 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The HIV-1 Pr55Gag precursor specifically selects genomic RNA (gRNA) from a large variety of cellular and spliced viral RNAs (svRNAs), however the molecular mechanisms of this selective recognition remains poorly understood. To gain better understanding of this process, we analyzed the interactions between Pr55Gag and a large panel of viral RNA (vRNA) fragments encompassing the main packaging signal (Psi) and its flanking regions by fluorescence spectroscopy. We showed that the gRNA harbors a high affinity binding site which is absent from svRNA species, suggesting that this site might be crucial for selecting the HIV-1 genome. Our stoichiometry analysis of protein/RNA complexes revealed that few copies of Pr55Gag specifically associate with the 5′ region of the gRNA. Besides, we found that gRNA dimerization significantly impacts Pr55Gag binding, and we confirmed that the internal loop of stem-loop 1 (SL1) in Psi is crucial for specific interaction with Pr55Gag. Our analysis of gRNA fragments of different length supports the existence of a long-range tertiary interaction involving sequences upstream and downstream of the Psi region. This long-range interaction might promote optimal exposure of SL1 for efficient Pr55Gag recognition. Altogether, our results shed light on the molecular mechanisms allowing the specific selection of gRNA by Pr55Gag among a variety of svRNAs, all harboring SL1 in their first common exon.
Introduction
All retroviruses, including HIV-1, selectively package their genomic RNA (gRNA) into virions from a large excess of cellular RNAs and a variety of partially or fully spliced viral RNAs (svRNAs).Citation1-7 The selective packaging of gRNA is based on cis-acting elements located in the 5′ untranslated region (UTR) of the gRNA and in the beginning of gag ().Citation5,6,8-13 Over the last years, the residues spanning the gag start codon (AUG) were proposed to base pair with the residues of the Unique-5´ region (U5) in the so-called U5-AUG interaction,Citation14-17 which would act as a structural switch regulating NCp7 binding to gRNA and packaging ().Citation17-19 The main packaging signal (Psi), a region composed of 4 stem-loops (SL), SL1 to SL4, is located downstream of U5 ().Citation10,11,20-24 SL1 is also known as the Dimerization Initiation Site (DIS) as it mediates the initial steps of gRNA dimerization through a kissing-loop interaction.Citation25-31 Interestingly, several studies indicate that the dimerization and encapsidation of HIV-1 gRNA are strongly interrelated processesCitation21,24,29,32-37 and this phenomenon can be also observed in other retroviruses.Citation4,6,38 SL2 contains the major splice donor (SD) site, SL3 has previously been assigned as the major determinant for specific gRNA packagingCitation5,10,11 and SL4, which exists in equilibrium with the U5-AUG long-range interaction, encompasses the gag AUG codon (). Regions upstream of Psi include the Tat Responsive Element (TAR) stem-loop required for efficient HIV-1 transcription, the Poly(A) hairpin, which contains the 5′ repressed copy of the polyadenylation signal, and the Primer Binding Site (PBS) domain, which binds a tRNAlys, 3 molecule that primes reverse transcription (). Those elements were also found to impact gRNA packaging,Citation39-42 although one should be cautious in the interpretation of these results since mutations in the leader RNA could induce misfolding, thus indirectly affecting the dimerization and the packaging.Citation43,44 Interestingly, svRNAs also contain the RNA motifs located upstream of SL2Citation45 that are known to act as positive packaging signals in the gRNA such as SL1.Citation24,40 Of note, we have previously shown that svRNAs can form homodimers and heterodimers with gRNA in vitro,Citation45 thus being theoretically competent for packaging.
Figure 1. Partners involved in HIV-1 genomic RNA packaging. (A) Schematic representation of the first 615 nucleotides at the 5′-end of HIV-1 gRNA and the main secondary structure elements. The blue square contains the Psi region (SL1-SL4). SL1 is also known as the Dimerization Initiation Site (DIS) as it mediates the initial steps of gRNA dimerization through a kissing-loop interaction. The major splice donor (SD) site in SL2 and the AUG translation initiation codon of the gag gene are indicated. The secondary structure of the dimer of the 5′ ends of HIV-1 gRNA showing U5-AUG conformation is on the right hand side.Citation17,19 (B) The Pr55Gag precursor and its domains: the matrix (MA), capsid (CA), nucleocapsid (NC), and the small proline rich p6 domain, as well as the spacer peptides SP1 and SP2 are shown. Positions of tryptophan residues (W) in the different domains of Pr55Gag are indicated.
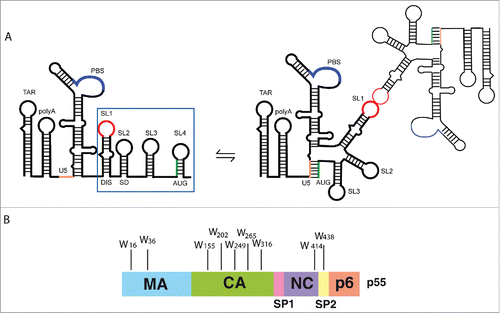
Specific gRNA packaging relies on interactions with the viral Pr55Gag precursor, which consists of 4 major domains, namely, matrix (MA), capsid (CA), nucleocapsid (NC), and p6, and 2 small spacer peptides (SP1 and SP2) (). The NC domain promotes gRNA incorporation into viral particles,Citation46-48 and displays a strong preference for Psi-containing RNAs.Citation2,49-51 Moreover, each structural element in Psi was previously found to behave as an independent binding site for the mature NCp7,Citation3,52-56 which is a RNA chaperone and governs nucleic acid destabilization and annealing of complementary sequences during the reverse transcription (for review see Citationrefs. 57,Citation58), and gRNA dimerization.Citation59-62 Interestingly, fast binding and dissociation kinetics also allow NCp7 to interact transiently with nucleic acids.Citation63 However, in vitro studies showed that Pr55Gag RNA chaperone activities differ from those of mature NCCitation64 and Pr55Gag has higher binding affinity for gRNA than NCp7.Citation2,65,66 Indeed, although NC is the major determinant for gRNA recognition, other domains within Pr55Gag contribute to the interaction with HIV-1 RNAs.Citation51,67 Interestingly, MA was found to bind RNA in vitroCitation51,68 and in the cytosol,Citation69 and this interaction is mostly driven by its highly basic region (HBR).Citation36,68,70,71 Moreover the absence of MA and CA domains reduces the binding specificity of the Pr55Gag precursor for gRNA and impairs virus production.Citation50,72 Both MA and CA domains likely contribute to Pr55Gag chaperone activity, and particularly the off-rate of Pr55Gag is much lower than that of NC.Citation66,67,73
A major difficulty in deciphering the mechanisms by which Pr55Gag selects HIV-1 gRNA has been the expression and purification of full-length Pr55Gag, which is notoriously sensitive to proteolytic cleavage during bacterial expression.Citation47 Most previous studies were therefore performed using truncated forms of Pr55Gag lacking the p6 domain (GagΔp6)Citation51,64,74-79 and/or fused to GSTCitation2,3,65,66,78,80 or the isolated NC domain.Citation64,81-83 We recently succeeded in purifying large amounts of intact full length Pr55Gag,Citation84 allowing us to characterize its interaction with a large panel of vRNA fragments.
We and others previously showed that Pr55Gag binds to elements within the 5′UTRCitation69,85,86 however the true equilibrium binding constants (Kd), and the stoichiometry of Pr55Gag/vRNA complexes were not determined. Here, we present a detailed analysis of Pr55Gag/vRNA interactions under equilibrium conditions using the natural fluorescence of the Pr55Gag Trp residues (). This approach allowed us to identify multiple classes of Pr55Gag binding sites within the 5′ region of the gRNA, and to determine the authentic Kd values for each of them. Remarkably, the highest affinity binding site was found to be completely absent in svRNA species, suggesting that this site is involved in specific discrimination and selection of gRNA by Pr55Gag. Interestingly, our stoichiometry analysis showed that about 6 Pr55Gag specifically associate with high affinity binding site(s) within a gRNA molecule, and this is consistent with the idea that cytoplasmic gRNA selection is ensured by a limited number of Pr55Gag molecules.Citation87-89 We also showed that SL1 deletions and mutants impairing gRNA dimerization have a significant impact on Pr55Gag binding, and we confirmed that the internal loop in SL1 () is a key recognition element for Pr55Gag binding. Finally, our analysis of gRNA fragments of different lengths supports the existence of a long-distance tertiary interaction involving genomic regions flanking Psi, and promoting the optimal exposure of the SL1 internal loop for efficient Pr55Gag recognition.
Results
In this study we used RNA fragments derived from both HIV-1 NL4.3 and MAL isolates. In our previous study,Citation85 as well as in the present work, we showed that identical mutations in NL4.3 and MAL isolates had very similar effects on Pr55Gag binding.
In order to analyze Pr55Gag binding to HIV-1 vRNA fragments, we took advantage of the intrinsic fluorescence of the protein which is due to 9 Trp residues distributed in MA, CA, NC and SP2 domains (). Because of their photophysical properties and their exquisite sensitivity to physicochemical environment, Trp residues constitute a useful tool to determine binding parameters in protein-nucleic acids systems.Citation90 To reduce unspecific protein/vRNA interactions, in vitro fluorescence spectroscopy assays were performed at 10 mM Mg2+ concentration (see Material and Methods Section).
SL1 contains a preferential binding site for Pr55Gag
Pr55Gag binding to individual stem-loops of the Psi region
Fluorescence spectroscopy is not affected by the RNA length, and it is thus possible to directly compare proteins binding to short oligonucleotides and to long RNA fragments. We first determined Pr55Gag binding parameters for chemically synthetized short RNAs corresponding to individual SLs of the Psi region (, and ). Interestingly, SL1 derived from the 2 isolates MAL (M35SL1 RNA) or NL4.3 (N35SL1 RNA) presented very similar affinities for Pr55Gag (). Notably, SL2, SL3 and SL4 displayed a 3 to 8-fold lower Pr55Gag binding affinity compared to SL1 (), suggesting that SL1 contains a specific binding site for Pr55Gag. Finally, the stoichiometry analysis (as described in Material and Methods) revealed that 2 to 3 Pr55Gag proteins bound the different SLs of the Psi region ().
Table 1. Pr55Gag binding to the individual stem-loops of the Psi region. Binding parameters were derived from the Scatchard modelCitation111 and stoichiometry analysis. RNAs derived from NL4.3 isolate have a name starting with “N.” Those derived from MAL isolate have a name starting with “M.”a Mean ± SD of at least 3 independent experiments.
Pr55Gag binding to the first 600/615 nucleotides of gRNA
We then extended our analysis to RNA fragments corresponding to the first 600/615 nucleotides of HIV-1 gRNA (N1-600WT and M1-615WT, ). The experimental binding curves were fitted with the one- and the 2- binding sites models (Fig. S1, Equation Equation4a and 4b, Material and Methods). Comparison of the resulting residuals together with the Scatchard plots of Pr55Gag binding to N1-600WT or M1-615WT RNAs, and to RNA fragments in which SL1 was fully or partially deleted, N1-600 ΔSL1 or M1-615 ΔSL1 (), supported the existence of 2 classes of binding sites exclusively in gRNA fragments. For those RNAs, we thus observed a high-affinity (Kd1, ) and a lower-affinity one (Kd2, ), with very similar affinities for both subtypes (), indicating that NL4.3 Pr55Gag binds equally to NL4.3 and MAL gRNA sequences. To investigate the role of SL1 in the context of this large RNA, we also tested RNA fragments in which SL1 was mutated in its apical loop (M1-615SL1sAL and N1-600SL1sAL, ) in order to prevent gRNA dimerizationCitation26,27 In line with these previous studies, we confirmed that RNA N1-600SL1sAL is unable to dimerize in the buffer and RNA concentration range used in the fluorescence assaysCitation85 (Fig. S2). Mutations in SL1 apical loop, similarly to the deletion of SL1, lead to the complete loss of the binding site(s) of highest affinity () and displayed a new class of binding sites with a significantly reduced affinity (Kd3, ) compared to wild-type RNAs (). Mutant RNAs for which the remaining SL elements of Psi were deleted (N1-600 ΔSL2, M1-615 ΔSL3 and M1-615 ΔSL4, ), led to the identification of 2 classes of binding sites, the highest affinity and the lowest affinity one (), suggesting that specific Pr55Gag binding sites do not reside in the region spanning SL2 to SL4.
Figure 2. Schematic representation of the HIV-1 MAL and NL4.3 RNA species, as well as NL4.3 svRNAs used in this study.
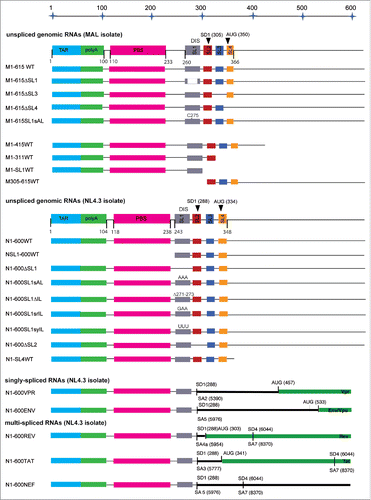
Figure 3. Pr55Gag interaction with N1-600WT and N1-600 ΔSL1 RNAs, and with M1-615WT and M1-615 SL1 ΔRNAs. (A) and (C) Increasing RNA concentrations were added to 100 nM Pr55Gag. The resulting binding curves corresponding to Pr55Gag interaction with N1-600 ΔSL1 or M1-615 SL1 ΔRNAs (red circles) were fitted according to the Scatchard model,Citation111 while the experimental curves resulting from Pr55Gag interaction with N1-600WT or M1-615WT ΔRNAs (black squares) were fitted with a Scatchard-like equation corresponding to a 2-binding sites model (). The residuals were plotted for each fit. (B) and (D). The Scatchard plots relative to N1-600WT and M1-615WT ΔRNAs did not display a linear pattern, confirming the presence of 2 classes of binding sites, while the plots relative to N1-600 ΔSL1 and M1-615 ΔSL1 RNAs yielded a linear pattern, confirming the presence of only one class of binding site.
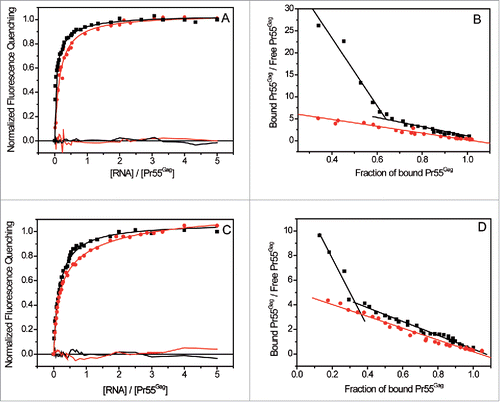
Table 2. Pr55Gag binding to the first 600/615 nucleotides of genomic and mutant RNAs: the impact of each SL of the Psi region. Binding parameters were derived from the Scatchard model.Citation111 Kdi (i = 1, 2, 3) corresponds to the different classes of Pr55Gag binding sites from the highest to lowest estimated affinity. Stoichiometry analysis revealed the number of bound Pr55Gag to the 3 different classes of binding sites. RNAs derived from NL4.3 isolate have a name starting with “N.” Those derived from MAL isolate have a name starting with “M.”a Mean ± SD of at least 3 independent experiments.
The analysis of stoichiometry systematically revealed that about 6 Pr55Gag bound the class of highest affinity in MAL and NL4.3 isolates (, and ). Importantly, SL1 deletion and apical loop substitutions impairing RNA dimerization reduced the number of interacting proteins with the second class of binding sites (Kd2) by about 2 to 3 fold (). This confirmed the impact of SL1 and of gRNA dimerization on Pr55Gag binding. In addition, deletion of SL2 led to a moderate effect compared to wild-type RNAs (about 5 bound Pr55Gag proteins, ), while SL3 and SL4 RNA mutants displayed only 3 proteins bound to the class of highest affinity () supporting the idea that these 2 SLs might contribute indirectly to Pr55Gag binding. Finally, all the tested RNAs displaying the third class of binding sites revealed one to two Pr55Gag proteins bound to the classes of lower affinity (Kd3, ).
Figure 4. Analysis of the stoichiometry of Pr55Gag binding to gRNA. Increasing RNA concentrations were added to 50 nM Pr55Gag. The experimental data (blue curve) expressed as normalized fluorescence quenching were reported vs the molar ratio of total [RNA] / [Pr55Gag]. Since two classes of Pr55Gag binding sites were detected in N1-600WT RNA, we traced the 2 fluorescence binding curves (red curves) each corresponding to a couple of parameters Kdn and Bn (n = 1,2, ). For each curve, we determined graphically the stoichiometry of the complexes of affinity Kd1 and Kd2 as previously described.Citation112
![Figure 4. Analysis of the stoichiometry of Pr55Gag binding to gRNA. Increasing RNA concentrations were added to 50 nM Pr55Gag. The experimental data (blue curve) expressed as normalized fluorescence quenching were reported vs the molar ratio of total [RNA] / [Pr55Gag]. Since two classes of Pr55Gag binding sites were detected in N1-600WT RNA, we traced the 2 fluorescence binding curves (red curves) each corresponding to a couple of parameters Kdn and Bn (n = 1,2, Table 2). For each curve, we determined graphically the stoichiometry of the complexes of affinity Kd1 and Kd2 as previously described.Citation112](/cms/asset/7279ea70-8528-4e4e-810d-22bd0b16a461/krnb_a_1256533_f0004_c.gif)
The internal loop of SL1 is crucial for the specific interaction with Pr55Gag
We next tested the impact of the AGG motif in the SL1 internal loop on Pr55Gag binding using RNA mutants of different length in which purines were deleted or substituted (AGG → GAA and AGG → UUU, ). In the SL1 context (35 nts), we observed only one class of binding sites and the deletion of the internal loop resulted in a 3-fold increase of Kd values (). However, this deletion surprisingly did not significatively impact on the number of associated proteins to RNA N35SL1 IL ().
Figure 5. The internal loop of SL1 is crucial for Pr55Gag binding. (A) Secondary structure of SL1 of HIV-1 NL4.3. Pr55Gag binding affinity was tested for wild-type SL1 sequence, and sequences in which the purines of the internal loop of SL1 were deleted or substituted (AGG → GAA (red), AGG → UUU (green)). (B) Increasing RNA concentrations were added to 100 nM Pr55Gag and experimental data relative to N1-600WT (black circles), and AGG → GAA substitution N1-600SL1srIL (red squares) RNAs were fitted according to the Scatchard model.Citation111 Increasing N1-600SL1 ΔIL RNA concentrations were added to 30 nM Pr55Gag, and resulting data were represented (blue triangles).
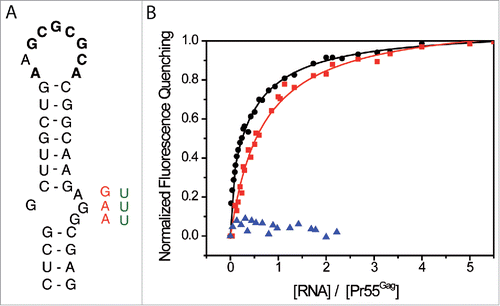
Table 3. Impact of deletion/mutations of the internal loop in SL1 on Pr55Gag binding. Binding parameters were derived from the Scatchard model.Citation111 For long RNA fragments we detected 3 classes of binding sites, Kdi (i = 1, 2, 3) corresponds to the different classes of Pr55Gag binding sites from the highest to lowest estimated affinity. Stoichiometry analysis revealed the number of Pr55Gag bound to the different classes of binding sites.a Mean ± SD of at least 3 independent experiments. n.d.: not determined.
A stronger effect was observed in the long RNA context (600 nts), and interestingly in this case, the substitution AGG → GAA (N1-600SL1srIL RNA, ) led to the complete loss of the class of higher affinity binding sites and introduced a third class of low affinity (Kd3, and ). Accordingly, stoichiometry analysis revealed that the number of associated Pr55Gag decreases to about 2 (N1-600SL1srIL, ). Finally the AGG → UUU mutation (N1-600SL1syIL, data not shown) and the deletion of the SL1 internal loop (N1-600SL IL) led to very limited fluorescence quenching (about 10-15%, ), and hence fluorescence curves could not be appropriately fitted by any model (). Altogether, these results clearly demonstrate the crucial role of the internal loop of SL1 for Pr55Gag binding. We previously showed that mutations of the SL1 internal loop did not significantly affect the RNA secondary structure and did not abrogate RNA dimerization.Citation85 Accordingly, we observed that N1-600SL1srIL dimerizes in the buffer used in the fluorescence assays (Fig. S2). Thus, mutations of the SL1 internal loop most likely affected Pr55Gag binding directly rather than via an indirect effect on RNA structure or on RNA dimerization.
gRNA contains a class of high-affinity binding sites for Pr55Gag which is absent from svRNA species and non-viral RNAs
Although several studies demonstrated that Pr55Gag efficiently binds gRNA, its binding properties for svRNAs remain poorly characterized.Citation2,3,65 In order to understand how Pr55Gag specifically selects the gRNA, we compared the binding of Pr55Gag to the first 600 nts of gRNA and svRNAs from the NL4.3 isolate (). In contrast with gRNA, which displayed 2 classes of binding sites, the one-binding site model was the most suitable to fit Pr55Gag binding data to svRNAs (). Moreover, all tested svRNAs bound Pr55Gag with similar affinity (). Supporting this major difference between gRNA and svRNAs, the fraction of bound protein versus unbound protein consistently yielded a linear pattern in the case of svRNAs, clearly indicating the presence of only one class of binding sites (). Conversely, a similar plot for N1-600WT RNA displayed 2 distinct linear patterns, thus confirming the presence of 2 classes of binding sites ().
Figure 6. Analysis of Pr55Gag interaction with gRNA and svRNAs: determination of the classes of binding sites. (A) Increasing RNAs concentrations were added to 100 nM Pr55Gag. The experimental curves resulting from Pr55Gag association to N1-600WT (black squares) were fitted with a Scatchard-like equation corresponding to a 2-binding sites model, while the experimental curves corresponding to Pr55Gag association to N1-600VPR (red circles) and N1-600TAT (green triangles) RNAs were fitted according to the Scatchard model ().Citation111 (B) The Scatchard plot relative to gRNA did not display a linear pattern, confirming the presence of 2 classes of binding sites, while the plots relative to svRNAs yielded a linear pattern, confirming the presence of only one class of binding site.
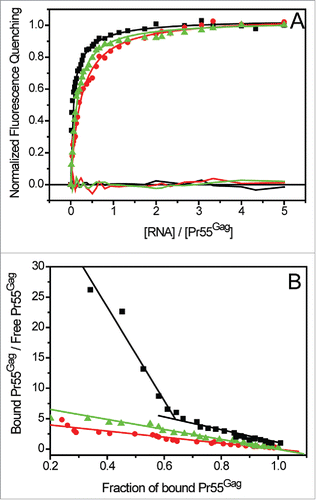
Table 4. Interaction of Pr55Gag with gRNA, svRNAs and non-viral RNAs. Binding parameters derived from the analysis of fluorescence titration curves were fitted by using the Scatchard model.Citation111 Kdi (i = 1, 2, 3) corresponds to the different classes of Pr55Gag binding sites from the highest to lowest estimated affinity. Stoichiometry analysis revealed the number of Pr55Gag bound to both classes of binding sites for each RNA molecule.a Mean ± SD of at least 3 independent experiments.
Altogether this analysis showed that the class of very high affinity binding sites found in gRNA is completely absent from svRNAs. Interestingly, Kd-values analysis revealed that the class of lower affinity in gRNA corresponds to the only class of Pr55Gag binding sites found in svRNAs (). Accordingly, the stoichiometry analysis showed that 2 to 3 Pr55Gag bind to svRNAs (), while about 2 proteins bind the class of lower affinity binding site(s) in gRNA (). Previous studies demonstrated that svRNAs efficiently dimerize,Citation45 and we checked that N1-600REV efficiently dimerized in the buffer used for fluorescence binding assays (Fig. S2), indicating that the lack of the high affinity binding site(s) in the svRNAs cannot be attributed to a dimerization defect. Finally, we tested Pr55Gag binding to several cellular non-viral RNAs of various lengths: 7SL RNA that is packaged into virions,Citation91,92 RNA fragments corresponding to the 3′UTR and the coding region of the cellular APOBEC3G mRNA that was observed to bind HIV-1 Vif,Citation93 the IRES located in the 5′UTR of the human glycyl-tRNA synthetase (GARS) mRNA,Citation94 the human selenoprotein M,Citation95 and the histone H4 mRNAs.Citation96,97 For all those RNAs, we observed only one class of lower affinity binding sites (Kd2, and Fig. S3), and the stoichiometry analysis showed that 2 to 3 Pr55Gag bind to those non-viral RNAs (). Taken together, our results suggest that the class of high-affinity binding sites for Pr55Gag is a specific feature of gRNA.
Influence of the regions flanking the Psi on efficient Pr55Gag binding
To test the role of sequences upstream of SL1 and downstream of SL4 on Pr55Gag binding, we analyzed a set of RNA constructs of different length encompassing the regions flanking Psi ( and ). Pr55Gag binding to M1-415WT RNA was rather similar to what observed for M1-615WT RNA (), and both fragments presented a class of high affinity binding sites (Kd1, ) and a class of lower affinity (Kd2, ). Accordingly, for those RNAs, about 6 Pr55Gag proteins bound to the class of high affinity, while about 2-3 proteins bound the lower affinity one (). Deletion of the region upstream of SL1 (NSL1-600WT RNA, ) reduced the overall affinity for Pr55Gag, since the Kd values of the class of high affinity were reduced by 2 to 4-fold compared to the previous RNAs. In addition for this RNA, our analysis displayed a third class of binding sites with lower affinity (Kd3, ). We observed about 6 proteins bound to the binding site(s) of higher affinity and only 2 proteins bound to the binding site(s) of lower affinity (). Notably, further truncation at the 3′ end of M1-615WT RNA resulted in a complete loss of the class of highest affinity (). For M1-SL1WT, M1-311WT (which span SL1 and SL2, ) and N1-SL4WT RNAs, the affinity of Pr55Gag was found to be quite similar to the lower affinity binding component observed for M1-415WT and M1-615WT RNAs (Kd2, ). We previously showed that RNAs resulting from the truncation at the 3′ end of M1-615WT RNA () efficiently dimerize,Citation85 and we compared the structures of the different vRNAs by selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE) to ensure that mutagenesis did not lead to RNA misfolding.Citation85 Similarly, M305-615 RNA, containing SL2-SL4 and the region downstream of SL4 up to nucleotide 615 (), displayed this same class of binding sites for Pr55Gag (). For all these constructs, we observed a stoichiometry of about 2 Pr55Gag proteins bound per RNA (). This analysis of M1-SL1, M1-311 and M1-SL4 RNAs showed that the presence of SL1 is thus not sufficient to ensure efficient Pr55Gag binding. Indeed, these RNAs displayed binding characteristics similar to svRNAs (). Taken together, our data support a model whereby the class of high affinity binding sites (Kd ∼2-5 nM, ) requires long-range interactions involving regions upstream of SL1 and downstream of SL4.
Table 5. Pr55Gag binding to a set of vRNAs of various length spanning regions upstream and downstream of the Psi. Binding parameters were derived by using the Scatchard model.Citation111 Kdi (i = 1, 2, 3) corresponds to the different classes of Pr55Gag binding sites from the highest to lowest estimated affinity. Stoichiometry analysis revealed the number of bound Pr55Gag to the different classes of binding sites. RNAs derived from MAL isolate have a name starting with “M.”a Mean ± SD of at least 3 independent experiments.
Discussion
To gain insight into the molecular mechanisms leading to the selective packaging of HIV-1 gRNA by Pr55Gag, we performed a detailed biophysical analysis based on the intrinsic fluorescence signal of the tryptophan residues distributed within the entire precursor Pr55Gag (B). Although a C-terminal 6His-tag was present in our recombinant protein, several lines of evidence suggest that in our system this effect remains weak to moderate. Even though it was recently reported that Pr55Gag binding to a short DNA fragment can be affected by the 6His-tag in low salts conditions,Citation98 at high ionic strengths all the titrations are essentially identical. This would demonstrate that the 6His-tag does not seem to alter Pr55Gag binding affinity nor stoichiometry in our high salt conditions. Moreover this same protein was previously used to analyze its interaction with RNA in vitro, and our data nicely fit with in viro/in cellula data (Table S1).Citation17,69,85,86 Finally, according to Yarus and coworkers, histidine binds RNAs that have an unpaired RAAGUGGG motif and a paired UAACG motif.Citation99 HIV-1 SL1 has a CAAGAGGC motif that is mostly paired and no UAACG motif; it is thus unlikely to bind histidine. We carefully characterized the binding parameters under equilibrium conditions between Pr55Gag and a large panel of vRNAs corresponding to the 5′ end of HIV-1 gRNA and svRNAs (). Even though it is difficult to establish if the classes of similar affinity observed in the different vRNAs correspond to identical Pr55Gag binding sites, our analysis on long RNA fragments (600 nts) revealed the presence of multiple classes of Pr55Gag binding sites: a class of high binding affinity (Kd1 < 10 nM), and an additional class of lower affinity that, for simplicity can be further splitted into 2 subclasses according to the range of the observed Kd values (Kd2 < 20 nM and Kd3 > 20 nM). More strikingly, we observed that the class of very high affinity is present exclusively in gRNA, and this feature might lead to the discrimination of gRNAs from svRNAs prior to packaging ( and , ). We also tested Pr55Gag binding to 7SL RNA, which is packaged into virionsCitation91,92 and RNA fragments corresponding to the 3′UTR and the coding region of the cellular APOBEC3G mRNA that was observed to bind HIV-1 Vif,Citation93 the IRES located in the 5′UTR of the human glycyl-tRNA synthetase (GARS) mRNA,Citation94 the selenoprotein M,Citation95 and the histone H4 mRNAs.Citation96,97 Our analysis revealed that none of those RNAs displayed high affinity binding site(s) ( and Fig. S3). Interestingly, Pr55Gag was previously found to interact with a transient structure of 7SL RNA leading to its packaging.Citation92 Our results would then suggest that the packaging of 7SL RNA and gRNA follows different pathways.
Previous dynamic light scattering data suggested that Pr55Gag likely oligomerizes in solution as a trimer,Citation85 and our stoichiometry analysis systematically revealed the association of about 6 Pr55Gag to the gRNA high-affinity binding site(s) (Kd1, ), while one to 3 proteins interact with the lower-affinity binding site(s) (Kd2, and Kd3, ), and about 3 Pr55Gag associate to the lower-affinity binding site(s) in svRNA molecules (Kd2, ). These data suggest not only that Pr55Gag oligomers could dissociate and rearrange subsequently to the interaction with vRNAs, but also that regions flanking the main binding site(s) drive the association of only a few additional Pr55Gag molecules. Thus, the overall analysis of large RNA fragments suggests that the specific selection of gRNA involves a very limited number of Pr55Gag molecules, in line with previous cellular studies.Citation88,89,100
We showed that SL1 is likely the primary Pr55Gag-binding site (), and our analysis clearly demonstrates that the purine rich internal loop in SL1 is a key determinant for Pr55Gag interaction () since substitutions (AGG → GAA or AGG → UUU, ) or deletion of this region dramatically impacted the interaction of Pr55Gag with vRNAs. Our results are in agreement with recent Pr55Gag footprinting,Citation85 and mutational interference mapping experiment (MIME).Citation86 Interestingly, this genomic site generated a consensus over the last years, since Pr55Gag binding sites in immature virions and NCp7 binding sites in mature virions,Citation17,69 fit with present findings and our previous in vitro data (Table S1).Citation85,86
Other studies showed that deletion of the lower part of SL1Citation101,102 or mutation of its internal loopCitation32 reduces gRNA packaging and viral infectivity. The effect of mutations in the SL1 internal loop on in vivo gRNA dimerizationCitation32,103-105 is probably a direct consequence of the weaker binding of Pr55Gag to the mutated SL1, the apical loop of which mediates gRNA dimerization. Our analysis of equilibrium binding constants and stoichiometry also pointed out the role of gRNA dimerization on efficient Pr55Gag binding (), suggesting that high affinity binding could require mutual interactions between Pr55Gag proteins bound to the 2 gRNA molecules, and this would in turn stabilize the gRNA dimer. Globally, fluorescence spectroscopy pointed out a stronger impact of gRNA dimerization on Pr55Gag binding compared to our previous study.Citation85 This is probably due to the fact that in filter binding assays, the overall lower RNA concentration leads to a diminished proportion of gRNA dimers. Interestingly, a recent ITC analysis on an RNA spanning the packaging signal showed that mutations in the apical loop in SL1 that prevent dimerization did not affect NCp7 binding.Citation106 In addition, ITC assays characterizing NCp7 interaction with the HIV-1 5′ leader RNA revealed elevated stoichiometry values (about 32 NCp7 molecules)Citation19 compared to the limited number of Pr55Gag molecules bound to gRNA that we observed by fluorescence spectroscopy. Altogether these findings underline the important differences existing between the full length Pr55Gag and its maturation product NCp7 with regard to their binding modes to HIV-1 gRNA.
Recent CLIP-seq data on identified regions spanning the end of SL2 and SL3 as interacting with Pr55Gag in cells,Citation69 and similarly in viro SHAPE data showed NCp7 association to SL2 and SL3 regions.Citation17 This is coherent with footprinting assays demonstrating structural rearrangement of SL3 upon Pr55Gag bindingCitation85 and with MIME analysis including SL3 in the Pr55Gag binding region (as summarized in Table S1).Citation86 According to our findings, those signals in Psi do not represent specific Pr55Gag binding sites, however SL3 and SL4 RNA mutants had an impact on the Pr55Gag binding stoichiometry (), showing that those SLs could contribute indirectly to Pr55Gag binding.
We further extended our analysis to RNA fragments of various lengths partially or entirely encompassing Psi (), and characterized true equilibrium binding constants and the stoichiometry of each class of Pr55Gag binding sites. Interestingly, we noticed that Pr55Gag bound M1-615WT RNA and M1-415WT RNA with rather similar efficiency (). Conversely, in RNAs that do not contain the region upstream of SL1 (NSL1-600WT, ), Pr55Gag overall binding affinity was reduced, although similar stoichiometry values were observed for all those RNAs (). Finally, in RNAs that do not span the entire Psi region (M1-SL1WT, M1-311WT and M1-SL4WT, ), the higher affinity binding site (Kd1) was completely absent and stoichiometry values importantly reduced (). The mere presence of SL1 in all of these vRNAs does not ensure optimal binding of Pr55Gag, in good agreement with our previous analysis.Citation85 Altogether our data support the existence of a long-range interaction involving sequences upstream of SL1 and downstream of SL4, that promotes Pr55Gag optimal binding to the high affinity site(s) in SL1. This result together with previous findings clearly points out how long-range interactions are necessary for gRNA specific encapsidation.Citation20,21,23,24,32,39-42,69,79,107,108 Even though further analysis will be necessary to identify the nucleotides that are involved, our model indicates that this interaction is not possible in svRNAs (which differ from gRNA downstream of SL2, ), and this could impact on Pr55Gag discrimination between unspliced gRNA and svRNAs. Moreover a recent NMR model of the leader region proposed that residues downstream of the major splice donor site would base pair and form a 3-way junction structure, which would be required for packaging of unspliced RNAs.Citation106 Despite the considerable differences between our and this study, the proposed model corroborates the idea that svRNAs are not selected for packaging since they cannot adopt the appropriate conformation.
Material and methods
Pr55Gag
Full-length non-myristilated Pr55Gag was expressed, purified, and characterized as recently describedCitation84,85
Plasmids, in vitro RNA transcription and purification
All plasmids used for in vitro transcription of RNAs used in this study have been described previously (Table S2).Citation26,29,45,85,109 The linearized plasmids were used as templates for the synthesis of RNAs by in vitro run off transcription using bacteriophage T7 RNA polymerase, followed by purification by size exclusion chromatography as described previously.Citation107
HIV-1 RNA oligonucleotides
RNA oligonucleotides corresponding to the individual stem-loops of the Psi region (SL1 to SL4) were produced by chemical synthesis and purified by reverse-phase HPLC and polyacrylamide gel electrophoresis (Integrated DNA Technologies, Inc.).
Non-viral RNA species
Plasmids used for in vitro transcription of RNAs corresponding to 7SL and to the 3′UTR and ORF of the cellular APOBEC3G mRNA have been described previously ().Citation93 The linearized plasmids were used as templates for the synthesis of RNAs by in vitro run off transcription using bacteriophage T7 RNA polymerase, followed by purification by size exclusion chromatography.Citation107
RNA fragments corresponding to the IRES located in the 5′UTR of the human glycyl-tRNA synthetase (GARS) mRNA,Citation94 and mRNAs of the selenoprotein MCitation95 and histone H4Citation96,97 were kindly provided by Drs. M. Frugier, J. Rudinger, C. Allmang and F. Martin (UPR9002, CNRS, Strasbourg University).
In vitro dimerization of HIV-1 RNA
Labeled RNAs were synthetized using [α32P] ATP (Amersham) as previously described.Citation110 The radioactive transcripts were purified on 6% denaturing polyacrylamide gels. To study the RNA dimerization dependence on RNA concentration (expressed in strands) and in the buffer typically used for fluorescence assays (see below), a fixed amount of radioactive RNA (5 nCi, 40 ng) was mixed to corresponding unlabeled RNA at a final concentrations ranging from 1 nM to 60 nM, and denaturated for 2 min at 90°C and snap-cooled for 2 min on ice. Proper RNA folding was then achieved by addition of 2-fold buffer (final concentration: 30 mM Tris–HCl (pH 7.5), 200 mM NaCl and 10 mM MgCl2). The samples were incubated for 15 min at 37°C and then analyzed on 0.8% agarose gel. Gels were run in 45 mM Tris-borate buffer supplemented with 0.1 mM MgCl2 for 4 h at 4°C. Agarose gels were fixed for 10 min in 10% tricloroacetic acid and dried for 45 min under vacuum at room temperature. Fuji imaging plates were exposed and scanned (Fujifilm Fla-5100). The area of the peaks corresponding to the monomeric and dimeric forms was quantified, and the percentage of the dimer was defined as the area of the dimer peak divided by the sum of the areas of the monomer and dimer peaks. Equilibrium dissociation constants Kd of the dimers were determined as the RNA concentration providing 50% of the dimer form.
Steady-State fluorescence spectroscopy
Prior to protein binding assays, RNAs (800 nM) were prepared in Milli-Q (Millipore) and folded as described above. Fluorescence measurements were recorded in quartz cells at 20 ± 0.1°C on a Fluoromax-4 fluorimeter (HORIBA Jobin-Yvon Inc., NJ, USA). The excitation wavelength was set at 295 nm for selective excitation of the Pr55Gag Trp residues. The emission wavelength was scanned from 305 to 520 nm; with an integration time of 0.1 s and excitation and emission bandwidths of 5 nm. In a typical titration, increasing amounts of RNA were added to 30, 50 or 100 nM Pr55Gag in the buffer (30 mM Tris–HCl (pH 7.5), 200 mM NaCl and 10 mM MgCl2), and the ratio between the [RNA] (expressed in strand) / [Pr55Gag] varied from 0 to 5. Addition of RNA solutions results in less than 1% change of the ionic strength of the experimental sample.Citation55 After the addition of each aliquot of RNA to the protein, the solution in the cuvette was mixed briefly and the fluorescence measured.
Fluorescence intensities were then corrected for buffer fluorescence and dilution effects.
To determine the binding parameters of Pr55Gag to the different RNA fragments, the fluorescence intensity measured for any added RNA concentration, I, was converted into the binding density, v corresponding to the nanomoles of protein bound, Pb, per nanomoles of nucleic acid concentration,
At:(1)
(1)
With I0 corresponding to the protein fluorescence intensity in the absence of RNA, If to the fluorescence intensity at the plateau when all the proteins are bound to the nucleic acid, and Pt corresponds to the total protein concentration. Since:(2)
(2)
(3)
(3)
Then one can easily calculate the concentration of bound, Pb, and free, protein, Pf, as functions of I, I0, If, Pt and At using Eq. Equation2(2)
(2) and Eq. Equation3
(3)
(3) . The experimental observed affinity, Kobs, and thus the dissociation constant, Kd mathematically corresponding to its inverse, was then computed by fitting the experimental data to equation:Citation111
(4a)
(4a)
Where n corresponds to the number of identical Pr55Gag binding sites to RNA. The parameters n and Kd were allowed to vary during fitting. In order to check for the presence of 2 classes of Pr55Gag binding sites for RNAs, we plotted the fraction of bound protein vs the unbound protein fraction. If the plot consistently yielded a linear pattern, we concluded that only one class of binding site(s) was present. Whenever we observed 2 linear patterns, fluorescence titration data were fitted with a Scatchard-like equation corresponding to a 2 binding sites model:(4b)
(4b)
With B1 + B2 = 1.
The analysis of fluorescence binding curves also allowed determination of the Pr55Gag binding stoichiometry, corresponding to the average number of proteins bound to one RNA molecule. The experimental data expressed as normalized fluorescence quenching were reported vs the molar ratio of total [RNA] expressed in strands. Stoichiometry can be graphically recovered by the intersection of the initial slope (the linear portion of the curves) at low [RNA] with the fluorescence plateau, as it was previously described.Citation112 When 2 different classes of binding sites were identified (Kdn; n = 1,2) as for example in the case for N1-600WT RNA, we traced the 2 corresponding fluorescence binding curves (red curves, ). For each curve, we determined graphically the stoichiometry of the complexes of affinity Kd1 and Kd2, as described above.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary_Data.docx
Download MS Word (39.5 MB)Acknowledgment
We are grateful to Drs C. Allmang, F. Martin, M. Frugier and J. Rudinger for the kind gift of non-viral RNAs.
Funding
This work was supported by grants from: the Agence Nationale de la Recherche sur le SIDA et les Hepatites Virales (ANRS) to SB, SIDACTION to RM, the US National Institutes of Health and Australian NHMRC and ARC grants to JM; and fellowships from the Egyptian Ministry of Higher Education and Scientific Research to EWAW, and the Initiative d'excellence (IDEX: “Par dela les frontieres, l'Universite de Strasbourg”) to RPS.
References
- Kuzembayeva M, Dilley K, Sardo L, Hu WS. Life of psi: how full-length HIV-1 RNAs become packaged genomes in the viral particles. Virology 2014; 454–5:362-70; PMID:24530126; http://dx.doi.org/10.1016/j.virol.2014.01.019
- Berkowitz RD, Luban J, Goff SP. Specific binding of human immunodeficiency virus type 1 gag polyprotein and nucleocapsid protein to viral RNAs detected by RNA mobility shift assays. J Virol 1993; 67:7190-200; PMID:8230441
- Clever J, Sassetti C, Parslow TG. RNA secondary structure and binding sites for gag gene products in the 5′ packaging signal of human immunodeficiency virus type 1. J Virol 1995; 69:2101-9; PMID:7884856
- Paillart JC, Shehu-Xhilaga M, Marquet R, Mak J. Dimerization of retroviral RNA genomes: an inseparable pair. Nat Rev Microbiol 2004; 2:461-72; PMID:15152202; http://dx.doi.org/10.1038/nrmicro903
- D'Souza V, Summers MF. How retroviruses select their genomes. Nat Rev Microbiol 2005; 3:643-55; PMID:16064056; http://dx.doi.org/10.1038/nrmicro1210
- Lu K, Heng X, Summers MF. Structural determinants and mechanism of HIV-1 genome packaging. J Mol Biol 2011; 410:609-33; PMID:21762803; http://dx.doi.org/10.1016/j.jmb.2011.04.029
- Rein A, Datta SA, Jones CP, Musier-Forsyth K. Diverse interactions of retroviral Gag proteins with RNAs. Trends Biochem Sci 2011; 36:373-80; PMID:21550256; http://dx.doi.org/10.1016/j.tibs.2011.04.001
- Berkhout B. Structure and function of the human immunodeficiency virus leader RNA. Prog Nucleic Acid Res Mol Biol 1996; 54:1-34; PMID:8768071; http://dx.doi.org/10.1016/S0079-6603(08)60359-1
- Russell RS, Hu J, Beriault V, Mouland AJ, Laughrea M, Kleiman L, Wainberg MA, Liang C. Sequences downstream of the 5′ splice donor site are required for both packaging and dimerization of human immunodeficiency virus type 1 RNA. J Virol 2003; 77:84-96; PMID:12477813; http://dx.doi.org/10.1128/JVI.77.1.84-96.2003
- Lever A, Gottlinger H, Haseltine W, Sodroski J. Identification of a sequence required for efficient packaging of human immunodeficiency virus type 1 RNA into virions. J Virol 1989; 63:4085-7; PMID:2760989
- Aldovini A, Young RA. Mutations of RNA and protein sequences involved in human immunodeficiency virus type 1 packaging result in production of noninfectious virus. J Virol 1990; 64:1920-6; PMID:2109098
- Luban J, Goff SP. Mutational analysis of cis-acting packaging signals in human immunodeficiency virus type 1 RNA. J Virol 1994; 68:3784-93; PMID:8189516
- McBride MS, Panganiban AT. Position dependence of functional hairpins important for human immunodeficiency virus type 1 RNA encapsidation in vivo. J Virol 1997; 71:2050-8; PMID:9032337
- Abbink TE, Berkhout B. A novel long distance base-pairing interaction in human immunodeficiency virus type 1 RNA occludes the Gag start codon. J Biol Chem 2003; 278:11601-11; PMID:12458192; http://dx.doi.org/10.1074/jbc.M210291200
- Abbink TE, Ooms M, Haasnoot PC, Berkhout B. The HIV-1 leader RNA conformational switch regulates RNA dimerization but does not regulate mRNA translation. Biochemistry 2005; 44:9058-66; PMID:15966729; http://dx.doi.org/10.1021/bi0502588
- Damgaard CK, Andersen ES, Knudsen B, Gorodkin J, Kjems J. RNA interactions in the 5′ region of the HIV-1 genome. J Mol Biol 2004; 336:369-79; PMID:14757051; http://dx.doi.org/10.1016/j.jmb.2003.12.010
- Wilkinson KA, Gorelick RJ, Vasa SM, Guex N, Rein A, Mathews DH, Giddings MC, Weeks KM. High-throughput SHAPE analysis reveals structures in HIV-1 genomic RNA strongly conserved across distinct biological states. PLoS Biol 2008; 6:e96; PMID:18447581; http://dx.doi.org/10.1371/journal.pbio.0060096
- Heng X, Kharytonchyk S, Garcia EL, Lu K, Divakaruni SS, LaCotti C, Edme K, Telesnitsky A, Summers MF. Identification of a minimal region of the HIV-1 5′-leader required for RNA dimerization, NC binding, and packaging. J Mol Biol 2012; 417:224-39; PMID:22306406; http://dx.doi.org/10.1016/j.jmb.2012.01.033
- Lu K, Heng X, Garyu L, Monti S, Garcia EL, Kharytonchyk S, Dorjsuren B, Kulandaivel G, Jones S, Hiremath A, et al. NMR detection of structures in the HIV-1 5′-leader RNA that regulate genome packaging. Science 2011; 334:242-5; PMID:21998393; http://dx.doi.org/10.1126/science.1210460
- Clavel F, Orenstein JM. A mutant of human immunodeficiency virus with reduced RNA packaging and abnormal particle morphology. J Virol 1990; 64:5230-4; PMID:2204725
- Berkhout B, van Wamel JL. Role of the DIS hairpin in replication of human immunodeficiency virus type 1. J Virol 1996; 70:6723-32; PMID:8794309
- Paillart JC, Berthoux L, Ottmann M, Darlix JL, Marquet R, Ehresmann B, Ehresmann C. A dual role of the putative RNA dimerization initiation site of human immunodeficiency virus type 1 in genomic RNA packaging and proviral DNA synthesis. J Virol 1996; 70:8348-54; PMID:8970954
- Harrison GP, Miele G, Hunter E, Lever AM. Functional analysis of the core human immunodeficiency virus type 1 packaging signal in a permissive cell line. J Virol 1998; 72:5886-96; PMID:9621050
- Houzet L, Paillart JC, Smagulova F, Maurel S, Morichaud Z, Marquet R, Mougel M. HIV controls the selective packaging of genomic, spliced viral and cellular RNAs into virions through different mechanisms. Nucleic Acids Res 2007; 35:2695-704; PMID:17426127; http://dx.doi.org/10.1093/nar/gkm153
- Skripkin E, Paillart JC, Marquet R, Ehresmann B, Ehresmann C. Identification of the primary site of the human immunodeficiency virus type 1 RNA dimerization in vitro. Proc Natl Acad Sci U S A 1994; 91:4945-9; PMID:8197162; http://dx.doi.org/10.1073/pnas.91.11.4945
- Paillart JC, Marquet R, Skripkin E, Ehresmann B, Ehresmann C. Mutational analysis of the bipartite dimer linkage structure of human immunodeficiency virus type 1 genomic RNA. J Biol Chem 1994; 269:27486-93; PMID:7961663
- Paillart JC, Skripkin E, Ehresmann B, Ehresmann C, Marquet R. A loop-loop “kissing” complex is the essential part of the dimer linkage of genomic HIV-1 RNA. Proc Natl Acad Sci U S A 1996; 93:5572-7; PMID:8643617; http://dx.doi.org/10.1073/pnas.93.11.5572
- Paillart JC, Skripkin E, Ehresmann B, Ehresmann C, Marquet R. The use of chemical modification interference and inverse PCR mutagenesis to identify the dimerization initiation site of HIV-1 genomic RNA. Pharm Acta Helv 1996; 71:21-8; PMID:8786995; http://dx.doi.org/10.1016/0031-6865(95)00048-8
- Paillart JC, Marquet R, Skripkin E, Ehresmann C, Ehresmann B. Dimerization of retroviral genomic RNAs: structural and functional implications. Biochimie 1996; 78:639-53; PMID:8955907; http://dx.doi.org/10.1016/S0300-9084(96)80010-1
- Berkhout B, Ooms M, Beerens N, Huthoff H, Southern E, Verhoef K. In vitro evidence that the untranslated leader of the HIV-1 genome is an RNA checkpoint that regulates multiple functions through conformational changes. J Biol Chem 2002; 277:19967-75; PMID:11896057; http://dx.doi.org/10.1074/jbc.M200950200
- van Bel N, Das AT, Cornelissen M, Abbink TE, Berkhout B. A short sequence motif in the 5′ leader of the HIV-1 genome modulates extended RNA dimer formation and virus replication. J Biol Chem 2014; 289:35061-74; PMID:25368321; http://dx.doi.org/10.1074/jbc.M114.621425
- Clever JL, Parslow TG. Mutant human immunodeficiency virus type 1 genomes with defects in RNA dimerization or encapsidation. J Virol 1997; 71:3407-14; PMID:9094610
- Laughrea M, Jette L, Mak J, Kleiman L, Liang C, Wainberg MA. Mutations in the kissing-loop hairpin of human immunodeficiency virus type 1 reduce viral infectivity as well as genomic RNA packaging and dimerization. J Virol 1997; 71:3397-406; PMID:9094609
- Russell RS, Liang C, Wainberg MA. Is HIV-1 RNA dimerization a prerequisite for packaging? Yes, no, probably? Retrovirology 2004; 1:23; PMID:15345057; http://dx.doi.org/10.1186/1742-4690-1-23
- Chen J, Rahman SA, Nikolaitchik OA, Grunwald D, Sardo L, Burdick RC, Plisov S, Liang E, Tai S, Pathak VK, et al. HIV-1 RNA genome dimerizes on the plasma membrane in the presence of Gag protein. Proc Natl Acad Sci U S A 2016; 113:E201-8; PMID:26712001; http://dx.doi.org/10.1073/pnas.1518572113
- Alfadhli A, McNett H, Tsagli S, Bachinger HP, Peyton DH, Barklis E. HIV-1 matrix protein binding to RNA. J Mol Biol 2011; 410:653-66; PMID:21762806; http://dx.doi.org/10.1016/j.jmb.2011.04.063
- Ooms M, Huthoff H, Russell R, Liang C, Berkhout B. A riboswitch regulates RNA dimerization and packaging in human immunodeficiency virus type 1 virions. J Virol 2004; 78:10814-9; PMID:15367648; http://dx.doi.org/10.1128/JVI.78.19.10814-10819.2004
- Aktar SJ, Vivet-Boudou V, Ali LM, Jabeen A, Kalloush RM, Richer D, Mustafa F, Marquet R, Rizvi TA. Structural basis of genomic RNA (gRNA) dimerization and packaging determinants of mouse mammary tumor virus (MMTV). Retrovirology 2014; 11:96; PMID:25394412; http://dx.doi.org/10.1186/s12977-014-0096-6
- Helga-Maria C, Hammarskjold ML, Rekosh D. An intact TAR element and cytoplasmic localization are necessary for efficient packaging of human immunodeficiency virus type 1 genomic RNA. J Virol 1999; 73:4127-35; PMID:10196309
- Didierlaurent L, Racine PJ, Houzet L, Chamontin C, Berkhout B, Mougel M. Role of HIV-1 RNA and protein determinants for the selective packaging of spliced and unspliced viral RNA and host U6 and 7SL RNA in virus particles. Nucleic Acids Res 2011; 39:8915-27; PMID:21791531; http://dx.doi.org/10.1093/nar/gkr577
- Russell RS, Hu J, Laughrea M, Wainberg MA, Liang C. Deficient dimerization of human immunodeficiency virus type 1 RNA caused by mutations of the u5 RNA sequences. Virology 2002; 303:152-63; PMID:12482667; http://dx.doi.org/10.1006/viro.2002.1592
- Clever JL, Miranda D, Jr., Parslow TG. RNA structure and packaging signals in the 5′ leader region of the human immunodeficiency virus type 1 genome. J Virol 2002; 76:12381-7; PMID:12414982; http://dx.doi.org/10.1128/JVI.76.23.12381-12387.2002
- Vrolijk MM, Ooms M, Harwig A, Das AT, Berkhout B. Destabilization of the TAR hairpin affects the structure and function of the HIV-1 leader RNA. Nucleic Acids Res 2008; 36:4352-63; PMID:18586822; http://dx.doi.org/10.1093/nar/gkn364
- Das AT, Vrolijk MM, Harwig A, Berkhout B. Opening of the TAR hairpin in the HIV-1 genome causes aberrant RNA dimerization and packaging. Retrovirology 2012; 9:59; PMID:22828074; http://dx.doi.org/10.1186/1742-4690-9-59
- Sinck L, Richer D, Howard J, Alexander M, Purcell DF, Marquet R, Paillart JC. In vitro dimerization of human immunodeficiency virus type 1 (HIV-1) spliced RNAs. RNA 2007; 13:2141-50; PMID:17925344; http://dx.doi.org/10.1261/rna.678307
- Cimarelli A, Sandin S, Hoglund S, Luban J. Basic residues in human immunodeficiency virus type 1 nucleocapsid promote virion assembly via interaction with RNA. J Virol 2000; 74:3046-57; PMID:10708419; http://dx.doi.org/10.1128/JVI.74.7.3046-3057.2000
- Campbell S, Rein A. In vitro assembly properties of human immunodeficiency virus type 1 Gag protein lacking the p6 domain. J Virol 1999; 73:2270-9; PMID:9971810
- Zhang Y, Qian H, Love Z, Barklis E. Analysis of the assembly function of the human immunodeficiency virus type 1 gag protein nucleocapsid domain. J Virol 1998; 72:1782-9; PMID:9499028
- Dannull J, Surovoy A, Jung G, Moelling K. Specific binding of HIV-1 nucleocapsid protein to PSI RNA in vitro requires N-terminal zinc finger and flanking basic amino acid residues. EMBO J 1994; 13:1525-33; PMID:8156990
- Geigenmuller U, Linial ML. Specific binding of human immunodeficiency virus type 1 (HIV-1) Gag-derived proteins to a 5′ HIV-1 genomic RNA sequence. J Virol 1996; 70:667-71; PMID:8523591
- Webb JA, Jones CP, Parent LJ, Rouzina I, Musier-Forsyth K. Distinct binding interactions of HIV-1 Gag to Psi and non-Psi RNAs: implications for viral genomic RNA packaging. RNA 2013; 19:1078-88; PMID:23798665; http://dx.doi.org/10.1261/rna.038869.113
- Amarasinghe GK, De Guzman RN, Turner RB, Summers MF. NMR structure of stem-loop SL2 of the HIV-1 psi RNA packaging signal reveals a novel A-U-A base-triple platform. J Mol Biol 2000; 299:145-56; PMID:10860728; http://dx.doi.org/10.1006/jmbi.2000.3710
- Amarasinghe GK, Zhou J, Miskimon M, Chancellor KJ, McDonald JA, Matthews AG, Miller RR, Rouse MD, Summers MF. Stem-loop SL4 of the HIV-1 psi RNA packaging signal exhibits weak affinity for the nucleocapsid protein. structural studies and implications for genome recognition. J Mol Biol 2001; 314:961-70; PMID:11743714; http://dx.doi.org/10.1006/jmbi.2000.5182
- Paoletti AC, Shubsda MF, Hudson BS, Borer PN. Affinities of the nucleocapsid protein for variants of SL3 RNA in HIV-1. Biochemistry 2002; 41:15423-8; PMID:12484783; http://dx.doi.org/10.1021/bi026307n
- Shubsda MF, Paoletti AC, Hudson BS, Borer PN. Affinities of packaging domain loops in HIV-1 RNA for the nucleocapsid protein. Biochemistry 2002; 41:5276-82; PMID:11955077; http://dx.doi.org/10.1021/bi016045+
- Athavale SS, Ouyang W, McPike MP, Hudson BS, Borer PN. Effects of the nature and concentration of salt on the interaction of the HIV-1 nucleocapsid protein with SL3 RNA. Biochemistry 2010; 49:3525-33; PMID:20359247; http://dx.doi.org/10.1021/bi901279e
- Godet J, Boudier C, Humbert N, Ivanyi-Nagy R, Darlix JL, Mely Y. Comparative nucleic acid chaperone properties of the nucleocapsid protein NCp7 and Tat protein of HIV-1. Virus Res 2012; 169:349-60; PMID:22743066; http://dx.doi.org/10.1016/j.virusres.2012.06.021
- Sleiman D, Goldschmidt V, Barraud P, Marquet R, Paillart JC, Tisne C. Initiation of HIV-1 reverse transcription and functional role of nucleocapsid-mediated tRNA/viral genome interactions. Virus Res 2012; 169:324-39; PMID:22721779; http://dx.doi.org/10.1016/j.virusres.2012.06.006
- Muriaux D, De Rocquigny H, Roques BP, Paoletti J. NCp7 activates HIV-1Lai RNA dimerization by converting a transient loop-loop complex into a stable dimer. J Biol Chem 1996; 271:33686-92; PMID:8969239; http://dx.doi.org/10.1074/jbc.271.52.33686
- Takahashi K, Baba S, Koyanagi Y, Yamamoto N, Takaku H, Kawai G. Two basic regions of NCp7 are sufficient for conformational conversion of HIV-1 dimerization initiation site from kissing-loop dimer to extended-duplex dimer. J Biol Chem 2001; 276:31274-8; PMID:11418609; http://dx.doi.org/10.1074/jbc.M104577200
- Kafaie J, Song R, Abrahamyan L, Mouland AJ, Laughrea M. Mapping of nucleocapsid residues important for HIV-1 genomic RNA dimerization and packaging. Virology 2008; 375:592-610; PMID:18343475; http://dx.doi.org/10.1016/j.virol.2008.02.001
- Jalalirad M, Laughrea M. Formation of immature and mature genomic RNA dimers in wild-type and protease-inactive HIV-1: differential roles of the Gag polyprotein, nucleocapsid proteins NCp15, NCp9, NCp7, and the dimerization initiation site. Virology 2010; 407:225-36; PMID:20828778; http://dx.doi.org/10.1016/j.virol.2010.08.013
- Cruceanu M, Gorelick RJ, Musier-Forsyth K, Rouzina I, Williams MC. Rapid kinetics of protein-nucleic acid interaction is a major component of HIV-1 nucleocapsid protein's nucleic acid chaperone function. J Mol Biol 2006; 363:867-77; PMID:16997322; http://dx.doi.org/10.1016/j.jmb.2006.08.070
- Wu T, Datta SA, Mitra M, Gorelick RJ, Rein A, Levin JG. Fundamental differences between the nucleic acid chaperone activities of HIV-1 nucleocapsid protein and Gag or Gag-derived proteins: biological implications. Virology 2010; 405:556-67; PMID:20655566; http://dx.doi.org/10.1016/j.virol.2010.06.042
- Damgaard CK, Dyhr-Mikkelsen H, Kjems J. Mapping the RNA binding sites for human immunodeficiency virus type-1 gag and NC proteins within the complete HIV-1 and −2 untranslated leader regions. Nucleic Acids Res 1998; 26:3667-76; PMID:9685481; http://dx.doi.org/10.1093/nar/26.16.3667
- Cruceanu M, Urbaneja MA, Hixson CV, Johnson DG, Datta SA, Fivash MJ, Stephen AG, Fisher RJ, Gorelick RJ, Casas-Finet JR, et al. Nucleic acid binding and chaperone properties of HIV-1 Gag and nucleocapsid proteins. Nucleic Acids Res 2006; 34:593-605; PMID:16449201; http://dx.doi.org/10.1093/nar/gkj458
- Rein A. Nucleic acid chaperone activity of retroviral Gag proteins. RNA Biol 2010; 7:700-5; PMID:21045546; http://dx.doi.org/10.4161/rna.7.6.13685
- Chukkapalli V, Oh SJ, Ono A. Opposing mechanisms involving RNA and lipids regulate HIV-1 Gag membrane binding through the highly basic region of the matrix domain. Proc Natl Acad Sci U S A 2010; 107:1600-5; PMID:20080620; http://dx.doi.org/10.1073/pnas.0908661107
- Kutluay SB, Zang T, Blanco-Melo D, Powell C, Jannain D, Errando M, Bieniasz PD. Global changes in the RNA binding specificity of HIV-1 gag regulate virion genesis. Cell 2014; 159:1096-109; PMID:25416948; http://dx.doi.org/10.1016/j.cell.2014.09.057
- Saad JS, Miller J, Tai J, Kim A, Ghanam RH, Summers MF. Structural basis for targeting HIV-1 Gag proteins to the plasma membrane for virus assembly. Proc Natl Acad Sci U S A 2006; 103:11364-9; PMID:16840558; http://dx.doi.org/10.1073/pnas.0602818103
- Shkriabai N, Datta SA, Zhao Z, Hess S, Rein A, Kvaratskhelia M. Interactions of HIV-1 Gag with assembly cofactors. Biochemistry 2006; 45:4077-83; PMID:16566581; http://dx.doi.org/10.1021/bi052308e
- Ott DE, Coren LV, Gagliardi TD. Redundant roles for nucleocapsid and matrix RNA-binding sequences in human immunodeficiency virus type 1 assembly. J Virol 2005; 79:13839-47; PMID:16254319; http://dx.doi.org/10.1128/JVI.79.22.13839-13847.2005
- Levin JG, Guo J, Rouzina I, Musier-Forsyth K. Nucleic acid chaperone activity of HIV-1 nucleocapsid protein: critical role in reverse transcription and molecular mechanism. Prog Nucleic Acid Res Mol Biol 2005; 80:217-86; PMID:16164976; http://dx.doi.org/10.1016/S0079-6603(05)80006-6
- Rye-McCurdy TD, Nadaraia-Hoke S, Gudleski-O'Regan N, Flanagan JM, Parent LJ, Musier-Forsyth K. Mechanistic differences between nucleic acid chaperone activities of the Gag proteins of Rous sarcoma virus and human immunodeficiency virus type 1 are attributed to the MA domain. J Virol 2014; 88:7852-61; PMID:24789780; http://dx.doi.org/10.1128/JVI.00736-14
- Jones CP, Datta SA, Rein A, Rouzina I, Musier-Forsyth K. Matrix domain modulates HIV-1 Gag's nucleic acid chaperone activity via inositol phosphate binding. J Virol 2011; 85:1594-603; PMID:21123373; http://dx.doi.org/10.1128/JVI.01809-10
- Munro JB, Nath A, Farber M, Datta SA, Rein A, Rhoades E, Mothes W. A conformational transition observed in single HIV-1 Gag molecules during in vitro assembly of virus-like particles. J Virol 2014; 88:3577-85; PMID:24403576; http://dx.doi.org/10.1128/JVI.03353-13
- Datta SA, Heinrich F, Raghunandan S, Krueger S, Curtis JE, Rein A, Nanda H. HIV-1 Gag extension: conformational changes require simultaneous interaction with membrane and nucleic acid. J Mol Biol 2011; 406:205-14; PMID:21134384; http://dx.doi.org/10.1016/j.jmb.2010.11.051
- Datta SA, Curtis JE, Ratcliff W, Clark PK, Crist RM, Lebowitz J, Krueger S, Rein A. Conformation of the HIV-1 Gag protein in solution. J Mol Biol 2007; 365:812-24; PMID:17097677; http://dx.doi.org/10.1016/j.jmb.2006.10.073
- Kenyon JC, Prestwood LJ, Lever AM. A novel combined RNA-protein interaction analysis distinguishes HIV-1 Gag protein binding sites from structural change in the viral RNA leader. Sci Rep 2015; 5:14369; PMID:26449409; http://dx.doi.org/10.1038/srep14369
- Datta SA, Zhao Z, Clark PK, Tarasov S, Alexandratos JN, Campbell SJ, Kvaratskhelia M, Lebowitz J, Rein A. Interactions between HIV-1 Gag molecules in solution: an inositol phosphate-mediated switch. J Mol Biol 2007; 365:799-811; PMID:17098251; http://dx.doi.org/10.1016/j.jmb.2006.10.072
- Feng YX, Copeland TD, Henderson LE, Gorelick RJ, Bosche WJ, Levin JG, Rein A. HIV-1 nucleocapsid protein induces “maturation” of dimeric retroviral RNA in vitro. Proc Natl Acad Sci U S A 1996; 93:7577-81; PMID:8755517; http://dx.doi.org/10.1073/pnas.93.15.7577
- Levin JG, Mitra M, Mascarenhas A, Musier-Forsyth K. Role of HIV-1 nucleocapsid protein in HIV-1 reverse transcription. RNA Biol 2010; 7:754-74; PMID:21160280; http://dx.doi.org/10.4161/rna.7.6.14115
- Kempf N, Postupalenko V, Bora S, Didier P, Arntz Y, de Rocquigny H, Mely Y. The HIV-1 nucleocapsid protein recruits negatively charged lipids to ensure its optimal binding to lipid membranes. J Virol 2015; 89:1756-67; PMID:25410868; http://dx.doi.org/10.1128/JVI.02931-14
- McKinstry WJ, Hijnen M, Tanwar HS, Sparrow LG, Nagarajan S, Pham ST, Mak J. Expression and purification of soluble recombinant full length HIV-1 Pr55(Gag) protein in Escherichia coli. Protein Expr Purif 2014; 100:10-8; PMID:24810910; http://dx.doi.org/10.1016/j.pep.2014.04.013
- Abd El-Wahab EW, Smyth RP, Mailler E, Bernacchi S, Vivet-Boudou V, Hijnen M, Jossinet F, Mak J, Paillart JC, Marquet R. Specific recognition of the HIV-1 genomic RNA by the Gag precursor. Nat Commun 2014; 5:4304; PMID:24986025; http://dx.doi.org/10.1038/ncomms5304
- Smyth RP, Despons L, Huili G, Bernacchi S, Hijnen M, Mak J, Jossinet F, Weixi L, Paillart JC, von Kleist M, et al. Mutational interference mapping experiment (MIME) for studying RNA structure and function. Nat Methods 2015; 106(45):19114-9; PMID:19861549; http://dx.doi.org/10.1038/ncomms5304
- Jouvenet N, Simon SM, Bieniasz PD. Imaging the interaction of HIV-1 genomes and Gag during assembly of individual viral particles. Proc Natl Acad Sci U S A 2009; 106:19114-9; http://dx.doi.org/doi:10.1073/pnas.0907364106
- Kutluay SB, Bieniasz PD. Analysis of the initiating events in HIV-1 particle assembly and genome packaging. PLoS Pathog 2010; 6:e1001200; PMID:21124996; http://dx.doi.org/10.1371/journal.ppat.1001200
- Hendrix J, Baumgartel V, Schrimpf W, Ivanchenko S, Digman MA, Gratton E, Krausslich HG, Muller B, Lamb DC. Live-cell observation of cytosolic HIV-1 assembly onset reveals RNA-interacting Gag oligomers. J Cell Biol 2015; 210:629-46; PMID:26283800; http://dx.doi.org/10.1083/jcb.201504006
- Chen Y, Barkley MD. Toward understanding tryptophan fluorescence in proteins. Biochemistry 1998; 37:9976-82; PMID:9665702; http://dx.doi.org/10.1021/bi980274n
- Onafuwa-Nuga AA, Telesnitsky A, King SR. 7SL RNA, but not the 54-kd signal recognition particle protein, is an abundant component of both infectious HIV-1 and minimal virus-like particles. RNA 2006; 12:542-6; PMID:16489186; http://dx.doi.org/10.1261/rna.2306306
- Keene SE, Telesnitsky A. cis-Acting determinants of 7SL RNA packaging by HIV-1. J Virol 2012; 86:7934-42; PMID:22593161; http://dx.doi.org/10.1128/JVI.00856-12
- Mercenne G, Bernacchi S, Richer D, Bec G, Henriet S, Paillart JC, Marquet R. HIV-1 Vif binds to APOBEC3G mRNA and inhibits its translation. Nucleic Acids Res 2010; 38:633-46; PMID:19910370; http://dx.doi.org/10.1093/nar/gkp1009
- Alexandrova J, Paulus C, Rudinger-Thirion J, Jossinet F, Frugier M. Elaborate uORF/IRES features control expression and localization of human glycyl-tRNA synthetase. RNA Biol 2015; 12:1301-13; PMID:26327585; http://dx.doi.org/10.1080/15476286.2015.1086866
- Wurth L, Gribling-Burrer AS, Verheggen C, Leichter M, Takeuchi A, Baudrey S, Martin F, Krol A, Bertrand E, Allmang C. Hypermethylated-capped selenoprotein mRNAs in mammals. Nucleic Acids Res 2014; 42:8663-77; PMID:25013170; http://dx.doi.org/10.1093/nar/gku580
- Martin F, Barends S, Jaeger S, Schaeffer L, Prongidi-Fix L, Eriani G. Cap-assisted internal initiation of translation of histone H4. Mol Cell 2011; 41:197-209; PMID:21255730; http://dx.doi.org/10.1016/j.molcel.2010.12.019
- Martin F, Menetret JF, Simonetti A, Myasnikov AG, Vicens Q, Prongidi-Fix L, Natchiar SK, Klaholz BP, Eriani G. Ribosomal 18S rRNA base pairs with mRNA during eukaryotic translation initiation. Nat Commun 2016; 7:12622; PMID:27554013; http://dx.doi.org/10.1038/ncomms12622
- Bewley MC, Reinhart L, Stake MS, Nadaraia-Hoke S, Parent LJ, Flanagan JM. A non-cleavable hexahistidine affinity tag at the carboxyl-terminus of the Pr55Gag polyprotein alters nucleic acid binding properties. Protein Expr Purif 2016; 130:137-145; PMID:27721079; http://dx.doi.org/10.1016/j.pep.2016.10.001
- Majerfeld I, Puthenvedu D, Yarus M. RNA affinity for molecular L-histidine; genetic code origins. J Mol Evol 2005; 61:226-35; PMID:15999244; http://dx.doi.org/10.1007/s00239-004-0360-9
- Jouvenet N, Bieniasz PD, Simon SM. Imaging the biogenesis of individual HIV-1 virions in live cells. Nature 2008; 454:236-40; PMID:18500329; http://dx.doi.org/10.1038/nature06998
- Liang C, Rong L, Laughrea M, Kleiman L, Wainberg MA. Compensatory point mutations in the human immunodeficiency virus type 1 Gag region that are distal from deletion mutations in the dimerization initiation site can restore viral replication. J Virol 1998; 72:6629-36; PMID:9658109
- Liang C, Rong L, Quan Y, Laughrea M, Kleiman L, Wainberg MA. Mutations within four distinct gag proteins are required to restore replication of human immunodeficiency virus type 1 after deletion mutagenesis within the dimerization initiation site. J Virol 1999; 73:7014-20; PMID:10400801
- Laughrea M, Shen N, Jette L, Wainberg MA. Variant effects of non-native kissing-loop hairpin palindromes on HIV replication and HIV RNA dimerization: role of stem-loop B in HIV replication and HIV RNA dimerization. Biochemistry 1999; 38:226-34; PMID:9890902; http://dx.doi.org/10.1021/bi981728j
- Shen N, Jette L, Liang C, Wainberg MA, Laughrea M. Impact of human immunodeficiency virus type 1 RNA dimerization on viral infectivity and of stem-loop B on RNA dimerization and reverse transcription and dissociation of dimerization from packaging. J Virol 2000; 74:5729-35; PMID:10823883; http://dx.doi.org/10.1128/JVI.74.12.5729-5735.2000
- Shen N, Jette L, Wainberg MA, Laughrea M. Role of stem B, loop B, and nucleotides next to the primer binding site and the kissing-loop domain in human immunodeficiency virus type 1 replication and genomic-RNA dimerization. J Virol 2001; 75:10543-9; PMID:11581429; http://dx.doi.org/10.1128/JVI.75.21.10543-10549.2001
- Keane SC, Heng X, Lu K, Kharytonchyk S, Ramakrishnan V, Carter G, Barton S, Hosic A, Florwick A, Santos J, et al. RNA structure. Structure of the HIV-1 RNA packaging signal. Science 2015; 348:917-21; PMID:25999508; http://dx.doi.org/10.1126/science.aaa9266
- Paillart JC, Skripkin E, Ehresmann B, Ehresmann C, Marquet R. In vitro evidence for a long range pseudoknot in the 5′-untranslated and matrix coding regions of HIV-1 genomic RNA. J Biol Chem 2002; 277:5995-6004; PMID:11744696; http://dx.doi.org/10.1074/jbc.M108972200
- Sakuragi J, Ode H, Sakuragi S, Shioda T, Sato H. A proposal for a new HIV-1 DLS structural model. Nucleic Acids Res 2012; 40:5012-22; PMID:22328732; http://dx.doi.org/10.1093/nar/gks156
- Lodmell JS, Paillart JC, Mignot D, Ehresmann B, Ehresmann C, Marquet R. Oligonucleotide-mediated inhibition of genomic RNA dimerization of HIV-1 strains MAL and LAI: a comparative analysis. Antisense Nucleic Acid Drug Dev 1998; 8:517-29; PMID:9918116; http://dx.doi.org/10.1089/oli.1.1998.8.517
- Marquet R, Baudin F, Gabus C, Darlix JL, Mougel M, Ehresmann C, Ehresmann B. Dimerization of human immunodeficiency virus (type 1) RNA: stimulation by cations and possible mechanism. Nucleic Acids Res 1991; 19:2349-57; PMID:1645868; http://dx.doi.org/10.1093/nar/19.9.2349
- Scatchard G. The attractions of proteins fo small molecules and ions. Ann NY Acad Sci 1949; 51:660-72; http://dx.doi.org/10.1111/j.1749-6632.1949.t00027297.x
- Bernacchi S, Henriet S, Dumas P, Paillart JC, Marquet R. RNA and DNA binding properties of HIV-1 Vif protein: a fluorescence study. J Biol Chem 2007; 282:26361-8; PMID:17609216; http://dx.doi.org/10.1074/jbc.M703122200
