ABSTRACT
The parasitic plant Cuscuta exchanges mRNAs with its hosts. Systemic mobility of mRNAs within plants is well documented, and has gained increasing attention as studies using grafted plant systems have revealed new aspects of mobile mRNA regulation and function. But parasitic plants take this phenomenon to a new level by forming seamless connections to a wide range of host species, and raising questions about how mRNAs might function after transfer to a different species. Cuscuta and other parasitic plant species also take siRNAs from their hosts, indicating that multiple types of RNA are capable of trans-specific movement. Parasitic plants are intriguing systems for studying RNA mobility, in part because such exchange opens new possibilities for control of parasitic weeds, but also because they provide a fresh perspective into understanding roles of RNAs in inter-organismal communication.
Introduction
Movement of RNA is increasingly recognized as a component of RNA function. Whether moving from cell to cell, over long distances within organisms, or between different species, mobile RNAs have potential to influence growth and development of recipient cells.Citation1,2 Recent research has demonstrated that RNAs are able to move between parasitic plant species and their hosts, which represents an extreme category of RNA mobility.Citation3 This work is at an early stage, but is informed by progress in understanding long distance RNA movement in plants as revealed in grafting studies.Citation4 This review focuses on Cuscuta, which is the parasitic plant species that has received the most research on RNA mobility. Discussion will emphasize recent work and implications from discoveries in RNA movement within plants.
Parasitism as a life strategy in plants has evolved at least 12 different times, and over 4,000 plant species are parasitic to some extent.Citation5,6 Given this diversity of origins, it is not surprising that parasitic plants encompass a wide range of morphologies and adaptations that enable them to locate and parasitize their hosts. Parasitic species vary in their level of host dependency from facultative to obligate. Facultative parasites can survive without a host, and resemble fully autotrophic plants in that they have green shoots and expanded leaves, but their roots are able to connect to host roots. Obligate parasites, in contrast, must connect with a host to grow and reproduce. Such parasites may exhibit extensive morphological modifications such as reduced or absent root systems, scale leaves, and lack of photosynthetic capacity. In extreme examples, parasitic plants may live primarily underground or internal to a host, emerging only to flower.
The key anatomic feature of parasitic plants is the haustorium, a term first used in 1813 to describe the physical and physiologic connection between a parasite and its host.Citation7 Haustoria exhibit great structural diversity, reflecting the variety of different evolutionary origins and levels of host dependence among parasitic plant species, although all haustoria contain a xylem-xylem connection that allows the parasite access to the host's water and dissolved nutrients.Citation8 Parasitic species vary in the extent to which they connect to host phloem, although all species appear to obtain some quantity of reduced carbon and nitrogen from their hosts. Certain parasite species have strong symplastic connections to their hosts and are able to import contents from host phloem.Citation9 The nature and function of these connections is central to understanding RNA exchange, but exact mechanisms of transfer remain poorly understood.
Parasitic plant species are engaged in a co-evolutionary relationship with their hosts. Parasites must be able to locate, invade and withdraw nutrients from their hosts. For obligate parasites this begins immediately following seed germination, and any parasite that fails to make a successful connection will perish from starvation. Parasitic plants tend to not kill their hosts outright because they need the hosts to accumulate sufficient resources to flower and set seed. Thus, parasitic plants are biotrophic pathogens, requiring a living host connection to provide water and nutrients, so another key function of the haustorium is to ensure that cell death does not occur in associated host tissues. This suggests that the parasites grow in coordination with their hosts and that mechanisms of communication operate between the 2 plant species. It is interesting to consider the signaling mechanisms that may occur to enable this interaction.
Among parasitic plants, Cuscuta species exhibit the best examples of exchange between host and parasite. Cuscuta are obligate stem parasites that take all their nutrition from their hosts, and are well known for their ability to transfer materials from hosts, taking not only simple photosynthates, but macromolecules such as proteins and RNAs (Summarized in ref.Citation3). Cuscuta seedlings must attach to a host within days of germinating, forming haustoria along points of contact where its thread-like stem coils around the host (). Cells of the haustoria grow invasively into the host tissue, passing between and through host cells to reach the vascular tissue of the host.Citation10 Haustorial cells that contact host xylem differentiate into xylem strands, while cells contacting host phloem differentiate into phloem/transfer cells.Citation11 In addition, parasite cells may form shared plasmodesmata with host cells, further extending the level of integration between host and parasite to symplastic continuity.Citation9,10 The regulation of exchange between host and parasite via joint plasmodesmata is unexplored, but may be important in understanding parasite-host interactions. Strong evidence indicates that phloem connections are active and important between Cuscuta and hosts,Citation9,12 although no anatomic evidence of sieve pore continuity has been documented.
Parasitism as a type of graft
Insight into parasitism may come from understanding graft associations. The haustorial connection has been called an “extraordinarily successful vegetative graft,”Citation13 and may involve many of the same mechanisms.Citation14 Plant grafting under natural conditions occurs widely and has long been a horticultural technique for improving production of certain crops.Citation15 Grafting has also been a valuable tool for studies of RNA mobility, whereby stocks and scions of different genotypes are brought together to trace RNA moving from one to the other. Recent examples of this include cucumber-pumpkin grafts,Citation16 grapevines,Citation2 Arabidopsis ecotypes,Citation4 and even Arabidopsis-tobacco (Nicotiana benthamiana) heterografts.Citation17 In such experiments the RNA sequences form the basis for identifying the source plant for a given mobile RNA, so the wider the genetic distance between grafting partners, the more confidence in tracing specific RNAs. From this point of view Cuscuta is an excellent “scion,” as it parasitizes a wide range of hosts from diverse plant families, with most Cuscuta transcripts being sufficiently distinct in sequence from their orthologues in hosts that they can be readily distinguished.
Although the Cuscuta haustorium shares some key features with graft unions, such as fused vascular systems that allow movement of RNAs, important differences exist. One difference is the remarkable ability of parasite haustoria to produce connections between plant species that are far more phylogenetically distant than is possible with horticultural graft compatibility. This indicates that haustorial connections involve additional factor(s) promoting integration with host tissues. Cuscuta has recently been shown to contain at least one compound that is recognized by a host receptor in a manner typical of host-pathogen interactions.Citation18 This indicates that parasitic plants and their hosts are engaged in a contest that is similar to other pathogen-host interactions, and that parasites may use effectors to suppress host defenses and facilitate parasite feeding. With this in mind it is interesting to explore host-parasite RNA exchange in light of recent work on RNA mobility across graft junctions.
mRNA mobility between Cuscuta and its hosts
Quantifying mRNA mobility
Cuscuta exchanges mRNAs with its hosts. A deep-sequencing study of C. pentagona parasitizing Arabidopsis thaliana found that about 1% of Illumina reads from parasite stem tissue near the region of host-parasite connection were of host origin.Citation19 Conversely, host stem tissue neighboring the region of parasite connection had 0.6% of reads derived from parasite RNA. These proportions may seem low at face value, but likely reflect the contents of foreign RNAs in a specific tissue of the recipient plants. Assuming that the foreign mRNAs are concentrated in phloem,Citation20,21 and that this tissue represents a small fraction of the volume of the stem, it is possible that the phloem of both host and parasite are actually rich in foreign mRNAs. The diversity of genes represented among mobile mRNAs is also interesting. For Arabidopsis-C. pentagona exchange, the numbers are relatively large, with over 9,000 different host transcripts represented among mRNAs derived from attached C. pentagona.Citation19 Over 2,000 different transcripts were detected in a similar experiment involving Arabidopsis-C. reflexa unions.Citation4 The numbers and scope of transcripts exchanged with C. pentagona was lower when tomato (Solanum lycopersicon) was the host as compared with Arabidopsis hosts,Citation19 indicating that specifics of the parasite-host interaction play a role in mRNA exchange.
The large number of genes with RNAs moving between hosts and Cuscuta is remarkable, but may not be out of line with recent findings of mRNA mobility in grafted plant systems. At least 2,006 genes producing mobile transcripts were identified from grafting studies of Arabidopsis ecotypes,Citation4 and this is certainly an underestimate because the genetic similarity of the Col-0 and Ped-0 ecotype grafting partners in this case allowed unambiguous identification of only 28% of Arabidopsis genes. In grafted grapevines (Vitis spp.) 3,333 different transcripts were identified as mobile, which may again be an underestimate for the same reasons as for the Arabidopsis ecotypes.Citation2 On the other hand, sequencing N. benthamiana scions from a heterograft to Arabidopsis stocks should have provided confident detection of mobile mRNAs, but resulted in only 138 Arabidopsis transcripts identified as mobile.Citation17
Regulation of mRNA mobility
The regulation of host-parasite mobility of RNA remains poorly understood. For RNA movement between Arabidopsis or tomato hosts and C. pentagona, a major factor correlating with mobility seems to be simply the abundance of a given mRNA in the region of the haustorium, with the most abundant transcripts having the higher likelihood of moving into the other species.Citation19 This is reasonable because there is generally a pronounced decrease in mRNA concentration across the haustorial-host interface such that approximately 1% of transcript numbers are detected in the recipient as compared with the source plant. Given this situation, transcripts with low expression to start will traverse haustorial interfaces at rates too low to be detectable in the recipient plant. This correlation between transcript abundance and mobility has also been reported for mRNAs moving across graft junctions in grafted systems.Citation2,4 Indeed, a detailed computational analysis of mobile mRNAs from Arabidopsis ecotype grafting confirmed that transcript abundance was highly correlated with mobility, but also that higher transcript stability and shorter transcript lengths were also factors contributing to mobility.Citation22 Although these data support a model in which most mobile RNAs move by a non-specific mechanism, they do not exclude the possibility that some mobile RNAs are specifically regulated, a model with compelling evidence of its own.
In contrast to the majority of mobile transcripts that seem to move in a non-specific manner, some transcripts show greater or lesser movement between host and parasite regardless of their abundance. A subset of mobile transcripts were detected as moving from Arabidopsis to C. pentagona at a higher rate than most other transcripts.Citation19 Quantification of specific mobile mRNAs from tomato to C. pentagona demonstrated the transcript for GIBBERELLIC ACID INSENSTIIVE (GAI), a well-characterized phloem-mobile transcript,Citation23 differed markedly from that of a cathepsin D proteinase inhibitor (PI).Citation20 For the GAI transcript, 0.002% of the amount detected in the host stem was present in the first 10 cm of Cuscuta, whereas 0.3% of the PI transcript was found in the parasite. Similar subsets of transcripts with markedly different graft transmission rates were reported for grapevine.Citation2
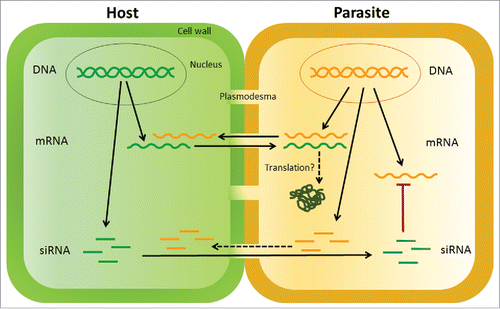
Another indication of regulation is the directionality of transcript movement. It was surprising to discover in the host-Cuscuta system that mRNAs are bidirectionally mobile between host and parasite ().Citation19 This contrasts with the idea of transport between host and parasite being predominantly unidirectional and following source-to-sink gradients, as reported for host-Cuscuta movement of carbon and nitrogen metabolites.Citation24 The possibility that mRNA exchange is directionally regulated in host-parasite interactions is supported by grafting studies that show clear directional movement. Grafting systems using Arabidopsis, cucurbits or grapevines all show bidirectional movement of certain transcripts, while other transcripts translocate specifically from stock to scion or vice versa.Citation2,4,16 Moreover, these studies show that mobility of specific transcripts is influenced by environmental conditions and specific stock/scion combinations, reinforcing the concept that phloem mobility of mRNA is used by plants to transmit precise signals to designated destinations. It will be interesting to learn how parasitic plants use this process in their interactions with hosts.
Figure 2. Schematic diagram of the types of RNA exchanges and functions that may occur between Cuscuta and its hosts. Host components are indicated in shades of green, and parasite components in yellow. Processes that have been experimentally demonstrated are indicated with solid arrows: bidirectional mRNA exchange and host-mediated silencing of parasite genes through siRNA transfer. Hypothesized processes are indicated by dashed arrows: movement of parasite siRNAs into hosts and translation of mobile transcripts. See text for discussion.
RNA trafficking in phloem is thought to play a role in plant development and environmental response.Citation1,25 This is supported by several cases in which movement of a gene transcript is associated with a phenotype change in the recipient tissue, including PFP-LeT6 (producing the Mouse-ears phenotype)Citation26 and GAICitation23 that both lead to alterations in leaf morphology in tomato scions grafted onto transgenic stocks expressing the mobile transcript. Transcripts of potato BEL5 move from leaves to stolons where they promote tuber formation.Citation27 Similarly, shoot-to-root movement of transcripts encoding a dominant mutant version of an auxin signaling factor (iaa18) suppressed growth of lateral roots.Citation28
The major barriers to such movement are the plasmodesmata that regulate entry and exit into sieve elements. Passage through plasmodesmata is facilitated by proteins that bind to mRNAs, creating ribonucleoprotein complexes.Citation29 The mRNA-protein interaction is thus part of the regulatory mechanism, and specific transcripts have been shown to associate with specific proteins. A recent example is the potato BEL5 gene, which encodes a mobile mRNA whose 3′ end is recognized by polypyrimidine tract-binding proteins that contribute to transcript mobility.Citation30 The result is a specific targeting mechanism that directs the BEL5 mRNA from leaves to stolons.Citation31 This illustrates how elements present in 3′ and 5′ ends of mRNAs can contribute to mobility, and in this case stem-loop conformations of RNA are implicated. But this mechanism does not easily explain movement of large numbers of mRNAs in a type of bulk flow as discussed above. However, the observation that tRNAs are abundant in phloem sap suggested that these could be involved in a more general mechanism for phloem mobility.Citation32 tRNAs have stem-loop conformations that may promote passage through plasmodesmata and they can interact with the 3′ ends of mRNAs to create a linked RNA that confers mobility on the complex. Furthermore, many mobile transcripts encode dicistronic tRNA at their 3′ or 5′ ends that are able to associate with their mRNAs and provide the structural motifs necessary for mobility. This system could allow a larger number of mRNAs to be mobile. The recent release of a database of plant mobile RNAs (PlaMoM) should facilitate searches for tRNA-like structure motifs in known mobile mRNAs and accelerate discovery of additional factors contributing to RNA systemic movement.Citation33
Translation of mobile mRNAs
A key question regarding mobile mRNAs in host-parasites systems is whether they serve any function. Perhaps the most obvious fate of mobile mRNAs would be translation into protein. This is reasonable considering that mechanisms for translation are highly conserved and it would be more surprising to discover that parasites (or hosts) have a mechanism for recognizing and specifically degrading mRNAs of another plant species. In the absence of such a mechanism, it would be expected that foreign mRNAs that move from sieve elements into companion cells or parenchyma cells would be translated like self mRNAs. Again, support for this hypothesis comes from grafting studies, including Arabidopsis ecotypes in which protein products from mobile mRNAs were detected in grafted tissues.Citation4 Another example, using a transgenic approach, fused the β-glucuronidase (GUS) reporter gene to IAA18 and detected GUS enzyme activity in wild-type Arabidopsis root-stocks grafted to the transgenic scions.Citation28 Since the GUS protein is not mobile, this suggests that the mobile mRNA was translated. Stronger evidence for translation was provided by grafting transgenic tobacco stocks expressing a mutant version of the protein DISRUPTION OF MEIOTIC CONTROL1 (DMC1) that leads to misshaped pollen in anthers in which the protein is expressed.Citation32 When this transgene was fused to a sequence that conferred mRNA mobility, grafted transgenic stocks conferred the deformed pollen phenotype on wild type scions, indicating translation and function of the protein in scions. In addition, GUS mRNAs that were fused to specific mobility-conferring tRNAs were able to move across graft unions and result in GUS enzyme activity in wild-type tissues, whereas neither GUS mRNA nor protein were mobile when expressed without mRNA mobility-conferring elements.
Small RNA mobility in parasite systems
Perhaps the most intriguing role for mobile RNAs in host-parasite interactions is as regulators of gene expression. This mechanism holds great potential impact as small interfering RNAs (siRNAs) can trigger amplification of a silencing signal through generation of secondary siRNAs and a cascade of silencing that propagates through an organism.Citation34 Within plants small RNAs are able to move systemically through phloem and affect gene expression in the destination tissue.Citation35 An exciting new development is the finding that small RNAs move between plants and fungi to act as modulators of defense or pathogen processes in the recipient organism.Citation36-38 Given the reports of small RNA movement between microbial, plant and animal systems,Citation38,39 the movement and function of miRNAs between parasitic plants and their hosts presents an attractive system for exploring this phenomenon.
Small RNAs move between hosts and parasitic plants when hosts are engineered to express parasite-specific RNA interference (RNAi) constructs (). This trans-specific transmission of a silencing signal has been shown to result in silencing of a Cuscuta gene. Here, tobacco (N. tabacum) was transformed with a silencing construct targeting C. pentagona SHOOT MERISTEMLESS-like (STM), a transcription factor expressed in haustorial tissues of the parasite.Citation40 The expression of this construct resulted in the transmission of siRNA into the parasite where it decreased expression of STM and led to the formation of aberrant haustorial cells. Additional examples of trans-specific gene silencing are found in other parasitic plant species. The facultative parasite Triphysaria versicolor engineered to express the GUS reporter gene showed silencing of GUS activity when it parasitized a host plant expressing a hairpin GUS RNAi construct.Citation41 Furthermore, this technique was used to inhibit T. versicolor growth by decreasing expression of acetyl CoA carboxylase, an essential gene in fatty acid synthesis.Citation42 Host-initiated gene silencing also decreased expression of the mannose 6-phosphate reductase gene in Orobanche (syn. Phelipanche) aegyptiaca growing on transgenic tomato expressing an RNAi construct targeting this gene transcript.Citation43 Taken together, these studies show that small RNA signals are able to traverse the haustorial connections in 2 different parasitic plant lineages, the Cuscuta and Orobanchaceae parasites. These approaches are aimed at developing the foundations for new control methods against parasitic weeds, but the implications are greater in terms of the potential interactions of small RNAs that could be exchanged between parasitic plants and their hosts.
Conclusions
The phenomenon of RNA mobility between parasitic plants and hosts has been known for less than a decade, and has not received wide attention for most of that time. However, the last few years have brought advances in understanding the scope of this exchange, which has also been informed by breakthroughs in characterizing RNA mobility within plants and revelations that small RNAs are moving – and functioning – between organisms.Citation38 Moreover, small RNAs have the potential to not only affect transient gene expression post-transcriptionally, but can direct persistent patterns of gene expression through modified DNA methylation patterns.Citation35,44 This suggests that Cuscuta could incur epigenetic changes related to the specific host it is parasitizing, or impose specific epigenetic changes on its host as another mechanism of host manipulation. Considering the far-reaching potential of mobile RNAs in general, research on this subject seems to be in its infancy.
Parasitic plants provide several advantages for studies of RNA movement as described above. However, research on parasitic plants has been limited by the fact that they don't fit the standards of most model systems; they require time and skill to grow, are recalcitrant to transformation, and are lacking in genetic resources. Progress has been made on some of these fronts, as transformation protocols exist for some species,Citation45-47 and transcriptomes are increasingly available,Citation48,49 but all are wild species with unrefined genetics and a relatively small community of researchers. In terms of RNA mobility and function, Cuscuta species are the most attractive for further development as a model system. A robust transformation protocol, complete genome, and defined varieties for researchers to use would substantially advance this research. It is possible to grow Cuscuta on model hosts such as Arabidopsis and tobacco, so the ability to transform and regenerate Cuscuta would enable exploration of the function of parasite genes and mobile RNAs in mediating host-parasite interactions.
Future research on RNA exchange in Cuscuta-host interactions should address questions of mobile RNA function. In light of the emerging role of small RNAs in inter-organismal interactions, and particularly in pathogenic fungi virulence,Citation36 it is important to investigate whether small RNAs are exchanged between host and Cuscuta. For all forms of RNA, it will be important to understand their significance in host-parasite interactions. Do they contribute to parasite success? Do they influence host specificity? Has Cuscuta evolved mechanisms to benefit from (or negate) the influx of host RNAs? It will be interesting to know whether the parasite RNA processing machinery differs from that of non-parasitic plants.
It is also important to explore mobile RNA in other parasitic species. Haustoria appear to share common themes in terms of structure and function, although differences are also apparent. Is RNA exchange among the common themes in haustoria-host interactions? The use of the trans-specific siRNA technology offers hope for the engineering of parasite resistant crops, but this may not work if the process varies substantially among parasite species. The outcome of this work would have broad implications for agriculture in parts of the world plagued by parasitic weed problems.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This research was supported by the NSF Plant Genome Research Program under grant IOS-1238057 and National Institute of Food and Agriculture Project No. 131997.
References
- Kragler F. RNA in the phloem: A crisis or a return on investment? Plant Science 2010; 178:99-104; PMID:20133178; http://dx.doi.org/10.1016/j.plantsci.2009.12.006
- Yang YZ, Mao LY, Jittayasothorn Y, Kang YM, Jiao C, Fei ZJ, Zhong GY. Messenger RNA exchange between scions and rootstocks in grafted grapevines. BMC Plant Biol 2015; 15:14; PMID:25603772; http://dx.doi.org/10.1186/s12870-014-0378-0
- Kim G, Westwood JH. Macromolecule exchange in Cuscuta–host plant interactions. Curr Op Plant Biol 2015; 26:20-25; PMID:26051214; http://dx.doi.org/10.1016/j.pbi.2015.05.012
- Thieme CJ, Rojas-Triana M, Stecyk E, Schudoma C, Zhang W, Yang L, Miñambres M, Walther D, Schulze WX, Paz-Ares J, et al. Endogenous Arabidopsis messenger RNAs transported to distant tissues. Nature Plants 2015; 1:1-7; PMID:27247031; http://dx.doi.org/10.1038/nplants.2015.25
- Tesitel J. Functional biology of parasitic plants: a review. Plant Ecol Evol 2016; 149:5-20; http://dx.doi.org/10.5091/plecevo.2016.1097
- Nickrent DL, Duff RJ, Colwell AE, Wolfe AD, Young ND, Steiner KE, de Pamphilis CW. Molecular phylogenetic and evolutionary studies of parasitic plants. In: Soltis DE, Soltis PS, Doyle JJ, editors. Molecular Systematics of Plants II: DNA sequencing, edn Boston: Kluwer Academic Publishers; 1998; 211-41; http://dx.doi.org/10.1007/978-1-4615-5419-6_8
- Kuijt J. Haustoria of phanerogamic parasites. Ann Rev Phytopathol 1977; 17:91-118; http://dx.doi.org/10.1146/annurev.py.15.090177.000515
- Irving LJ, Cameron DD. You are what you eat: Interactions between root parasitic plants and their hosts. editors. Advances in Botanical Research, Vol 50, edn London: Academic Press Ltd; 2009; 87-138; http://dx.doi.org/10.1016/s0065-2296(08)00803-3
- Birschwilks M, Haupt S, Hofius D, Neumann S. Transfer of phloem-mobile substances from the host plants to the holoparasite Cuscuta sp. J Exp Bot 2006; 57:911-21; PMID:16467411; http://dx.doi.org/10.1093/jxb/erj076
- Vaughn KC. Dodder hyphae invade the host: a structural and immunocytochemical characterization. Protoplasma 2003; 220:189-200; PMID:12664283; http://dx.doi.org/10.1007/s00709-002-0038-3
- Vaughn KC. Conversion of the searching hyphae of dodder into xylic and phloic hyphae: A cytochemical and immunocytochemical investigation. Intl J Plant Sci 2006; 167:1099-1114; http://dx.doi.org/10.1086/507872
- Birschwilks M, Sauer N, Scheel D, Neumann S. Arabidopsis thaliana is a susceptible host plant for the holoparasite Cuscuta spec. Planta 2007; 226:1231-41; PMID:17598126; http://dx.doi.org/10.1007/s00425-007-0571-6
- Kuijt J. Tissue compatibility and the haustoria of parasitic angiosperms. In: Moore R, editors. Vegetative Compatibility Responses in Plants, edn Waco, TX: Baylor University; 1983; 1-12.
- Melnyk CW, Meyerowitz EM. Plant grafting. Curr Biol 2015; 25:R183-8; PMID:25734263; http://dx.doi.org/10.1016/j.cub.2015.01.029
- Mudge K, Janick J, Scofield S, Goldschmidt EE. A history of grafting. In: Janick J, ed. Horticultural Reviews, Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2009; 437-93; http://dx.doi.org/10.1002/9780470593776.ch9.
- Zhang Z, Zheng Y, Ham B-K, Chen J, Yoshida A, Kochian LV, Fei Z, Lucas WJ. Vascular-mediated signalling involved in early phosphate stress response in plants. Nature Plants 2016; 2:16033; PMID:27249565; http://dx.doi.org/10.1038/nplants.2016.33
- Notaguchi M, Higashiyama T, Suzuki T. Identification of mRNAs that move over long distances using an RNA-seq analysis of Arabidopsis/Nicotiana benthamiana heterografts. Plant Cell Physiol 2014; 56:311-21; PMID:25527829; http://dx.doi.org/10.1093/pcp/pcu210
- Hegenauer V, Fürst U, Kaiser B, Smoker M, Zipfel C, Felix G, Stahl M, Albert M. Detection of the plant parasite Cuscuta reflexa by a tomato cell surface receptor. Science 2016; 353:478-81; PMID:27471302; http://dx.doi.org/10.1126/science.aaf3919
- Kim G, LeBlanc ML, Wafula EK, dePamphilis CW, Westwood JH. Genomic-scale exchange of mRNA between a parasitic plant and its hosts. Science 2014; 345:808-811; PMID:25124438; http://dx.doi.org/10.1126/science.1253122
- LeBlanc M, Kim G, Patel B, Stromberg V, Westwood J. Quantification of tomato and Arabidopsis mobile RNAs trafficking into the parasitic plant Cuscuta pentagona. New Phytol 2013; 200:1225-33; PMID:23914903; http://dx.doi.org/10.1111/nph.12439
- David-Schwartz R, Runo S, Townsley B, Machuka J, Sinha N. Long-distance transport of mRNA via parenchyma cells and phloem across the host-parasite junction in Cuscuta. New Phytol 2008; 179:1133-41; PMID:18631294; http://dx.doi.org/10.1111/j.1469-8137.2008.02540.x
- Calderwood A, Kopriva S, Morris RJ. Transcript abundance axplains mRNA mobility data in Arabidopsis thaliana. Plant Cell 2016; 28:610-5; PMID:26952566; http://dx.doi.org/10.1105/tpc.15.00956
- Haywood V, Yu T-S, Huang N-C, Lucas WJ. Phloem long-distance trafficking of GIBBERELLIC ACID-INSENSITIVE RNA regulates leaf development. Plant J 2005; 42:49-68; PMID:15773853; http://dx.doi.org/10.1111/j.1365-313X.2005.02351.x
- Jeschke WD, Baumel P, Rath N, Czygan F-C, Proksch P. Modeling of the flows and partitioning of carbon and nitrogen in the holoparasite Cuscuta reflexa Roxb. and its host Lupinus albus L. II. Flows between host and parasite and within the parasitized host. J Exp Bot 1994; 45:801-812; http://dx.doi.org/10.1093/jxb/45.6.801
- Turgeon R, Wolf S. Phloem transport: Cellular pathways and molecular trafficking. Ann Rev Plant Biol 2009; 60:207-21; PMID:19025382; http://dx.doi.org/10.1146/annurev.arplant.043008.092045
- Kim M, Canio W, Kessler S, Sinha N. Developmental changes due to long-distance movement of a homeobox fusion transcript in tomato. Science 2001; 293:287-9; PMID:11452121; http://dx.doi.org/10.1126/science.1059805
- Hannapel DJ. A model system of development regulated by the long-distance transport of mRNA. J Integ Plant Biol 2010; 52:40-52; PMID:20074139; http://dx.doi.org/10.1111/j.1744-7909.2010.00911.x
- Notaguchi M, Wolf S, Lucas WJ. Phloem-mobile Aux/ IAA transcripts target to the root tip and modify root architecture. J Integ Plant Biol 2012; 54:760-72; PMID:22925478; http://dx.doi.org/10.1111/j.1744-7909.2012.01155.x
- Lucas WJ, Groover A, Lichtenberger R, Furuta K, Yadav S-R, Helariutta Y, He X-Q, Fukuda H, Kang J, Brady SM, et al. The plant vascular system: Evolution, development and functions. J Integ Plant Biol 2013; 55:294-388; PMID:23462277; http://dx.doi.org/10.1111/jipb.12041
- Cho SK, Sharma P, Butler NM, Kang I-H, Shah S, Rao AG, Hannapel DJ. Polypyrimidine tract-binding proteins of potato mediate tuberization through an interaction with StBEL5 RNA. J Exp Bot 2015; 66:6835-47; PMID:26283046; http://dx.doi.org/10.1093/jxb/erv389
- Hannapel DJ, Sharma P, Lin T. Phloem-mobile messenger RNAs and root development. Front Plant Sci 2013; 4:1-12; PMID:23882275; http://dx.doi.org/10.3389/fpls.2013.00257
- Zhang WN, Thieme CJ, Kollwig G, Apelt F, Yang L, Winter N, Andresen N, Walther D, Kragler F. tRNA-related sequences trigger systemic mRNA transport in plants. Plant Cell 2016; 28:1237-49; PMID:27268430; http://dx.doi.org/10.1105/tpc.15.01056
- Guan D, Yan B, Thieme C, Hua J, Zhu H, Boheler KR, Zhao Z, Kragler F, Xia Y, Zhang S. PlaMoM: a comprehensive database compiles plant mobile macromolecules. Nucleic Acids Res 2016; 4; 45(D1):D1021-D1028; PMID:27924044; http://dx.doi.org/10.1093/nar/gkw988
- Axtell MJ. Classification and comparison of small RNAs from plants. Ann Rev Plant Biol 2013; 64:137-59; http://dx.doi.org/10.1146/annurev-arplant-050312-120043
- Molnar A, Melnyk CW, Bassett A, Hardcastle TJ, Dunn R, Baulcombe DC. Small silencing RNAs in plants are mobile and direct epigenetic modification in recipient cells. Science 2010; 328:872-5; PMID:20413459; http://dx.doi.org/10.1126/science.1187959
- Wang M, Weiberg A, Lin F-M, Thomma BPHJ, Huang H-D, Jin H. Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nature Plants 2016; 2:16151; PMID:27643635; http://dx.doi.org/10.1038/nplants.2016.151
- Weiberg A, Wang M, Lin F-M, Zhao H, Zhang Z, Kaloshian I, Huang H-D, Jin H. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 2013; 342:118-23; PMID:24092744; http://dx.doi.org/10.1126/science.1239705
- Weiberg A, Bellinger M, Jin H. Conversations between kingdoms: small RNAs. Curr Op Biotechnol 2015; 32:207-15; PMID:25622136; http://dx.doi.org/10.1016/j.copbio.2014.12.025
- Han L, Luan YS. Horizontal transfer of small RNAs to and from plants. Front Plant Sci 2015; 6:7. PMID:25688249; http://dx.doi.org/10.3389/fpis.2015.01173
- Alakonya A, Kumar R, Koenig D, Kimura S, Townsley B, Runo S, Garces HM, Kang J, Yanez A, David-Schwartz R, et al. Interspecific RNA interference of SHOOT MERISTEMLESS-like disrupts Cuscuta pentagona plant parasitism. Plant Cell 2012; 24:3153-66; PMID:22822208; http://dx.doi.org/10.1105/tpc.112.099994
- Tomilov AA, Tomilova NB, Wroblewski T, Michelmore R, Yoder JI. Trans-specific gene silencing between host and parasitic plants. Plant J 2008; 56:389-397; PMID:18643992; http://dx.doi.org/10.1111/j.1365-313X.2008.03613.x
- Bandaranayake PCG, Yoder JI. Trans-specific gene silencing of acetyl-CoA carboxylase in a root-parasitic plant. Mol Plant-Microbe Interact 2013; 26:575-84; PMID:23383721; http://dx.doi.org/10.1094/MPMI-12-12-0297-R
- Aly R, Cholakh H, Joel DM, Leibman D, Steinitz B, Zelcer A, Naglis A, Yarden O, Gal-On A. Gene silencing of mannose 6-phosphate reductase in the parasitic weed Orobanche aegyptiaca through the production of homologous dsRNA sequences in the host plant. Plant Biotech J 2009; 7:487-98; PMID:19490480; http://dx.doi.org/10.1111/j.1467-7652.2009.00418.x
- Lewsey MG, Hardcastle TJ, Melnyk CW, Molnar A, Valli A, Urich MA, Nery JR, Baulcombe DC, Ecker JR. Mobile small RNAs regulate genome-wide DNA methylation. Proc Natl Acad Sci U S A 2016; 113:E801-10; PMID:26787884; http://dx.doi.org/10.1073/pnas.1515072113
- Fernández-Aparicio M, Rubiales D, Bandaranayake P, Yoder J, Westwood J. Transformation and regeneration of the holoparasitic plant Phelipanche aegyptiaca. Plant Methods 2011; 7:36; PMID:22067615; http://dx.doi.org/10.1186/1746-4811-7-36
- Ishida JK, Yoshida S, Ito M, Namba S, Shirasu K. Agrobacterium rhizogenes-mediated transformation of the parasitic plant Phtheirospermum japonicum. PLoS ONE 2011; 6:e25802; PMID:21991355; http://dx.doi.org/10.1371/journal.pone.0025802
- Tomilov A, Tomilova N, Yoder JI. Agrobacterium tumefaciens and Agrobacterium rhizogenes transformed roots of the parasitic plant Triphysaria versicolor retain parasitic competence. Planta 2007; 225:1059-71; PMID:17053892; http://dx.doi.org/10.1007/s00425-006-0415-9
- Westwood J, dePamphilis C, Das M, Fernandez-Aparicio M, Honaas L, Timko M, Wafula E, Wickett N, Yoder J. The parasitic plant genome project: New tools for understanding the biology of Orobanche and Striga. Weed Sci 2012; 60:295-306; http://dx.doi.org/10.1614/WS-D-11-00113.1
- Ranjan A, Ichihashi Y, Farhi M, Zumstein K, Townsley B, David-Schwartz R, Sinha NR. De novo assembly and characterization of the transcriptome of the parasitic weed Cuscuta pentagona identifies genes associated with plant parasitism. Plant Physiol 2014; 166:1186-99; PMID:24399359; http://dx.doi.org/10.1104/pp.113.234864

