ABSTRACT
Ribosome biogenesis requires a variety of trans-acting factors in order to produce functional ribosomal subunits. In human cells, the complex formed by the proteins hNob1 and hPno1 is crucial to the site 3 cleavage occurring at the 3ʹ-end of 18S pre-rRNA. However, the properties and activity of this complex are still poorly understood. We present here a detailed characterization of hNob1 organization and its interaction with hPno1. We redefine the boundaries of the endonuclease PIN domain present in hNob1 and we further delineate the precise interacting modules required for complex formation in hNob1 and hPno1. Altogether, our data contributes to a better understanding of the complex biology required during the site 3 cleavage step in ribosome biogenesis.
Introduction
Ribosome biogenesis is one of the most complex pathway existing in eukaryotic cells. This is due to the high number of factors involved and to the variety of the enzymatic reactions occurring along the entire pathway [Citation1]. In addition, the pathway is physically segregated between the nucleus and the cytoplasm by the nuclear membrane, which acts as a natural checkpoint. In recent years, a number of studies have revealed with atomic details the architecture of pre-ribosomal particles such as the 90S, the pre-60S and the pre-40S ribosomes prior or after their export to the cytoplasm [Citation2–Citation9]. It is noteworthy that most studies have been performed using fungal pre-ribosomal particles, and therefore may differ from the situation encountered in human cells. The study of human ribosome biogenesis is directly relevant for understanding the set of mammalian diseases affecting this pathway and collectively referred to as ribosomopathies [Citation10]. In this context, dedicated studies of the structure and function of the mammalian factors are highly relevant.
The recent structural studies on fungal ribosome biogenesis revealed the location of numerous trans-acting factors on the pre-ribosomal particles allowing for a comprehensive understanding of their biological role [Citation11]. However, some critical factors are only partially visible or even absent from these snapshot images of the ribosome assembly process. For example, comprehensive structural data is lacking for Rrp5, a protein involved in both the pre-40S and the pre-60S synthesis pathway and for human Nob1 (hereafter hNob1), the endonuclease required for the cleavage at site 3 during the last step of pre-40S maturation. hNob1 is often associated with its natural protein partner hPno1/hDim2, which in turn regulates Nob1’s catalytic activity [Citation12]. The position of the yeast Pno1 protein on late pre-ribosome particles has been recently identified [Citation5], but Nob1’s location on the ribosomal particle in any organism was until very recently unknown [Citation13]. In addition, it appears that functional differences exist for Nob1 depending on the organism and thus studies on orthologues are of specific interest. As an example, the protein Fap7 has been shown to inhibit Nob1’s endonuclease activity in Pyrococcus horikoshii [Citation14], while the Fap7 protein promotes the same endonucleolytic activity in human [Citation15]. The hNob1 is therefore a paradigm of an essential trans-acting protein for which limited molecular information is available.
The structure of the Nob1 protein from P. horikoshii is available, but does not allow for full modelling of the mammalian protein due to various large sequence insertions, which do not exist in the archaeal protein [Citation16]. hNob1 harbors a similar organization to the archaeal orthologue and consists of PIN (PilT N terminus) endonuclease and zinc ribbon domains, separated by linker region that is poorly conserved with the exception of its last C-terminal residues [Citation16,Citation17]. In lower eukaryotic species, the linker has a minimal length of ~ 15 residues while it exceeds ~ 100 residues in human. The conserved C-terminal linker region was shown to be critical for the Nob1/Pno1 complex formation in Chaetomium thermophilum [Citation18]. The presence of an active PIN domain required to cleave the pre-rRNA was demonstrated via structure prediction and mutational studies more than a decade ago while the precise function of the zinc ribbon is unknown [Citation19]. A recent review classifying the PIN domain-like superfamily [Citation20] defined the folding landscape of PIN domains as always adopting a prototypical β1-α1-α2-β2-α3-β3-α4-β4-α5-β5 topology [Citation20]. Their overall structure forms a five-stranded β-sheet packed by five α-helices with possible additional secondary structure elements although these extra elements do not change the global organization [Citation20]. Moreover, the four active site residues in the majority of the cases are located in the secondary structure element β1, α3, α4 and β4. Surprisingly, one of the amino acid predicted to be essential for the yeast Nob1 PIN domain activity, D110, appeared dispensable in yeast complementation experiments [Citation19]. In addition, PIN domains generally display low affinity for their target RNA, leading to the hypothesis that hNob1 requires additional stabilization upon interacting with its cleavage site on ribosomal rRNA. This hypothesis would explain the inability to reconstitute the cleavage reaction in vitro with hNob1 only [Citation21]. Even if the zinc ribbon may be involved in stabilizing pre-rRNA contacts, one likely candidate for this role is the hNob1 partner hPno1. hPno1 contains two hnRNP K-homology (KH) domains and these domains are known to possess RNA-binding ability [Citation22]. Additional proteins such as CINAP/Fap7 could also be implicated in this multi-factor reaction [Citation14].
Here, we present a detailed molecular analysis of the interaction between the human trans-acting factor hNob1 and its binding partner hPno1. We revisit and propose a new delineation for the hNob1 PIN domain on the basis of biochemical data. We further demonstrate that the interaction between hNob1 and hPno1 requires the conserved sequence in hNob1 that precedes the second half of the PIN domain, along with the KH-like domain of hPno1. We have described the minimal interaction surface between the two proteins as a single amino acid substitution abrogates the complex formation. We also observe that the non-conserved extensions found in hNob1 C-terminus and hPno1 N-terminus are, in our in vitro conditions, dispensable.
Results
In the last two decades, a number of studies have contributed to our understanding of Nob1’s function in ribosome biogenesis. Most of our knowledge comes from seminal research performed by the group of D. Tollervey using the yeast protein [Citation17]. Based on sequence alignment of the Nob1 protein family, it was proposed that Nob1 would contain a PIN domain and a zinc ribbon separated by a linker of variable length [Citation17]. Almost a decade later, the NMR structure of the orthologous Nob1 protein from P. horikoshii was solved and the atomic data confirmed the presence of a PIN domain and of a zinc ribbon domain separated by an unstructured linker [Citation16].
Careful analysis of the Nob1 sequence alignment, 3D-structural modelling using the web server PHYRE2 [Citation23] and recent PIN superfamily definition [Citation20] consistently suggest that the PIN domain present in the hNob1 protein (and likely in yeast) must be redefined. The domain should rather be divided in two pieces separated by a long linker () [Citation16]. The first ‘half’ would contain the required secondary structure elements β1-α1-α2-β2-α3-β3-α4 (β1-α1-α2-β2-α3-α4-β3-α5-α6 according to the predicted secondary structure elements present in the human sequence). The second part would cover the last elements β4-α5-β5, the missing elements required to form a functional PIN domain according to the recent classification (β4-α7-β5 according to our prediction; ).
Figure 1. Human Nob1 domain organization. (a) Sequence alignment and secondary structure prediction of Nob1 homologues from major clades showing conservation along the protein sequence performed with ESPript [Citation30]. Coloring scheme is as follow: Fully conserved residues have a red background, partially conserved amino acids are shown as bold letters over a yellow box. The alignment has the following UniProt entries: human, Q9ULX3; mouse, Q8BW10; zebrafish, F1R4C1; african clawed frog, Q6VEU0; fruit fly, Q9W2Y4; baker’s yeast, Q08444; C. thermophilum, G0S762 and P. horikoshii, O58440. The first 79 amino acids of C. thermophilum sequence have been removed due to non-conservation with the other homologues and space limitation. Secondary structure element prediction from PHYRE is indicated above the alignment and numbered. The sequence corresponding to the proposed first and second half of the PIN domain is boxed in green and salmon, respectively. The proposed PIN domain active site are indicated by red dots while the other residues reported to be involved in the active site are indicated by blue stars. Three black asterisk indicate the mutated residues in panel E. The zinc ribbon domain is outlined in dark blue. (b) PIN domain structural prediction is shown in cartoon representation and colored in green and salmon according to domain limits defined in panel A. Individual secondary structure elements are labelled. Active site residues are shown as colored sticks (cyan, carbon; red, oxygen) and labelled. Mutated residues are shown as sticks and labelled in italic. (c) Schematic view of the endonuclease hNob1 domain organization colored as in panel A. The different hNob1 fragments used for domain boundary definition are shown below. (d) The two halves of the PIN domain interact together. Ni-NTA purification of lysate from cells co-expressing two Nob1 fragments showing complex formation between fragment 1–134 and 206–314. This complex is not observed between fragments 1–134 and 249–314, thus excluding a stable interaction between the zinc ribbon and the first half of the PIN domain. (e) Interface mutations affect PIN domain formation. Ni-NTA purification of lysate from cells co-expressing two Nob1 fragments. The non-histidine tagged fragments was carrying the mutation D83K, L87S or Y91A. Mutation of hNob1 (1–134) L87S abolishes interaction with the fragment 206–314.
![Figure 1. Human Nob1 domain organization. (a) Sequence alignment and secondary structure prediction of Nob1 homologues from major clades showing conservation along the protein sequence performed with ESPript [Citation30]. Coloring scheme is as follow: Fully conserved residues have a red background, partially conserved amino acids are shown as bold letters over a yellow box. The alignment has the following UniProt entries: human, Q9ULX3; mouse, Q8BW10; zebrafish, F1R4C1; african clawed frog, Q6VEU0; fruit fly, Q9W2Y4; baker’s yeast, Q08444; C. thermophilum, G0S762 and P. horikoshii, O58440. The first 79 amino acids of C. thermophilum sequence have been removed due to non-conservation with the other homologues and space limitation. Secondary structure element prediction from PHYRE is indicated above the alignment and numbered. The sequence corresponding to the proposed first and second half of the PIN domain is boxed in green and salmon, respectively. The proposed PIN domain active site are indicated by red dots while the other residues reported to be involved in the active site are indicated by blue stars. Three black asterisk indicate the mutated residues in panel E. The zinc ribbon domain is outlined in dark blue. (b) PIN domain structural prediction is shown in cartoon representation and colored in green and salmon according to domain limits defined in panel A. Individual secondary structure elements are labelled. Active site residues are shown as colored sticks (cyan, carbon; red, oxygen) and labelled. Mutated residues are shown as sticks and labelled in italic. (c) Schematic view of the endonuclease hNob1 domain organization colored as in panel A. The different hNob1 fragments used for domain boundary definition are shown below. (d) The two halves of the PIN domain interact together. Ni-NTA purification of lysate from cells co-expressing two Nob1 fragments showing complex formation between fragment 1–134 and 206–314. This complex is not observed between fragments 1–134 and 249–314, thus excluding a stable interaction between the zinc ribbon and the first half of the PIN domain. (e) Interface mutations affect PIN domain formation. Ni-NTA purification of lysate from cells co-expressing two Nob1 fragments. The non-histidine tagged fragments was carrying the mutation D83K, L87S or Y91A. Mutation of hNob1 (1–134) L87S abolishes interaction with the fragment 206–314.](/cms/asset/be0dbf37-03e8-4994-ae87-e6c747fc229f/krnb_a_1517013_f0001_oc.jpg)
Interestingly, such a split organization changes the identity of the putative active site residues. When the PIN domain was defined within the yeast sequence, residues D15, E43, D84, D92 and D110 (residues D10, E36, D75, D83 and S101 in the human sequence) were proposed as conserved acidic residues possibly important for the enzymatic activity [Citation17,Citation19]. Since then, several studies have experimentally validated D15, E43 and D92 as critical for the yeast Nob1 activity, while D84 and D110 were apparently not required [Citation19,Citation24]. Our predicted hNob1 PIN domain model fits the secondary structure requirements for a functional PIN domain of the VapC PIN superfamily (). According to our new definition, the residues D75 and S101 (corresponding to residues D84 and D110 in the yeast protein) are not predicted to be located in the active site (). This is likely the reason why the growth of S. cerevisiae cells was unaffected by the replacement of endogenous Nob1 protein with the mutated D110N Nob1 [Citation19]. Based on our secondary structure prediction and sequence alignment, we predicted the β4-α5-β5 secondary structure elements between residues 234 and 255 (), labelled β4-α7-β5 in hNob1). With this revised arrangement, the fourth active site residues would correspond to D238 rather than S101 (D271 and D110 respectively in the yeast protein, ().
We next validated this computational prediction using coexpression experiments with multiple hNob1 constructs (). We first demonstrate that hNob1 fragment encompassing residues 1–134 can interact stably with hNob1 residues 206–314 (). However, such an interaction is becoming unstable if we remove the stretch from residues 206 to 249, strongly suggesting that the interaction surface between the two proposed ‘halves’ are located within the protein fragment covering the residues 206 to 249 (). In order to challenge our hypothesis, we generated point mutations to destabilize the bi-partite PIN domain. Hence, D83K, L87S and Y91A point mutants targeting the interface between the two proposed PIN domain fragments were introduced based on the predicted model (). hNob1 (1–134) carrying the mutation L87S is unable to form a stable complex with the hNob1 fragment 206–314 () whereas D83K or Y91A mutations do not apparently affect the complex formation. We thus show that the PIN domain of hNob1 is formed by two ‘halves’ separated by a largely dispensable loop. Such a modular organization has been very recently confirmed by the cryo-EM based structural analysis of human pre-40S particles [Citation13].
To gain further insight into the human Nob1 and Pno1 interaction, we decided to precisely map the recognition surface. We performed coexpression experiments using full-length hPno1 with various truncations of hNob1. We initiated the experiment using the full-length proteins and showed an efficient and stable interaction despite partial degradation of hNob1 (). As a negative control, we also verified that untagged hPno1 alone did not show nonspecific binding to Ni-NTA beads (data not shown). Since the C-terminal part of hNob1 is predicted to be disordered and is not conserved through evolution (), we supposed that the observed degradation of the full-length (FL) protein occurred within this region. We next deleted the entire region that follows the zinc ribbon and reassessed the impact of this deletion on the stability of the complex. The binding to hPno1 was unaffected by the truncation (construct 1–314, ). Next, we further shortened the protein by removing the zinc ribbon domain (resulting in a construct from residues 1 to 255) and showed that it had no effect on hPno1 association (). Thus, the entire C-terminal region including the zinc ribbon is dispensable in vitro for complex formation. We attempted to use the construct encompassing the first half of hNob1 PIN domain but we could not obtain the protein (data not shown). Instead, we used a construct containing only the linker located inside the PIN domain (construct 104–230) and coexpressed it with hPno1. Co-purification was observed for this construct, indicating that the interaction between hNob1 and hPno1 is at least partially mediated by the linker ().
Figure 2. Definition of the minimal fragment of hNob1 or hPno1 required for complex formation. (a) Schematic view of hNob1 fragments (upper part) used for the mapping of the interaction. Co-expression of the indicated hNob1 constructs with or without untagged hPno1 (bottom part). Protein are identified next to the SDS-PAGE. (b) Schematic view of hPno1 fragments (upper part) used for the mapping of the interaction. Co-expression of the indicated untagged hPno1 constructs with histidine-tagged hNob1 (bottom part). Small scale Ni-NTA purifications are described in Material and Methods.
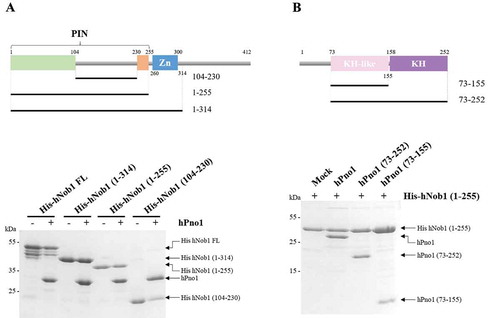
We then turned our interest towards the binding site of hNob1 on hPno1. Pno1 is a conserved protein, which contains two KH domains [Citation25]. The first KH domain of hPno1 is termed ‘KH-like’ in the literature because it does not contain the critical GXXG motif known to mediate ssRNA association [Citation26]. Over the years, several studies performed in yeast have looked into the association of Nob1 and Pno1 [Citation18,Citation26]. In particular, Nob1 binding sites on the Pno1 protein were identified on the KH-like domain [Citation26]. Several mutations located at different places on the KH-like domain including the 310-helix fragment normally devoted to RNA association have been shown to modulate or abolish Nob1 association [Citation26]. We performed similar experiments with the human proteins by testing the capacity of the full-length hPno1 protein (hPno1 construct 1–252) to bind to hNob1 fragment encompassing the PIN domain (hNob1 construct 1–255; ). In absence of pre-rRNA, the two proteins can form a stable complex (). We further tested the importance of the N-terminal non-conserved extension present in the hPno1 protein (residues 1 to 73) and showed that its deletion does not impair complex formation (). Similarly, to hNob1, the evolutionary non-conserved region of hPno1 is dispensable for complex formation. Finally, we truncated hPno1 to the KH-like domain (residues 73–155) and showed that it is sufficient to observe near WT-level of interaction with hNob1 ().
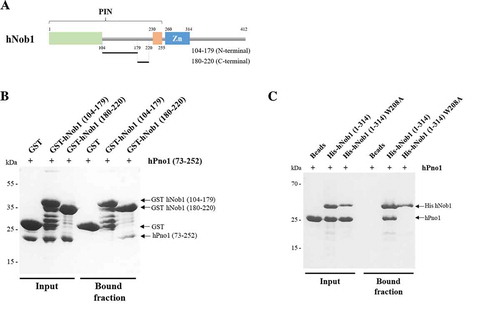
To map as precisely as possible, the site of interaction between the two proteins, we decided to find the responsible residues on each protein. We previously observed that the internal loop present in the PIN domain is necessary and sufficient for a stable association of hPno1 (construct 104–230, ). The loop was artificially divided in two fragments based on sequence composition () and used to test hPno1 association by pull-down experiments (). The fragment from 104 to 179 is poorly conserved in evolution, while the sequence of the fragment 180 to 220 is highly conserved through evolution (). To facilitate the stable expression of the linker sequences, we fused these two sequences to a glutathione S-transferase tag. After purification of the GST-fused sequences, we incubated them with the N-terminal deleted hPno1 construct (). The C-terminal part of the linker (residues 180–220) was able to retain hPno1 on the beads while the fragment 104–179 failed to interact (). These data therefore suggest that the PIN domain, the zinc ribbon and the large unstructured C-terminal region are dispensable for the interaction with hPno1. Based on sequence conservation (), a stretch of three residues, i.e. from 208 to 210 (amino acid sequence: WIT), was targeted for mutation to possibly impact the complex formation. We mutated the Tryptophan 208 into an Alanine in the core of this highly-conserved linker region and reassessed complex formation by pull-down experiments using independently purified mutated hNob1 (W208A) and hPno1 proteins. hNob1 mutant protein was readily expressed but did not stably bind to hPno1 (). We thus concluded that the formation of the human complex relies on a short sequence motif found in the loop present between the bipartite PIN domain, a situation reminiscent to the one recently identified in the fungal complex () [Citation18].
Figure 3. hNob1 interaction with hPno1 depends on the tryptophan at position 208 in hNob1 and on the KH-like domain of hPno1. (a) Schematic view of the different parts of the hNob1 internal loop used to define the minimal region of interaction with hPno1. (b) Pull-down assay of GST-tagged hNob1 linker constructs with hPno1 showing that the C-terminal part of the linker is sufficient for association. (c) Pull down assay of His-tagged hNob1 (1–314) with or without the mutation W208A. Pull-down experiments shown in panel B and C were performed as described in Material and Methods.
Finally, we turned to hPno1 and using a sequence conservation mapping performed by the Consurf server [Citation27] identified a hot spot of conservation located between residues 105 to 110 (data not shown). Unfortunately, point mutations in this region led to poor stability and/or solubility of hPno1, which prevented us from testing the capacity of the mutated hPno1 protein to bind to hNob1. Recently, the same peptide was shown to mediate numerous contacts with hNob1 [Citation13].
Discussion
Nob1/Pno1 is the key complex responsible for the final maturation step of the 18S pre-rRNA. Despite numerous studies on the yeast proteins, information is still lacking on their exact function and on the formation of the complex especially within the context of human ribosome biogenesis. With the aim to fill this lack of knowledge, we first observed that the boundaries of the PIN domain defined a decade ago for hNob1 should be modified to reflect the published biochemical data [Citation16,Citation19]. We demonstrate that the PIN domain extends further into the hNob1 primary sequence by using bioinformatics and biochemical methods (). With this new organization of the PIN domain, a long partially conserved sequence has now become an internal loop of the PIN domain corresponding to approximately residues 96 to 232. However, the unstructured loop may not be a simple ‘separator’ between the newly defined bi-partite PIN domain.
We then set out to define the determinant of the interaction within the hNob1/hPno1 complex by using co-expression and pull-down assays. Several deletion or point mutation constructs were generated and used to characterize in vitro the minimal protein fragments compulsory to observe a stable complex. Using this approach, the region of contact between the two proteins could be reduced to a short linear fragment located between residues 206 and 220 for hNob1 and to the KH-like domain of hPno1. Further fine mapping of hPno1 was precluded by an inability to obtain stable mutant protein using our experimental setup.
By redefining the limits of the PIN domain of hNob1 and the location of the interaction surface with hPno1, we now postulate that hPno1 may regulate the endonuclease activity of hNob1. It is possible that the binding of Pno1 sterically hinders the PIN domain active site or modulates the accessibility of the PIN domain for its substrate 18S pre-rRNA. Recent data obtained with the C. thermophilum Nob1 and Pno1 proteins demonstrate that co-expressing ctPno1 with two ctNob1 constructs encompassing residues 1–169 and 250–354 can form a stable complex [Citation18]. The two ctNob1 constructs can be found in the same complex either by sandwiching ctPno1 or via a direct interaction between them. These constructs correspond to residues 1–115 and 188–291 in hNob1. According to our experiments, the construct 1–115 covers the first half of the PIN domain, thus insufficient to bind stably to hPno1 in vitro (). The second construct, i.e. residues 188–291, contains the other half of the PIN domain plus the zinc ribbon. Taken together, our data help explain why ctNob1/ctPno1 tri-partite complex has been reconstituted by Sturm and colleagues [Citation18]. Their ctNob1 constructs effectively covered the two halves of the PIN domain, an observation undetected at that time.
In our hands, the zinc ribbon does not interact stably with the first half of the PIN domain (). Although we did not observe any decrease in complex formation when deleting this region from hNob1 in vitro (), it is possible that in vivo, the zinc ribbon and/or the C-terminal region participate in complex formation with Pno1. In addition to potentially helping the interaction with hPno1, the close proximity between the zinc ribbon and the active site of the PIN domain makes us suggest that the zinc ribbon could be important to regulate the hNob1 endonucleolytic activity.
In conclusion, the human protein hPno1 binds to hNob1 using a similar surface as compared to the yeast counterpart [Citation26]. hNob1 contacts hPno1 using residues between 206 and 220. Within the hNob1 linker position 104 to 232, a single stretch of amino acids shows a significant degree of conservation (). Three residues are strictly conserved from Archaea to Human, i.e. the sequence WIT. We demonstrate by single residue mutation the key participation of the amino acid W208 for hNob1/hPno1 complex formation [Citation18]. These data suggest a highly conserved mechanism such as a ‘knobs-into-holes’ type of interaction between the two proteins even though each organism may present some specificities represented by the surrounding sequence variations. During the preparation of this manuscript, Ameismeier and colleagues reported the cryo-EM based model of several late stage pre-40S particles either purified from native source or reconstituted where they validate the present conclusion on the model of hNob1 and its mode of interaction with hPno1 [Citation13].
A key question remains regarding hNob1’s activity on the 18S pre-rRNA. Indeed, information on hNob1 recognition and cleavage of 18S pre-rRNA are missing since hNob1’s active site is located at more than 30 Å away from the newly defined PIN domain active site [Citation13]. However, a previous study had shown that Pno1 and Nob1 could be cross-linked to overlapping region of the pre-18S rRNA, clearly suggesting that both proteins are able to reach the future 18S rRNA 3ʹ-end [Citation28]. The role of other individual components in 18S pre-rRNA cleavage may in future be provided by additional structures of pre-40S intermediates or by structures of individual protein complexes together with their target RNA. Alternatively, one cannot exclude that additional players such as the CINAP/Fap7 protein or the Rps14 protein, may be required to induce the obligatory local rearrangements leading to the close proximity between hNob1 PIN domain active site and pre-rRNA allowing the direct observation of the site 3 cleavage configuration. More data will therefore be necessary to fully comprehend the role of the Nob1/Pno1 complex during this critical step of ribosome production.
Materials and methods
PCR amplification and cloning
Plasmids expressing hNob1 and hPno1 were built by inserting the PCR-amplified sequences of hNob1 or hPno1 into modified pET vectors allowing for coexpression and with or without an N-terminal hexa-Histidine or Glutathione S-Transferase (GST) Tag. Cloning was performed with a modified sequence ligation independent cloning (LIC) protocol and plasmids were verified by DNA sequencing.
Protein expression and purification
hNob1 and hPno1 constructs were individually transformed or cotransformed into Escherichia coli BL21 Rosetta 2 cells. Bacteria were cultured in TB medium at 37°C and protein expression was induced with 0.2 mM isopropyl 1-thio-β-D-galactopyranoside overnight at 15°C. Cells were pelleted down by centrifugation and resuspended with 1 mL of ice cold Lysis buffer (50 mM Tris-HCl pH 8, 300 mM NaCl, 0.1% Triton X100, 5 mM β-mercaptoethanol and 10% glycerol w/v). Cells were lysed by sonication. The soluble fraction was separated from the crude extract by centrifugation at 14,000 rpm for 30 min at 4°C. Cleared lysate was incubated with 50 µL Ni-NTA beads SIGMA (H0537) for 2 h at 4°C on a rotating wheel. Beads were spun down to remove the unbound material and washed five times with Wash buffer (50 mM Tris-HCl pH 8, 300 mM NaCl, 20 mM imidazole, 5 mM β-mercaptoethanol and 10% glycerol w/v). His-tagged proteins were eluted by competition with Elution buffer (Wash buffer plus 250 mM imidazole). Eluted samples were analyzed by SDS PAGE and stained with Coomassie blue. GST-fused proteins were purified on GST beads SIGMA (G4510) following same purification protocol with the exception that the Wash buffer did not contain imidazole. Protein elution was carried out with Wash buffer supplemented with 20 mM reduced glutathione.
Pull down assay
Proteins were purified as indicated above. Histidine-tagged full length hPno1 and a construct from residues 73–252 were subjected to a cleavage step with TEV protease during an overnight incubation at 16°C. Cleaved tag, TEV proteases and undigested proteins were removed by reloading the sample on Ni-NTA and collecting the flow-through fraction. Tagged hNob1 constructs were added to hPno1 in a 1:1 molar ratio. The mixture volume was adjusted to 1 mL with binding buffer (50 mM Na2HPO4/NaH2PO4, pH 7.5, 500 mM NaCl, 5 mM β-mercaptoethanol and 10% glycerol w/v). Samples were incubated at 4°C for 1 h and afterwards 30 µL of NiNTA beads was added to the mixture and further incubated on a rotating wheel for 1 h at 4°C. Unbound material (flow-through) was collected after centrifugation for 30 s at 2,000 g. Beads were washed five times with Wash buffer 2 (50 mM Na2HPO4/NaH2PO4, pH 7.5, 500 mM NaCl, 20 mM imidazole, 5 mM β-Mercaptoethanol and 10% glycerol w/v) before elution with 30 µL of Elution buffer 2 (Wash buffer 2 supplemented with 250 mM imidazole). Input and bound fractions were analyzed by SDS PAGE and stained with Coomassie blue. GST pull-downs were performed similarly except that buffers did not contain imidazole and the elution buffer was supplemented with 20 mM of reduced glutathione.
Bioinformatics analysis of hNob1 or hPno1
Protein sequences for hNob1 (UniProt entry Q9ULX3) and hPno1 (UniProt entry Q9NRX1) were uploaded to the online server PHYRE2 for structure prediction [Citation23] and to the ConSurf server for sequence conservation prediction [Citation27].
The structural model obtained for hNob1 was used to prepare the panel B from and subsequently design the truncation/point mutation boundaries. The most closely related model found by PHYRE is the atomic structure of Nob1 from P. horikoshii (PDB code 2LCQ) [Citation16].
The sequence conservation score within the Pno1 protein family was calculated using 150 sequences from homologous proteins taken in the UniRef90. The conservation score was then mapped onto the surface of the archaeal Pno1 structure from P. horikoshii (PDB code 2E3U) [Citation29].
Acknowledgments
We thank Cameron Mackereth and Fabien Darfeuille for critical reading of the manuscript. We further acknowledge Simon Lebaron, Marie-Françoise O’Donohue, Lucile Maleuvre and Manon Coursières for experiments, which unfortunately could not be included. This work was funded by ANR grant (ANR Blanc 2010 grant RIBOPRE40S (ANR-10-BLAN-1224) and RIBOMAN ANR-16-CE11-0029 to S.F.), the INSERM and the University of Bordeaux.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Barandun J, Hunziker M, Klinge S. Assembly and structure of the SSU processome-a nucleolar precursor of the small ribosomal subunit. Curr Opin Struct Biol. 2018;49:85–93.
- Scaiola A, Peña C, Weisser M, et al. Structure of a eukaryotic cytoplasmic pre-40S ribosomal subunit. EMBO J. 2018;37(7):e98499.
- Kater L, Thoms M, Barrio-Garcia C, et al. Visualizing the assembly pathway of nucleolar Pre-60S ribosomes. Cell. 2017;171(7):1599–1610.e14.
- Ma C, Wu S, Li N, et al. Structural snapshot of cytoplasmic pre-60S ribosomal particles bound by Nmd3, Lsg1, Tif6 and Reh1. Nat Struct Mol Biol. 2017;24(3):214–220.
- Heuer A, Thomson E, Schmidt C, et al. Cryo-EM structure of a late pre-40S ribosomal subunit from Saccharomyces cerevisiae. Elife. 2017;6.
- Kornprobst M, Turk M, Kellner N, et al. Architecture of the 90S pre-ribosome: a structural view on the birth of the eukaryotic ribosome. Cell. 2016;166(2):380–393.
- Sun Q, Zhu X, Qi J, et al. Molecular architecture of the 90S small subunit pre-ribosome. Elife. 2017;6e22086. DOI:10.7554/eLife.22086.
- Barandun J, Chaker-Margot M, Hunziker M, et al. The complete structure of the small-subunit processome. Nat Struct Mol Biol. 2017;24(11):944–953.
- Sanghai ZA, Miller L, Molloy KR, et al. Modular assembly of the nucleolar pre-60S ribosomal subunit. Nature. 2018;556(7699):126–129.
- Narla A, Ebert BL. Ribosomopathies: human disorders of ribosome dysfunction. Blood. 2010;115(16):3196–3205.
- Peña C, Hurt E, Panse VG. Eukaryotic ribosome assembly, transport and quality control. Nat Struct Mol Biol. 2017;24:689–699.
- Tone Y, Toh-E A. Nob1p is required for biogenesis of the 26S proteasome and degraded upon its maturation in Saccharomyces cerevisiae. Genes Dev. 2002;16(24):3142–3157.
- Ameismeier M, Cheng J, Berninghausen O, et al. Visualizing late states of human 40S ribosomal subunit maturation. Nature. 2018;558(7709):249–253.
- Hellmich UA, Weis BL, Lioutikov A, et al. Essential ribosome assembly factor Fap7 regulates a hierarchy of RNA-protein interactions during small ribosomal subunit biogenesis. Proc Natl Acad Sci USA. 2013;110(38):15253–15258.
- Bai D, Zhang J, Li T, et al. The ATPase hCINAP regulates 18S rRNA processing and is essential for embryogenesis and tumour growth. Nat Commun. 2016;7:12310.
- Veith T, Martin R, Wurm JP, et al. Structural and functional analysis of the archaeal endonuclease Nob1. Nucleic Acids Res. 2012;40(7):3259–3274.
- Fatica A, Oeffinger M, Dlakić M, et al. Nob1p is required for cleavage of the 3ʹ end of 18S rRNA. Mol Cell Biol. 2003;23(5):1798–1807.
- Sturm M, Cheng J, Baßler J, et al. Interdependent action of KH domain proteins Krr1 and Dim2 drive the 40S platform assembly. Nat Commun. 2017;8(1).
- Fatica A, Tollervey D, Dlakić M. PIN domain of Nob1p is required for D-site cleavage in 20S pre-rRNA. RNA. 2004;10(11):1698–1701.
- Matelska D, Steczkiewicz K, Ginalski K. Comprehensive classification of the PIN domain-like superfamily. Nucleic Acids Res. 2017;45(12):6995–7020.
- Lamanna AC, Karbstein K. Nob1 binds the single-stranded cleavage site D at the 3ʹ-end of 18S rRNA with its PIN domain. Proc Natl Acad Sci USA. 2009;106(34):14259–14264.
- Valverde R, Edwards L, Regan L. Structure and function of KH domains. FEBS J. 2008;275(11):2712–2726.
- Kelley LA, Mezulis S, Yates CM, et al. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10(6):845–858.
- Pertschy B, Schneider C, Gnädig M, et al. RNA Helicase Prp43 and Its Co-factor Pfa1 Promote 20 to 18 S rRNA processing catalyzed by the endonuclease Nob1. J Biol Chem. 2009;284(50):35079–35091.
- Nicastro G, Taylor IA, Ramos A. KH-RNA interactions: back in the groove. Curr Opin Struct Biol. 2015;30:63–70.
- Woolls HA, Lamanna AC, Karbstein K. Roles of Dim2 in ribosome assembly. J Biol Chem. 2011;286(4):2578–2586.
- Ashkenazy H, Abadi S, Martz E, et al. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016;44(W1):W344–50.
- Turowski TW, Lebaron S, Zhang E, et al. Rio1 mediates ATP-dependent final maturation of 40S ribosomal subunits. Nucleic Acids Res. 2014 Oct;42(19):12189–12199.
- Jia MZ, Ohtsuka J, Lee WC, et al. Crystal structure of Dim2p: a preribosomal RNA processing factor, from Pyrococcus horikoshii OT3 at 2.30 A. Proteins. 2007;69(2):428–432.
- Robert X, Gouet P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014;42(W1):W320–W324.
