ABSTRACT
Location of the translation initiation codon generally requires scanning of the 43S ribosomal preinitiation complex (43S PIC) from the 5′ of the mRNA. Associated RNA helicases can facilitate movement of the 43S PIC by removing secondary structure present in the 5′ UTR of mRNA, which is required for codon inspection. The canonical RNA-dependent helicase eIF4A is directly involved in this process, as part of the eIF4F complex (eIF4G + eIF4A + eIF4E) that associates first with mRNA and then recruits the 43S PIC to initiate scanning. The topology and operational mechanism of the scanning PIC are probably the least understood aspects of the initiation step. Recent findings from translation of alphavirus mRNA, together with new biochemical and structural data of the 43S PIC, suggest a role for the ES6S region of 40S as the gateway for mRNA entry during scanning. The presence of eIF4G-eIF4A complex in this region, interacting with the incoming mRNA, supports a model where eIF4A could work ahead of the scanning complex during translation initiation. Here we present additional data supporting this model.
Introduction
The scanning model proposed 30 years ago by M. Kozak describes the path followed by 40S ribosomal subunits to reach the initiation codon (usually AUG) in most mammalian mRNAs. According to this model, 40S subunits are recruited to the cap structure at the 5′ end of mRNA to initiate the movement (scanning) toward the 3′ end.Citation1 Canonical cap-dependent initiation requires the binding of the eIF4F complex to the mRNA cap structure (activation), which promotes the attachment of 40S subunit. Before mRNA binding, the 40S subunit first associates with a ternary complex (Met-tRNAi bound to eIF2), eIF3, eIF5, eIF1 and eIF1A to form the 43S ribosomal preinitiation complex (43S PIC) that can select and accommodate the first AUG of mRNA in a good sequence context (AUGi).Citation2,Citation3 However, scanning by the 43S PIC is limited due to the presence of RNA secondary structure, which can slow down the advance of the complex. For many mRNAs, efficient scanning requires the participation of RNA helicases that unwind the secondary structure, driving migration of the 43S complex along the 5′ UTR.Citation4 The canonical RNA helicase eIF4A binds mRNA as part of the eIF4F complex, which subsequently associates with the 43S PIC through interaction with eIF3.Citation5-Citation8 The level of dependence on eIF4F activity is proportional to the degree of secondary structure found in the 5′ UTR of a given mRNA, with the exception of some viruses that can recruit the 43S PIC directly to highly structured regions (IRES) that are present in their mRNAs.Citation2,Citation9-Citation11
The mechanism of ribosomal attachment to capped mRNAs and the topology of the scanning complex (43S PIC + 4F + mRNA) are still a matter of debate. Based on the biochemical activity of the eIF4A helicase and structural data from the eIF4F complex, 2 conceptually different models have been proposed to explain how the scanning complex moves along the 5′ UTR of mRNAs. In the first model, eIF4F bound to eIF3 is placed near the exit channel of the 40S ribosomal subunit such that the eIF4A helicase can contact both the incoming 3′ mRNA sequences and the sequences located 5′ of the PIC.Citation12,Citation13 This model would involve a U-turn orientation of mRNA around the 40S subunit neck, such that the eIF4A helicase could work on both the leading and trailing edges of the scanning PIC, thus promoting its advance. A second model considers the scanning PIC as a simple Brownian ratchet driven by thermal diffusion. In this model, eIF4F would work exclusively as a clamp behind the scanning PIC, preventing 3′-to-5′ backsliding of the complex and thus imposing unidirectional 5′-to-3′ movement.Citation14
Recent advances in single-particle cryo-electron microscopy (cryo-EM) techniques have revealed the topology and conformational insights of the 43S PIC with unprecedented resolution.Citation15,Citation16 However, the exact location of eIF4F complex binding to the 43S PIC during scanning is still unknown. Some studies placed eIF4F above the 40S subunit platform near the mRNA exit channel,Citation12 whereas other reports suggested that eIF4F could place around the neck of the 40S solvent side, spanning from the entry to the exit channel.Citation17 Since eIF4A probably alternates cycles of binding and dissociation from mRNA,Citation17-Citation19 this dynamic behavior may be responsible for limiting the resolution of scanning PIC topology by current cryo-EM.
Interaction of SV mRNA with the ES6S region and eIF4A in a stalled 48S complex
Historically, the study of viral mRNA translation has shed much light on the fundamentals of translation initiation in mammals.Citation20 Recently, translation of subgenomic 26S mRNA of Alphavirus (Sindbis, SV; Semliki Forest virus, SFV) has gained attention given its low requeriments for initiation factors (eIFs) in infected cells, including eIF2.Citation21,Citation22 Alphaviral 26S mRNAs, which encode the structural proteins required for virion assembly, are efficiently translated from 4–6 hours post-infection in cultured cells.Citation21,Citation23 The 26S mRNA is 5′ capped with an unstructured 5′ UTR that is 50 nt in length, which precedes an initiation codon in an optimal sequence context (ACCAUGA) as expected for a highly translated mRNA. A distinctive feature of the SV/SFV 26S mRNA is the presence of a stable stem loop structure (DLP) located 25–31 nt downstream of the initiation codon that promotes 43S PIC stalling.Citation21,Citation24 Presence of the DLP improves translation of 26S mRNA in infected cells by allowing the 43S PIC to locate on the AUGi even in the absence of functional eIF2, a strategy that overcomes PKR-dependent eIF2α phosphorylation in infected cells.Citation21,Citation25 The activity of the DLP is position-dependent and requires a minimum stability (ΔG° = −15−20 kcal mol−1) at the base of the stem.Citation26 Using minimal versions of SV and SFV 26S mRNAs labeled with thio-UTP at specific positions, we were able to map by UV cross-linking the interaction of the DLP structure with 18S rRNA and eIFs in a stalled 48S complex assembled in RRL in the presence of non-hydrolyzable GTP analog (GMP-PNP).Citation26 A typical cross-linking assay is depicted in , showing the contacts of the DLP structure with helices of the ES6S region in 18S rRNA that are projected from the solvent side of the 40S ribosomal subunit (). Since previous reports showed that the eIF4G middle domain could contact helices of the ES6S region in a 48S complex assembled with picornaviral mRNAs,Citation27 we analyzed the DLP-protein contacts by cross-linking 48S complexes assembled with SV and SFV mRNAs. Interestingly, a prominent band of 48 kDa contacting the base of the DLP was specifically detected in 48S complex, but not in 80S complex (). This protein band was identified as the helicase eIF4A by immunoprecipitation with specific antibodies (), and by using a natural inhibitor (hippuristanol)Citation28 that specifically blocks the RNA-binding activity of eIF4A ().
Figure 1. Contact of the 5′ region of mRNA CDS with the solvent side of 40S ribosome and eIFs in a 48S complex. (A) Experimental outline of cross-linking assays using a minimal version of Sindbis virus (SV) mRNA bearing photoactivatable 4-thio UTP residues at the indicated positions in the DLP structure (rounded dots, SV-U1; squared dots, SV-U2). [32P]-labeled mRNAs were used for the assembly of 48S complexes in RRL in the presence of GMP-PNP as described recently.Citation26 Ribosomal fraction was purified by sedimentation through a sucrose cushion at 200,000xg for 3h and analyzed. (B) An aliquot of the sample was used to analyze mRNA-18S rRNA cross-linking by primer extension with RT as described previously.Citation26 The presence of extension arrests corresponding to the ES6S region of 18S rRNA is indicated. The rest of the sample was digested with RNAse A and U1, and the resulting [32P]-cross-linked proteins were analyzed by SDS-PAGE followed by autoradiography. (C) Protein bands detected in 48S complex or in 80S complex assembled in the presence of GMP-PNP and cycloheximide, respectively. (D) The identity of a 48 kDa band was confirmed by immunoprecipitation under denaturing conditionsCitation32 using an anti-eIF4A antibody (St. Johns, STJ27247) or a control antiserum against RPS2 (Santa Cruz Biotech. ). (E) The eIF4A protein band was further confirmed by treatment with hippuristanol, a specific inhibitor that prevents the RNA-binding activity of eIF4A.Citation28 The intensity of p52 band greatly varied among experiments, depending on the RRL batch used and the ultracentrifugation conditions. The molecular weight markers are shown. WRF: whole ribosomal fraction. is adapted from Toribio et al. (2016).Citation26
![Figure 1. Contact of the 5′ region of mRNA CDS with the solvent side of 40S ribosome and eIFs in a 48S complex. (A) Experimental outline of cross-linking assays using a minimal version of Sindbis virus (SV) mRNA bearing photoactivatable 4-thio UTP residues at the indicated positions in the DLP structure (rounded dots, SV-U1; squared dots, SV-U2). [32P]-labeled mRNAs were used for the assembly of 48S complexes in RRL in the presence of GMP-PNP as described recently.Citation26 Ribosomal fraction was purified by sedimentation through a sucrose cushion at 200,000xg for 3h and analyzed. (B) An aliquot of the sample was used to analyze mRNA-18S rRNA cross-linking by primer extension with RT as described previously.Citation26 The presence of extension arrests corresponding to the ES6S region of 18S rRNA is indicated. The rest of the sample was digested with RNAse A and U1, and the resulting [32P]-cross-linked proteins were analyzed by SDS-PAGE followed by autoradiography. (C) Protein bands detected in 48S complex or in 80S complex assembled in the presence of GMP-PNP and cycloheximide, respectively. (D) The identity of a 48 kDa band was confirmed by immunoprecipitation under denaturing conditionsCitation32 using an anti-eIF4A antibody (St. Johns, STJ27247) or a control antiserum against RPS2 (Santa Cruz Biotech. ). (E) The eIF4A protein band was further confirmed by treatment with hippuristanol, a specific inhibitor that prevents the RNA-binding activity of eIF4A.Citation28 The intensity of p52 band greatly varied among experiments, depending on the RRL batch used and the ultracentrifugation conditions. The molecular weight markers are shown. WRF: whole ribosomal fraction. Fig. 1B is adapted from Toribio et al. (2016).Citation26](/cms/asset/34efbe77-827d-4cc7-926a-c37033998c22/krnb_a_1247146_f0001_b.gif)
Toward a topological model of PIC scanning
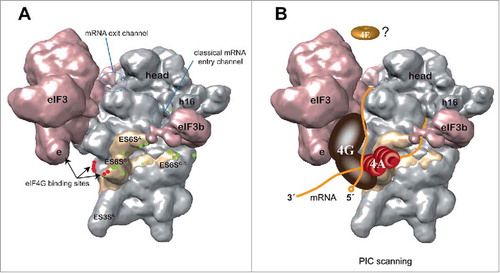
Based on data from the alphavirus mRNA 48S complex,Citation26 we propose a model for PIC scanning that integrates structural data from 40S crystals, cryo-EM reconstructions of a minimal 43S PIC, together with biochemical and genetic data of eIF activities and interactions (). According to this model, mRNA is threaded through the ES6S region before reaching the classical entrance of mRNA into the 40S cleft. Interestingly, computational analysis suggested the existence of sticky regions in ES6S helices complementary to short sequences that are present in the 5´UTR and coding region of eukaryotic mRNAs.Citation29 Whether this complementary could promote the attachment of mRNA to 40S is an attractive possibility worth exploring. For SV and SFV, the DLP structure becomes trapped in the ES6S region during scanning, an event that allows 40S subunits to locate on the AUGi of SV/SFV 26S mRNAs in infected cells.Citation26 Of note, interaction of mRNA with the ES6S region was also observed in 48S complexes assembled with variants of SV and SFV mRNA whose DLPs had been destroyed by point mutations, showing that the DLP structure itself does not impose binding of mRNA to the ES6S region.Citation26 However, no cross-linking of eIF4A to mRNA was detected in 48S complexes assembled with SV ΔDLP mRNA (data not shown), showing that the DLP structure likely stabilizes eIF4A-RNA interactions by jamming the activity of eIF4A, thus allowing the detection of these interactions in a 48S complex for the first time. Since eIF4A is recruited to eIF4F via 2 independent binding sites in eIF4G,Citation17,Citation30 eIF4A working on the ES6S region during PIC scanning would be compatible with previous data showing the interaction of the eIF4G middle domain with ES6S helices and eIF3e,Citation6 a subunit of eIF3 that is oriented toward the ES6S region in the 43S complex. Thus, helicase eIF4A, probably acting with eIF4B and eIF4H coactivators, could bind and unwind the secondary structure of mRNA in the ES6S region, before penetrating the narrow mRNA channel as a single strand. This model is consistent with eIF4A working at the leading edge of the scanning PIC, rather than acting as a simple clamp on the trailing edge to prevent 40S backsliding. This model also agrees with recent data from Kumar et al. (2016) using in vitro-reconstituted initiation complexes on capped β-globin mRNA, suggesting that mRNA is threaded into the 40S cleft from the solvent side assisted by the eIF4E-eIF4G-eIF3-40S chain of interactions.Citation13 This would involve dissociation of eIF4E from the 5′ cap once the 43S PIC attaches to mRNA,Citation13 a result that had been previously predicted.Citation31
Figure 2. Model of PIC scanning based on data from an alphavirus mRNA 48S complex. (A) Solvent side view of a 43S PIC model (EMD-5658)Citation16 showing the cross-linking sites (green dots) of alphavirus mRNA DLP with the ES6S region of 18S rRNA (pale yellow). The data are derived from experiments such as those shown in . The ES6S region is made up of 3 main RNA stems as indicated. The binding sites of eIF4G to the eIF3e subunitCitation6 and ES6 helices (red dots)Citation27 are also indicated. The entry and exit regions define the minimal channel where mRNA is threaded for decoding (60S subunit side, not visible). (B) A model of PIC scanning that integrates most data published to date. According to this model, mRNA enters the ribosome through (or near) the ES6S region where eIF4A bound to eIF4G may be placed, interacting with the incoming region (3′) of mRNA for secondary structure unwinding. The elongated structure of scaffold eIF4G may span from the right arm of eIF3 (e subunit) to ES6S, 2 regions that make contact with the middle domain of eIF4G.Citation17 The probable dissociation of eIF4E from the cap structure of mRNA upon 48S assembly is based on recent data.Citation13
Future challenges
Verification of the present model will require structural determination of the position of eIF4F within the scanning complex with greater confidence. The presumed dynamic nature of the eIF4F complex bound to PIC and mRNA has probably hampered the use of cryo-EM techniques for 3-dimensional reconstruction of the 43S PIC bound to natural mRNA. The use of DLP or DLP-like structures in mRNA that stabilize the 48S complex by trapping eIF4A (and eIF4F), may help overcome this limitation. In addition, the use of modern single-molecule techniques based on optical tweezers combined with FRET analysis may reveal the transient interactions that occur within the eIF4F complex, shedding more light on the topology of the scanning PIC at work.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This project was supported by a grant from Ministerio de de Economía y Competitividad (BFU-2013-45003-R). Institutional support from the Fundación Ramón Areces is also acknowledged.
References
- Kozak M. Evaluation of the “scanning model” for initiation of protein synthesis in eucaryotes. Cell 1980; 22:7-8; PMID:7000367; http://dx.doi.org/10.1016/0092-8674(80)90148-8
- Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol 2010; 11:113-27; PMID:20094052; http://dx.doi.org/10.1038/nrm2838
- Hinnebusch AG. Molecular mechanism of scanning and start codon selection in eukaryotes. Microbiol Mol Biol Rev 2011; 75:434-67, first page of table of contents; PMID:21885680; http://dx.doi.org/10.1128/MMBR.00008-11
- Parsyan A, Svitkin Y, Shahbazian D, Gkogkas C, Lasko P, Merrick WC, Sonenberg N. mRNA helicases: the tacticians of translational control. Nat Rev Mol Cell Biol 2011; 12:235-45; PMID:21427765; http://dx.doi.org/10.1038/nrm3083
- Imataka H, Sonenberg N. Human eukaryotic translation initiation factor 4G (eIF4G) possesses two separate and independent binding sites for eIF4A. Mol Cell Biol 1997; 17:6940-7; PMID:9372926; http://dx.doi.org/10.1128/MCB.17.12.6940
- LeFebvre AK, Korneeva NL, Trutschl M, Cvek U, Duzan RD, Bradley CA, Hershey JW, Rhoads RE. Translation initiation factor eIF4G-1 binds to eIF3 through the eIF3e subunit. J Biol Chem 2006; 281:22917-32; PMID:16766523; http://dx.doi.org/10.1074/jbc.M605418200
- Garcia-Garcia C, Frieda KL, Feoktistova K, Fraser CS, Block SM. RNA BIOCHEMISTRY. Factor-dependent processivity in human eIF4A DEAD-box helicase. Science 2015; 348:1486-8; PMID:26113725; http://dx.doi.org/10.1126/science.aaa5089
- Korneeva NL, Lamphear BJ, Hennigan FL, Merrick WC, Rhoads RE. Characterization of the two eIF4A-binding sites on human eIF4G-1. J Biol Chem 2001; 276:2872-9; PMID:11060291; http://dx.doi.org/10.1074/jbc.M006345200
- Kozak M. Circumstances and mechanisms of inhibition of translation by secondary structure in eucaryotic mRNAs. Mol Cell Biol 1989; 9:5134-42; PMID:2601712; http://dx.doi.org/10.1128/MCB.9.11.5134
- Pelletier J, Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5′ noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell 1985; 40:515-26; PMID:2982496; http://dx.doi.org/10.1016/0092-8674(85)90200-4
- Pelletier J, Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature 1988; 334:320-5; PMID:2839775; http://dx.doi.org/10.1038/334320a0
- Marintchev A, Edmonds KA, Marintcheva B, Hendrickson E, Oberer M, Suzuki C, Herdy B, Sonenberg N, Wagner G. Topology and regulation of the human eIF4A/4G/4H helicase complex in translation initiation. Cell 2009; 136:447-60; PMID:19203580; http://dx.doi.org/10.1016/j.cell.2009.01.014
- Kumar P, Hellen CU, Pestova TV. Toward the mechanism of eIF4F-mediated ribosomal attachment to mammalian capped mRNAs. Genes Dev 2016; 30:1573-88; PMID:27401559; http://dx.doi.org/10.1101/gad.282418.116
- Spirin AS. How does a scanning ribosomal particle move along the 5′-untranslated region of eukaryotic mRNA? Brownian Ratchet model. Biochemistry 2009; 48:10688-92; PMID:19835415; http://dx.doi.org/10.1021/bi901379a
- des Georges A, Dhote V, Kuhn L, Hellen CU, Pestova TV, Frank J, Hashem Y. Structure of mammalian eIF3 in the context of the 43S preinitiation complex. Nature 2015; 525:491-5; PMID:26344199; http://dx.doi.org/10.1038/nature14891
- Hashem Y, des Georges A, Dhote V, Langlois R, Liao HY, Grassucci RA, Hellen CU, Pestova TV, Frank J. Structure of the mammalian ribosomal 43S preinitiation complex bound to the scanning factor DHX29. Cell 2013; 153:1108-19; PMID:23706745; http://dx.doi.org/10.1016/j.cell.2013.04.036
- Nielsen KH, Behrens MA, He Y, Oliveira CL, Jensen LS, Hoffmann SV, Pedersen JS, Andersen GR. Synergistic activation of eIF4A by eIF4B and eIF4G. Nucleic Acids Res 2011; 39:2678-89; PMID:21113024; http://dx.doi.org/10.1093/nar/gkq1206
- Ray BK, Lawson TG, Kramer JC, Cladaras MH, Grifo JA, Abramson RD, Merrick WC, Thach RE. ATP-dependent unwinding of messenger RNA structure by eukaryotic initiation factors. J Biol Chem 1985; 260:7651-8; PMID:3838990
- Rogers GW, Jr., Lima WF, Merrick WC. Further characterization of the helicase activity of eIF4A. Substrate specificity. J Biol Chem 2001; 276:12598-608; PMID:11278350; http://dx.doi.org/10.1074/jbc.M007560200
- Walsh D, Mathews MB, Mohr I. Tinkering with translation: protein synthesis in virus-infected cells. Cold Spring Harb Perspect Biol 2013; 5:a012351; PMID:23209131; http://dx.doi.org/10.1101/cshperspect.a012351
- Ventoso I, Sanz MA, Molina S, Berlanga JJ, Carrasco L, Esteban M. Translational resistance of late alphavirus mRNA to eIF2alpha phosphorylation: a strategy to overcome the antiviral effect of protein kinase PKR. Genes Dev 2006; 20:87-100; PMID:16391235; http://dx.doi.org/10.1101/gad.357006
- Castello A, Sanz MA, Molina S, Carrasco L. Translation of Sindbis virus 26S mRNA does not require intact eukariotic initiation factor 4G. J Mol Biol 2006; 355:942-56; PMID:16343528; http://dx.doi.org/10.1016/j.jmb.2005.11.024
- Strauss JH, Strauss EG. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev 1994; 58:491-562; PMID:7968923
- Frolov I, Schlesinger S. Translation of Sindbis virus mRNA: analysis of sequences downstream of the initiating AUG codon that enhance translation. J Virol 1996; 70:1182-90; PMID:8551579
- Ventoso I. Adaptive changes in alphavirus mRNA translation allowed colonization of vertebrate hosts. J Virol 2012; 86:9484-94; PMID:22761388; http://dx.doi.org/10.1128/JVI.01114-12
- Toribio R, Diaz-Lopez I, Boskovic J, Ventoso I. An RNA trapping mechanism in Alphavirus mRNA promotes ribosome stalling and translation initiation. Nucleic Acids Res 2016; 44:4368-80; PMID:26984530; http://dx.doi.org/10.1093/nar/gkw172
- Yu Y, Abaeva IS, Marintchev A, Pestova TV, Hellen CU. Common conformational changes induced in type 2 picornavirus IRESs by cognate trans-acting factors. Nucleic Acids Res 2011; 39:4851-65; PMID:21306989; http://dx.doi.org/10.1093/nar/gkr045
- Bordeleau ME, Mori A, Oberer M, Lindqvist L, Chard LS, Higa T, Belsham GJ, Wagner G, Tanaka J, Pelletier J. Functional characterization of IRESes by an inhibitor of the RNA helicase eIF4A. Nat Chem Biol 2006; 2:213-20; PMID:16532013; http://dx.doi.org/10.1038/nchembio776
- Panek J, Kolar M, Vohradsky J, Shivaya Valasek L. An evolutionary conserved pattern of 18S rRNA sequence complementarity to mRNA 5′ UTRs and its implications for eukaryotic gene translation regulation. Nucleic Acids Res 2013; 41:7625-34; PMID:23804757; http://dx.doi.org/10.1093/nar/gkt548
- Morino S, Imataka H, Svitkin YV, Pestova TV, Sonenberg N. Eukaryotic translation initiation factor 4E (eIF4E) binding site and the middle one-third of eIF4GI constitute the core domain for cap-dependent translation, and the C-terminal one-third functions as a modulatory region. Mol Cell Biol 2000; 20:468-77; PMID:10611225; http://dx.doi.org/10.1128/MCB.20.2.468-477.2000
- Merrick WC. eIF4F: a retrospective. J Biol Chem 2015; 290:24091-9; PMID:26324716; http://dx.doi.org/10.1074/jbc.R115.675280
- Lee AS, Kranzusch PJ, Cate JH. eIF3 targets cell-proliferation messenger RNAs for translational activation or repression. Nature 2015; 522:111-4; PMID:25849773; http://dx.doi.org/10.1038/nature14267
