ABSTRACT
Cold adaptation is an evolutionary process that has dramatic impact on enzymatic activity. Increased flexibility of the protein structure represents the main evolutionary strategy for efficient catalysis and reaction rates in the cold, but is achieved at the expense of structural stability. This results in a significant activity-stability tradeoff, as it was observed for several metabolic enzymes. In polymerases, however, not only reaction rates, but also fidelity plays an important role, as these enzymes have to synthesize copies of DNA and RNA as exact as possible. Here, we investigate the effects of cold adaptation on the highly accurate CCA-adding enzyme, an RNA polymerase that uses an internal amino acid motif within the flexible catalytic core as a template to synthesize the CCA triplet at tRNA 3′-ends. As the relative orientation of these residues determines nucleotide selection, we characterized how cold adaptation impacts template reading and fidelity. In a comparative analysis of closely related psychro-, meso-, and thermophilic enzymes, the cold-adapted polymerase shows a remarkable error rate during CCA synthesis in vitro as well as in vivo. Accordingly, CCA-adding activity at low temperatures is not only achieved at the expense of structural stability, but also results in a reduced polymerization fidelity.
Introduction
The majority of the earth's habitats including deep-sea and polar regions are either temporarily or permanently exposed to temperatures below 5°C.Citation1-Citation4 Yet, these habitats are populated by a wide diversity of cold-adapted organisms of all three domains of life.Citation3-Citation5 Such psychrophiles are defined by optimal growth rates at less than 15 to 20°CCitation6 and belong to the most abundant group of organisms on earth.Citation2 Similar to survival at high temperatures, thriving in the cold requires specific cellular and molecular adaptations. In particular, a cell's metabolism depends to a large extent on enzymes that are able to compensate for drastically reduced chemical reaction rates at low temperatures.Citation1,Citation5,Citation7-Citation10
A common adaptation strategy of psychrophilic enzymes is the destabilization of the enzyme/substrate complex to decrease the required activation energy. As a result, the majority of psychrophilic enzymes have a higher K M value (which, by approximation, can be interpreted as lower substrate binding affinity) at low temperatures in order to achieve a higher kcat (reaction rate) compared to enzymes from meso- and thermophilic organisms.Citation8,Citation11,Citation12 In most cases, this destabilization of the enzyme/substrate complex is the result of a higher degree of structural flexibility of the protein.Citation2,Citation11,Citation13-Citation15 However, the increased flexibility leads to a rapid denaturation at higher temperatures, rendering cold-adapted enzymes thermolabile. Thus, structural flexibility is achieved at the expense of stability. This relation between activity, flexibility as well as stability is referred to as activity-stability tradeoff.Citation4,Citation8,Citation12,Citation16
Most investigations on psychrophilic adaptation were conducted on metabolic enzymes such as cellulases, amylases and others with considerable activity at low temperatures and a greatly reduced thermal stability.Citation17,Citation18 DNA and RNA polymerases, however, are not only characterized by stability and turnover number. In nucleic acid synthesis, processivity and fidelity are equally important parameters. Furthermore, conformational flexibility seems to be an essential feature in the polymerization reaction even at moderate temperatures, as it was demonstrated for transcription initiation of the bacterial DNA-dependent RNA polymerase or the nucleotide addition by the CCA-adding enzyme synthesizing the CCA triplet at the tRNA 3′-end.Citation19-Citation22 Hence, it is conceivable that cold adaptation by increasing the structural flexibility in these enzymes has an impact on their polymerization activity. Yet, as nucleotide selection/incorporation is mainly determined by base complementarity with the DNA template, an impact on the fidelity of the conventional RNA polymerase is not very likely, as only base pairing stability, but not specificity, in DNA-RNA interaction is temperature-dependent. In addition, as this enzyme is composed of several subunits (α2ββ’ω), analysis of cold adaptation of individual components is difficult. As a consequence, only very limited data are available for psychrophilic RNA polymerases.Citation23
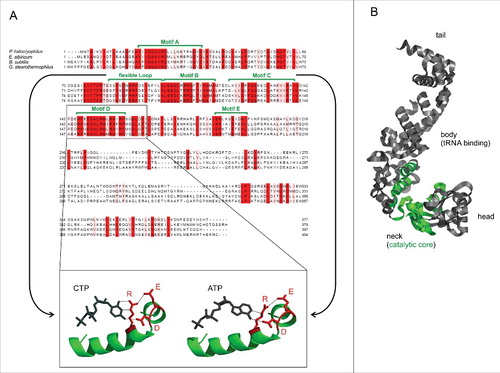
In contrast, the specialized CCA-adding enzyme represents a good model RNA polymerase for such investigations. First, it acts as a monomer and is present as a single isoform in the cell.Citation24 Second, it is not using an external nucleic acid as a template, but depends on a set of highly conserved amino acid residues in the catalytic core that form Watson-Crick-like hydrogen bonds to CTP and ATP ().Citation25 Here, exact positioning of these templating amino acids in the flexible core determines the base-pairing specificity. In addition, the enzyme contains several further flexible regions involved in coordinated movements during the individual reaction steps.Citation22,Citation24-Citation27 However, such a defined elasticity in sequence-specific polymerization seems to be at odds with a relaxed structural flexibility in cold adaptation. To investigate whether these contradicting requirements have a consequence on nucleotide incorporation efficiency and especially polymerization fidelity, we examined the reactivity of CCA-adding enzymes adapted to different temperatures.
Figure 1. Sequence and structural presentation of CCA-adding enzymes. (A) Sequence alignment with highly conserved residues in red. Motifs A to E of the catalytic coreCitation25 are indicated in green. The flexible loop element involved in switching the nucleotide specificity from CTP towards ATP is also labeled.Citation26 The boxed elements represent the templating amino acid residues in motif D, forming hydrogen bonds to the bound CTP and ATP, respectively.Citation25 The relative positioning of E, D and R determine the nucleotide specificity. (B) Crystal structure of the GstCCA enzyme.Citation25 From N- to C-terminus, the enzyme consists of head, neck, body and tail domains. While the body region binds the tRNA substrate, the neck domain contains the motifs of the catalytic core (green).
Results
CCA-adding enzymes in Bacillales are closely related, but show different temperature requirements
A typical feature of enzymes from temperature-adapted organisms is their significant catalytic activity in a defined temperature range - psychrophilic enzymes are active in the cold, while their thermophilic counterparts show catalysis at considerable high temperatures.Citation28-Citation31 To find out whether the CCA-adding enzymes of four closely related species of Bacillales exhibit such typical temperature-adaptations, we investigated their thermal stability and catalytic reactivity. In this genus, the CCA triplet is encoded only in part of the tRNA genes, while other genes lack this sequence.Citation32 In these cases, the CCA triplet has to be synthesized posttranscriptionally by the CCA-adding enzyme.Citation33-Citation35 We focused on the corresponding enzymes of Exiguobacterium sibiricum (strain 255-15) that is able to tolerate temperatures of −2.5°CCitation36 and Planococcus halocryophilus (strain Or1), growing at −15°C, one of the lowest temperatures demonstrated.Citation37 As meso- and thermophilic counterparts, CCA-adding enzymes of Bacillus subtilis subsp. subtilis and Geobacillus stearothermophilus were chosen. All enzymes show a high conservation of the catalytic core,Citation25,Citation26 and an overall sequence identity of 37–45% indicates their close evolutionary relation (). Further, computational predictions suggest the typical structure of bacterial CCA-adding enzymes for all candidates (; Figure S1).
Recombinant enzymes were expressed in E. coli and tested for activity in a range from 0 to 90°C. In vitro transcribed radioactively labelled tRNAPhe from Saccharomyces cerevisiae was used as a substrate, representing one of the best characterized tRNAs with a structure of the non-modified in vitro transcript highly similar to the native tRNA.Citation38,Citation39 Accordingly, this tRNA is frequently used as a standard substrate for in vitro tRNA processing reactions.Citation22,Citation40-Citation43 Furthermore, its structural robustness renders it an ideal substrate for the investigated broad temperature range. Products were separated on a denaturing polyacrylamide gel, and their signal intensity was determined densitometrically and plotted against the individual incubation temperatures (). To monitor and avoid any reaction plateau effects, incubations were performed under non-saturating conditions for 5 min, where an almost complete CCA addition is only observed at the optimal reaction temperature of each enzyme. For normalization, the maximum intensity of the final reaction products, corresponding to a tRNA with complete CCA end, was set to 100%. The cold-adapted EsiCCA and PhaCCA exhibit a temperature optimum for catalysis between 20 and 30°C, but are also active at 0 to 10°C. The mesophilic BsuCCA is less active at low temperatures and shows a maximum at 40°C. At 50°C, both cold-adapted and mesophilic enzymes show a sharp drop of activity. The thermophilic GstCCA, however, shows almost no activity at 0 to 30°C, while the reaction optimum is located at 60°C. A further increase of temperature reduces the enzymatic activity, and at 90°C, the enzyme is completely inactive. In the kinetic analysis, we focused on the incorporation of the terminal A residue on a tRNA ending with CC, representing the rate-limiting step in CCA-addition that requires a series of conformational rearrangements in the enzyme.Citation22,Citation42,Citation44 Hence, KM as well as kcat were determined for ATP as a substrate. The obtained parameters represent apparent values, as the solubility properties of the tRNA substrate do not allow the use of excessive saturating concentrations.Citation20,Citation45-Citation47 Both KM and kcat are in agreement with the temperature-dependent activities of the enzymes, with the highest kcat values at the corresponding optimal reaction temperatures (; Figure S2).
Figure 2. Temperature dependent activity and stability of CCA-adding enzymes and chimeras. (A) Temperature-dependent CCA-incorporation on yeast tRNAPhe under non-saturating conditions was recorded densitometrically in independent triplicates. For each enzyme (GstCCA, red; BsuCCA, green; EsiCCA, blue; PhaCCA, cyan), a representative gel picture is shown. Reactions occurred under non-saturating conditions, where only partial CCA-addition is observed at most temperatures. As an example, GstCCA shows a CCA incorporation at 93% efficiency only at the temperature optimal for this enzyme (60°C). Hence, the chosen conditions clearly allow a relative quantitation of the reaction. Reaction products corresponding to complete CCA incorporation were plotted against the temperature. Error bars indicate standard deviation. For normalization, the maximum product signal for each enzyme was set to 100%. The temperature activity curves show that EsiCCA (blue) and PhaCCA (cyan) are cold-adapted enzymes, while BsuCCA (green) and GstCCA (red) represent mesophilic and thermophilic proteins, respectively. (B) Melting behavior of CCA-adding enzymes indicates their individual temperature adaptation. CD-spectroscopy was recorded at 222 nm from 0 to 90°C. Distinct unfolding can be observed at 33°C and 38°C for EsiCCA and PhaCCA, respectively. BsuCCA unfolds at 44°C, while GstCCA shows a melting temperature of 73°C. (C) Temperature-dependent activity spectrum of enzyme chimeras GstEsiCCA (dark grey) and EsiGstCCA (light grey). While parental enzymes (blue and red) show typical cold- or heat-adapted activities, chimeric enzymes show a temperature optimum similar to the psychrophilic EsiCCA enzyme (blue). These data demonstrate that the psychrophilic enzyme part dictates the temperature profile of the polymerization reaction. (D) Melting behavior of chimeras. Due to a composition of psychro- and mesophilic parts, a two-step melting curve was expected. However, both chimeras show an intermediate single-step unfolding with TM values of 57°C and 56°C, comparable to that of mesophilic enzymes.
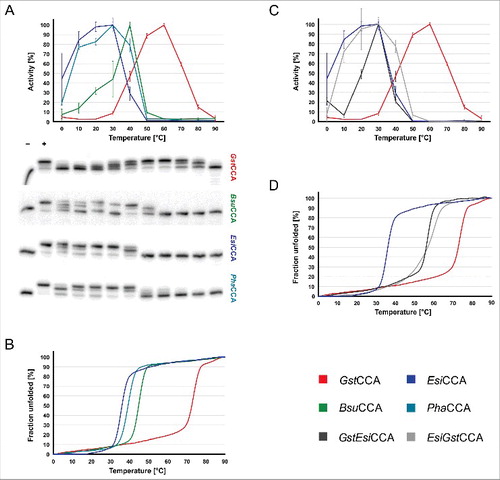
Table 1. Apparent kinetic parameters of CCA-adding enzymes at different temperatures.
To determine temperature-dependent melting characteristics of the individual enzymes, thermal unfolding was measured. CD spectroscopy at 222 nm is frequently used to follow the temperature-dependent denaturation of proteins, monitoring the unfolding of alpha-helical elements.Citation48-Citation51 As tertiary interactions in the 3D structure are usually destroyed before the secondary structures,Citation52-Citation55 the unfolding of alpha-helical elements definitely indicates denaturation and functional loss of a protein. Hence, these measurements give a conservative estimation of a proteins melting temperature, allowing to discriminate psychrophilic CCA-adding enzymes from their thermostable counterparts based on the unfolding of secondary structures. As shown in , the obtained denaturation curves reveal a distinct unfolding behavior of all enzymes with melting temperatures (TM; corresponding to 50% unfolding) of 33°C (EsiCCA), 38°C (PhaCCA), 44°C (BsuCCA) and 73°C (GstCCA), respectively. The discrepancy between maximum activity (50-60°C) and thermal denaturation (73°C) of GstCCA can be explained by the thermal instability of the tRNA substrate that lacks stabilizing modifications and exhibits a melting temperature of 65°C.Citation56 Thus, all four candidates clearly exhibit adaptation to the corresponding temperatures, and EsiCCA as well as PhaCCA show the highest activity at temperatures between 0 and 20°C.
A characteristic feature of many cold-adapted enzymes is a locally increased thermal instability of the active site, whereas regions not involved in catalysis can be as stable as mesophilic enzymes.Citation11 Hence, we investigated whether the active site alone is responsible for cold-adaptation. With melting temperatures of 33°C and 73°C (), the psychrophilic EsiCCA and the thermophilic GstCCA show the most pronounced thermal adaptation of the investigated enzyme candidates. Accordingly, these enzymes were expected to show the clearest differences in temperature-dependent activity and were therefore chosen for further analysis. N- and C-termini (carrying the catalytic core and the tRNA binding region, respectively) were reciprocally exchanged, leading to chimeras EsiGstCCA and GstEsiCCA (Figure S1). The chimeric enzymes show a reaction temperature optimum comparable to that of the parental cold-adapted enzyme, irrespective of whether the psychrophilic part was the N- or the C-terminal region (). This indicates that i) both N- and C-termini contribute to cold adaptation and ii) the psychrophilic part restricts the activity of the chimeras to low temperatures.
According to their composition, the chimeras were expected to show a two-step thermal denaturation of their alpha-helical elements, where the cold-adapted part unfolds first, while the thermostable part denatures at a higher temperature. Surprisingly, both EsiGstCCA and GstEsiCCA show a single-step denaturation curve with melting temperatures of 55 to 57°C (). Hence, although catalyzing CCA-addition at a temperature characteristic for the psychrophilic part, the unfolding behavior of the chimeras is comparable to that of a mesophilic enzyme, indicating a mutual influence in thermal stability of the different enzyme regions.
CCA-addition with impaired fidelity
A hallmark of CCA-addition is the remarkably high fidelity of tRNA nucleotidyltransferases during polymerization. With their specific amino acid template, these enzymes do not only show an efficient discrimination against GTP and UTP, but reliably incorporate C and A residues in the correct number and order.Citation24,Citation25,Citation57,Citation58 In this reaction, coordinated movements in the NTP binding pocket define the nucleotide selection and adjust the catalytic core to accommodate the growing tRNA 3′-end, dictating the number of the added nucleotides.Citation22,Citation59 As it is conceivable that an increased flexibility of the catalytic core has an impact on the fidelity of this reaction, a direct comparison of a cold-adapted (flexible) and a thermostable (rigid) version of the CCA-adding enzymes was conducted. Analogous to the investigation of the chimeric enzymes, we focused on EsiCCA and GstCCA, the two enzymes with the lowest and highest melting temperature as the most pronounced thermal adaptation.
Both recombinant enzymes were incubated under standard conditionsCitation22,Citation26 at 0 and 20°C in the presence of NTPs with a radioactively labeled in vitro transcribed yeast tRNAPhe lacking the CCA-end. The reaction products were separated on a denaturing polyacrylamide gel and visualized by autoradiography (). For GstCCA, the resulting autoradiogram shows shifted reaction products that indicate the addition of three nucleotides, corresponding to the incorporation of a complete CCA sequence at both temperatures. Similarly, incubation with EsiCCA also leads to main reaction products that are elongated by the polymerization of the CCA terminus. However, and in contrast to the GstCCA-catalyzed reaction, additional bands above the main product signal are visible, indicating the incorporation of further nucleotides besides the CCA triplet. To investigate the 3′ ends of the reaction products in more detail, the corresponding bands were excised as indicated, reverse transcribed into cDNA, cloned, and 78 to 83 individual clones analyzed by sequencing (). For investigating the error rate of GstCCA, the same region of the gel was eluted. Here, 72 to 75 clones were sequenced.
Figure 3. The psychrophilic EsiCCA enzyme shows an increased nucleotide misincorporation. (A) CCA-addition at 0 and 20°C. Similar to the E. coli enzyme (EcoCCA), GstCCA shows an addition of three residues, indicated by the shift of the product band in the denaturing PAA gel. EsiCCA incorporates additional residues, leading to new reaction bands with lower electrophoretic mobility. The boxed bands were eluted and nucleotide addition on the substrate tRNA was analyzed by sequencing. (B) 23.1–26.4% of the EsiCCA reaction products (blue) carry additional C and A residues, leading in most cases to tRNAs ending with CCACC (A). In contrast, only 2.8–4% of the GstCCA reaction products (red) carry such erroneous 3′-ends (B).
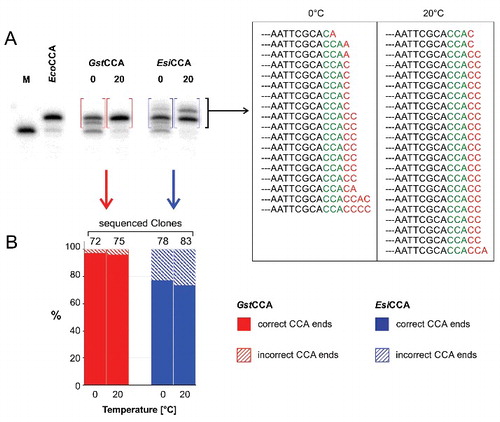
In the reaction products of the thermostable Gst enzyme, only 2 and 3 erroneous CCA-ends were observed at 0 and 20°C, respectively, carrying extra nucleotides C (3 clones), CA (1 clone) or AAC (1 clone). This corresponds to an error rate of 2.8 to 4%, comparable to results on the human or E. coli CCA-adding enzymes.Citation58 EsiCCA, however, showed a considerable fraction of tRNA products with additional incorporations of C and A residues in various number and order, ranging from single nucleotide additions (A or C; 9 clones) to the incorporation of two (CC, CA; 27 clones), three (CCA; 1 clone) or even four residues (CCAC, CCCC; 2 clones). In most cases, the additional sequences consisted of two C residues, resembling a partial addition of a second CCA terminus. As the rate-limiting step in CCA-addition is the incorporation of the terminal A residue,Citation22,Citation42,Citation44 it is very likely that the rather high number of CCACC ends is a consequence of this enzyme feature. In total, this represents misincorporations in 23.1 to 26.4% at 0 and 20°C, respectively.
As tRNAs carrying erroneous 3′-termini are not functional in translation, the question arises whether the increased error rate during CCA-addition is also detectable in vivo in psychrophilic bacteria. To answer this question, the psychro-, meso- and thermophilic organisms were grown in three independent experiments. Subsequently, fractions consisting of small RNAs with a size between 50 and 200 nts were isolated, and tRNA 3′-ends were analyzed by RNA-Seq in a high-throughput approach. It is well documented that non-charged, deacylated tRNAs accumulate under stress conditions like limited nutrition, as it is observed in the stationary growth phase.Citation60,Citation61 In such a situation, the CCA-end is subjected to an increased turnover event, where 3′-terminal nucleotides are removed by hydrolysis or RNase T activity and subsequently restored by the CCA-adding enzyme.Citation58,Citation62,Citation63 To take this end turnover and tRNA repair into account in our analysis, individual samples were taken in exponential as well as stationary phase. In total, 865,733 to 3,567,406 tRNA reads per organism in the exponential phase, and 611,810 to 2,643,369 tRNA reads in the stationary phase were analyzed.
Furthermore, in Bacillales, not all the tRNA genes encode the CCA-end. Using the genomic reference sequences for each investigated species, we identified that between 48.2% (G. stearothermophilus) and 77.1% (P. halocryophilus) of the tRNAs carry a genetically encoded CCA triplet, while in 22.9 to 51.8% of the tRNA genes in these species, this triplet is not encoded (Fig. S3). For B. subtilis and E. sibiricum, similar distributions of CCA-carrying and CCA-free tRNA genes were observed. Consequently, the tRNAs lacking a CCA end depend on the activity of the CCA-adding enzyme for 3′-end maturation, while in tRNAs with encoded CCA triplet, the CCA-adding enzyme is exclusively required for maintenance and repair of this sequence.Citation58,Citation64,Citation65 As it is possible that the erroneous polymerization activity of CCA-adding enzyme affects maturation (complete CCA synthesis) and tRNA repair (predominantly A addition) to different extents, both total tRNA as well as tRNA fraction with posttranscriptionally added CCA end were considered separately in the analysis ().
Figure 4. tRNAs with erroneous CCA ends accumulate in psychrophilic species. (A) 3′-end analysis of tRNAs with posttranscriptionally added CCA ends. In exponential as well as stationary phase, P. halocryophilus and E. sibiricum tRNAs show a highly significant increase in erroneous CCA ends compared to B. subtilis and G. stearothermophilus. Indicated numbers represent percentage values. (B) The same is true for the total tRNA pool, where tRNAs with genomically encoded CCA ends are included. This indicates that not only the de novo CCA-addition, but also the repair and maintenance of CCA ends is affected. For statistical analysis, a Mann-Whitney test (*p < 0.05, **p < 0.01, ***p < 0,001) was performed on data for each tRNA isoacceptor from three replicates (with the exception for B. subtilis stationary phase, where two replicates were analyzed).
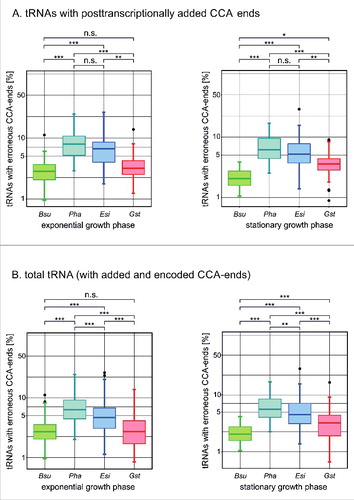
Examination of the 3′-end composition in tRNA with added CCA ends revealed that in both exponential and stationary phase, between 5 and 7% of the tRNAs in the psychrophilic species showed misincorporations of C and A residues in addition to the conventional CCA end, similar to the in vitro observation. In the meso- and thermophilic species, however, significantly less transcripts (2 to 3%) carried such incorrect ends (). When total tRNA (with both encoded as well as posttranscriptionally added CCA ends) was analyzed, a comparable and also significantly increased amount of erroneous CCA addition with identical misincorporations of the same type was observed ().
Hence, the in vivo data present a highly significant increase in erroneous CCA ends in E. sibiricum and P. halocryophilus, similar to the in vitro obtained sequences shown in . Accordingly, the in vitro as well as the in vivo investigations indicate that cold-adapted CCA-adding enzymes show a higher error rate resulting in extra nucleotide incorporations compared to enzymes adapted to moderate or high temperatures. Obviously, in both functions of the CCA-adding enzyme - the de novo CCA-addition as well as the restoration of CCA triplets - the accuracy of the polymerization reaction is considerably impaired.
Discussion
Closely related enzymes with minor changes for cold adaptation
Approximately 85% of the earth's biosphere are exposed to temperatures below 5°C and host a wide variety of cold-adapted microorganisms.Citation1-Citation5 An important prerequisite for survival at low temperatures is the evolution of enzymes that maintain their specific activities under these conditionsCitation4 (and references therein). While investigations of such cold adaptations were predominantly done on metabolic enzymes,Citation10,Citation66,Citation67 cell growth at low temperatures is also dependent on functional transcription and translation. Here, we focused on an essential RNA polymerase, the CCA-adding enzyme. During the addition of the CCA triplet to the tRNA 3′-end, the enzyme executes several coordinated movements in order to determine the sequence and number of incorporated nucleotides.Citation22,Citation24-Citation27 As a defined flexibility is a prerequisite for such domain movements, it is conceivable that the increased flexibility in cold-adapted enzyme versions affects polymerization and nucleotide selection. For a meaningful comparative study of temperature adaptation in proteins, one has to consider several key criteria10: First, a balanced ratio between similarity and sufficient evolutionary distance (<50% sequence identity) is important to ensure that temperature adaptation could have occurred.Citation68 Second, the evolutionary distance has to be kept as low as possible (>30% sequence identity) to minimize effects of genetic drift.Citation10 Third, catalytically important residues should be conserved. With a sequence identity of 37–45% and highly conserved catalytic core motifs, the selected CCA-adding enzymes from the four Bacillales species are ideally suited.
Representing an essential step in tRNA maturation, CCA-addition is adapted to the actual environmental temperatures of the individual organisms (, S2). At 4°C, EsiCCA and PhaCCA show KM values for ATP incorporation that are 64- to 72-fold higher than that of GstCCA. While KM is not equivalent to the binding constant KD , it approximately represents binding affinity. Hence, the increased KM values suggest a reduced affinity of these enzymes for ATP. In addition, both psychrophilic enzymes show a strongly reduced thermal stability (), and it can be concluded that both reduced affinity and unfolding are the result of an increased structural flexibility. Interestingly, the chimeric proteins are catalytically active in the cold, but show mesophilic unfolding (, ). The psychrophilic activity is explained by the individual functions of N- and C-termini. The N-terminus contains the complete catalytic core - hence, temperature-dependent catalysis of the chimera is identical to that of the parental psychrophilic enzyme. The C-terminus is involved in tRNA interaction, and the fact that CCA-addition of GstEsiCCA is restricted to low temperatures indicates that this chimeric enzyme binds a substrate tRNA only in the cold. Consequently, nucleotide addition can also occur only at low temperature, although the catalytic core would allow a more efficient polymerization at elevated temperatures.
For both chimeric enzymes, a two-step denaturation process was expected, where the psychrophilic part unfolds first, while the thermostable part denatures at higher temperatures. The observed single-step unfolding seems to be the consequence of the close evolutionary relation of the parental enzymes, so that even in the chimeras, interactions between N- and C-termini persist, where the thermophilic part stabilizes the psychrophilic region to a certain extent. Yet, the replaced heat-adapted region is missing, leading to a decreased stability of the Gst enzyme part. This unfolding as well as the functionality of the chimeras is a clear indication that cold-adaptation is the cumulative result of several individual small changes in all parts of the protein that - due to the close relation of the enzymes - retain a domain compatibility. This is supported by the high conservation of the catalytic core motifs and the occurrence of only small local changes in the predicted C-terminal structure of EsiCCA, where several short alpha-helical elements are reduced or missing, contributing to the increased flexibility (Figure S1). This reduced α-helical content in the Esi C-terminus is also visible in the CD-spectroscopic analysis, where the characteristic minimum at 222 nm is absent in the corresponding chimera (Figure S4). As the chimeras show that local cold adaptations are obviously distributed over the whole enzyme structure, it is impossible to investigate the specific contribution of individual small changes to psychrophilic activity.
A new tradeoff: Cold adaptation has an impact on polymerization fidelity
A striking feature of CCA-adding enzymes is their high fidelity in polymerization. These enzymes do not only show an efficient discrimination against GTP and UTP, but reliably incorporate C and A residues in the correct number and order.Citation24,Citation25,Citation57,Citation58 In the polymerization reaction, CCA-adding enzymes undergo several rearrangements to accommodate the growing tRNA 3′-end in the catalytic core and to switch the single nucleotide binding pocket from CTP binding to ATP interaction.Citation20-Citation22,Citation25 These rearrangements require a defined structural elasticity. In the cold-adapted enzymes, however, a further increase in flexibility obviously leads to a catalytic core that accommodates more than just three added nucleotides at the tRNA 3′-end, resulting in an increased error rate ( & ).
Several elements involved in such structural rearrangements were already described in the catalytic core of CCA-adding enzymes. Between motifs A and B, a flexible loop region is located that adjusts the NTP-recognizing amino acids in motif D according to the growing primer and the nature of the nucleotide to be incorporated ().Citation25,Citation26,Citation69 As these amino acids are also present in the psychrophilic enzymes, EsiCCA and PhaCCA still incorporate exclusively C and A residues. For the first nucleotide addition, the catalytic core is pre-formed for CTP specificity, impeding the addition of other nucleotides.Citation25 Then, a small motif consisting of a basic and an acidic amino acid (B/A motif) binds and positions the growing CCA-end (C74 and C75) that then stacks with the incoming ATP.Citation20,Citation26 As a consequence, even the flexible psychrophilic CCA-adding enzymes first incorporate a regular and correct CCA triplet. However, the increased flexibility of the catalytic core seems to have dramatic consequences for the termination of the polymerization reaction. Motif C and two α-helices in the neck domain contribute to the coordinated motion of the head domain and the catalytic core during polymerization, and it is very likely that these elements in concert regulate the number of incorporated nucleotides at the tRNA 3′-end.Citation22,Citation59 In the cold-adapted enzymes, an increased overall flexibility of these elements obviously leads to the incorporation of additional C and A residues. However, as no crystal structure of psychrophilic CCA-adding enzymes is available, it is difficult to predict whether the described elements indeed have a cold-adapted increased flexibility that leads to the observed rate of misincorporation. As head and neck domains form an intricate network of hydrogen bonds and salt bridges, the analysis of chimeras with single element replacements and the interpretation of their activity is at high risk, as indicated above.
Interestingly, the amount of erroneous CCA ends with extra nucleotides is not only observed in vitro, but is also significantly increased in vivo in the psychrophilic species (). While in vitro a stable and unmodified yeast tRNAPhe transcript was used as a substrate, the in vivo analysis investigated the whole cold-adapted and modified tRNA pool of E. sibiricum and P. halocryophilus. Yet, both in vitro and in vivo tRNA substrates show a strong increase in nucleotide misincorporations, indicating that temperature-adaptation as well as modification status of the tRNA substrate has no impact on the fidelity of CCA addition. Because this high error rate is observed for tRNAs with encoded CCA-end as well as for tRNAs with posttranscriptionally added CCA triplet, it seems that both de novo synthesis and CCA-end repair are equally affected. Furthermore, this inaccurate nucleotide addition is independent of the charging state of the tRNAs, as tRNA pools from exponential as well as stationary growth phase have very similar error rates.
It is very likely that this erroneous CCA-addition has an impact on the third - equally important - function of CCA-adding enzymes. For hypomodified and structurally destabilized - probably nonfunctional - tRNAs, a quality control mechanism was described, where the CCA-adding enzyme forces the tRNA acceptor stem to refold into a structure that mimics a CCA-free tRNA.Citation27,Citation70,Citation71 On this isomerized tRNA, the enzyme then adds a second round of CCA, and the resulting CCACCA sequence is regarded as a specific degradation tag to remove such unstable transcripts from the tRNA pool, avoiding their harmful participation in translation. Hence, it is possible that the erroneous addition of CCACCA due to the low fidelity of cold-adapted CCA-adding enzymes is corrected by the tRNA quality control mechanism. As a result, intact tRNAs inactivated by the CCACCA tag would be degraded.
On the other hand, the quality control mechanism might represent an important prerequisite for efficient cold adaptation. In psychrophilic organisms, not only enzymes, but also tRNAs are adapted to a cold environment. It is documented that in psychrophilic bacteria, tRNAs carry an increased level of dihydrouridine, while other - structure-stabilizing - modifications are only found at low amounts.Citation72-Citation74 Representing the only known base that is not planar due to its saturated ring system, the dihydrouridine modification introduces a local flexibility in the tRNA structure. Hence, tRNAs follow the same strategy as proteins in order to be functional in the cold. As such a structural flexibility reduces the overall stability of the transcript, it is very likely that such tRNAs have a higher tendency to misfold, forcing the addition of a second CCA sequence. Subsequently, the tRNA degradation machinery removes these misfolded and tagged transcripts and keeps their abundance at a reduced level in order to avoid detrimental effects on translation and - consequently - on the general fitness of the cell. Such a control mechanism is supported by the observation that the error rate of CCA-addition in vitro () is much higher than that observed in vivo (). Accordingly, in such a scenario, the cold adaptation of tRNAs might be the result of a co-evolution with psychrophilic CCA-adding enzymes, where the increased - actually erroneous - CCACCA synthesis is required to remove cold-adapted but misfolded tRNAs from the cellular pool.
Taken together, and in contrast to metabolic enzymes, the price to be paid for cold adaption of this specialized RNA polymerase is not only a reduced overall reaction rate and thermal destabilization (activity-stability), but also a reduced fidelity in polymerization. Yet, it is possible that this impact on fidelity is not just a tradeoff that results from the increased overall enzymatic flexibility, but actually represents an improved quality control to monitor the structural intactness of flexible tRNA as an essential feature in cold adaptation. Regardless whether the increased error rate is a prerequisite or consequence of cold adaptation, the psychrophilic E. sibiricum and P. halocryophilus obviously found a way to deal with such an inaccurate but nevertheless essential CCA-addition.
Materials and methods
Construction of recombinant clones
Genes of CCA-adding enzymes from G. stearothermophilus (DSM-22), B. subtilis subsp. subtilis (DSM-10), E. sibiricum 255-15 (DSM-17290) and P. halocryophilus Or1 (DSM-24743) were PCR-amplified from genomic DNA and cloned into pET-30 Ek/LIC, resulting in constructs with N-terminal His6-tag. For generation of chimeric enzymes, DNA fragments encoding the corresponding enzyme part were amplified by PCR and partial genes were fused by overlap extension PCR and cloned into pET-30 Ek/LIC.
Protein expression and purification
Enzymes were expressed with N-terminal His6-tag in E. coli BL21(DE3) cca::cam lacking the endogenous CCA-adding enzyme. For standard induction (GstCCA), expression was induced at OD600 of 0.6 by the addition of IPTG to a final concentration of 1 mM for 3 h at 37°C. For cold induction of EsiCCA and PhaCCA, cells were grown at 37°C to an OD600 of 1.2-1.5. The culture was diluted 1:2 with 4°C cold medium supplemented with IPTG (final concentration 1 mM). Expression was performed for additional 16–18 h at 18°C. Cells were harvested and lysed using a FastPrep24 (6 m/s, 20–30 s; MP Biochemicals) in 20 mM Tris/HCl (pH 6.9-7.5), 500 mM NaCl, 5 mM MgCl2. In the case of chimeric enzymes, glycerol was added to a final concentration of 10% to increase stability in solution.
After centrifugation (20 min at 16000 x g) and filtration (0.45 µm), recombinant proteins were purified on a HisTrap FF column (GE Healthcare) using ÄKTApurifier 100 or ÄKTApure 25 (GE Healthcare) and eluted with 500 mM imidazole. Peak fractions were applied to a XK16/60 Superdex 75 pg column (GE Healthcare), and buffer was exchanged during size exclusion chromatography (20 mM Tris/HCl (pH 6.9-7.5), 200 mM NaCl, 5 mM MgCl2). Enzyme preparations for activity assays and kinetic analysis were stored in the presence of 40% (v/v) glycerol at −20°C, proteins used in CD spectroscopy were stored at 4°C.
tRNA preparation
Yeast tRNAPhe lacking the CCA terminus or ending with CC were prepared as described.Citation22,Citation75,Citation76 For radioactive labeling of tRNA, α-32P-ATP (3000 Ci/mmol) was added to transcription reactions.
In vitro CCA incorporation and kinetic analysis
Standard CCA addition and kinetic assays were performed as described.Citation42,Citation58,Citation69 Temperature-dependent activities were measured in 20 µl reaction volume (5 µM tRNA, 30 mM HEPES/KOH (pH 7.6), 30 mM KCl, 6 mM MgCl2, 1 mM NTP, 2 mM DTT) at 0 to 90°C using limited enzyme amounts that result in ∼90% efficiency of CCA-addition only at optimal temperatures. These conditions were determined in time series and varying enzyme concentrations. After 5 min incubation, reactions were stopped by ethanol-precipitation, separated by denaturing PAGE and visualized by autoradiography using a Typhoon 9410 phosphorimager (GE Healthcare). Quantification of three independent experiments was performed densitometrically with ImageQuant Software (GE Healthcare) and plotted against temperature.
Steady-state kinetic analysis of AMP incorporation was performed in the presence of 5 µM in vitro transcribed yeast tRNAPhe-CC and 3.5 µCi α-32P-ATP (3000 Ci/mmol) at various temperatures as described.Citation22,Citation26,Citation42,Citation45 ATP was titrated in various ranges depending on enzyme and temperature (0.2 µM – 5 mM). Reactions were stopped by addition of 20 µl EDTA (50 mM) and spotted onto DE81 filters (GE Healthcare). Filters were washed with 80 ml 0.3 M NH4 formate, 10 mM pyrophosphate and remaining radioactivity was measured in a scintillation counter (MicroBeta 2, Perkin Elmer). Kinetic parameters of three independent experiments for each combination of enzyme and temperature were analyzed using curve fitting by nonlinear regression (GraphPadPrism). Due to the solubility properties of RNA, the kinetic values represent apparent values, as the tRNA could not be used at excessive saturating concentration when ATP was titrated according to the literature.Citation20,Citation45-Citation47
CD-spectroscopy
CD spectra were recorded using a J-715 CD spectroscope (JASCO) and PTFE-plug sealed cuvettes with a path length of 2 mm. The response time was set to 4’’ and the scan speed to 50 nm/min (scan interval: 0.2 nm) and 50°C/h (0.2°C), respectively. CD-spectra were recorded from 260 to 200 nm at a concentration of 1 µM and the determined optimal reaction temperature for each enzyme (25°C, 40°C and 55°C) in 20 mM Tris/HCl (pH 6.9-7.5), 200 mM NaCl and 5 mM MgCl2. Enzyme chimeras were analyzed at 25°C. Temperature dependent unfolding was observed between 0 and 90°C at a fixed wavelength of 222 nm. For each setup, spectra were averaged over three scans. For temperature unfolding, the resulting curves were smoothed using the Means-Movement algorithm taking previous and following 4 data points into account (Jasco Spectra Manager).
Computational analysis of protein structures
For alignment of primary sequences, Clustal Omega hosted at mobyle.pasteur.frCitation77 was used with default parameters. Structural models of PhaCCA, EsiCCA and BsuCCA were predicted using the I-TASSER serverCitation78 and superimposed with the crystal structure of GstCCA (1MIV;Citation25) using Pymol (https://www.pymol.org/).
Cultivation of Bacillales species and RNA isolation
G. stearothermophilus (DSM-22), B. subtilis subsp. subtilis (DSM-10), E. sibiricum 255-15 (DSM-17290) and P. halocryophilus Or1 (DSM-24743) were grown at 55°C, 37°C, or 25°C in nutrient medium (medium 1) or trypticase soy yeast extract medium (medium 92), respectively, as recommended by the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ; https://www.dsmz.de/) and according to the corresponding publications.Citation79-Citation82 Main cultures were inoculated from precultures to an OD600 of 0.2.
Cultures were grown in three independent replicates. RNA was isolated from samples taken at exponential and stationary phase using TRIzol according to the suppliers’ instructions (Life Technologies). Additionally, cells lysis was enhanced using a FastPrep24 (6 m/s, 30’’, MP Biomedicals). High molecular weight RNA was removed using high salt precipitation with 5 M LiCl, and the supernatant was desalted on G-25 gel filtration spin columns (PD-10, GE Healthcare). Ribosomal RNA was depleted using a RiboZero-Kit for gram-positive bacteria (EpiCentre). The resulting preparations containing low molecular weight RNA were ethanol precipitated.
Illumina MiSeq-based tRNA deep sequencing
A pre-adenylated 3′-blocked oligonucleotide (NEB #E2610) was ligated to the 3′-end of the isolated RNA using truncated T4-RNA-Ligase 2 (NEB #M0242) according to the supplier's instructions, and the reaction was stopped after 2 h by incubation at 65°C for 10 min. Ligation products were reverse transcribed using RevertAid (Thermo Scientific) as instructed, using 32P-5’-labeled primer complementary to the adapter. cDNA was separated from RT-primers on a 10% denaturing PAA gel. The 3′-end of the isolated cDNA was ligated to a pre-adenylated 3′-blocked oligonucleotide using 5’-App-DNA/RNA-Ligase (NEB) at reaction conditions for ssDNA ligation according to the supplier (10 mM Bis-Tris-Propane-HCl, pH 7.5, 10 mM MgCl2, 1 mM DTT and 5 mM MnCl2). Primers complementary to the ligated adapters were used to introduce bar codes for Illumina sequencing. PCR-products were separated on native PAA gels and stained with methylene blue. Products with apparent sizes of 120 – 250 bp were eluted and sequenced in a multiplex setup in a single lane of an Illumina MiSeq device for each replicate.
Computational analysis of RNA Seq data
Genomic reference sequences were downloaded from the NCBI website (https://www.ncbi.nlm.nih.gov/nuccore/) for each genome: B. subtilis (NC_000964.3), E. sibiricum (NC_010556.1), G. stearothermophilus (NZ_JYNW00000000.1), and P. halocryophilus (NZ_CP016537.2).
Transfer RNAs were annotated within the four reference genomes using tRNAscan-SE 1.3.1.Citation83 Predictions for tRNAs were manually curated, cleaned from pseudo-genes, and all CCA tails were clipped. Adapters in deep sequencing data were clipped using cutadapt 1.11,Citation84 and clipped reads were associated with a certain tRNA gene if they contained a correct 15 nt long 3′-end. In the read sequence, all nucleotides preceding this 15 nt subsequence were identified as tails of tRNAs. Reads containing genomically encoded nucleotides within the tails were categorized as unprocessed transcripts, while others were sorted for correct (CCA) and incorrect post-transcriptional added tails.
Since deep sequencing reads may contain partially tRNA reads, and for methodical reasons start at the 3′-end, each tRNA gene is associated with its 15 nt 3′-end sequence. This size allows usually for a unique identification of tRNAs, and is short enough to end before most conserved modifications of the T-loop are reached. Since several tRNA genes encode for isogenic tRNA, multiple hits of each read could be observed within a group of isoacceptors and were counted multiple times. To avoid misrepresentation, only counts associated with each distinct isoacceptor were used for the statistical analysis, so that every read is used only once for the analysis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Abbreviations
| Bsu | = | Bacillus subtilis |
| Esi | = | Exiguobacterium sibiricum |
| Gst | = | Geobacillus stearothermophilus |
| Pha | = | Planococcus halocryophilus |
| PCR | = | polymerase chain reaction |
Suppl_mat_Cold_adaptation_of_tRNA_nucleotidyltransferases.zip
Download Zip (9.1 MB)Acknowledgements
We thank Sonja Bonin and Tobias Friedrich for expert technical assistance and George Feller and Brighton Samatanga for valuable discussion. We are greatly indebted to Gunter Meister for RNA deep sequencing.
Additional information
Funding
References
- Feller G. Psychrophilic enzymes: from folding to function and biotechnology. Scientifica (Cairo). 2013;2013:512840.
- Maayer P de, Anderson D, Cary C, Cowan DA. Some like it cold: understanding the survival strategies of psychrophiles. EMBO Rep. 2014;15:508-517. doi:10.1002/embr.201338170
- Sarmiento F, Peralta R, Blamey JM. Cold and Hot Extremozymes: Industrial Relevance and Current Trends. Front Bioeng Biotechnol. 2015;3:148. doi:10.3389/fbioe.2015.00148
- Siddiqui KS. Some like it hot, some like it cold: Temperature dependent biotechnological applications and improvements in extremophilic enzymes. Biotechnol Adv. 2015;33:1912-1922. doi:10.1016/j.biotechadv.2015.11.001
- Struvay C, Feller G. Optimization to low temperature activity in psychrophilic enzymes. Int J Mol Sci. 2012;13:11643-11665. doi:10.3390/ijms130911643
- Morita R. Psychrophilic Bacteria. Bacteriol Rev. 1975;39:144-167.
- Feller G. Protein stability and enzyme activity at extreme biological temperatures. J Phys Condens Matter 2010;22:323101. doi:10.1088/0953-8984/22/32/323101
- Siddiqui KS, Williams TJ, Wilkins D, Yau S, Allen MA, Brown MV, Lauro FM, Cavicchioli R. Psychrophiles. Annu. Rev. Earth Planet. Sci. 2013;41:87-115. doi:10.1146/annurev-earth-040610-133514
- Mitsuya D, Tanaka S-i, Matsumura H, Urano N, Takano K, Ogasahara K, Takehira M, Yutani K, Ishida M. Strategy for cold adaptation of the tryptophan synthase alpha subunit from the psychrophile Shewanella frigidimarina K14-2: crystal structure and physicochemical properties. J Biol Chem. 2014;155:73-82.
- Kovacic F, Mandrysch A, Poojari C, Strodel B, Jaeger K-E. Structural features determining thermal adaptation of esterases. Protein Eng Des Sel. 2016;29:65-76. doi:10.1093/protein/gzv061
- Feller G, Gerday C. Psychrophilic enzymes: Hot topics in cold adaptation. Nat Rev Microbiol. 2003;1:200-208. doi:10.1038/nrmicro773
- Siddiqui KS, Cavicchioli R. Cold-adapted enzymes. Annu Rev Biochem. 2006;75:403-433. doi:10.1146/annurev.biochem.75.103004.142723
- D'Amico S, Collins T, Marx J-C, Feller G, Gerday C. Psychrophilic microorganisms: challenges for life. EMBO Rep. 2006;7:385-389. doi:10.1038/sj.embor.7400662
- Roulling F, Piette F, Cipolla A, Struvay C, Feller G. Psychrophilic Enzymes: Cool Responses to Chilly Problems. Tokyo, New York: Springer; 2011.
- Yang L-L, Tang S-K, Huang Y, Zhi X-Y. Low Temperature Adaptation Is Not the Opposite Process of High Temperature Adaptation in Terms of Changes in Amino Acid Composition. Genome Biol Evol 2015;7:3426-3433. doi:10.1093/gbe/evv232
- D'Amico S, Marx J-C, Gerday C, Feller G. Activity-stability relationships in extremophilic enzymes. J Biol Chem. 2003;278:7891-7896. doi:10.1074/jbc.M212508200
- Garsoux G, Lamotte J, Gerday C, Feller G. Kinetic and structural optimization to catalysis at low temperatures in a psychrophilic cellulase from the Antarctic bacterium Pseudoalteromonas haloplanktis. Biochem J. 2004;384:247-253.
- Yang G, Aprile L, Turturo V, Pucciarelli S, Pucciarelli S, Miceli C. Characterization and comparative analysis of psychrophilic and mesophilic alpha-amylases from Euplotes species: a contribution to the understanding of enzyme thermal adaptation. Biochem Biophys Res Commun. 2013;438:715-720. doi:10.1016/j.bbrc.2013.07.113
- Darst SA, Opalka N, Chacon P, Polyakov A, Richter C, Zhang G, Wriggers W. Conformational flexibility of bacterial RNA polymerase. Proc Natl Acad Sci U S A. 2002;99:4296-4301. doi:10.1073/pnas.052054099
- Tomita K, Fukai S, Ishitani R, Ueda T, Takeuchi N, Vassylyev DG, Nureki O. Structural basis for template-independent RNA polymerization. Nature. 2004;430:700-704. doi:10.1038/nature02712
- Kim S, Liu C, Halkidis K, Gamper HB, Hou Y-M. Distinct kinetic determinants for the stepwise CCA addition to tRNA. RNA. 2009;15:1827-1836. doi:10.1261/rna.1669109
- Ernst FGM, Rickert C, Bluschke A, Betat H, Steinhoff H-J, Mörl M. Domain movements during CCA-addition: A new function for motif C in the catalytic core of the human tRNA nucleotidyltransferases. RNA Biol. 2015;12:435-446. doi:10.1080/15476286.2015.1018502
- Uma S, Jadhav RS, Seshu Kumar G, Shivaji S, Ray MK. A RNA polymerase with transcriptional activity at 0°C from the Antarctic bacterium Pseudomonas syringae. FEBS Lett. 1999;453:313-317. doi:10.1016/S0014-5793(99)00660-2
- Betat H, Rammelt C, Mörl M. tRNA nucleotidyltransferases: ancient catalysts with an unusual mechanism of polymerization. Cell Mol Life Sci. 2010;67:1447-1463. doi:10.1007/s00018-010-0271-4
- Li F, Xiong Y, Wang JM, Cho HD, Tomita K, Weiner AM, Steitz TA. Crystal structures of the Bacillus stearothermophilus CCA-adding enzyme and its complexes with ATP or CTP. Cell. 2002;111:815-824. doi:10.1016/S0092-8674(02)01115-7
- Neuenfeldt A, Just A, Betat H, Mörl M. Evolution of tRNA nucleotidyltransferase: A small deletion generated CC-adding enzymes. Proc Natl Acad Sci U S A. 2008;105:7953-7958. doi:10.1073/pnas.0801971105
- Kuhn C-D, Wilusz JE, Zheng Y, Beal PA, Joshua-Tor L. On-Enzyme Refolding Permits Small RNA and tRNA Surveillance by the CCA-Adding Enzyme. Cell. 2015;160:644-658. doi:10.1016/j.cell.2015.01.005
- Berlemont R, Pipers D, Delsaute M, Angiono F, Feller G, Galleni M, Power P. Exploring the Antarctic soil metagenome as a source of novel cold-adapted enzymes and genetic mobile elements. Rev Argent Microbiol. 2011;43:94-103.
- Rabert C, Gutiérrez-Moraga A, Navarrete A, Navarrete-Campos D, Bravo L, Gidekel M. Expression of a Deschampsia antarctica Desv. polypeptide with lipase activity in a Pichia pastoris vector. Int J Mol Sci. 2014;15:2359-2367. doi:10.3390/ijms15022359
- Kikani BA, Singh SP. Enzyme stability, thermodynamics and secondary structures of α-amylase as probed by the CD spectroscopy. Int J Biol Macromol. 2015;81:450-460. doi:10.1016/j.ijbiomac.2015.08.032
- Yamada C, Sawano K, Iwase N, Matsuoka M, Arakawa T, Nishida S, Fushinobu S. Isolation and characterization of a thermostable lipase from Bacillus thermoamylovorans NB501. J Gen Appl Microbiol. 2017;62:313-319.
- Jühling F, Mörl M, Hartmann RK, Sprinzl M, Stadler PF, Putz J. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009;37:D159-162. doi:10.1093/nar/gkn772
- Deutscher MP. Ribonucleases, tRNA nucleotidyltransferase, and the 3′ processing of tRNA. Prog Nucleic Acid Res Mol Biol. 1990;39:209-240.
- Yue D, Maizels N, Weiner AM. CCA-adding enzymes and poly(A) polymerases are all members of the same nucleotidyltransferase superfamily: characterization of the CCA-adding enzyme from the archaeal hyperthermophile Sulfolobus shibatae. RNA. 1996;2:895-908.
- Schürer H, Schiffer S, Marchfelder A, Mörl M. This is the end: processing, editing and repair at the tRNA 3′-terminus. Biol Chem. 2001;382:1147-1156. doi:10.1515/BC.2001.144
- Rodrigues DF, Goris J, Vishnivetskaya T, Gilichinsky D, Thomashow MF, Tiedje JM. Characterization of Exiguobacterium isolates from the Siberian permafrost. Description of Exiguobacterium sibiricum sp nov. Extremophiles. 2006;10:285-294. doi:10.1007/s00792-005-0497-5
- Mykytczuk NCS, Foote SJ, Omelon CR, Southam G, Greer CW, Whyte LG. Bacterial growth at-15 degrees C; molecular insights from the permafrost bacterium Planococcus halocryophilus Or1. ISME J. 2013;7:1211-1226. doi:10.1038/ismej.2013.8
- Shi H, Moore PB. The crystal structure of yeast phenylalanine tRNA at 1.93 A resolution: a classic structure revisited. RNA. 2000;6:1091-1105. doi:10.1017/S1355838200000364
- Byrne RT, Konevega AL, Rodnina MV, Antson AA. The crystal structure of unmodified tRNAPhe from Escherichia coli. Nucleic Acids Res. 2010;38:4154-4162. doi:10.1093/nar/gkq133
- Oommen A, Li XQ, Gegenheimer P. Cleavage specificity of chloroplast and nuclear tRNA 3′-processing nucleases. Mol Cell Biol. 1992;12:865-875. doi:10.1128/MCB.12.2.865
- Loria A, Pan T. The 3′ substrate determinants for the catalytic efficiency of the Bacillus subtilis RNase P holoenzyme suggest autolytic processing of the RNase P RNA in vivo. RNA. 2000;6:1413-1422. doi:10.1017/S1355838200000959
- Hoffmeier A, Betat H, Bluschke A, Günther R, Junghanns S, Hofmann HJ, Mörl M. Unusual evolution of a catalytic core element in CCA-adding enzymes. Nucleic Acids Res. 2010;38:4436-4447. doi:10.1093/nar/gkq176
- Tretbar S, Neuenfeldt A, Betat H, Mörl M. An inhibitory C-terminal region dictates the specificity of A-adding enzymes. Proc Natl Acad Sci U S A. 2011;108:21040-21045. doi:10.1073/pnas.1116117108
- Scheibe M, Bonin S, Hajnsdorf E, Betat H, Mörl M. Hfq stimulates the activity of the CCA-adding enzyme. BMC Mol Biol. 2007;8:92. doi:10.1186/1471-2199-8-92
- Betat H, Rammelt C, Martin G, Mörl M. Exchange of regions between bacterial poly(A) polymerase and the CCA-Adding enzyme generates altered specificities. Mol Cell. 2004;15:389-398. doi:10.1016/j.molcel.2004.06.026
- Cho HD, Verlinde CL, Weiner AM. Archaeal CCA-adding enzymes: central role of a highly conserved beta-turn motif in RNA polymerization without translocation. J Biol Chem. 2005;280:9555-9566. doi:10.1074/jbc.M412594200
- Tomita K, Ishitani R, Fukai S, Nureki O. Complete crystallographic analysis of the dynamics of CCA sequence addition. Nature. 2006;443:956-960. doi:10.1038/nature05204
- Consalvi V, Chiaraluce R, Giangiacomo L, Scandurra R, Christova P, Karshikoff A, Knapp S, Ladenstein R. Thermal unfolding and conformational stability of the recombinant domain II of glutamate dehydrogenase from the hyperthermophile Thermotoga maritima. Protein Eng Des Sel 2000;13:501-507. doi:10.1093/protein/13.7.501
- Greenfield NJ. Using circular dichroism collected as a function of temperature to determine the thermodynamics of protein unfolding and binding interactions. Nat Protoc. 2006;1:2527-2535.
- Greenfield NJ. Using circular dichroism spectra to estimate protein secondary structure. Nat Protoc. 2006;1:2876-2890.
- Wi AR, Jeon S-J, Kim S, Park HJ, Kim D, Han SJ, Yim JH, Kim H-W. Characterization and a point mutational approach of a psychrophilic lipase from an arctic bacterium, Bacillus pumilus. Biotechnol Lett. 2014;36:1295-1302. doi:10.1007/s10529-014-1475-8
- Williams MA, Thornton JM, Goodfellow JM. Modelling protein unfolding: hen egg-white lysozyme. Protein Eng. 1997;10:895-903. doi:10.1093/protein/10.8.895
- Feng Z, Ha JH, Loh SN. Identifying the site of initial tertiary structure disruption during apomyoglobin unfolding. Biochemistry. 1999;38:14433-14439. doi:10.1021/bi991933e
- Nelson ED, Grishin NV. Efficient expansion, folding, and unfolding of proteins. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;70:51906. doi:10.1103/PhysRevE.70.051906
- Jha Santosh Kumar, Udgaoankar Jayant B. Free energy barriers in protein folding and unfolding reactions. Curr Sci. 2010;99:457-475.
- Schuabb C, Berghaus M, Rosin C, Winter R. Exploring the Free Energy and Conformational Landscape of tRNA at High Temperature and Pressure. ChemPhysChem 2015;16:138-146.
- Weiner AM. tRNA maturation: RNA polymerization without a nucleic acid template. Curr Biol. 2004;14:R883-5. doi:10.1016/j.cub.2004.09.069
- Lizano E, Scheibe M, Rammelt C, Betat H, Mörl M. A comparative analysis of CCA-adding enzymes from human and E. coli: Differences in CCA addition and tRNA 3 ′-end repair. Biochimie. 2008;90:762-772.
- Toh Y, Takeshita D, Numata T, Fukai S, Nureki O, Tomita K. Mechanism for the definition of elongation and termination by the class II CCA-adding enzyme. EMBO J. 2009;28:3353-3365. doi:10.1038/emboj.2009.260
- Starosta AL, Lassak J, Jung K, Wilson DN. The bacterial translation stress response. FEMS Microbiol Rev. 2014;38:1172-1201. doi:10.1111/1574-6976.12083
- Pletnev P, Osterman I, Sergiev P, Bogdanov A, Dontsova O. Survival guide: Escherichia coli in the stationary phase. Acta Naturae. 2015;7:22-33.
- Deutscher MP, Marlor CW, Zaniewski R. RNase T is responsible for the end-turnover of tRNA in Escherichia coli. Proc Natl Acad Sci U S A. 1985;82:6427-6430. doi:10.1073/pnas.82.19.6427
- Padmanabha KP, Deutscher MP. RNase T affects Escherichia coli growth and recovery from metabolic stress. J Bacteriol. 1991;173:1376-1381. doi:10.1128/jb.173.4.1376-1381.1991
- Zhu L, Deutscher MP. tRNA nucleotidyltransferase is not essential for Escherichia coli viability. EMBO J. 1987;6:2473-2477.
- Reuven NB, Zhou Z, Deutscher MP. Functional overlap of tRNA nucleotidyltransferase, poly(A) polymerase I, and polynucleotide phosphorylase. J Biol Chem. 1997;272:33255-33259. doi:10.1074/jbc.272.52.33255
- Tiberti M, Papaleo E. Dynamic properties of extremophilic subtilisin-like serine-proteases. J Struct Biol. 2011;174:69-83. doi:10.1016/j.jsb.2011.01.006
- Dick M, Weiergraber OH, Classen T, Bisterfeld C, Bramski J, Gohlke H, Pietruszka J. Trading off stability against activity in extremophilic aldolases. Sci Rep. 2016;6:17908. doi:10.1038/srep17908
- Rost B. Protein structures sustain evolutionary drift. Fold Des. 1997;2:S19-S24. doi:10.1016/S1359-0278(97)00059-X
- Just A, Butter F, Trenkmann M, Heitkam T, Mörl M, Betat H. A comparative analysis of two conserved motifs in bacterial poly(A) polymerase and CCA-adding enzyme. Nucleic Acids Res. 2008;36:5212-5220. doi:10.1093/nar/gkn494
- Wilusz JE, Whipple JM, Phizicky EM, Sharp PA. tRNAs marked with CCACCA are targeted for degradation. Science. 2011;334:817-821. doi:10.1126/science.1213671
- Betat H, Mörl M. The CCA-adding enzyme: A central scrutinizer in tRNA quality control. Bioessays. 2015;37:975-982. doi:10.1002/bies.201500043
- Dalluge JJ, Hashizume T, Sopchik AE, McCloskey JA, Davis DR. Conformational flexibility in RNA: the role of dihydrouridine. Nucleic Acids Res. 1996;24:1073-1079. doi:10.1093/nar/24.6.1073
- Dalluge JJ, Hamamoto T, Horikoshi K, Morita RY, Stetter KO, McCloskey JA. Posttranscriptional modification of tRNA in psychrophilic bacteria. J. Bacteriol. 1997;179:1918-1923. doi:10.1128/jb.179.6.1918-1923.1997
- Lorenz C, Lünse CE, Mörl M. tRNA Modifications: Impact on Structure and Thermal Adaptation. Biomolecules. 2017;7:E35.
- Schürer H, Lang K, Schuster J, Mörl M. A universal method to produce in vitro transcripts with homogeneous 3 ′ ends. Nucleic Acids Res. 2002;30:e56.
- Mörl M, Lizano E, Willkomm DK, Hartmann RK. Production of RNAs with Homogeneous 5′- and 3′-Ends. In: Hartmann RK, Bindereif A, Schön A, Westhof E, editors. Handbook of RNA biochemistry. Weinheim, Chichester: Wiley-VCH; John Wiley [distributor]; 2012; p. 22-35.
- Neron B, Menager H, Maufrais C, Joly N, Maupetit J, Letort S, Carrere S, Tuffery P, Letondal C. Mobyle: a new full web bioinformatics framework. Bioinformatics. 2009;25:3005-3011.
- Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40.
- Nakamura LK, Roberts MS, Cohan FM. Relationship of Bacillus subtilis clades associated with strains 168 and W23: a proposal for Bacillus subtilis subsp subtilis subsp nov and Bacillus subtilis subsp spizizenii subsp nov. Int J Syst Bacteriol. 1999;49:1211-1215.
- Rodrigues DF, Ivanova N, He Z, Huebner M, Zhou J, Tiedje JM. Architecture of thermal adaptation in an Exiguobacterium sibiricum strain isolated from 3 million year old permafrost: A genome and transcriptome approach. BMC Genomics. 2008;9:547.
- Mykytczuk NCS, Wilhelm RC, Whyte LG. Planococcus halocryophilus sp nov., an extreme sub-zero species from high Arctic permafrost. Int J Syst Bacteriol. 2012;62:1937-1944.
- Coorevits A, Dinsdale AE, Halket G, Lebbe L, Vos P de, van Landschoot A, Logan NA. Taxonomic revision of the genus Geobacillus: emendation of Geobacillus, G. stearothermophilus, G. jurassicus, G. toebii, G. thermodenitrificans and G. thermoglucosidans (nom. corrig., formerly ‘thermoglucosidasius’); transfer of Bacillus thermantarcticus to the genus as G. thermantarcticus comb. nov; proposal of Caldibacillus debilis gen. nov., comb. nov; transfer of G. tepidamans to Anoxybacillus as A. tepidamans comb. nov; and proposal of Anoxybacillus caldiproteolyticus sp. nov. Int J Syst Evol Microbiol. 2012;62:1470-1485.
- Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955-964.
- Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:10. doi:10.14806/ej.17.1.200
