ABSTRACT
Polycomb repressive complex 2 (PRC2) and its methylation of histone 3 at lysine 27 (H3K27me3) play a crucial role in epigenetic regulation of normal development and malignancy. Several factors regulate the recruitment of PRC2 and affects its chromatin modification function. Over the past years, emerging discoveries have portrayed the association of RNA (protein-coding and non-coding) with PRC2 as a critical factor in understanding PRC2 function. With PRC2 being a macromolecular complex of interest in development and diseases, further studies are needed to relate the rapidly evolving PRC2:RNA biology in that scenario. In this review, we summarize the current understanding of different modes of RNA binding by PRC2, and further discuss perspectives, key questions and therapeutic applications of PRC2 binding to RNAs.
Introduction
Polycomb group of proteins modulate the expression of development-related genes in the early stage through regulation of chromatin structure. The group obtained its name Polycomb (Pc) from the improper body segmentation in Drosophila mutants, where it was considered to be the negative regulator of homeostatic genes related to segmentation [Citation1]. The polycomb group (PcG) of proteins have been shown to be crucial in early development and adulthood through their spatial expression and its ability to modify chromatin [Citation2,Citation3]. In mammals, PcG consists of the polycomb repressive complex 1 (PRC1) and PRC2.
PRC2, which modifies the chromatin to maintain the genes in their repressive state during development, has been reported to be dysregulated in several cancer types [Citation4,Citation5]. PRC2 complex has four core subunits – enhancer of zeste homolog 1 or 2 (EZH1/2), suppressor of zeste 12 (SUZ12), embryonic ectoderm development (EED) and retinoblastoma binding protein 4/7 (RBBP4/7). The repressive function of PRC2 comes from its ability to mono, di- and trimethylate lysine 27 on histone 3 (H3K27me3). This methyltransferase activity is catalyzed by EZH1/2 which is modulated by SUZ12 and EED, while RBBP4/7 serves as the histone binding protein. There is a huge abundance of methylated H3K27 in the genome; in embryonic stem cells 50% of H3K27 are di-methylated while 15% are mono- and tri-methylated [Citation6]. The role of H3K27me3 has only been partly solved. It is thought that H3K27me2 is of limited importance for gene repression acting as a precursor molecule for H3K27me3, while H3K27me1 is the result of demethylation [Citation7]. The H3K27me3 acts as a recognition site for EED, which facilitates the positive feedback loop for PRC2. The site also acts as the docking site of CBX of PRC1 complex to form the repressive complex [Citation8,Citation9], though contradictory studies regarding dependency of PRC1 on PRC2 have challenged that notion [Citation10–Citation12]. Apart from the four major subunits the complex also contains accessory subunits – adipocyte enhancer-binding protein 2 (AEBP2), orthologues of drosophila Polycomb like (PHF1, MTF2, PHF19), jumonji and AT-rich interaction domain containing 2 (JARID2), PRC2 associated LCOR isoform 1 and 2 (PALI1/2) and Elongin BC and PRC2 associated protein (EPOP) [Citation7,Citation13–Citation15].
PRC2 in Drosophila gets recruited to chromatin by identifying the Polycomb response elements (PREs) in DNA sequences [Citation16,Citation17]. This led to the search for such functionally similar sequences in mammals’ DNA. Vertebrates’ PREs, however, are not conserved and hence have only recently been discovered [Citation18–Citation21]. Multiple protein factors also have been associated with the recruitment of PRC2 to its target. One such promising molecule was YY1, a mammalian orthologue of Drosophila PRE DNA binding protein PHO [Citation22], but genome wide analysis studies showed no overlap between YY1 and PRC2 targets [Citation23]. PRC2 has also been observed to be associated with GC rich regions (also known as CpG islands), with increased association in repressed genes and decreased association near promoters and enhancers [Citation24–Citation26]. Despite these possibilities, they do not completely explain the specificity and dynamics of PRC2 widespread chromatin association. For instance, several studies have disputed the CpG island hypothesis [Citation19,Citation27,Citation28]. Interestingly, the subunits EZH2, SUZ12, AEBP2 and JARID2 all show affinity to RNA, which led to the speculation that PRC2:RNA binding could regulate PRC2 recruitment to chromatin [Citation29–Citation33]. While the RNA binding activity of PRC2 could be one of the most promising regulatory factor for its association with chromatin, the mechanism of PRC2:RNA binding still remains elusive.
lncRNA/short ncRNA and PRC2
The proof of PRC involvement with RNA in gene regulation came first from its association with long non-coding RNA (lncRNAs) where it has been shown to be recruited to mediate suppression either in cis or in trans. One of the first lncRNA to be associated with PRC2 was Xist. The Xist gene is associated with X-chromosome inactivation (XCI) in mammals, which is needed to equalize the X gene dosage between females and males [Citation34]. Xist gene was found to have the ability to bypass the nuclear export and localize on the same chromosome from which it is transcribed, thus mediating a cis-regulatory signal. Immunofluorescence studies first showed the association of Xist with PRC2, where EED was found to be highly enriched on the inactive X-chromosome [Citation35,Citation36]. Further proof came when EZH2 expression was shown to coincide with Xist expression on the inactive X-chromosome area, which was found to be highly enriched with H3K27me3 marks deposited by PRC2 [Citation37,Citation38]. Later, biochemical analysis of PRC2 and Xist interaction revealed the presence of RepA, a 1.6kb short transcript in the intron 1 of Xist [Citation29]. Xist RNA was shown via RNA immunoprecipitation (RIP) to co-precipitate with PRC2 proteins, and qualitative electrophoretic mobility shift assays (EMSA) showed the binding of RepA to EZH2, a heterodimer of EZH2-EED and a heterotrimeric complex of EZH2-EED-SUZ12. This led to the proposition of a specific RNA binding motif of PRC2, which is an A-repeat region that forms a two-hairpin secondary structure [Citation29,Citation39]. The same studies showed that PRC2 subunits are also capable of binding with a part of Tsix RNA, an antisense transcript of Xist that is being expressed in the active X-chromosome. These observations led to the development of a model where XCI is induced by Xist recruitment of PRC2 which then deposits H3K27me3, while in pre-XCI cells PRC2 action is blocked by Tsix [Citation29].
Another lncRNA that expanded the nature of PRC2 association with RNA was HOTAIR, which is located on the HOXC locus. Suppression of HOTAIR using RNA interference (RNAi) was observed to result in the activation of genes in the HOXD locus, suggesting a trans-acting gene regulation. RIP studies identified higher association of HOTAIR with PRC2 than any other control RNA, showing that HOTAIR recruits PRC2 to mediate suppression of gene expression on the HOXD locus by deposition of H3K27me3 [Citation40,Citation41]. While recent papers have casted doubt on this model [Citation42,Citation43], such discrepancies in findings could be due to cell-type variations or the different levels of HOTAIR transcript among many other experimental factors.
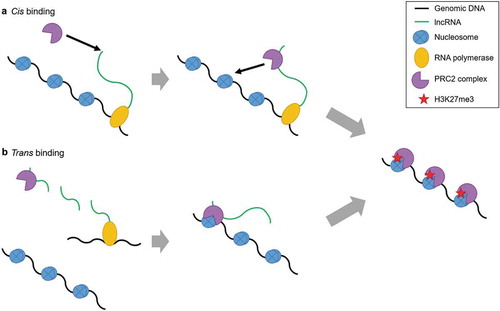
Since then, several studies have associated other lncRNAs (Kcq1ot1, Braveheart, MALAT1, PINC, Air, ANRIL) with PRC2, which led to the belief that recognition of a specific lncRNA leads PRC2 to the chromatin [Citation44]. Combining the RIP experiments with microarray analysis revealed PRC2’s association with 20% of human lncRNAs, a breakthrough study that showed PCR2’s association with multiple RNAs [Citation45]. Later, similar experiments were performed to identify short ncRNAs transcribed upstream of PRC2 target genes that attached with PRC2 [Citation32]. Based on the RNA folding data, it was observed that PRC2 recruitment takes place by interaction with a two-hairpin motif, similar to the one predicted in the Xist:PRC2 study, which was found in 71% of short ncRNAs transcribed from PRC2 silenced genes [Citation29,Citation32]. This model was later challenged, and it was proven that the two-hairpin motif is insufficient for PRC2 recruitment [Citation46]. The collective evidences of the association of PRC2 with various lncRNA led to the proposition of the early general model of PRC2:RNA binding (). This model predicted that PRC2 gets recruited by specific protein-RNA interactions with the lncRNA either through PRC2 core subunits or mediated by cofactors like JARID2. The binding initiates the deposition of PRC2 mediated H3K27me3, which creates a feedback loop for PRC2 to remain bound and maintain the repression.
Figure 1. lncRNA mediated PRC2 binding. RNA immunoprecipitation studies showed the first association of RNA with PRC2. The binding of lncRNA with PRC2 was thought to be the reason behind PRC2 recruitment, and this gave rise to the idea of lncRNA specific binding of PRC2. lncRNA guided PRC2 to the target gene locus where it would identify repressive chromatin markers (H3K27me3/H2AK119ub) and cause gene repression. PRC2 was seen to deposit H3K27me3 on chromatin and cause gene repression either in cis (A) as was seen in case of Xist and RepA for XCI and in trans (B) as was seen in HOTAIR-mediated suppression of HOXD locus.
Promiscuity and models of RNA-mediated PRC2 recruitment
Though the general model of binding specificity of PRC2 to lncRNA gives a compelling idea behind PRC2 recruitment, doubts were soon cast on this model. The lncRNA:PRC2 model speculates that PRC2 deposits H3K27me3 on the target loci of the repressed gene [Citation29,Citation32,Citation40]. According to the model, there should be a high enrichment of H3K27me3 mark around the promoters of the genes for the lncRNAs. However, included in the 9000 PRC2-associated RNAs identified by genome-wide RIP-seq were mRNAs for housekeeping genes and highly expressed RNAs that are not regulated by PRC2 [Citation30,Citation47]. Furthermore, studies found that the dissociation constants Kd between PRC2 and irrelevant transcripts from ciliates and bacteria were comparable to known PRC2-targeted lncRNAs [Citation47]. Striking, EZH2 enrichment was positively correlated by RNA transcription and active chromatin marks in EZH2 RIP-seq data sets [Citation47]. To test the specificity of PRC2 to lncRNA, in vitro RNA EMSA studies were performed between the PRC2 subunits (EZH2, EED, SUZ12), the known associated RNAs (Xist, RepA and HOTAIR) and RNA of foreign origin (maltose binding protein (MBP) mRNA of E. coli and the P4–P6 domain of Tetrahymena ribozyme). PRC2 bound preferentially to cognate RNAs over the non-specific RNA, but the binding was highly dependent on the buffer conditions [Citation46]. PRC2-RepA binding was evident in both TBE buffer [Citation47] and THEM buffer [Citation31], but binding with P4–P6 was only seen in TBE [Citation46]. Moreover, when a new buffer was introduced [Citation46] PRC2 showed similar affinity for RepA, MBP 1–300 and P4–P6. The alteration of PRC2:RNA binding specificity and affinity with different experimental conditions thus limited the relevance of PRC2 RNA binding specificity.
‘Junk mail’ model
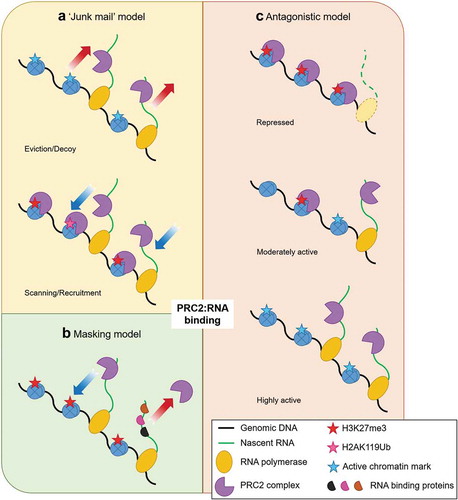
To improve on RIP, photoactivatable ribonucleotide-enhanced crosslinking and immunoprecipitation (PAR-CLIP) was performed, which identified association of EZH2 with the 5ʹ region of 774 nascent RNAs [Citation48]. Unbiased genome wide studies identified the wide association of PRC2 with RNA, which showed its promiscuous binding and led to the suggestion of the RNA-mediated scanning and eviction model, also known as the ‘junk mail’ model (). Promiscuous binding meant that PRC2 binds to several RNAs without the need for a specific RNA binding motif. This model proposed that the promiscuous binding of PRC2 serves as a checkpoint for the genes that escaped epigenetic silencing [Citation47]. At active genes, the promiscuous binding of RNA causes the eviction of PRC2 from chromatin or the elongating RNA acts as a decoy; the presence of active chromatin marks H3K4me3 and H3k36me3 reduces the affinity of PRC2 to bind to chromosomes (). On the other hand, if any gene requires silencing, binding of PRC2 to RNA brings it closer to the chromatin marks H2AK119ub deposited by PRC1 [Citation49–Citation51] and H3K27me3 by PRC2 itself [Citation52], which signals for PRC2 mediated gene repression.
Figure 2. Model systems for RNA-mediated recruitment of PRC2. PRC2 was shown to have promiscuous binding rather than having any specificity for lncRNAs. The promiscuous nature of PRC2 was thought to be a way for PRC2 to bind with RNA and scan the genome for genes that escaped silencing (scanning), and in active genes elongating RNA acts as a decoy for PRC2 (eviction). PRC2 remained in a deactivated/poised state when bound to RNA with its HMTase activity inhibited and would deposit H3K27me3 only when it recognized repressive markers (H3K27me3/H2AK119ub). This was known as the ‘junk mail’ model (A). Due to the odds in binding seen in vitro and in vivo, it was thought that the intrinsic promiscuity of PRC2 to bind with RNA meant that it must compete with other RBPs. RNAs that were not bound to any other proteins had a higher chance to bind with PRC2. This was known was the ‘masking model’ (B). Recent iCLIP studies showed that the RNA and chromatin compete with each other to bind with PRC2, thus having an antagonistic relationship (C). In moderately active gene (C, middle) both RNA and chromatin compete, but in active genes (C, bottom) the continuous formation of RNA drives PRC2 from chromatin while in repressed genes (C, top) loss of RNA cause the chromatin to bind with PRC2.
Masking model
Apart from the ‘junk mail’ model, another model was proposed which states that PRC2 prefers to bind with transcripts that are exposed and not bound to any other proteins. This model was termed as the ‘masking model’ (). It explained the binding preferences of PRC2 in vivo where the RNAs compete with each other to bind to PRC2, and the transcript without any bound proteins have a higher chance to outcompete other RNAs [Citation53].
RNA-chromatin antagonistic model
To improve on the sensitivity of PRC2 and RNA binding, individual nucleotide resolution crosslinking and immunoprecipitation (iCLIP) was performed. It was revealed that PRC2 binds to nascent RNAs at almost all active genes. The previously identified EZH2-bound RNAs (ezRNAs) are longer and have higher expression, which explained their increased association with PRC2. Degrading the RNA in cells increased the recruitment of PRC2 to chromatin, while release of PRC2 from chromatin resulted in increased PRC2:RNA binding [Citation54]. An earlier study also found that inhibition of transcription led to SUZ12 chromatin recruitment and that a mutual exclusion between PRC2 binding and active transcription exists [Citation55]. This suggested a model where RNA and chromatin competes for binding with PRC2, thus having an antagonistic relationship [Citation54] (). RNA and chromatin actively compete at genes that are lowly expressed, while at highly expressed genes, the increased levels of nascent RNA shift the balance towards PRC2:RNA binding. In cases where a gene is silenced during any biochemical process, the RNA level goes down and facilitates recruitment of PRC2 to the CpG islands to form the repressive complex mediated by EED’s positive feedback loop [Citation52] and CBX-containing PRC1 [Citation49,Citation50].
Findings from studies on RNA inhibition of PRC2 catalytic activity
In studying the role of RNA in PRC2 recruitment, Kaneko et al. [Citation48] noted that genes producing ezRNAs tended to have lower H3K27me3 levels. It was postulated that such an observation could be due to RNA inhibiting PRC2 activity or that RNA was evicting PRC2 from chromatin, thus preventing H3K27me3 deposition (the antagonistic model). In the following year, they along with others performed HMTase assays that unanimously suggested inhibition of PRC2 catalytic activity by RNA [Citation31,Citation53,Citation56] thus lending support to the former view. However, these studies did not look into automethylation of the EZH2 subunit [Citation57,Citation58]. Besides observing similar decrease in H3 methylation in the presence of RNA in HMTase assays, Wang et al. (2017) also noted that RNA had minimal effect on EZH2 automethylation [Citation59]. These findings suggested that RNA was not an active-site inhibitor of PRC2 methyltransferase. Most importantly, it was shown that increasing concentrations of RNA were able to strip PRC2 from nucleosomes [Citation59], which further corroborated the RNA-chromatin antagonistic model. In light of current evidences, the antagonistic model seems to hold true which would exclude the ‘junk mail’ model as the former does not agree with the latter. The ‘junk mail’ model posits that RNA has dual roles in both acting as a checkpoint by recruiting PRC2 to lowly expressed/target genes and as a decoy/natural sink at active genes. On the other hand, the antagonistic model proposes that RNA acts solely as a decoy by inhibiting PRC2 interaction with chromatin and hence clashes with the ‘junk mail’ model.
Nevertheless, evidences of RNA being able to recruit PRC2 should not be dismissed and should be reconciled with the antagonistic model. To this, Ringrose [Citation60] proposed a kinetic mathematical model in which PRC2 recruitment or eviction is determined by the rate at which RNA is released from the locus. A slow release rate of RNA (blocked state) meant that the majority of the RNA remains attached to chromatin and thus PRC2 bound to the RNA is kept in close proximity to the chromatin (recruitment), allowing for repression to happen. On the other hand, a high release rate of RNA meant that most of the RNA is freed from chromatin. Hence, any RNA-bound PRC2 is also consequently removed and new RNA can continuously be produced, further titrating away PRC2 from the region [Citation60]. This model, confirmed through computer simulations, while similar to the ‘junk’ mail model, did not require RNA to have catalytic inhibitory effects on PRC2 for recruitment. At the same time, it is also able to account for the examples of PRC2 recruitment by RNA.
Tying kinetic mathematical model back to lncRNA and promiscuity
Several studies have implicated lncRNA-mediated PRC2 recruitment in cancer [Citation61–Citation63], which is still valid with the RNA-chromatin competition for PRC2 binding picture in mind. However, the kinetic mathematical model did not support any lncRNA specificity. It is also possible that the lncRNA:PRC2 interaction may not be the only recruitment factor, as involvement of lncRNA with other repressor proteins may recruit PRC2 as well [Citation64,Citation65].
Even though the RNA-chromatin antagonistic relationship explains the loss of binding of PRC2 at CpG islands next to promoters/enhancers [Citation26] and the reduction of H3K27me3 [Citation31,Citation53,Citation56], it does not address the promiscuous nature of PRC2:RNA binding. Previously, it was observed that the Kd of PRC2 and RNA continually decreased when extra bases were added to the 5ʹ end of the E. coli MBP mRNA [Citation47], suggesting that affinity increases with the length of RNA. In particular, a linear dependence exists between log(Kd) and log(RNA length) which indicates that PRC2 binding sites are abundant within RNA. However, this raises the complexity of finding a RNA recognition motif (RRM) within PRC2. A reverse approach was taken to identify the PRC2-binding motif within RNA, which led to the identification of short tracts of G’s and G-quadruplexes that were capable of sustaining PRC2 and RNA binding both in vitro and in vivo [Citation33]. This interaction is conserved over vast evolutionary distance as it was observed for chameleon PRC2 as well [Citation66]. The binding preference of PRC2 with RNA increases from the spaced G’s or G-tracts in single stand to G-quadruplexes, a structure where G-tracts bind via Hoogsteen base pairing. Surprisingly, the affinity substantially reduces for duplex RNA. Nonetheless, the abundance of G-tracts within the transcriptome rationalizes the promiscuous RNA binding by PRC2.
Molecular picture behind PRC2:RNA binding
Even though PRC2 shows promiscuous binding to RNA, separating its RRM from its chromatin binding motif would enable wide understanding of RNA mediated epigenetic silencing and gene inhibitory functions. Crystal structures of the catalytic core of PRC2 (EZH2, EED and VEFS domain from SUZ12) have been obtained from fungus Chaetomium thermophilum (ctPRC2) [Citation67], chimeric PRC2 containing EZH2 from American chameleon (Anolis carolinensis) and other subunits from human (hsPRC2) [Citation68,Citation69], but none of these studies were able to identify a canonical RRM. Later, domain deletion and subsequent site-directed mutagenesis pinpointed four residues in the BAM domain of EZH2 in ctPRC2 for RNA binding. However, only one of the four residues is conserved in hsPRC2, suggesting that ctPRC2 and hsPRC2 achieve specificity for G-quadruplex RNA differently [Citation66]. Hydrogen deuterium exchange mass spectroscopy (HDX-MS) revealed that sites 290–308 (E1) and 338–350 (E2) on hsEED are potentially involved in RNA binding [Citation66]. HDX-MS followed by alanine scanning mutagenesis/sequence substitution on hsEZH2 also located binding sites in the N-terminal helix (F32, R34, D36 and F39), residues 489–494 (PRKKKR) in the CXC domain, and K656 in the I-SET helix. Mutations at these sites reduced the RNA binding affinity of 3 m PRC2, a minimal complex of the PRC2 catalytic lobe that consists of the core subunits EZH2, EED and the VEFS domain from SUZ12 (EZH2-EED-SUZ12 VEFS) [Citation66]. These findings indicate that PRC2 binds RNA through several different binding sites that are spread throughout the complex. Such non-canonical RNA binding property might be common in chromatin-binding proteins as it was also observed that DNA methyltransferase 3 was able to interact with RNA at its catalytic site as well as an allosteric site [Citation70].
While the VEFS domain of SUZ12 has been extensively studied for RNA interaction, a newly obtained cryo-EM structure of hsPRC2 with its cofactors AEBP2 and JARID2 hinted that other SUZ12 domains are equally important [Citation71]. The ‘neck’ region, which is a C2H2-type zinc-finger domain, was shown to form extensive hydrophobic interactions with EZH2 SAL motif, EED and the VEFS domain of SUZ12. In addition, a SUZ12 domain rich in β-sheet structure that makes up the ‘foot’ of the hsPRC2 complex resembled an RRM-like fold [Citation71]. HDX experiments further confirmed that this domain contributes to RNA binding [Citation71]. These show that the ‘neck’ region and the β-sheet rich domain of SUZ12 may also play a crucial role in the RNA binding abilities of PRC2. Mutations in the ‘neck’ and VEFS domain have been associated with cancer [Citation72,Citation73], and hence SUZ12 could be a potential target for cancer therapy.
Besides the core subunits, the cofactors AEBP2 and JARID2 have also been implicated in RNA binding. Comparing between a four-subunit complex consisting of EZH2, SUZ12, EED and RBBP4 and a five-subunit complex with an additional AEBP2 cofactor revealed a two-fold decrease in the Kd for RNA binding. The five-subunit complex also displayed a higher Hill coefficient value, which confirms AEBP2 has a positive binding cooperativity in PRC2 [Citation47]. Later, it was noted through crosslinking experiments that AEBP2 contacts RNA as well [Citation33] fits well with the finding of positive binding cooperativity. JARID2 also contacts RNA directly through a 30-amino acid region, from residues 332–358, which is not involved in JARID2 interaction with PRC2 in vivo [Citation74].
While studies of each PRC2 subunits’ interactions with RNA have been done, an understanding of how the subunits interact in the complex to influence PRC2:RNA binding is also needed. For instance, EED do not bind RNA, but when complexed with the other subunits, it decreased their RNA binding affinity to both cognate and nonspecific RNAs [Citation31]. On the other hand, both SUZ12 and JARID2 has been shown to bind RNA, but they also attenuate RNA binding for EZH2 or whole PRC2 and EZH2-SUZ12-EED subcomplexes respectively [Citation31]. These findings thus demonstrate that interactions of subunits with each other can refine and lend greater specificity to overall PRC2:RNA interactions. Considering that subunits are ultimately complexed into PRC2 to function, more studies on this area would definitely shed light on PRC2:RNA binding patterns.
Conclusions, questions and future directions
The interaction between PRC2 and RNA has retained a great deal of attention. The view that nascent RNAs are just linker molecules is changing as emerging evidences suggest that both coding and non-coding nascent RNAs hold importance in transcription regulation and chromatin modification. It has been established that PRC2 interacts with nascent RNA at essentially all active genes and that mutual antagonism between RNA binding and chromatin association helps shape the pattern of PRC2 recruitment to chromatin. Further experiments are needed to ascertain the critical role of nascent RNAs in directing chromatin binding proteins, and whether there exists any specific role of nascent RNA despite the observed promiscuous binding.
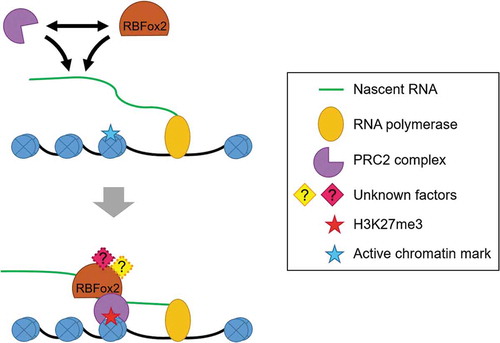
Although EZH2-EED-SUZ12 is capable of binding to the nascent RNA, there are likely to be several other factors including RNA binding proteins (RBPs) that modulate such binding. One such RBP that was found to interact with nascent RNA and PRC2 to direct PRC2 to the genome was RNA binding protein fox 1 homologue 2 (RBFox2). RBFox2 which was found to be part of large RNP (ribonucleoproteins) that facilitated the binding of Xist Rep A to PRC2 [Citation65]. RBfox2 is thought to initially interact with nascent RNA and then recruit PRC2 to the chromatin via protein-protein interactions [Citation75]. Thus, RBFox2 may be responsible for modulating the balance that exists behind the RNA levels and chromatin state and regulate their antagonistic nature to PRC2. With the increasing evidences of RBPs association with the regulatory RNA to control transcription, we propose that RBFox2 may be just one of the proteins or a part of a complex that interacts with nascent RNA to modulate PRC2 (). This would open up a new avenue of targeting PRC2 in diseases, where RNPs and RBPs associated with PRC2 could be potential targets.
Figure 3. RBPs modulating the binding of PRC2 on genome. Several evidences have pointed out the association of RBPs with RNA and their role in regulating gene expression. RBFox2, a pre-mRNA splicing regulator, was found to be associated with nascent RNA and helped in the recruitment of PRC2 to active genes by protein-protein interaction. RBFox2 may act as a part of a complex or it may be one of the several RBPs that binds with PRC2.
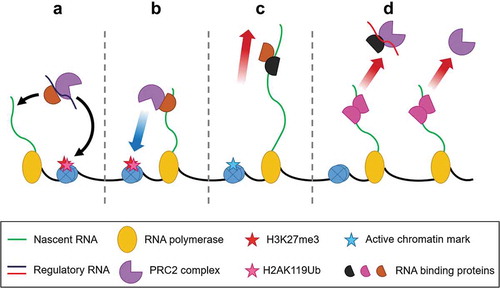
It is still under investigation whether there exists any specific site within PRC2 subunits and cofactors that regulates a specific protein-protein interaction with RBPs. We speculate that a threefold site-specific interaction may occur between PRC2, RBPs and RNA, which may lead to PRC2 RNA binding specificity (). In a study conducted to relate PRC2 RNA binding activity to other RBPs, iCLIP was performed for PRC2 and the splicing regulators FUS and HNRNPC. Through the experiment, it was revealed that some specific peaks of PRC2 binding overlapped with peaks of FUS or HNRNPC [Citation54]. Interestingly, PRC2 has a distinctive RNA crosslinking signature, binding to unspliced nascent RNA at essentially all active genes, and enrichment of PRC2 was observed primarily at exon-intron boundaries within RNAs [Citation54]. It was found that for PRC2 subunit SUZ12, the number of exons in the transcript, rather than its length, is a more dominant determinant of binding. These observations might suggest a mechanism whereby hindrance of PRC2 silencing may require spliced RNA, sensed by SUZ12 interaction [Citation54]. Consistently, recent studies have uncovered a regulatory layer between co-transcriptional RNA splicing and chromatin [Citation76–Citation79]. Therefore, it will be important to test whether PRC2 binding to these nascent RNA might serve functions other than chromatin targeting, particularly the possibility of RNA splicing regulation by PRC2.
Figure 4. Speculated RNA:RBP:PRC2 interactions. The interactions between PRC2 and RNA can be postulated to be modulated by proteins that have affinity for PRC2 and RNA. In repressed genes, the proteins binding to a regulatory RNA can bring PRC2 to the genetic loci for trans repression (A) or PRC2 can bind to the proteins with nascent RNA and deposit H2K27me3 (B). In the case of active genes, even if PRC2 binds with RBPs, the elongating RNA takes PRC2 away from the chromatin and the active chromatin marks does not allow chromatin binding (C). Moreover, the proteins bound with the nascent RNA can cause a steric hindrance for PRC2 to bind or that the proteins bound to nascent RNA can inhibit PRC2 joining (D).
Although PRC2 binds nascent RNA nonselectively, it is believed that enrichment of the binding should be at defined locations that are related to a specific RNA structure. Wang et al. discovered that PRC2 has a high affinity for G-quadruplex RNA, and enrichment of the motif at PRC2 target genes provides a means for RNA-mediated regulation [Citation33]. RNA G-quadruplex has been implicated in the alternative splicing of pre-mRNAs with roles both in global and transcript-specific regulation of mRNA synthesis [Citation80,Citation81]. It has been demonstrated that RNA G-quadruplexes are found associated with both exon splicing enhancers and intron splicing enhancers and silencers [Citation82]. RNA G-quadruplex formation and stabilization with ligands has been shown to affect the splicing of many different mRNAs, some of which are important in certain cancers. A recent high-throughput study of G-quadruplex formation in the human genome showed a high density of G-quadruplexes located near splicing sites [Citation83]. From our perspective, it would be particularly interesting to study whether there exists a potential role of binding interaction between PRC2 and G-quadruplex in RNA splicing.
Nevertheless, the evolutionarily conserved nature of PRC2 binding preference for G-quadruplets, even with varied binding sites, hints of PRC2:RNA binding having crucial role as mutations in the RNA binding sites have been found in cancers [Citation72,Citation73]. While inhibitors targeting the histone methyltransferase activity of PRC2 has been developed for cancers with gain-of-function PRC2 or EZH2 overexpression, there has yet to be methods to stimulate PRC2 enzymatic activity. Venturing into this area would hold therapeutic value for cancers where PRC2 activity is inhibited such as in certain glioma and leukemia [Citation5]. This could be achieved by disrupting the inhibitory interaction between PRC2 and RNA [Citation84]. The G-quadruplex motif or the RNA binding sites in EZH2 and EED can open doors to new therapeutic approaches. Indeed, molecules targeting the G-quadruplexes have been considered to be a potential anticancer strategy [Citation84,Citation85] and have been shown to inhibit the PRC2:RNA binding in vitro [Citation33]. It is also hypothetically possible to relieve PRC2 of its antagonistic interaction with RNA via the use of antisense oligonucleotides that target specific transcripts that PRC2 binds to [Citation84]. RNA mimics of the G-quadruplex could also be employed to inhibit PRC2 catalytic activity. These new strategies to inhibit the RNA-binding activity of PRC2 or its target transcripts could be used to tune the ability of PRC2 to repress polycomb-target genes and may bring about a substantial advance in epigenetic therapies for relevant cancer patient population.
Authors’ contributions
J Yan J, B Dutta, Y.T. Hee reviewed the literature and wrote the manuscript. W.J. Chng reviewed the manuscript.
Acknowledgments
We would like to thank Reinhard Brunmeir for his help in proofreading the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276(5688):565–570.
- Schuettengruber B, Cavalli G. Recruitment of polycomb group complexes and their role in the dynamic regulation of cell fate choice. Development. 2009;136(21):3531–3542.
- Di Croce L, Helin K. Transcriptional regulation by Polycomb group proteins. Nat Struct Mol Biol. 2013;20(10):1147–1155.
- Helin K, Dhanak D. Chromatin proteins and modifications as drug targets. Nature. 2013;502(7472):480–488.
- Kim KH, Roberts CW. Targeting EZH2 in cancer. Nat Med. 2016;22(2):128–134.
- Juan AH, Wang S, Ko KD, et al. Roles of H3K27me2 and H3K27me3 examined during fate specification of embryonic stem cells. Cell Rep. 2016;17(5):1369–1382.
- Margueron R, Reinberg D. The polycomb complex PRC2 and its mark in life. Nature. 2011;469(7330):343–349.
- Fischle W, Wang Y, Jacobs SA, et al. Molecular basis for the discrimination of repressive methyl-lysine marks in histone H3 by polycomb and HP1 chromodomains. Genes Dev. 2003;17(15):1870–1881.
- Wang L, Brown JL, Cao R, et al. Hierarchical recruitment of polycomb group silencing complexes. Mol Cell. 2004;14(5):637–646.
- Yu M, Mazor T, Huang H, et al. Direct recruitment of polycomb repressive complex 1 to chromatin by core binding transcription factors. Mol Cell. 2012;45(3):330–343.
- Dietrich N, Lerdrup M, Landt E, et al. REST-mediated recruitment of polycomb repressor complexes in mammalian cells. PLoS Genet. 2012;8(3):e1002494.
- Schoeftner S, Sengupta AK, Kubicek S, et al. Recruitment of PRC1 function at the initiation of X inactivation independent of PRC2 and silencing. Embo J. 2006;25(13):3110–3122.
- Conway E, Jerman E, Healy E, et al. A family of vertebrate-specific polycombs encoded by the LCOR/LCORL genes balance PRC2 subtype activities. Mol Cell. 2018;70(3):408–421 e8.
- Beringer M, Pisano P, Di Carlo V, et al. EPOP functionally links Elongin and polycomb in pluripotent stem cells. Mol Cell. 2016;64(4):645–658.
- Liefke R, Karwacki-Neisius V, Shi Y. EPOP interacts with Elongin BC and USP7 to modulate the chromatin landscape. Mol Cell. 2016;64(4):659–672.
- Chan CS, Rastelli L, Pirrotta V. A polycomb response element in the Ubx gene that determines an epigenetically inherited state of repression. Embo J. 1994;13(11):2553–2564.
- Simon J, Chiang A, Bender W, et al. Elements of the Drosophila bithorax complex that mediate repression by polycomb group products. Dev Biol. 1993;158(1):131–144.
- Cuddapah S, Roh TY, Cui K, et al. A novel human polycomb binding site acts as a functional polycomb response element in Drosophila. PLoS One. 2012;7(5):e36365.
- Sing A, Pannell D, Karaiskakis A, et al. A vertebrate polycomb response element governs segmentation of the posterior hindbrain. Cell. 2009;138(5):885–897.
- Woo CJ, Kharchenko PV, Daheron L, et al. A region of the human HOXD cluster that confers polycomb-group responsiveness. Cell. 2010;140(1):99–110.
- Du J, Kirk B, Zeng J, et al. Three classes of response elements for human PRC2 and MLL1/2-Trithorax complexes. Nucleic Acids Res. 2018;46:8848–8864.
- Wilkinson FH, Park K, Atchison ML. Polycomb recruitment to DNA in vivo by the YY1 REPO domain. Proc Natl Acad Sci USA. 2006;103(51):19296–19301.
- Xi H, Yu Y, Fu Y, et al. Analysis of overrepresented motifs in human core promoters reveals dual regulatory roles of YY1. Genome Res. 2007;17(6):798–806.
- Tanay A, O’Donnell AH, Damelin M, et al. Hyperconserved CpG domains underlie polycomb-binding sites. Proc Natl Acad Sci USA. 2007;104(13):5521–5526.
- Ku M, Koche RP, Rheinbay E, et al. Genomewide analysis of PRC1 and PRC2 occupancy identifies two classes of bivalent domains. PLoS Genet. 2008;4(10):e1000242.
- Jermann P, Hoerner L, Burger L, et al. Short sequences can efficiently recruit histone H3 lysine 27 trimethylation in the absence of enhancer activity and DNA methylation. Proc Natl Acad Sci USA. 2014;111(33):E3415–21.
- Schorderet P, Lonfat N, Darbellay F, et al. A genetic approach to the recruitment of PRC2 at the HoxD locus. PLoS Genet. 2013;9(11):e1003951.
- van Heeringen SJ, Akkers RC, van Kruijsbergen I, et al. Principles of nucleation of H3K27 methylation during embryonic development. Genome Res. 2014;24(3):401–410.
- Zhao J, Sun BK, Erwin JA, et al. Polycomb proteins targeted by a short repeat RNA to the mouse X-chromosome. Science. 2008;322(5902):750–756.
- Zhao J, Ohsumi TK, Kung JT, et al. Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol Cell. 2010;40(6):939–953.
- Cifuentes-Rojas C, Hernandez AJ, Sarma K, et al. Regulatory interactions between RNA and polycomb repressive complex 2. Mol Cell. 2014;55(2):171–185.
- Kanhere A, Viiri K, Araujo CC, et al. Short RNAs are transcribed from repressed polycomb target genes and interact with polycomb repressive complex-2. Mol Cell. 2010;38(5):675–688.
- Wang X, Goodrich KJ, Gooding AR, et al. Targeting of polycomb repressive complex 2 to RNA by short repeats of consecutive guanines. Mol Cell. 2017;65(6):1056–1067 e5.
- Lyon MF. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. 1961;190:372.
- Duthie SM, Nesterova TB, Formstone EJ, et al. Xist RNA exhibits a banded localization on the inactive X chromosome and is excluded from autosomal material in cis. Hum Mol Genet. 1999;8(2):195–204.
- Mak W, Baxter J, Silva J, et al. Mitotically stable association of polycomb group proteins eed and enx1 with the inactive x chromosome in trophoblast stem cells. Curr Biol. 2002;12(12):1016–1020.
- Plath K, Fang J, Mlynarczyk-Evans SK, et al. Role of histone H3 lysine 27 methylation in X inactivation. Science. 2003;300(5616):131–135.
- Silva J, Mak W, Zvetkova I, et al. Establishment of histone h3 methylation on the inactive X chromosome requires transient recruitment of Eed-Enx1 polycomb group complexes. Dev Cell. 2003;4(4):481–495.
- Wutz A, Rasmussen TP, Jaenisch R. Chromosomal silencing and localization are mediated by different domains of Xist RNA. Nat Genet. 2002;30(2):167–174.
- Rinn JL, Kertesz M, Wang JK, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129(7):1311–1323.
- Li L, Liu B, Wapinski OL, et al. Targeted disruption of hotair leads to homeotic transformation and gene derepression. Cell Rep. 2013;5(1):3–12.
- Amândio AR, Necsulea A, Joye E, et al. Hotair is dispensible for mouse development. PLoS Genet. 2016;12(12):e1006232.
- Portoso M, Ragazzini R, Brencic Z, et al. PRC2 is dispensable for HOTAIR-mediated transcriptional repression. Embo J. 2017;36(8):981–994.
- Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43(6):904–914.
- Khalil AM, Guttman M, Huarte M, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci USA. 2009;106(28):11667–11672.
- Davidovich C, Wang X, Cifuentes-Rojas C, et al. Toward a consensus on the binding specificity and promiscuity of PRC2 for RNA. Mol Cell. 2015;57(3):552–558.
- Davidovich C, Zheng L, Goodrich KJ, et al. Promiscuous RNA binding by polycomb repressive complex 2. Nat Struct Mol Biol. 2013;20(11):1250–1257.
- Kaneko S, Son J, Shen SS, et al. PRC2 binds active promoters and contacts nascent RNAs in embryonic stem cells. Nat Struct Mol Biol. 2013;20(11):1258–1264.
- Cooper S, Dienstbier M, Hassan R, et al. Targeting polycomb to pericentric heterochromatin in embryonic stem cells reveals a role for H2AK119u1 in PRC2 recruitment. Cell Rep. 2014;7(5):1456–1470.
- Kalb R, Latwiel S, Baymaz HI, et al. Histone H2A monoubiquitination promotes histone H3 methylation in polycomb repression. Nat Struct Mol Biol. 2014;21(6):569–571.
- Blackledge NP, Farcas AM, Kondo T, et al. Variant PRC1 complex-dependent H2A ubiquitylation drives PRC2 recruitment and polycomb domain formation. Cell. 2014;157(6):1445–1459.
- Margueron R, Justin N, Ohno K, et al. Role of the polycomb protein EED in the propagation of repressive histone marks. Nature. 2009;461(7265):762–767.
- Herzog VA, Lempradl A, Trupke J, et al. A strand-specific switch in noncoding transcription switches the function of a polycomb/trithorax response element. Nat Genet. 2014;46(9):973–981.
- Beltran M, Yates CM, Skalska L, et al. The interaction of PRC2 with RNA or chromatin is mutually antagonistic. Genome Res. 2016;26(7):896–907.
- Riising EM, Comet I, Leblanc B, et al. Gene silencing triggers polycomb repressive complex 2 recruitment to CpG islands genome wide. Mol Cell. 2014;55(3):347–360.
- Kaneko S, Son J, Bonasio R, et al. Nascent RNA interaction keeps PRC2 activity poised and in check. Genes Dev. 2014;28(18):1983–1988.
- Müller J, Hart CM, Francis NJ, et al. Histone methyltransferase activity of a Drosophila polycomb group repressor complex. Cell. 2002;111(2):197–208.
- Sanulli S, Justin N, Teissandier A, et al. Jarid2 methylation via the PRC2 complex regulates H3K27me3 deposition during cell differentiation. Mol Cell. 2015;57(5):769–783.
- Wang X, Paucek RD, Gooding AR, et al. Molecular analysis of PRC2 recruitment to DNA in chromatin and its inhibition by RNA. Nat Struct Mol Biol. 2017;24(12):1028–1038.
- Ringrose L. Noncoding RNAs in polycomb and trithorax regulation: A quantitative perspective. Annu Rev Genet. 2017;51:385–411.
- Chen W-M, Huang M-D, Sun D-P, et al. Long intergenic non-coding RNA 00152 promotes tumor cell cycle progression by binding to EZH2 and repressing p15 and p21 in gastric cancer. Oncotarget. 2016;7(9):9773–9787.
- Lin P-C, Huang H-D, Chang -C-C, et al. Long noncoding RNA TUG1 is downregulated in non-small cell lung cancer and can regulate CELF1 on binding to PRC2. BMC Cancer. 2016;16(1):583.
- Su J, Zhang E, Han L, et al. Long noncoding RNA BLACAT1 indicates a poor prognosis of colorectal cancer and affects cell proliferation by epigenetically silencing of p15. Cell Death Dis. 2017;8:e2665.
- Brockdorff N. Noncoding RNA and polycomb recruitment. Rna. 2013;19(4):429–442.
- Chu C, Zhang QC, Da Rocha ST, et al. Systematic discovery of Xist RNA binding proteins. Cell. 2015;161(2):404–416.
- Long Y, Bolanos B, Gong L, et al. Conserved RNA-binding specificity of polycomb repressive complex 2 is achieved by dispersed amino acid patches in EZH2. Elife. 2017;6:e31558.
- Jiao L, Liu X. Structural basis of histone H3K27 trimethylation by an active polycomb repressive complex 2. Science. 2015;350(6258):aac4383.
- Justin N, Zhang Y, Tarricone C, et al. Structural basis of oncogenic histone H3K27M inhibition of human polycomb repressive complex 2. Nat Commun. 2016;7:11316.
- Brooun A, Gajiwala KS, Deng Y-L, et al. Polycomb repressive complex 2 structure with inhibitor reveals a mechanism of activation and drug resistance. Nat Commun. 2016;7:11384.
- Holz-Schietinger C, Reich NO. RNA modulation of the human DNA methyltransferase 3A. Nucleic Acids Res. 2012;40(17):8550–8557.
- Kasinath V, Faini M, Poepsel S, et al. Structures of human PRC2 with its cofactors AEBP2 and JARID2. Science. 2018;359(6378):940–944.
- Brecqueville M, Cervera N, Adelaide J, et al. Mutations and deletions of the SUZ12 polycomb gene in myeloproliferative neoplasms. Blood Cancer J. 2011;1(8):e33.
- Mouradov D, Sloggett C, Jorissen RN, et al. Colorectal cancer cell lines are representative models of the main molecular subtypes of primary cancer. Cancer Res. 2014;74(12):3238–3247.
- Kaneko S, Bonasio R, Saldana-Meyer R, et al. Interactions between JARID2 and noncoding RNAs regulate PRC2 recruitment to chromatin. Mol Cell. 2014;53(2):290–300.
- Wei C, Xiao R, Chen L, et al. RBFox2 binds nascent RNA to globally regulate polycomb complex 2 targeting in mammalian genomes. Mol Cell. 2016;62(6):875–889.
- Gonzalez I, Munita R, Agirre E, et al. A lncRNA regulates alternative splicing via establishment of a splicing-specific chromatin signature. Nat Struct Mol Biol. 2015;22(5):370–376.
- Fox-Walsh K, Fu XD. Chromatin: the final frontier in splicing regulation? Dev Cell. 2010;18(3):336–338.
- Luco RF, Allo M, Schor IE, et al. Epigenetics in alternative pre-mRNA splicing. Cell. 2011;144(1):16–26.
- Luco RF, Misteli T. More than a splicing code: integrating the role of RNA, chromatin and non-coding RNA in alternative splicing regulation. Curr Opin Genet Dev. 2011;21(4):366–372.
- Fay MM, Lyons SM, Ivanov P. RNA G-quadruplexes in biology: principles and molecular mechanisms. J Mol Biol. 2017;429(14):2127–2147.
- Rouleau S, Jodoin R, Garant JM, et al. RNA G-quadruplexes as key motifs of the transcriptome. Adv Biochem Eng Biotechnol. 2017.
- Soemedi R, Cygan KJ, Rhine CL, et al. The effects of structure on pre-mRNA processing and stability. Methods. 2017;125:36–44.
- Chambers VS, Marsico G, Boutell JM, et al. High-throughput sequencing of DNA G-quadruplex structures in the human genome. Nat Biotechnol. 2015;33(8):877–881.
- Wang X, Davidovich C. Targeting PRC2: RNA offers new opportunities. Oncotarget. 2017;8(64):107346–107347.
- Balasubramanian S, Hurley LH, Neidle S. Targeting G-quadruplexes in gene promoters: a novel anticancer strategy? Nat Rev Drug Discov. 2011;10(4):261–275.
