ABSTRACT
Arabidopsis thaliana aminohydrolase (AtADAL) has been shown to be involved in the metabolism of N6-methyl-AMP, a proposed intermediate during m6A-modified RNA metabolism, which can be subsequently incorporated into newly synthesized RNA by Pol II. It has been proposed that AtADAL will prevent N6-methyl-AMP reuse and catabolize it to inosine monophosphate (IMP). Here, we have solved the crystal structures of AtADAL in the apo form and in complex with GMP and IMP in the presence of Zn2+. We have identified the substrate-binding pocket of AtADAL and compared it with that for adenosine deaminase (ADA), adenine deaminase (ADE) and AMP deaminase (AMPD) from multiple species. The comparisons reveal that plant ADAL1 may have the potential ability to catalyze different alkyl-group substituted substrates.
Introduction
Adenyl-deaminase family proteins play a key role in purine metabolism and can be classified into five subfamilies, including Adenosine Deaminase (ADA), ADA-Like (ADAL), Adenosine Deaminase-related Growth Factor (ADGF), Adenine Deaminase (ADE), and AMP Deaminase (AMPD) [Citation1]. In humans, two ADA1 enzymes are coupled with CD26, a membrane glycoprotein, to form a 280-kDa protein complex required for degradation of extracellular adenosine [Citation2]. ADA2 is a homodimer that belongs to ADGF subfamily in invertebrates, which is also known as CECR1 (cat eye syndrome critical region protein 1) [Citation3,Citation4]. There are three types of AMPDs expressed in particular tissues: AMPD1 in muscle, AMPD2 in liver and AMPD3 in erythrocytes [Citation5]. AMPD and ADE also share some of the conserved ADA catalytic residues [Citation6,Citation7], but ADE was only discovered in prokaryotes and fungi, with presumably higher organisms not requiring ADE for function [Citation6]. A reported phylogenetic study discovered a novel group, ADAL, with further analysis indicating that this group is similar to canonical ADA [Citation3,Citation8–Citation10], given that it contains all the conserved catalytic residues as those observed in ADA and ADGF [Citation1]. In addition, sequence studies show that ADAL proteins clearly form a distinct cluster with ADA and ADE [Citation1].
It has been speculated that the physiological role of ADAL involves participation in the purine nucleotide salvage pathways [Citation11]. In vitro expressed human ADAL1 can remove different alkyl-groups from N6-substituted purines, as well as O6-substituted purines and 2-aminopurine nucleotides [Citation11]. Recently, Arabidopsis ADAL, also known as MAPDA, was demonstrated to act as a specific deaminase of N6-methyl-AMP, thereby converting N6-methyl-AMP to inosine monophosphate (IMP) in the cytosol [Citation12] (). Neither adenosine, nor AMP, N6-methyl-adenosine, N6-methyl-ATP and O6-methyl-guanosine are the substrates of AtADAL [Citation12].
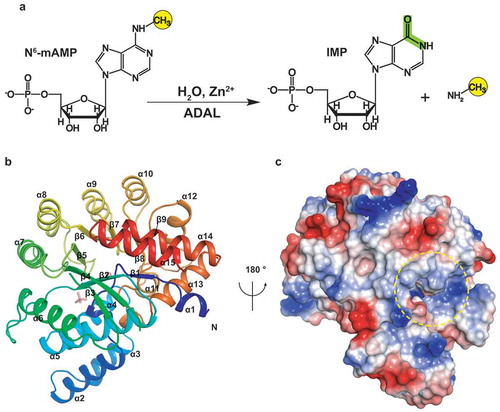
Figure 1. The overall structure of AtADAL. (A) Schematic representation of the reaction for AtADAL deaminase. (B) The overall structure of AtADAL shown in a ribbon representation colored by spectrum. (C) An electrostatic surface view of the structure in panel B rotated 180°. The pocket is indicated by yellow circle
m6A is the most abundant RNA modification amongst over 100 chemical modifications in eukaryotes, with the pattern of m6A distribution dynamically regulated by ‘reader’, ‘writer’ and ‘eraser’ proteins [Citation13,Citation14]. The m6A modification is established by the core methyltransferase complex METTL3-METTL14 [Citation15,Citation16], assisted by accessory proteins [Citation16–Citation24]. Many ‘reader’ proteins have been shown to carry out m6A-dependent functions with YTH homology domain-containing proteins, such as YT521-B, to specifically recognize m6A modifications with high binding affinities through an aromatic cage capture mechanism [Citation16,Citation25–Citation29], whereas HNRNPC, HNRNPG, and HNRNPA2/B1 were found to recognize the m6A-modified RNA through a ‘m6A-switch’ mechanism [Citation30–Citation32]. In addition, IGF2BP1 has also been experimentally validated to bind the m6A modification [Citation33]. Two demethylases, FTO and ALKBH5, have been demonstrated to take part in the removal of the m6A modification [Citation34,Citation35]. Notably, N6-methyl-AMP has been shown to be released together with other mononucleotides during RNA metabolism in the cytosol [Citation12]. Plants may require N6-methyl-AMP deaminase ADAL to catabolize N6-methyl-AMP to IMP in vivo by hydrolytically removing the aminomethyl group, because N6-methyl-AMP can be further phosphorylated to N6-mATP, which could be incorporated into newly synthesized RNA by RNA polymerase II [Citation12].
Although the structures of ADA family members ADA, ADGF, ADE and AMPD have been reported, the three-dimensional structure of ADAL has not been determined to date [Citation7,Citation36–Citation38], with no direct structural evidence available that explains and validates the preference for N6-methyl-AMP by AtADAL. Here, we have solved the crystal structure of AtADAL in the apo- and product-bound forms. Our structural studies illustrate the specificity of ADAL for binding GMP and IMP and for distinguishing different substrates through substrate binding pocket recognition and phosphate anchoring mechanisms. Furthermore, comparative analysis of different bound ligands has also revealed insights into the molecular basis guiding efforts at specific drug design based on different available structures of ADAL complexes.
Results
Overall structure of AtADAL and Zn2+ binding site
We solved the crystal structure of full-length apo-AtADAL at 1.75Å resolution (), with one molecule of AtADAL in the asymmetric unit. The crystals belong to the P212121 space group (). Despite very low sequence identities to the known structures of the adenyl-deaminase family, the three-dimensional structure of AtADAL is highly similar to the structure of ADA from mouse (PDB code: 1A4M) [Citation36] that was used to solve the apo-AtADAL structure by molecular replacement. AtADAL adopts a β/α-barrel structure, consisting of 9 parallel β-strands and 15 peripheral α-helices that surround the central β-strands (). There is a deep pocket in the AtADAL with a positive-charged surface (). ADAL belongs to the TIM-barrel structural scaffolds, that include many enzymes with diverse catalytic functions and very low sequence similarity [Citation39].
Table 1. Data collection and refinement statistics
Ion binding site
Like other adenyl-deaminase family proteins, AtADAL also contains divalent metal ions at the active site. The divalent Zn2+ cation is bound tightly by His13, His15, His217 and Asp295, together with hydrogen-bonding to a water molecule located about 1.5 Å away (). This water is also hydrogen-bonded to the side chain of His240 and Asp295, which together with the other four Zn2+-binding ligands, makes for a pentacoordinated divalent cation (). Sequence alignment of ADALs with ADAs and ADEs revealed that these four amino acids involved in Zn2+ coordination are highly conserved amongst these enzymes (). It has been established mechanistically that the Zn2+ polarizes the water molecule bounded by the side chain of His240 and Asp295, which in turn attacks the substrate to form a tetrahedral intermediate at the C6 position of the purine ring [Citation7]. Many studies have shown that upon Zn2+ removal, or mutation of any conserved amino acids involved in Zn2+ coordination, leads to the loss of the enzymatic activity, suggesting the importance of Zn2+ in the catalytic function of adenyl-deaminase family proteins [Citation40].
Figure 2. The Zn2+ ion-binding site and substrate-binding pocket. (A) The Zn2+ion binding site. The residues that participate in Zn2+ ion binding are shown in light-blue in a stick representation. The Zn2+ is shown as an orange sphere and the water molecule as a red sphere. Omit FO-FC electron density contoured level is 1.5 σ level at 1.75 Å resolution. (B) Sequence alignment of the Zn2+ ion binding site residues among plant and human ADAL, yeast ADE, and human and mouse ADA. (C) Electrostatic surface representation of the substrate pocket. The phosphate is shown in a stick representation. and the pocket is indicated by a yellow circle. (D) Interactions involved in recognition of the phosphate group. Omit FO-FC electron density contoured level is 1.5 σ level at 1.75 Å resolution
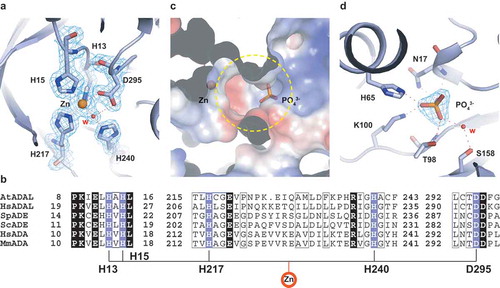
AtADAL contains a deep funnel-shaped cavity () with the zinc ion positioned at the bottom of the cavity (). Interestingly, we also observed a phosphate group bound in the vicinity of the pocket under the buffer conditions used for crystallization (, ). The bound phosphate forms hydrogen bonds with the side chain of Lys100, His65, and through a water-mediated hydrogen bond to the side chain of Ser158, as well as with the main chain and the side chain of Asn17 and Thr98 ().
Bound GMP and IMP in AtADAL substrate pocket
To further elucidate the structural basis underlying substrate binding properties and catalytic activities of ADAL proteins, we mutated key residue Asp295 to Asn29 (D295N) to prevent cleavage, and crystallized this AtADAL mutant in complex with N6-methyl-AMP. To our surprise, we observed a GMP molecule bound in the active site (, ). Given that it is very highly unlikely that N6-methyl-AMP could be converted to GMP by the catalytically dead AtADAL mutant, it appears likely that purified AtADAL expressed from E. coli contained endogenous GMP. We collected several high-resolution data sets on the AtADAL(D295N)-GMP complex (up to 1.9 Å; contains one molecule in the asymmetric unit) and confirmed the presence of bound GMP (). A structural comparison between apo-AtADAL and the AtADAL(D295N)-GMP complex exhibited an RMSD of 0.16 Å, implicating almost identical structures ().
Figure 3. Structure of GMP bound AtADAL complex. (A) Superposition of the structures between apo-AtADAL (green) and GMP bound AtADAL complex (light blue). (B) Substrate binding status for GMP. Omit FO-FC electron density contoured level is 1.5 σ at 1.9 Å resolution. The residues involved in chelating Zn2+ ion are colored in light blue while bound GMP is shown in wheat in a stick presentation. (C) Detailed interactions between AtADAL pocket residues and the base ring of GMP. The hydrogen bonds are shown as dashed black lines and water molecules are shown as red spheres. (D) Detailed intermolecular contacts for ribose and phosphate groups of GMP with AtADAL residues lining the pocket in the complex
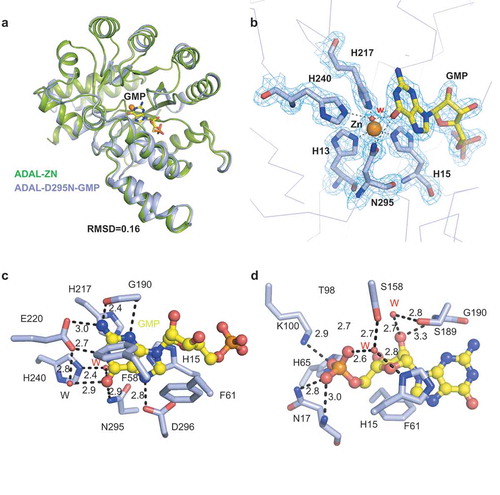
The bound GMP is anchored in its pocket and clamped in a hydrophobic environment composed of two phenylalanines (Phe58 and Phe61) on one side and two histidines (His15 and His217) on the other side (). All heteroatoms of the guanine ring are recognized through a network of hydrogen bonding interactions, including N1 (paired with side chain of Glu220), NH2 (paired with side chain of Glu220 and main chain of Gly90), O6 (paired with side chain of Asn295 and water-mediated pairing with His240 and Glu220), N7 (paired with the side chain of Asp296) and N3 (paired with the main chain of Gly190) as shown in . In addition, both the base and sugar groups of bound GMP are also anchored through a hydrogen bonding and stacking network, including 2ʹ-OH (paired with main chain of Gly190 and water-mediated pairing Ser189) and sugar ring (stacked with side chain of Phe61) as shown in . The high similarity between the structures of AtADAL and other ADAs proteins suggest that AtADAL may exhibit a similar enzymatic mechanism as ADA [Citation7,Citation41]. By analogy with mouse ADA1 enzymes, it is conceivable that His240 of AtADAL might be capable of abstracting a proton from the attacking water, whereas the side chain of Asp295 might serve as a proton donor to the N1 atom of the N6-methyl-AMP substrate, thereby promoting the nucleophilic attack at the C6 atom by reducing the N1 to C6 double bond character [Citation7].
We have also determined the structure of AtADAL(D295N) bound to IMP (), with only minor changes in the overall structure compared with that bound to GMP (Supplementary Figure 1A, 1B).
Modeling multiple substrates in the substrate pocket
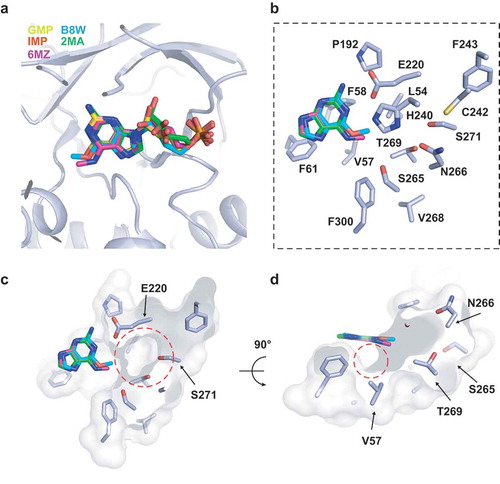
Previous studies had shown that 6-methyl-guanosine-5ʹ-monophosphate was also a good substrate of HsADAL [Citation11], thus we docked N6-methyl-AMP (6MZ), 6-methyl-guanosine-5ʹ-monophosphate (B8W) and 2-methyladenosine-5ʹ-monophosphate (2MA) into the active site based on the structure of AtADAL(D295N) bound to GMP. 2MA was used as a control (). The modeling results showed that these three substrates had almost identical locations in the pocket (), but B8W has a relative lower score amongst three based on docking simulation with Schrodinger suite (Supplementary Table 1), indicating that B8W is also a suitable substrate for AtADAL. Moreover, we identified a large pocket (lined by Val57, Phe58, Leu54 and Phe300, Glu220, Phe243, Cys242 et al.) (), which has the potential to accommodate the nucleoside base and its 6ʹ- substituted group of the substrate (). The depth of this pocket may determine the size of the 6ʹ-substituted group. There is also a small hydrophobic interspace between the substrate 6ʹ-substituted group and AtADAL Val57, which might accommodate the reaction intermediate generated during catalysis () [Citation41].
Figure 4. Molecular docking for the 6ʹ-purine derivatives on the structure of AtADAL-D295N-GMP. (A) The binding site of different 6ʹ-purine derivates by docking with Schrodinger suite. (B) Amino acids that lining the 6ʹ-purine derivatives binding pocket in AtADAL-D295N. (C) An electrostatic surface view of the depth of the binding pocket circled in red dashes. (D) An electrostatic surface view of the structure in panel C rotated by 90°. A small hydrophobic interspace, which might accommodate the reaction intermediate, is circled in red dashes
Comparison of ligand pocket in AtADAL with related pockets in HsADA1, HsADA2 and PaADE
In order to further investigate the molecular basis for the selectivity of AtADAL for N6-methylated AMP, we superimposed the structures of AtADAL with those published structures of adenyl deaminase family proteins in substrate bound states, including HsADA1 with 2ʹ-deoxyadenosine (PDB code: 3IAR), HsADA2 with coformycin (PDB code: 3LGG) and PaADE with adenine (PDB code: 3PAO) (, , ). There are three major differences during the comparisons of these three structures. Firstly, the conformational difference of Glu220 between AtADAL and other three enzymes stands out. Glu220 in AtADAL forms optimal hydrogen bonds with the N1 and N2 positions of the Watson-Crick edge of bound GMP and IMP (), whereas this Glu220 adopts a different rotamer in other structures so it can only bind to the N1 position of adenine (, , ), which indicates that 2ʹ-aminopurine substrate could be only recognized by the ADAL family.
Figure 5. Structural comparisons reveal substrate binding preference of AtADAL. (A–C) Superposition of the structures of AtADAL (in light blue) with HsADA1-2ʹ-deoxyadenosine complex (PDB code: 3IAR, in teal) (A), HsADA2-coformycin complex (PDB code: 3LGG, in green) (B), and PaADE-adenine complex (PDB code: 3PAO, in violet) (C). (D–L) Detailed comparisons of base specific substrate binding. The colors are same as in panels A-C. The differences associated with base-specific recognitions are highlighted by red circle or black arrows
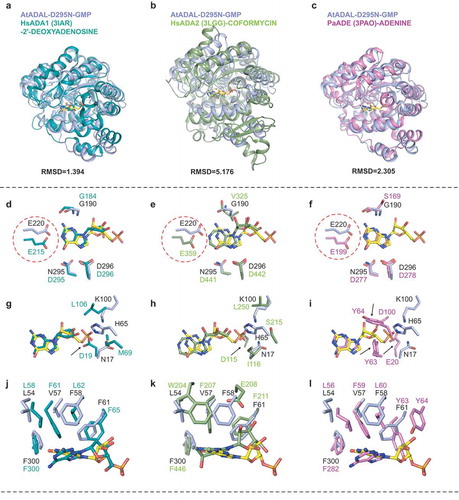
Secondly, the phosphate anion is bound by many residues that includes Asn17, His65, Thr98, Lys100 and Ser158 in the structure of apo-AtADAL (). Sequence comparison indicated that the majority of these residues are conserved between ADALs, but Asn17 in AtADAL was replaced by negatively-charged Asp in ADA1 and Glu in ADE, thereby impacting the phosphate binding. While in ADA2, Asn17 is replaced by a large hydrophobic residue (I116), which together with an adjacent negatively-charged residue (Asp115) will also clash with the phosphate group (, , , Supplementary Figure 2). Other residues that are involved in phosphate recognition including AtADAL Thr98 and Lys100, are changed to Asn and Gln in ADE and Ser and His in HsADA1 (Supplementary Figure 2). Moreover, almost no positively-charged residues can be seen around the phosphate recognition region in HsADA1 and HsADA2, although they have enough space to accommodate a phosphate group (, , , Supplementary Figure 2). Two conserved tyrosine residues (Tyr63 and Tyr64) in PaADE not only hindered the recognition of phosphate group, but also clashed with the ribose group, thereby explaining ADEs’ preference for adenine (, Supplementary Figure 2). Therefore,monophosphate substrate could only be recognized in the ADAL family instead of ADA or ADE.
Thirdly, the size of the substrate pocket has shrunk in HsADA1, HsADA2 or PaADE1, because Val57 in AtADAL is replaced by an equivalent phenylalanine in HsADA1 (Phe61), HsADA2 (Phe207) and PaADE (Phe59), respectively (, , ). By contrast, Phe58 in AtADAL is replaced by Leu62 in HsADA1, Glu208 in HsADA2 and Leu60 in PaADE, respectively (, , , Supplementary Figure 2). It has been reported that AtADAL(V57F) mutant showed a dramatic reduction in the catalytic efficiency (20.6-fold lower than wild-type) [Citation12]. We also noticed that besides Val57 and Phe58, another residue Leu54, corresponding to Leu58 in HsADA1, to Leu56 in PaADE and to Trp204 in HsADA2, with these amino acids moving closer to the base of the substrate (, , ). These comparisons suggest that Leu54, Val57 and Phe58 of AtADAL likely act together to enlarge the space for the reaction intermediate of the 6ʹ-substituted leaving group, indicating that ADAL could catalyze larger 6ʹ-substituted group substrate than other family members.
It was reported that ADGF/ADA2 proteins decrease the binding affinity for adenosine on proceeding from lower to higher species [Citation1]. This loss of affinity strongly correlates with the increasing hydrophobicity of the entrance of the binding pocket, suggesting that improved anchoring of the substrate correlates with higher enzymatic catalytic activities [Citation1]. This is in consistent with the higher catalytic activity of ADAL for alkyl-groups of 6-purine monophosphates because of its stringent substrate-binding pocket [Citation11]. In summary, according to our results, the amino acids located on α4 in AtADAL represents a pivotal region for substrate selectivity (Supplementary Figure 2).
Discussion
Previous structure-activity relationship (SAR) studies indicated that human ADAL1 enzymes hydrolyze different N6- and O6-substituted purine and 2-aminopurine nucleotides, including N6-methyl, O6-methyl, O6-ethyl, and 6-halogen substitutions [Citation42–Citation44]. Recent papers also proposed that AtADAL1 might be specifically involved in the catabolism of N6-methylated adenosine nucleotides produced by the degradation of m6A-modified nucleic acids [Citation45–Citation47]. Our structures of AtADAL(D295N)-GMP and AtADAL(D295N)-IMP complexes identified that AtADAL1 contains residues involved in the rigid binding of the phosphate moiety. Moreover, based on our structures, we speculate that the large substrate binding pocket of AtADAL may accommodate multiple alkyl-groups substituted substrates.
AMPD has a similar catalytic function to ADAL and shares half of the evolutionarily conserved sequence residues [Citation48]. The structure of the AMPD domain of embryonic factor 1 (FAC1) from Arabidopsis thaliana in complex with coformycin 5ʹ-phosphate (PDB code: 2A3L) showed that AMPD also recognizes the 5ʹ-monophosphate substrate (Supplementary Figure 3a, c) [Citation38]. However, the structural comparison of ADAL1 with FAC1 showed that two enzymes utilize different residues for anchoring the phosphate (Supplementary Figure 3c). Lys100 in AtADAL is changed to Lys466 in AtFAC1, and together with Tyr467 determines the orientation of the phosphate group in AtAMPD (Supplementary Figure 3C). Val57 in AtADAL is changed to lysine (Lys462) in AtFAC1, which contributes to the recognition of the phosphate group of coformycin 5ʹ-phosphate (Supplementary Figure 3C).
The substate-binding pocket in AtADAL provides an explanation for the substrate binding specificity of ADAL proteins. AtADAL and HsADAL share high sequence identity, especially for residues lining the substrate-binding pocket (Supplementary Figure 2). Although the catalytic domain and catalytic sites of ADAL, ADA1, ADA2 and AMPD proteins are similar, the structures of their hydrophobic binding pockets and phosphate anchor pockets are very different. During the preparation of this manuscript, a paper reported the structure of the same protein was published on Nucleic Acids Research [Citation49]. In that paper, Jia et al. described the structure of AtADAL in the apo form and in the ligand-bound form. Generally, two papers have the same conclusion regarding the underlying molecular basis of ADAL recognition and catalysis.
Materials & methods
Protein expression and purification
The plasmid containing the DNA fragments encoding the full-length AtADAL (NCBI Reference Sequence: NM_116726.4) was ordered from Shanghai Generay Biotech Co., Ltd. The target gene was cloned into a modified pET28a-SUMO vector. The plasmid encodes a His6-SUMO tag at the N-terminus following Ulp1 protease site. The plasmid was transformed into Escherichia coli strain BL21 (DE3) grown in LB medium supplemented with 50 mg/ml kanamycin. The recombinant protein expression was induced by 0.2 mM IPTG at 37°C, followed by 16–18 h incubation at 18°C. The cell pellets were resuspended in buffer containing 20 mM Tris-HCl pH 8.0, 500 mM NaCl, 25 mM imidazole pH 8.0 and lysed using the high press and further clarified by centrifugation at 17,000 rpm. Supernatants were purified with Ni-NTA resin (GE Healthcare), the target protein was washed with lysis buffer and then eluted with a buffer containing 20 mM Tris-HCl, pH 8.0, 500 mM NaCl and 500 mM imidazole pH 8.0. Ulp1 protease was added to remove the N-terminal His6-SUMO and dialyzed with lysis buffer containing 20 mM Tris pH 8.0, 500 mM NaCl. The mixture was applied to another Ni-NTA resin to remove the protease and uncleaved proteins with the buffer containing 20 mM Tris pH 8.0, 500 mM NaCl, 25 mM imidazole pH 8.0. Eluted proteins were concentrated by centrifugal ultrafiltration, loaded onto a pre-equilibrated HiLoad 16/60 Superdex 200-pg column in an Äkta-purifier (GE Healthcare), eluted at a flow rate of 1 ml/min with the buffer containing 10 mM Tris pH 8.0, 100 mM NaCl. Purified fractions were pooled together and concentrated by centrifugal ultrafiltration before use. The AtADAL(D295N) mutant was amplified by overlap-PCR from the wild-type plasmid and cloned into the same vector. The method for purifying this mutant is the same as that for the wild-type protein.
Crystallization
The wild-type AtADAL in 10 mM Tris pH 8.0, 100 mM NaCl buffer was concentrated to 10 mg/ml. AtADAL was crystallized in the solution containing 0.8 M potassium phosphate (dibasic), 0.1 M HEPES/sodium hydroxide pH 7.5, 0.8 M sodium phosphate (monobasic). The crystals were cryoprotected by adding the crystallization solution supplemented with 25% glycerol and flash frozen in liquid nitrogen. The crystals of AtADAL(D295N)-GMP complex in the same buffer as wild-type protein were grown in the solution containing 20% PEG 8,000; 0.1 M MES pH 6.0; 0.2 M calcium acetate. The crystals of AtADAL(D295N)-IMP were grown in solution containing 0.2 M ammonium acetate, 0.1 M Tris pH 8.5, 25% w/v polyethylene glycol 3,350.
X-ray data collection and refinement
The X-ray diffraction data were collected at Shanghai SSRF beamlines BL18U and BL19U1. The data sets of diffraction images were integrated and scaled using the HKL2000 suite [Citation50]. The structure was phased using the molecular replacement method using the mouse adenosine deaminase structure (1A4M) as a phasing model by PHASER in the CCP4i suite [Citation51]. The model was improved using alternate cycles of manual building in Coot [Citation52] and refinement in REFMAC5 [Citation53], and the final structure was refined with the PHENIX [Citation54]. The datasets of the complex structure of AtADAL with bound GMP and IMP were processed as mentioned with the phasing model of the structure of wild-type AtADAL.
Docking experiment
The coordinates of the small molecules were downloaded from RCSB PDB and manually put into the structure of AtADAL-D295N-GMP. The molecular docking and dynamic were carried out with the Schrodinger suite (Schrödinger, LLC) according to the standard protocol.
Accession codes
Atomic coordinates and structure factor amplitudes have been deposited with the PDB with accession codes 6IV5 for apo-AtADAL, 6J23 for AtADAL(D295N)-GMP complex and 6J4T for AtADAL(D295N)-IMP complex.
Supplemental Material
Download MS Word (1.7 MB)Acknowledgments
We thank the BL18U1 and BL19U beamlines staff at the Shanghai Synchrotron Radiation Facility for assistance during data collection. We thank Dr. Yanchao Liu (Shanghai Blueray Biopharma Co., Ltd.) for docking analysis and Dr. Dinshaw J. Patel for critical reading of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplementary material for this article can be accessed here.
Additional information
Funding
References
- Maier SA, Galellis JR, McDermid HE. Phylogenetic analysis reveals a novel protein family closely related to adenosine deaminase. J Mol Evol. 2005;61:776–794.
- Franco R, Casado V, Ciruela F, et al. Cell surface adenosine deaminase: much more than an ectoenzyme. Prog Neurobiol. 1997;52:283–294.
- Akalal DB, Schein CH, Nagle GT. Mollusk-derived growth factor and the new subfamily of adenosine deaminase-related growth factors. Curr Pharm Des. 2004;10:3893–3900.
- McDermid HE, Duncan AM, Brasch KR, et al. Characterization of the supernumerary chromosome in cat eye syndrome. Science. 1986;232:646–648.
- Gross M. Molecular biology of AMP deaminase deficiency. Pharm World Sci. 1994;16:55–61.
- Ribard C, Rochet M, Labedan B, et al. Sub-families of alpha/beta barrel enzymes: a new adenine deaminase family. J Mol Biol. 2003;334:1117–1131.
- Wilson DK, Rudolph FB, Quiocho FA. Atomic structure of adenosine deaminase complexed with a transition-state analog: understanding catalysis and immunodeficiency mutations. Science. 1991;252:1278–1284.
- Maier SA, Podemski L, Graham SW, et al. Characterization of the adenosine deaminase-related growth factor (ADGF) gene family in Drosophila. Gene. 2001;280:27–36.
- Zurovec M, Dolezal T, Gazi M, et al. Adenosine deaminase-related growth factors stimulate cell proliferation in Drosophila by depleting extracellular adenosine. Proc Natl Acad Sci U S A. 2002;99:4403–4408.
- Akalal DB, Bottenstein JE, Lee SH, et al. Aplysia mollusk-derived growth factor is a mitogen with adenosine deaminase activity and is expressed in the developing central nervous system. Brain Res Mol Brain Res. 2003;117:228–236.
- Murakami E, Bao H, Mosley RT, et al. Adenosine deaminase-like protein 1 (ADAL1): characterization and substrate specificity in the hydrolysis of N(6)- or O(6)-substituted purine or 2-aminopurine nucleoside monophosphates. J Med Chem. 2011;54:5902–5914.
- Chen M, Urs MJ, Sanchez-Gonzalez I, et al. m(6)A RNA degradation products are catabolized by an evolutionarily conserved N(6)-Methyl-AMP deaminase in plant and mammalian cells. Plant Cell. 2018;30:1511–1522.
- Wu B, Li L, Huang Y, et al. Readers, writers and erasers of N(6)-methylated adenosine modification. Curr Opin Struct Biol. 2017;47:67–76.
- Yang Y, Hsu PJ, Chen YS, et al. Dynamic transcriptomic m(6)A decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018;28:616–624.
- Liu J, Yue Y, Han D, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–95.
- Ping XL, Sun BF, Wang L, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189.
- Schwartz S, Mumbach MR, Jovanovic M, et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5ʹ sites. Cell Rep. 2014;8:284–296.
- Haussmann IU, Bodi Z, Sanchez-Moran E, et al. m(6)A potentiates Sxl alternative pre-mRNA splicing for robust Drosophila sex determination. Nature. 2016;540:301–304.
- Patil DP, Chen CK, Pickering BF, et al. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537:369–373.
- Ruzicka K, Zhang M, Campilho A, et al. Identification of factors required for m(6) A mRNA methylation in Arabidopsis reveals a role for the conserved E3 ubiquitin ligase HAKAI. New Phytol. 2017;215:157–172.
- Guo J, Tang HW, Li J, et al. Xio is a component of the Drosophila sex determination pathway and RNA N(6)-methyladenosine methyltransferase complex. Proc Natl Acad Sci U S A. 2018;115:3674–3679.
- Knuckles P, Lence T, Haussmann IU, et al. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m(6)A machinery component Wtap/Fl(2)d. Genes Dev. 2018;32:415–429.
- Wen J, Lv R, Ma H, et al. Zc3h13 regulates nuclear RNA m(6)A methylation and mouse embryonic stem cell self-renewal. Mol Cell. 2018;69(1028–1038):e1026.
- Yue Y, Liu J, Cui X, et al. VIRMA mediates preferential m(6)A mRNA methylation in 3ʹUTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 2018;4:10.
- Schwartz S, Agarwala SD, Mumbach MR, et al. High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell. 2013;155:1409–1421.
- Li F, Zhao D, Wu J, et al. Structure of the YTH domain of human YTHDF2 in complex with an m(6)A mononucleotide reveals an aromatic cage for m(6)A recognition. Cell Res. 2014;24:1490–1492.
- Luo S, Tong L. Molecular basis for the recognition of methylated adenines in RNA by the eukaryotic YTH domain. Proc Natl Acad Sci U S A. 2014;111:13834–13839.
- Theler D, Dominguez C, Blatter M, et al. Solution structure of the YTH domain in complex with N6-methyladenosine RNA: a reader of methylated RNA. Nucleic Acids Res. 2014;42:13911–13919.
- Xu C, Wang X, Liu K, et al. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat Chem Biol. 2014;10:927–929.
- Alarcon CR, Goodarzi H, Lee H, et al. HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell. 2015;162:1299–1308.
- Liu N, Dai Q, Zheng G, et al. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518:560–564.
- Liu N, Zhou KI, Parisien M, et al. N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 2017;45:6051–6063.
- Huang H, Weng H, Sun W, et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20:285–295.
- Jia G, Fu Y, Zhao X, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–887.
- Zheng G, Dahl JA, Niu Y, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29.
- Wang Z, Quiocho FA. Complexes of adenosine deaminase with two potent inhibitors: X-ray structures in four independent molecules at pH of maximum activity. Biochemistry. 1998;37:8314–8324.
- Zavialov AV, Yu X, Spillmann D, et al. Structural basis for the growth factor activity of human adenosine deaminase ADA2. J Biol Chem. 2010;285:12367–12377.
- Han BW, Bingman CA, Mahnke DK, et al. Membrane association, mechanism of action, and structure of Arabidopsis embryonic factor 1 (FAC1). J Biol Chem. 2006;281:14939–14947.
- Wierenga RK. The TIM-barrel fold: a versatile framework for efficient enzymes. FEBS Lett. 2001;492:193–198.
- Niu W, Shu Q, Chen Z, et al. The role of Zn2+ on the structure and stability of murine adenosine deaminase. J Phys Chem B. 2010;114:16156–16165.
- Wilson DK, Quiocho FA. A pre-transition-state mimic of an enzyme: X-ray structure of adenosine deaminase with bound 1-deazaadenosine and zinc-activated water. Biochemistry. 1993;32:1689–1694.
- Murakami K, Shirasaka T, Yoshioka H, et al. Escherichia coli mediated biosynthesis and in vitro anti-HIV activity of lipophilic 6-halo-2ʹ,3ʹ-dideoxypurine nucleosides. J Med Chem. 1991;34:1606–1612.
- Porter DJ, Spector T. Alternative substrates for calf intestinal adenosine deaminase. A pre-steady-state kinetic analysis. J Biol Chem. 1993;268:2480–2485.
- Pegg AE, Swann PF. Metabolism of O6-alkyldeoxyguanosines and their effect on removal of O6-methylguanine from rat liver DNA. Biochim Biophys Acta. 1979;565:241–252.
- Schinkmanova M, Votruba I, Holy A. N6-methyl-AMP aminohydrolase activates N6-substituted purine acyclic nucleoside phosphonates. Biochem Pharmacol. 2006;71:1370–1376.
- Ratel D, Ravanat JL, Berger F, et al. N6-methyladenine: the other methylated base of DNA. Bioessays. 2006;28:309–315.
- Ratel D, Ravanat JL, Charles MP, et al. Undetectable levels of N6-methyl adenine in mouse DNA: cloning and analysis of PRED28, a gene coding for a putative mammalian DNA adenine methyltransferase. FEBS Lett. 2006;580:3179–3184.
- Chang ZY, Nygaard P, Chinault AC, et al. Deduced amino acid sequence of Escherichia coli adenosine deaminase reveals evolutionarily conserved amino acid residues: implications for catalytic function. Biochemistry. 1991;30:2273–2280.
- Jia Q, Xie W. Alternative conformation induced by substrate binding for Arabidopsis thalianaN6-methyl-AMP deaminase. Nucleic Acids Res. 2019;47:3233–3243.
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326.
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, et al. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674.
- Emsley P, Lohkamp B, Scott WG, et al. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501.
- Murshudov GN, Skubak P, Lebedev AA, et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr. 2011;67:355–367.
- Adams PD, Afonine PV, Bunkoczi G, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221.
