor single stranded at the ligation junction. This setup allowed us to achieve, though in an intermolecular set-up, ligation yields of up to 45%.Citation38
T4 RNA ligase 2 (T4 Rnl2). The enzyme ligates RNA acceptor strands (3′-OH group) with RNA- or DNA-donor strands (5′-phosphate group), preferentially in nicked dsRNA substrates.Citation60,61 It is a close relative of T4 Rnl1 and was first described by Ho and Shuman in 2002.Citation62 We have successfully used T4 Rnl2 to circularize a 104nt linear precursor RNA with donor and acceptor ends positioned in a double stranded region.Citation29 Recently, circular RNAs ranging in size from 129 to 387 nucleotides were prepared by intramolecular ligation of the respective linear precursor in the presence of a 20nt DNA helper oligonucleotide.Citation63
In summary, RNA circularization by enzymatic ligation is a valuable option. All three enzymes mentioned above are ATP-dependent and catalyze the joining of RNA termini with 5′-phosphate and 3′-OH via 3 nucleotidyltransfer steps: (i) the enzyme reacts with ATP to form a covalent ligase-AMP intermediate; (ii) AMP of the intermediate is transferred to the 5′-phosphorylated RNA terminus forming 5′-RNA-adenylate (AppRNA); (iii) AppRNA is attacked by the 3′-hydroxyl group to form a phosphodiester bond, whereby AMP is liberated. Ligation can proceed in an intramolecular fashion leading to circRNA. Formation of side products by intermolecular ligation cannot be completely ruled out. However, the intrinsic secondary structure of the RNA to be ligated in conjunction with the clever choice of the ligation site and the application of helper oligonucleotides may assist intramolecular ligation, thus favoring RNA circularization.
Chemical strategies
Chemical ligation of nucleic acid strands in inter- or intramolecular fashion allows formation of natural phosphodiester bonds. However, in addition the chemist's toolbox provides a large number of powerful reactions that may be used for joining 2 appropriately functionalized fragments or termini of one and the same molecule. Whereas natural phosphodiester bond formation is favorable in terms of generating an unmodified linkage as it occurs in natural biomolecules, yields are often unsatisfactory. On the contrary, more efficient reactions can be achieved by specific functionalization/activation of reacting ends, which however creates unnatural linkages in the ligated/circularized molecule.
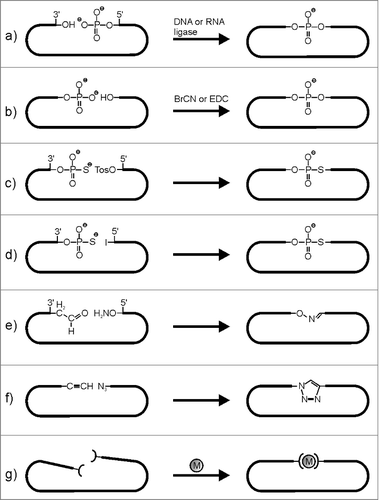
Natural phosphodiester linkages. As an alternative to enzymatic ligation, phosphodiester formation can be achieved also by pure chemical strategies, and it is up to the experimental design, if ligation proceeds inter- or intramolecularly. Small circles can be prepared by chemical cyclization of protected oligonucleotides in solution or still at solid support.Citation43,64 For longer sequences however, template-based strategies as explained above and illustrated in are needed. Natural phosphodiester bonds are formed by condensation of 5′-phosphate with 3′-OH or vice versa in the presence of phosphate activating agents, such as cyanogen bromide (BrCN) or water-soluble ethyl-3-(3′-dimethylaminopropyl)-carbodiimide (EDC)(). Chemical ligation was originally developed for linking DNA strands, and since then has been used quite extensively for the synthesis of DNA circles.Citation43 The most significant problem associated with chemical ligation of RNA is the formation of 2′, 5′-phosphodiester bonds, due to phosphate migration. To circumvent this problem, 2′-deoxynucleotides may be used at the 3′-end of oligonucleotides to be ligated/circularized. As a representative example, Wang et al. synthesized a 34mer circular RNA by chemical ligation of the 5′-phosphorylated linear precursor with BrCN/imidazole/NiCitation2+. Favorable orientation of the 2 reacting ends was achieved by triplex formation with a short DNA oligonucleotide (as shown in ).Citation65
Non-natural linkages. There are a number of alternative chemistries for oligonucleotide ligation, which however, rely on specific functionalization of the reacting ends, and as mentioned above, in consequence yield unnatural linkages at the ligation junction (). Nevertheless, such alternative chemistries have been used, because of high efficiency and the possibility to be carried out at larger scale. Furthermore, one non-natural linkage within a circular DNA or RNA may well be tolerated without considerable effects on the function. Basically, the required functionalities for chemical ligation/circularization are introduced during oligonucleotide synthesis or alternatively, are conjugated post-synthetically. Circularization then is initiated by either self-templating effects or helper oligonucleotides as described above, bringing the 3′- and 5′-end in close proximity. Whereas a number of reports describe the synthesis of circular DNAs with a non-natural linkage at the ligation site, application of such specific protocols in the field of circRNA synthesis is still rather rare. For example, DNA has been circularized using SN2- reactions between 5′-termini with tosylate or iodo leaving group and 3′-phosphorothioates ().Citation66,67 Reaction of bifunctionalized oligonucleotides with terminal 5′-oxyamine and 3′-aldehyde delivered circDNA with oxime linkage ().Citation68 Oxime circularization was also used for circularization of RNA, although only small circles (tetramers) were prepared.Citation69 Other circularization procedures involve copper catalyzed azide alkyne cycloaddition (CuAAC)Citation70,71 (), strategies for photoligation,Citation72 or conjugation of metal binding ligands such as terpyridine to the 5′- and 3′-end of the oligonucleotide to be circularized ().Citation73 The latter method was used to produce a circular 2′-OMe-RNA. Terpyridine was coupled to the 5′- and 3′-end of a short linear RNA by phosphoramidite chemistry and circularization was achieved by addition of Zn2+ or Fe2+.
Many chemical ligation protocols still suffer from rather low yields and as described above often require specific functionalization of the linear precursor. It remains questionable if the available strategies can be used for circularization of large circular RNA molecules, and if they can compete with enzymatic ligation. Click circularization by CuAACCitation70,71 is certainly one of the most powerful methods for linking the 2 ends of a linear RNA bearing a terminal alkyne and azide function (). However, these functionalities first need to be introduced, and the resulting triazole linkage has to be tolerated. Thus, the choice of the circularization strategy should always consider the intended application of the circular product.
Ribozymes for RNA circularization
A recent study of human circRNAs revealed that these molecules are usually composed of 1–5 exons.Citation4 Considering that about 80 % of exons are in the size range of 20 to 200 bp,Citation74 biologically relevant circular RNAs may greatly vary in size, being composed of up to 1000 bases. Thus, in addition to enzymatic and chemical strategies described above that are more favorable to the synthesis of smaller RNA circles, methods for production of large circular RNAs are needed.
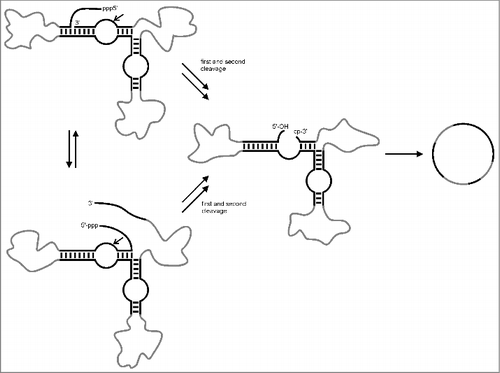
Many ribozymes occurring in nature are capable of catalyzing phosphodiester bond formation within specific sequence context. However, this natural occurring activity has rarely been used for engineering into tools that would support RNA ligation/circularization at preparative scale. Previously, we have engineered hairpin ribozyme variants that fold into 2 alternative cleavage active conformations, thus cutting off their 5′- and 3′-terminus, and thereby producing a 5′-OH group and a 3′-terminal 2′,3′-cyclic phosphate.Citation29,75,Citation76 These characteristic ends are required for ligation, which then can take place in an intramolecular manner as depicted in . The driving force of this reaction is the characteristic cleavage-ligation equilibrium of the hairpin ribozyme: Ligation proceeds via ring opening of the cyclic phosphate, thus being enthalpically favored over cleavage. Entropically, ligation is disfavored, owing to the decrease in degrees of conformational freedom upon connecting the 2 fragments. However, the entropic cost of ligation is rather small and can be compensated by the favorable enthalpic contribution. In addition, ligation is about 2 times faster than cleavage.Citation77,78 Thus, the internal equilibrium of the hairpin ribozyme is shifted toward ligation. Translated into practical use this means that the 2 activities can be controlled by structural modulation. Hairpin ribozymes that form a stable structure, such that fragments remain bound, favor ligation, whereas hairpin ribozymes that are less stable, such that cleavage fragments can easily dissociate, favor cleavage. We have demonstrated hairpin ribozyme supported RNA circularization for the generation of 83mers.Citation29,75,Citation76 However, as shown in , the hairpin ribozyme structure may be embedded in RNA sequences of variable length (gray lines), thus paving the way toward preparation of large RNA circles. A disadvantage of the procedure is that the generated circRNAs are not stable due to the dynamic equilibrium of hairpin ribozyme catalyzed cleavage and ligation. Previously, we have shown that hairpin ribozyme activity can be allosterically controlled by external cofactors.Citation79,80 Therefore, a possible scenario of hairpin ribozyme supported RNA circularization could exist of enzymatic or chemical synthesis of linear precursors containing the hairpin ribozyme structure at the ligation junction, but requiring an additional cofactor, such as an oligonucleotide effector or an allosteric activator, for induction of ligation. After reaction, the cofactor can be removed, thus switching off hairpin ribozyme activity and leaving behind the ligated RNA as stable circle.
Figure 3. Hairpin ribozyme supported RNA circularization. The in vitro transcribed linear precursor folds into 2 alternative cleavage-active conformations, removing first the 3′-end and then the 5′-end or vice versa. The resulting structure contains a 5′-OH and a 2′, 3′-cyclic phosphate as required for ligation. As a result a circular RNA is formed. For detailed information see text. Black: minimal hairpin ribozyme structure; gray: any desired sequence.
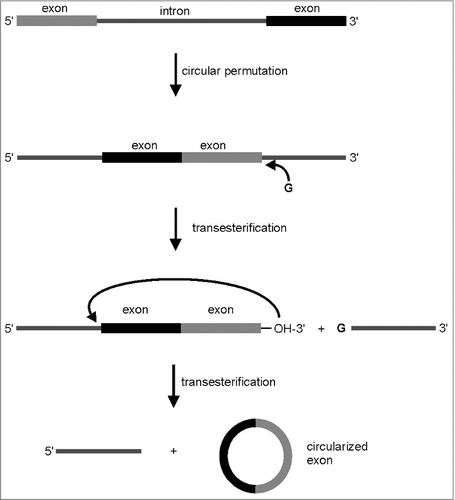
Another method for ribozyme-supported production of circular RNAs relies on a spontaneous group I intron self-splicing system (). A circularly permuted group I intron precursor RNA consisting of end-to-end fused exons that interrupt half intron sequences, self-splices to generate a circular RNA.Citation81 As it was demonstrated later, foreign sequences can be integrated into the exon of such a permuted self-splicing system, thus allowing the synthesis of circRNAs with desired sequence elements.Citation82 Based on the permuted intron exon (PIE) method, a variety of circular RNAs up to the length of 1130 nts have been generated in vitro as well as in vivo, mostly in E.coli cells.Citation4,83-86 Because RNA ligation for circularization is catalyzed by the intronic RNA, the system is also known as RNA cyclase ribozyme.Citation82 There is an early report on PIE mediated production of circular RNA in yeast. The circularly permuted group I intron precursor RNA was expressed as a eucaryotic mRNA from a regulated promotor, and the accumulation of circRNAs was demonstrated.Citation82 However, it remains questionable if the PIE method is reliably applicable in eucaryotic cells, because so far further evidence is missing. There are also reports on modified group II introns for inverse splicing that generates circRNA.Citation87,88 In a way similar to the PIE method, exons need to be arranged in a consecutive way with the branch point upstream and intronic sequences up- and downstream of the exons. Group II intron self-splicing systems designed in this manner have shown good circularization efficiency and are favorable in terms of complete exon sequence variability. However, due to the mechanism of group II intron self-splicing, the produced circRNA have a 2′, 5′-phosphodiester linkage at the ligation junction.
Summary
The appearance of circRNAs in all kingdoms of life implies that these abundant stable RNA species have important biological functions, which however are not yet fully understood. The growing interest in circRNA function has put demand on chemists to develop strategies for synthesis of circular RNA. Enzymatic and chemical strategies for RNA ligation have been adapted to proceed at intramolecular level, assisted by self-templating effects or helper oligonucleotides. In addition, naturally occurring ribozymes have been engineered to support circularization of linear precursors in self-processing reaction schemes. The choice of the specific protocol should be made on the basis of where the circRNA should be produced (in vitro or in vivo), the nature of the RNA components (modified versus natural nucleotides), and the size of the circRNA. Ribozyme supported self-circularization (hairpin ribozyme or cyclase ribozyme) allows in vivo production of circRNA, whereas chemical and enzymatic procedures can be applied exclusively in vitro. Natural linear RNAs can easily be produced by in vitro transcription; the integration of modified nucleotides or other functionalities however, requires chemical synthesis of linear precursors followed by chemical or enzymatic ring closure. Circularization of linear precursors is entropically disfavored, in particular when it comes to circles of larger size. Therefore, self-splicing scenarios that as a result of intrinsic mechanistic features deliver circular RNAs are advantageous over enzymatic/chemical ring closure reactions for the synthesis of large circRNAs. On the contrary, smaller RNA circles can be easily generated by intramolecular ligation.
The field of investigation into biogenesis and function of circRNA will continue and it is on the chemists to refine RNA circularization strategies or even to develop new protocols to deliver circRNA models on demand.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One 2012; 7:e30733; PMID:22319583; https://doi.org/10.1371/journal.pone.0030733
- Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO. Cell-type specific features of circular RNA expression. PLoS Genet 2013; 9:e1003777; PMID:24039610; https://doi.org/10.1371/journal.pgen.1003777
- Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013; 19:141-57; PMID:23249747; https://doi.org/10.1261/rna.035667.112
- Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013; 495:333-8; PMID:23446348; https://doi.org/10.1038/nature11928
- Guo JU, Agarwal V, Guo H, Bartel DP. Expanded identification and characterization of mammalian circular RNAs. Genome Biol 2014; 15:409; PMID:25070500; https://doi.org/10.1186/s13059-014-0409-z
- Zhang Y, Zhang XO, Chen T, Xiang JF, Yin, Q.F., Xing YH. Circular intronic long noncoding RNAs. Mol Cell 2013; 51:792-806; PMID:24035497; https://doi.org/10.1016/j.molcel.2013.08.017
- Li Z, Huang C, Bao C, Chen L, Lin M, Wang X, Zhong G, Yu B, Hu W, Dai L, et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol 2015; 22:256-64; PMID:25664725; https://doi.org/10.1038/nsmb.2959
- Danan M, Schwartz S, Edelheit S, Sorek R. Transcriptome-wide discovery of circular RNAs in Archaea. Nucleic Acids Res 2012; 40:3131-42; PMID:22140119; https://doi.org/10.1093/nar/gkr1009
- Flores R, Grubb D, Elleuch A, Nohales MA, Delgado S, Gago S. Rolling-circle replication of viroids, viroid-like satellite RNAs and hepatitis delta virus: variations on a theme. RNA Biol 2011; 8:200-6; PMID:21358283; https://doi.org/10.4161/rna.8.2.14238
- Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol 2014; 32:453-61; PMID:24811520; https://doi.org/10.1038/nbt.2890
- Qu S, Yang X, Li X, Wang J, Gao Y, Shang R, Sun W, Dou K, Li H. Circular RNA: A new star of noncoding RNAs. Cancer Lett 2015; 365:141-8; PMID:26052092; https://doi.org/10.1016/j.canlet.2015.06.003
- Lu T, Cui L, Zhou Y, Zhu C, Fan D, Gong H, Zhao Q, Zhou C, Zhao Y, Lu D, et al. Transcriptome-wide investigation of circular RNAs in rice. RNA 2015; 21:2076-87; PMID:26464523; https://doi.org/10.1261/rna.052282.115
- Broadbent KM, Broadbent JC, Ribacke U, Wirth D, Rinn JL, Sabeti PC. Strand-specific RNA sequencing in Plasmodium falciparum malaria identifies developmentally regulated long non-coding RNA and circular RNA. BMC Genomics 2015; 16:454; PMID:26070627; https://doi.org/10.1186/s12864-015-1603-4
- Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell 2014; 56:55-66; PMID:25242144; https://doi.org/10.1016/j.molcel.2014.08.019
- Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature 2013; 495:384-8; PMID:23446346; https://doi.org/10.1038/nature11993
- Burd CE, Jeck WR, Liu Y, Sanoff HK, Wang Z, Sharpless NE. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet 2010; 6:e1001233; PMID:21151960; https://doi.org/10.1371/journal.pgen.1001233
- Hansen TB, Kjems J, Damgaard CK. Circular RNA and miR-7 in cancer. Cancer Res 2013; 73:5609-12; PMID:24014594; https://doi.org/10.1158/0008-5472.CAN-13-1568
- Li F, Zhang L, Li W, Deng J, Zheng J, An M, Lu J, Zhou Y. Circular RNA ITCH has inhibitory effect on ESCC by suppressing the Wnt/beta-catenin pathway. Oncotarget 2015; 6:6001-13; PMID:25749389; https://doi.org/10.18632/oncotarget.3469
- Westholm JO, Miura P, Olson S, Shenker S, Joseph B, Sanfilippo P, Celniker SE, Graveley BR, Lai EC. Genome-wide analysis of drosophila circular RNAs reveals their structural and sequence properties and age-dependent neural accumulation. Cell Rep 2014; 9:1966-80; PMID:25544350; https://doi.org/10.1016/j.celrep.2014.10.062
- Bahn JH, Zhang Q, Li F, Chan TM, Lin X, Kim Y, Wong DT, Xiao X. The landscape of microRNA, Piwi-interacting RNA, and circular RNA in human saliva. Clin Chem 2015; 61:221-30; PMID:25376581; https://doi.org/10.1373/clinchem.2014.230433
- Li P, Chen S, Chen H, Mo X, Li T, Shao Y, Xiao B, Guo J. Using circular RNA as a novel type of biomarker in the screening of gastric cancer. Clin Chim Acta 2015; 444:132-6; PMID:25689795; https://doi.org/10.1016/j.cca.2015.02.018
- Petkovic S, Muller S. RNA circularization strategies in vivo and in vitro. Nucleic Acids Res 2015; 43:2454-65; PMID:25662225; https://doi.org/10.1093/nar/gkv045
- Frommer J, Hieronymus R, Selvi Arunachalam T, Heeren S, Jenckel M, Strahl A, Appel B, Muller S. Preparation of modified long-mer RNAs and analysis of FMN binding to the ypaA aptamer from B. subtilis. RNA Biol 2014; 11:609-23; PMID:24755604; https://doi.org/10.4161/rna.28526
- El-Sagheer AH, Brown T. New strategy for the synthesis of chemically modified RNA constructs exemplified by hairpin and hammerhead ribozymes. Proc Natl Acad Sci U S A 2010; 107:15329-34; PMID:20713730; https://doi.org/10.1073/pnas.1006447107
- Gao M, Gnutt D, Orban A, Appel B, Righetti F, Winter R, Narberhaus F, Muller S, Ebbinghaus S. RNA Hairpin Folding in the Crowded Cell. Angew Chem Int Ed Engl 2016; 55:3224-8; PMID:26833452; https://doi.org/10.1002/anie.201510847
- Sampson JR, Uhlenbeck OC. Biochemical and physical characterization of an unmodified yeast phenylalanine transfer RNA transcribed in vitro. Proc Natl Acad Sci USA 1988; 85:1033-7; PMID:3277187; https://doi.org/10.1073/pnas.85.4.1033
- Pitulle C, Kleineidam RG, Sproat B, Krupp G. Initiator oligonucleotides for the combination of chemical and enzymatic RNA synthesis. Gene 1992; 112:101-5; PMID:1372580; https://doi.org/10.1016/0378-1119(92)90309-D
- Wolf J, Dombos V, Appel B, Muller S. Synthesis of guanosine 5′-conjugates and their use as initiator molecules for transcription priming. Org Biomol Chem 2008; 6:899-907; PMID:18292882; https://doi.org/10.1039/b716151d
- Petkovic S, Muller S. RNA self-processing: formation of cyclic species and concatemers from a small engineered RNA. FEBS Lett 2013; 587:2435-40; PMID:23796421; https://doi.org/10.1016/j.febslet.2013.06.013
- Winston SE, Fuller SA, Evelegh MJ, Hurrell JG. Conjugation of enzymes to antibodies. Curr Protoc Mol Biol 2001; Chapter 11:Unit 11 1; PMID:18265067; https://doi.org/10.1002/0471142727.mb1101s50
- Rublack N, Muller S. Synthesis of a bifunctional cytidine derivative and its conjugation to RNA for in vitro selection of a cytidine deaminase ribozyme. Beilstein J Org Chem 2014; 10:1906-13; PMID:25246949; https://doi.org/10.3762/bjoc.10.198
- Kuznetsova SA, Merenkova IN, Kanevsky IE, Shabarova ZA, Blumenfeld M. Efficient synthesis of DNA dumbbells using template-induced chemical ligation in double-stranded polynucleotides closed by minihairpin fragments. Antisense Nucleic Acid Drug Dev 1999; 9:95-100; PMID:10192294; https://doi.org/10.1089/oli.1.1999.9.95
- Ashley GW, Kushlan DM. Chemical synthesis of oligodeoxynucleotide dumbbells. Biochemistry 1991; 30:2927-33; PMID:2007128; https://doi.org/10.1021/bi00225a028
- Rentzeperis D, Ho J, Marky LA. Contribution of loops and nicks to the formation of DNA dumbbells: melting behavior and ligand binding. Biochemistry 1993; 32:2564-72; PMID:8448114; https://doi.org/10.1021/bi00061a014
- Nilsson M, Malmgren H, Samiotaki M, Kwiatkowski M, Chowdhary BP, Landegren U. Padlock probes: circularizing oligonucleotides for localized DNA detection. Science 1994; 265:2085-8; PMID:7522346; https://doi.org/10.1126/science.7522346
- Dolinnaya NG, Blumenfeld M, Merenkova IN, Oretskaya TS, Krynetskaya NF, Ivanovskaya MG, Vasseur M, Shabarova ZA. Oligonucleotide circularization by template-directed chemical ligation. Nucleic Acids Res 1993; 21:5403-7; PMID:8265356; https://doi.org/10.1093/nar/21.23.5403
- Shabarova ZA, Fedorova OA, Dolinnaya NG, Gottikh MB. Derivatization and template-guided ligation of oligodeoxyribonucleotides using cyanogen bromide and N-substituted morpholines. Orig Life Evol Biosph 1997; 27:555-66; PMID:11536842; https://doi.org/10.1023/A:1006577107354
- Turunen JJ, Pavlova LV, Hengesbach M, Helm M, Müller S, Hartmann RK, Frilander MJ. RNA ligation. In: Hartmann RK, Bindereif A, Schön A, Westhof E, eds. Handbook of RNA Biochemistry. Weinheim, Germany: Wiley-VCH, 2014; 45-88 https://doi.org/10.1002/9783527647064; ISBN: 978-3-527-32764-5
- Lohman GJ, Tabor S, Nichols NM. DNA ligases. Curr Protoc Mol Biol 2011; Chapter 3:Unit 3 14; PMID:21472697; https://doi.org/10.1002/0471142727.mb0314s94
- Kurschat WC, Muller J, Wombacher R, Helm M. Optimizing splinted ligation of highly structured small RNAs. RNA 2005; 11:1909-14; PMID:16251384; https://doi.org/10.1261/rna.2170705
- Moore MJ, Sharp PA. Site-specific modification of pre-mRNA: the 2′-hydroxyl groups at the splice sites. Science 1992; 256:992-7; PMID:1589782; https://doi.org/10.1126/science.1589782
- Moore MJ. Joining RNA molecules with T4 DNA ligase. Methods Mol Biol 1999; 118:11-9; PMID:10549511; https://doi.org/10.1385/1-59259-676-2:11
- Gaglione M, Di Fabio G, Messere A. Current methods in synthesis of cyclic oligonucleotides and analoga. Curr Org Chem 2012; 16:1371-89; https://doi.org/10.2174/138527212800672673
- Chen CY, Sarnow P. Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science 1995; 268:415-7; PMID:7536344; https://doi.org/10.1126/science.7536344
- Park WS, Miyano-Kurosaki N, Abe T, Takai K, Yamamoto N, Takaku H. Inhibition of HIV-1 replication by a new type of circular dumbbell RNA/DNA chimeric oligonucleotides. Biochem Biophys Res Commun 2000; 270:953-60; PMID:10772932; https://doi.org/10.1006/bbrc.2000.2542
- Middleton T, Herlihy WC, Schimmel PR, Munro HN. Synthesis and purification of oligoribonucleotides using T4 RNA ligase and reverse-phase chromatography. Anal Biochem 1985; 144:110-7; PMID:3985307; https://doi.org/10.1016/0003-2697(85)90091-0
- Bain JD, Switzer C. Regioselective ligation of oligoribonucleotides using DNA splints. Nucleic Acids Res 1992; 20:4372; PMID:1380699; https://doi.org/10.1093/nar/20.16.4372
- England TE, Uhlenbeck OC. 3′-terminal labelling of RNA with T4 RNA ligase. Nature 1978; 275:560-1; PMID:692735; https://doi.org/10.1038/275560a0
- England TE, Uhlenbeck OC. Enzymatic oligoribonucleotide synthesis with T4 RNA ligase. Biochemistry 1978; 17:2069-76; PMID:667012; https://doi.org/10.1021/bi00604a008
- Romaniuk E, McLaughlin LW, Neilson T, Romaniuk PJ. The effect of acceptor oligoribonucleotide sequence on the T4 RNA ligase reaction. Eur J Biochem 1982; 125:639-43; PMID:7117259; https://doi.org/10.1111/j.1432-1033.1982.tb06730.x
- Beaudry D, Perreault JP. An efficient strategy for the synthesis of circular RNA molecules. Nucleic Acids Res 1995; 23:3064-6; PMID:7544891; https://doi.org/10.1093/nar/23.15.3064
- Abe N, Abe H, Ito Y. Dumbbell-shaped nanocircular RNAs for RNA interference. J Am Chem Soc 2007; 129:15108-9; PMID:18001025; https://doi.org/10.1021/ja0754453
- Abe N, Abe H, Nagai C, Harada M, Hatakeyama H, Harashima H, Ohshiro T, Nishihara M, Furukawa K, Maeda M, et al. Synthesis, structure, and biological activity of dumbbell-shaped nanocircular RNAs for RNA interference. Bioconjug Chem 2011; 22:2082-92; PMID:21899349; https://doi.org/10.1021/bc2003154
- Abe N, Abe H, Ohshiro T, Nakashima Y, Maeda M, Ito Y. Synthesis and characterization of small circular double-stranded RNAs. Chem Commun (Camb) 2011; 47:2125-7; PMID:21180718; https://doi.org/10.1039/C0CC04551A
- Ohtsuki T, Kawai G, Watanabe K. Stable isotope-edited NMR analysis of Ascaris suum mitochondrial tRNAMet having a TV-replacement loop. J Biochem 1998; 124:28-34; PMID:9644241; https://doi.org/10.1093/oxfordjournals.jbchem.a022092
- Persson T, Cuzic S, Hartmann RK. Catalysis by RNase P RNA: unique features and unprecedented active site plasticity. J Biol Chem 2003; 278:43394-401; PMID:12904300; https://doi.org/10.1074/jbc.M305939200
- Rigden JE, Rezaian MA. In vitro synthesis of an infectious viroid: analysis of the infectivity of monomeric linear CEV. Virology 1992; 186:201-6; PMID:1727598; https://doi.org/10.1016/0042-6822(92)90074-Y
- Wang L, Ruffner DE. Oligoribonucleotide circularization by ‘template-mediated’ ligation with T4 RNA ligase: synthesis of circular hammerhead ribozymes. Nucleic Acids Res 1998; 26:2502-4; PMID:9580707; https://doi.org/10.1093/nar/26.10.2502
- Steger J, Graber D, Moroder H, Geiermann AS, Aigner M, Micura R. Efficient access to nonhydrolyzable initiator tRNA based on the synthesis of 3′-azido-3′-deoxyadenosine RNA. Angew Chem Int Ed Engl 2010; 49:7470-2; PMID:21038451; https://doi.org/10.1002/anie.201003424
- Nandakumar J, Shuman S. How an RNA ligase discriminates RNA versus DNA damage. Mol Cell 2004; 16:211-21; PMID:15494308; https://doi.org/10.1016/j.molcel.2004.09.022
- Bullard DR, Bowater RP. Direct comparison of nick-joining activity of the nucleic acid ligases from bacteriophage T4. Biochem J 2006; 398:135-44; PMID:16671895; https://doi.org/10.1042/BJ20060313
- Ho CK, Shuman S. Bacteriophage T4 RNA ligase 2 (gp24.1) exemplifies a family of RNA ligases found in all phylogenetic domains. Proc Natl Acad Sci USA 2002; 99:12709-14; PMID:12228725; https://doi.org/10.1073/pnas.192184699
- Abe N, Matsumoto K, Nishihara M, Nakano Y, Shibata A, Maruyama H, Shuto S, Matsuda A, Yoshida M, Ito Y, et al. Rolling Circle Translation of Circular RNA in Living Human Cells. Sci Rep 2015; 5:16435; PMID:26553571; https://doi.org/10.1038/srep16435
- Micura R. Cyclic oligoribonucleotides (RNA) by solid-phase synthesis. Chemistry 1999; 5:2077-82; https://doi.org/10.1002/(SICI)1521-3765(19990702)5:7%3c2077::AID-CHEM2077%3e3.0.CO;2-U
- Wang S, Kool ET. Circular RNA oligonucleotides. Synthesis, nucleic acid binding properties, and a comparison with circular DNAs. Nucleic Acids Res 1994; 22:2326-33; PMID:7518582; https://doi.org/10.1093/nar/22.12.2326
- Herrlein MK, Nelson JS, Letsinger RL. A covalent lock for self-assembledoligonucleotide conjugates. J Am Chem Soc 1995; 117:10151-2; https://doi.org/10.1021/ja00145a042
- Xu Y, Kool ET. A novel %'-iodonucleoside allows efficient nonenzymatic ligation of singlestranded and duplex DNAs. Tetrahedron Lett 1997; 38:5595-8; PMID:19924262; https://doi.org/10.1016/S0040-4039(97)01266-5
- Sturm MB, Roday S, Schramm VL. Circular DNA and DNA/RNA hybrid molecules as scaffolds for ricin inhibitor design. J Am Chem Soc 2007; 129:5544-50; PMID:17417841; https://doi.org/10.1021/ja068054h
- Edupuganti OP, Defrancq E, Dumy P. Head-to-tail oxime cyclization of oligodeoxynucleotides for the efficient synthesis of circular DNA analogues. J Org Chem 2003; 68:8708-10; PMID:14575507; https://doi.org/10.1021/jo035064h
- El-Sagheer AH, Kumar R, Findlow S, Werner JM, Lane AN, Brown T. A very stable cyclic DNA miniduplex with just two base pairs. Chembiochem 2008; 9:50-2; PMID:18058775; https://doi.org/10.1002/cbic.200700538
- Kumar R, El-Sagheer A, Tumpane J, Lincoln P, Wilhelmsson LM, Brown T. Template-directed oligonucleotide strand ligation, covalent intramolecular DNA circularization and catenation using click chemistry. J Am Chem Soc 2007; 129:6859-64; PMID:17488075; https://doi.org/10.1021/ja070273v
- Liu J, Taylor JS. Template-directed photoligation of oligodeoxyribonucleotides via 4-thiothymidine. Nucleic Acids Res 1998; 26:3300-4; PMID:9628933; https://doi.org/10.1093/nar/26.13.3300
- Zapata L, Bathany K, Schmitter JM, Moreau S. Metal-assisted hybridization of oligonucleotides, evaluation of circular 2′-O-Me RNA as ligands for the TAR RNA target. Eur J Org Chem 2003; 3:1022-8; https://doi.org/10.1002/ejoc.200390143
- Sakharkar MK, Chow VT, Kangueane P. Distributions of exons and introns in the human genome. In Silico Biol 2004; 4:387-93; PMID:15217358
- Pieper S, Vauleon S, Muller S. RNA self-processing towards changed topology and sequence oligomerization. Biol Chem 2007; 388:743-6; PMID:17570827; https://doi.org/10.1515/BC.2007.067
- Petkovic S, Badelt S, Block S, Flamm C, Delcea M, Hofacker I, Müller S. Sequence-controlled RNA self-processing: computational design, biochemical analysis and visualization by AFM. RNA 2015; 21:1249-60; PMID:25999318; https://doi.org/10.1261/rna.047670.114
- Nesbitt SM, Erlacher HA, Fedor MJ. The internal equilibrium of the hairpin ribozyme: temperature, ion and pH effects. J Mol Biol 1999; 286:1009-24; PMID:10047478; https://doi.org/10.1006/jmbi.1999.2543
- Fedor MJ. Tertiary structure stabilization promotes hairpin ribozyme ligation. Biochemistry 1999; 38:11040-50; PMID:10460159; https://doi.org/10.1021/bi991069q
- Strohbach D, Novak N, Muller S. Redox-active riboswitching: allosteric regulation of ribozyme activity by ligand-shape control. Angew Chem Int Ed Engl 2006; 45:2127-9; PMID:16502442; https://doi.org/10.1002/anie.200503820
- Vauleon S, Muller S. External regulation of hairpin ribozyme activity by an oligonucleotide effector. Chembiochem 2003; 4:220-4; PMID:12616637; https://doi.org/10.1002/cbic.200390035
- Puttaraju M, Been MD. Group I permuted intron-exon (PIE) sequences self-splice to produce circular exons. Nucleic Acids Res 1992; 20:5357-64; PMID:1279519; https://doi.org/10.1093/nar/20.20.5357
- Ford E, Ares M, Jr. Synthesis of circular RNA in bacteria and yeast using RNA cyclase ribozymes derived from a group I intron of phage T4. Proc Natl Acad Sci USA 1994; 91:3117-21; PMID:7512723; https://doi.org/10.1073/pnas.91.8.3117
- Umekage S, Kikuchi Y. In vivo circular RNA production using a constitutive promoter for high-level expression. J Biosci Bioeng 2009; 108:354-6; PMID:19716528; https://doi.org/10.1016/j.jbiosc.2009.04.011
- Umekage S, Kikuchi Y. In vitro and in vivo production and purification of circular RNA aptamer. J Biotechnol 2009; 139:265-72; PMID:19138712; https://doi.org/10.1016/j.jbiotec.2008.12.012
- Puttaraju M, Perrotta AT, Been MD. A circular trans-acting hepatitis delta virus ribozyme. Nucleic Acids Res 1993; 21:4253-8; PMID:7692400; https://doi.org/10.1093/nar/21.18.4253
- Puttaraju M, Been MD. Circular ribozymes generated in Escherichia coli using group I self-splicing permuted intron-exon sequences. J Biol Chem 1996; 271:26081-7; PMID:8824250; https://doi.org/10.1074/jbc.271.42.26081
- Mikheeva S, Hakim-Zargar M, Carlson D, Jarrell K. Use of an engineered ribozyme to produce a circular human exon. Nucleic Acids Res 1997; 25:5085-94; PMID:9396820; https://doi.org/10.1093/nar/25.24.5085
- Jarrell KA. Inverse splicing of a group II intron. Proc Natl Acad Sci U S A 1993; 90:8624-7; PMID:8378340; https://doi.org/10.1073/pnas.90.18.8624