ABSTRACT
Long non-coding RNAs (lncRNAs), initially recognized as byproducts of the transcription process, have been proven to play crucial modulatory roles in preserving overall homoeostasis of cells and tissues. Furthermore, aberrant levels of these transcripts have been shown to contribute many diseases, including cancer. Among these, many aspects of ovarian cancer biology have been found to be regulated by lncRNAs, including cancer initiation, progression and dissemination. In this review, we summarize recent studies to highlight the various roles of lncRNAs in ovary in normal and pathological conditions, immune system, diagnosis, prognosis, and therapy. We address lncRNAs that have been extensively studied in ovarian cancer and their contribution to cellular dynamics.
Introduction
Ovarian cancer is the most common lethal gynaecologic cancer [Citation1]. Risk factors for ovarian cancer include genetic factors, such as germline BRCA1 and BRCA2 mutations, ethnic background since both incidence and death rates are higher in non-hispanic white individuals, early age at menarche, late-onset menopause, total number of menstrual cycles, nulliparity or low parity, not breastfeeding, dietary habits consisting high meat, fat and alcohol consumption, inadequate physical activity, above-average height and weight, as well as use of tobacco [Citation2–Citation8].
Diagnosis at advanced stages, resistance to chemotherapy, and predisposition to metastasis in addition to low socioeconomic profile are main contributors to the low survival rate of ovarian cancer patients [Citation9,Citation10]. Current clinical treatments mainly involve surgical debulking alongside with platinum- and taxane-based chemotherapy; however, more efficient and less toxic approaches are needed [Citation11]. Consequently, researchers have begun identifying targets that can regulate oncogenes and tumour suppressor genes directly or indirectly.
The findings of Human Genome Project (HGP) suggested that majority of the genome does not encode proteins [Citation12]. The GENCODE 7 dataset revealed 20,687 protein-coding loci of which exons of the protein-coding genes to constitute 2.94% of the genome meanwhile 9640 long noncoding RNA loci were detected [Citation13]. In contrast to short non-coding RNAs that are made up of fewer than 200 nucleotides in length; lncRNAs are transcripts that possess >200 nucleotides, most of which are not translated into proteins. According to estimates from the Encyclopaedia of DNA Elements (ENCODE) project, more than 28,000 lncRNAs are encoded by the human genome and constitute the most functionally diverse class of non-coding transcripts [Citation14].
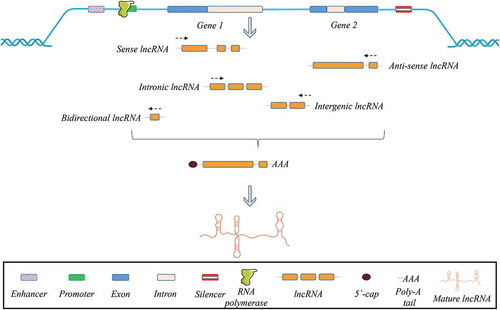
Approximately 98% of non-coding regions in the human genome are interspersed between coding regions [Citation15]. The lncRNAs transcribed from intergenic regions which do not overlap with coding genes are categorized as intergenic lncRNAs, meanwhile those transcribed completely from intronic regions of protein-coding genes are called intronic lncRNAs [Citation16,Citation17]. The lncRNAs can be encoded in the same direction as host mRNAs (sense), in the opposite direction (anti-sense), or in the opposite direction of its adjacent protein-coding gene (bidirectional) [Citation16]. Classification of the lncRNAs based on their location and origin is depicted in .
Figure 1. Classification of lncRNAs according to their direction of transcription and origin of the region.
LncRNAs have been proven to have regulatory roles in chromatin dynamics, embryogenesis, development, differentiation, gene expression, and protein stability [Citation18–Citation21]. Increasing number of studies report that lncRNAs are aberrantly expressed or mutated in an assorted lineages of cancer [Citation22]. It has been suggested that certain lncRNAs have an inhibitory or triggering effect on certain aspects of ovarian cancer, including cell viability, tumour development, immune response, metastasis, and chemoresistance [Citation23–Citation25]. Since lncRNAs have a major influence in ovarian cancer, they can be regarded as potential indicators of prognosis and diagnosis, as well as targets for treatment. In this review, we emphasize those lncRNAs that have been found to have a predominant role in malignant transformation, tumour progression, acquisition of drug resistance, and potential therapeutic value in ovarian cancer therapy.
Biogenesis
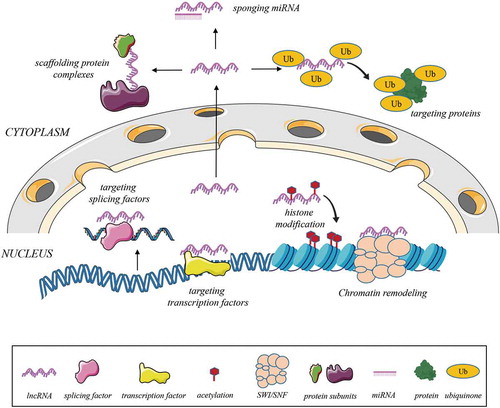
The biogenesis of lncRNAs occurs within the nucleus of the cells similar to the synthesis of protein-coding RNAs. Similar to what has been observed with mRNAs, many lncRNAs are transcribed by the enzyme RNA polymerase II and a smaller number are transcribed by RNA polymerase III. After transcription, most lncRNAs undergo a capping and polyadenylation process [Citation26–Citation28]. Almost all lncRNAs possess canonical splicing sites that lead to distinct isoforms frequently consisting of two exons [Citation29]. After biogenesis and processing, lncRNAs can be retained in the nucleus, whereas some can be transported to cytoplasm [Citation30]. Particular characteristics of lncRNAs are defined by their primary sequence motifs, yet secondary structure including loops, helices and bulges and tertiary structure formed by interaction of multiple helices, knots, loops or different structures composed by sequence-specific or backbone interactions designate their stability, biological function and compartmentalization [Citation31–Citation34].
Inside the nucleus, by modulating histone modification, lncRNAs are able to regulate the cell cycle and proliferation [Citation35]. In this context, alteration in the N-terminal region of histones by lncRNAs was demonstrated to be one of the determinants of initiation and/or progression of cancer [Citation36]. Likewise, transcription factors targeted by specific lncRNAs may lead to up- or downregulation of various genes associated with cancer including p53, SP1, and E2F1 [Citation37]. In the nucleus, lncRNAs can also operate on alternative splicing of transcribed mRNAs by mechanisms such as interaction with particular splicing factors [Citation38]. Moreover, it has been suggested that a group of lncRNAs are recognized by splicing factors, culminating in either regulation of post-translational modifications or orchestration of the relationship between splicing factors and mRNAs [Citation39,Citation40]. A subset of lncRNAs have also been discovered to partake in chromatin remodelling in an ATP-dependent manner by influencing the construction of nucleosomes through nucleosome remodelling complexes such as SWItch/Sucrose Non-Fermentable (SWI/SNF) [Citation41].
In the cytoplasm, lncRNAs may prevent the function of miRNAs by sponging or repress/stimulate the activity of various proteins via different routes [Citation42–Citation44]. An overview of the biogenesis and functions of lncRNAs is shown in .
Diversity in lncRNA function in ovary
Bioactive roles of lncRNAs in normal physiological conditions
LncRNAs show diverse expression profiles in both normal physiology and pathophysiology. In a profiling report of human tissue samples, it was proved that expression levels of number of lncRNAs differ in healthy ovary, cyst-bearing ovary and malignant epithelial ovarian cancer. Altered lncRNAs in cancerous cells were responsible of the regulation of cell cycle, division and proliferation [Citation45].
The lncRNA Nuclear Enriched Abundant Transcript (NEAT) 1 has been found to be specifically crucial in development of corpus luteum. The study conducted by Nakagawa et al. showed that fertility was significantly subsided in NEAT 1 knockout (KO) female mice when compared to wild-type or heterozygous mice. Investigation of the expression of certain genes which are essential for generation, function and regression of corpus luteum indicated that NEAT 1 is fundamental for regulation of luteal genes such as Lhcgr, Star and Akr1c18. It was suggested that NEAT 1 might upregulate transcription factors including Nr5a1 and Sp1 and thus Star to form corpus luteum. Furthermore, it was pointed out that while progesterone level ascended during early pregnancy in wild-type mice, at 3.5 days post coitum (dpc), 50% of NEAT 1 KO mice failed to demonstrate higher serum progesterone level [Citation46].
Modulation of expression of specific lncRNAs was also reported to be important for oocyte and early embryo development. Xu and his team observed that alteration in the level of various lncRNAs in human cumulus cells contribute to the processes of oocyte and embryo development. When three pairs of low and high-quality embryos obtained from patients which underwent in vitro fertilization (IVF) treatment were analysed via microarray; 124 and 509 lncRNAs were significantly upregulated and suppressed, respectively [Citation47].
A study published in 2018 focused on the correlation in between ovarian ageing and expression of long non-coding RNAs. Transcriptome profiling of ovarian samples acquired from young and middle-aged mice revealed that the expression of the lncRNA growth arrest-specific 5 (Gas5) significantly downregulated in middle-aged ovaries when compared to ovary samples from young mice. Interestingly, in the same study, they showed Gas5 was inhibited in the ovaries of C57BL/6J mice when they were fed with standard American diet suggesting that dietary habits have influence on the function of ovary [Citation48].
Roles of lncRNAs in ovarian pathophysiology
Hypoxia
Hypoxia can develop as a result of events such as inflammation, tissue ischaemia, or tumours with large size [Citation49]. In cancer, hypoxia-inducible factor (HIF)-1α and −2α proteins heterodimerize with HIF-1β and eventually bind to hypoxia-response elements (HREs) in the promoter regions of multiple genes associated with invasion, metastasis, and angiogenesis [Citation50]. According to the recent reports, many lncRNAs respond to stress factors including hypoxia which can play a major role in chemoresistance in ovarian cancer [Citation51,Citation52].
In hypoxia, lncRNAs can be either HIF-dependent or -independent. An antisense transcript of eNOS named sONE has been found to be HIF-independent, whereas other hypoxia-related lnRNAs are HIF-dependent, directly or indirectly [Citation53,Citation54]. Directly HIF-dependent lncRNAs contain HREs in their promoter regions, as confirmed in lncRNAs such as HOX transcript antisense intergenic RNA (HOTAIR) [Citation55].
In a study conducted by Wang et al., it was demonstrated that cyclin-dependent kinase inhibitor 2B-antisense 1 (CDKN2B-AS1) was significantly overexpressed in different ovarian cancer cell lines. It was suggested that this lncRNA inhibits miR-411-3p and supports tumour growth through miR-411-3p/HIF1a/VEGF/P38 pathway [Citation56]. In another article, data regarding LINK-A pointed out that the lncRNA was upregulated in ovarian cancer patients both in tumour tissue and in serum. The overexpression was linked to migration, invasion and stimulation of HIF-1 [Citation57].
Invasion, angiogenesis, and metastasis
Metastasis is defined as a cellular event that is initiated by local cell invasion from a primary tumour site followed by the formation of new blood vessels, migration to the tumour microenvironment, transport via blood and lymph to distant organs, and finally colonization and proliferation in the new location [Citation58].
In 2017, Yim et al. published a study about the oncogenic effect and the role of metastasis of homeobox protein A cluster antisense RNA 1 (HOXA-AS1) subtype 11 in ovarian cancer. They showed that HOXA-AS1 is highly expressed in 129 serous ovarian cancer tissue samples as compared with those of healthy tissue. Moreover, they proved that lncRNA led the epithelial ovarian cancer cell lines to rapid proliferation, invasion, and migration by triggering related genes such as MMP-9, E-cadherin, vimentin, snail, twist, and B-catenin [Citation59]. Regulation of PTEN through the lncRNA maternally expressed gene 3 (MEG3) was shown to inhibit cell proliferation, promote apoptosis and block cell cycle in ovarian cancer cell lines as well as in a xenograft model [Citation60]. Colon Cancer Associated Transcript 2 (CCAT2) which was first described by Ling et al. is associated with increased invasion and metastasis capability of ovarian cancer cell lines in addition to patient tumour samples [Citation61,Citation62].
lncRNA and immune modulation
Expression of genes involved in the immune system is consequential for an organism to fight against pathogens while restraining autoimmunity. Focus of the recent studies have been slithered through function of RNAs from proteins, which are well-defined components essential for an intact immune system and include, for example, transcription factors, surface receptors and cytokines also participate in gene expression [Citation63]. Indeed, certain lncRNAs have been shown to take part in diverse aspects of the immune system involving activation or suppression [Citation64,Citation65].
In a study concerning roles of Gas5 in ovarian cancer, downregulation of the lncRNA led to inhibition of inflammasome formation and pyroptosis meanwhile overexpression caused an opposite effect both in vitro and in vivo [Citation66]. Zhao and his team identified the role of LINC00092 as a contributor to proliferation and metastasis of ovarian cancer cells mediated by cancer-associated fibroblasts (CAF). Tissues obtained from serous ovarian cancer patients, SKOV3 and A2780 ovarian cancer cell lines in addition to an in vivo model exhibited a profile of which LINC00092 was stimulated following induction of CAF-secreted C-X-C motif chemokine ligand 14 (CXCL14) [Citation67].
lncRNAs in ovarian cancer
H19
H19 is one of the lncRNAs primordially described that is encoded by the H19 locus located on chr11p15.5 [Citation68]. The H19 gene is transcribed into the lncRNA by RNA polymerase II enzyme before polyadenylation, gaining 5ʹ cap and finally spliced into 2.3-kb RNA. H19 is known to be paternally imprinted and maternally expressed [Citation69]. Its expression is suppressed after the full development of the embryo and birth except for particular tissues such as in the uterus and breast [Citation70]. Overexpression of H19 was reported for a range of cancers such as ovarian cancer [Citation71,Citation72].
In ovarian cancer, H19 was demonstrated to trigger migration and invasion of tumour cells when highly expressed [Citation73]. Li et al. showed that H19 directly bound to miR-370-3p and eventually promoted TGF-β–associated epithelial mesenchymal transition (EMT) by exhibiting characteristics of competing for endogenous RNA (ceRNA) [Citation71].
An open-label, dose-escalation phase 1/2a clinical trial that included 14 subjects was established to harness H19 overexpression for targeted therapy. The subjects were chosen to have recurrent advanced stage ovarian cancer or to be primary peritoneal carcinoma patients which are resistant to platinum-based chemotherapy. After 6 weeks of monitoring, the subjects were divided into three groups, according to treatment dose: 60 mg, 120 mg, or 240 mg of intraperitoneal DTA-H19 (BC-819) which is a DNA plasmid which encodes the A fragment of Diphtheria Toxin (DTA) controlled by the promoter sequence of H19 gene promoter. H19 lncRNA was identified in the ascites fluid in 90% of the patients with use of in situ hybridization. Treatment of ectopically developed tumours with DTA-H19 resulted in 40% repression of tumour size. During the study, toxicity due to treatment dose was not encountered with any concentration administered. Therefore, it was concluded that DTA-H19 is well tolerated by patients and can be considered as a novel approach against advanced as well as drug-resistant ovarian and/or peritoneal carcinoma. In addition, overall survival (OS) rates were examined and were in accordance with previous studies, ranging from a median of 6.3 to 15.0 months. Since the best response was observed with stable ascites and tumour size, this treatment could be more beneficial for less advanced ovarian/peritoneal tumours [Citation74].
HOTAIR
HOTAIR is a 2.2-kb lncRNA that is transcribed from antisense strands of genes HOXC12 and HOXC11 localized on chromosome 12.q13.13. Correlative to H19, HOTAIR is transcribed by RNA polymerase II, and subsequent to splicing, it acquires a poly-A tail and a 5ʹ-cap [Citation75]. It was initially reported by Rinn et al. in 2007 and since then, its effects on tumour generation, invasion, metastasis, and drug resistance have been disclosed [Citation75–Citation77].
In previous studies, HOTAIR was designated as an important factor in chemoresistance. For instance, Yu et al. showed that HOTAIR knockdown led to increased sensitivity towards cisplatin and caused autophagy in ovarian cancer. In addition, the HOTAIR knockdown procedure could increase sensitivity of ovarian cancer cells against cisplatin [Citation78].
Ozes et al. described a mechanism of action of HOTAIR, in which it suppressed nuclear factor kappa-light-chain-enhancer of activated B cells (NF-ᴋB) inhibitor, NF-ᴋB in B-cell inhibitor, alpha (IK-βα) by directly binding to the chromosomal region near IK-βα in ovarian cancer cells. Afterwards, activation of NF-ᴋB triggered MMP-9 and pro-survival protein interleukin 6 (IL-6), which in return induced apoptosis inhibitors such as B-cell lymphoma 2 (Bcl-2), B-cell lymphoma-extra large (Bcl-xL), and X-linked inhibitor of apoptosis protein (XIAP) while inhibiting tumour suppressor protein p53 and promoting epithelial mesenchymal transition [Citation79].
An alternative mechanism reported by Li et al. was HOTAIR upregulation to activate canonical Wnt (Wnt/β-catenin) signalling pathway [Citation80]. It was observed that HOTAIR upregulated cyclin D1, cyclin-dependent kinase 4 (CDK4), and β-catenin. Wnt is known to lead β-catenin to migrate in the cytoplasm and following translocation into the nucleus to activate transcription factors of TCF/LEF family. Members of this family are acknowledged to participate in cell proliferation, progression of cell cycle phases, EMT, invasion, and migration [Citation80,Citation81].
MALAT-1
MALAT-1 is a lncRNA featuring >8,000 nucleotides and is encoded by the non-coding NEAT 2 gene located on chr11q13. It was first described in 2003 in early-stage non-small-cell lung carcinoma (NSCLC) patients as a marker of prognosis, since it was observed that escalation in expression of MALAT-1 was explicitly associated with poor OS rates and metastasis [Citation82]. Similar to HOTAIR, MALAT-1 also affects the Wnt/β-catenin signalling pathway; therefore, downregulation of MALAT-1 could be a useful strategy in terms of inhibiting ovarian cancer cell viability, invasion, and migration [Citation83].
MALAT-1 was showed to act as a miRNA sponge to support incipience and progression of cancer [Citation84]. MALAT-1 inhibited miR-211, which has a role in downregulating PHD finger protein 19 (PHF19) in order to impede proliferation, migration, xenograft growth, and endorsed apoptosis in ovarian cancer cells HEY-T30 and SKOV3 [Citation85]. MARCH7, an ubiquitin E3 ligase that belongs to the membrane-associated RING-CH (MARCH) family, has functions in various pathways such as those associated with the immune system, membrane trafficking, and cancer development [Citation86,Citation87]. According to Hu et al., interaction between MALAT-1 and MARCH7 via inhibition of miR-200a resulted in suppression of the TGF-β-smad2/3 pathway in SKOV3 and A2780 cells to promote metastasis [Citation88]. Finally, in a study by Shi et al., significant overexpression of miR-218 was observed after MALAT-1 knockdown in certain choriocarcinoma cell lines. Eventually, cell viability was decreased, cell cycle arrest was triggered at the G0/G1 phase, and the ratio of p27/cyclin D1 was increased [Citation89].
X inactivate-specific transcript (XIST)
XIST is a 17-kb transcript expressed in the inactive X chromosome [Citation90]. In female mammals, one of the X chromosomes is silenced in cells with the aim of providing tantamount X-linked gene ratio in between different sexes. Kawakami et al. showed that the loss of an inactive X chromosome is frequently associated with multiplication of active X chromosomes in female-derived neoplasms. Although the lncRNA is present in all female somatic cells, expression of XIST was identified as depleted in breast, ovarian, and cervical cancer cell lines [Citation91].
Wang et al. verified that overexpression of XIST decreased proliferation and invasion rate of CAOV3 and OVCAR3 ovarian cancer cell lines and tumour size in a mouse model. Lentivirus-mediated stimulation of XIST led to sponging effect on hsa-miR-214-3p which is a direct target of the lncRNA. Furthermore, the upregulation resulted in decreased cisplatin resistance in an in vivo model as showed via the shrinkage of the tumour [Citation92]. In a comprehensive study, RNA expression levels were investigated in primary and recurrent tumour samples from the same ovarian cancer patients as well as various ovarian and breast cancer cell lines. XIST was observed to be the most differentially expressed as it was suppressed significantly in the recurrent tumour samples and the low expression level of XIST was associated resistance to paclitaxel. It was concluded that since there is a high correlation in between XIST regulation and patient response to chemotherapy, XIST might be considered as a biomarker for patient response towards first-line chemotherapy [Citation93].
Plasmacytoma variant translocation 1 (PVT1) PVT1
PVT1 comprises 1716 nucleotides and is located on chr8q24.21. It was discovered in murine leukaemia virus-induced T lymphoma cells [Citation94]. It was designated as an oncogene since upregulation of the gene was witnessed in certain cancers [Citation95,Citation96]. Zou et al. reported that after the knockdown process of PVT1, sex-determining region Y-box 2 (SOX2) oncogene was also suppressed in ovarian cancer cell lines in vitro. PVT1 could enhance the invasion and proliferation of ovarian cancer cells through upregulating SOX2 [Citation95].
Several studies have pointed to the interaction between PVT1 and miRNAs. One study published in 2018 determined that the PVT1 level was elevated in ovarian tumour tissues compared with normal ovarian tissues obtained from cancer patients. PVT1 supported progression of ovarian cancer by silencing miR-214. The suggested mechanism of action was that PVT1 mediated the binding of enhancer of zeste homolog 2 (EZH2), a histone-lysine N-methyltransferase, to the promoter region of miR-214 and subsequently suppressed the miRNA. The promotion of ovarian cancer progression by PVT1 involved in regulation of the EMT and PVT1 interaction with EZH2 repressed miR-214 expression in ovarian cancer cells [Citation97]. Similarly, miR-133a was found to be suppressed in ovarian cancer, and the inhibition was correlated with clinical stages, poor prognosis, and metastasis capability [Citation98]. Yang and colleagues acknowledged that PVT1 bound to miR-133a directly in ovarian cancer cells, which suggested that the impact of PVT1 was at least in part due to its interaction with miR-133a [Citation99].
UCA1
UCA1 is another lncRNA that can be categorized as an oncogene localized in the sense strand of the chr19p13.12, with a length of 1439 bp. Initially, UCA1 was believed to be overexpressed only in bladder cancer [Citation100]. Mounting evidence suggests that UCA1 contributes to drug resistance in ovarian and other cancers [Citation101,Citation102]. Wang et al. showed that UCA1 directly repressed miR-129, whereas overexpression of miR-129 ameliorated paclitaxel (PTX) resistance in PTX-resistant SKOV3 and HeyA-8 (SKOV3/PTX and HeyA-8/PTX) cells. The reasons for this effect were in part due to miR-129 being a direct target for ATP binding cassette subfamily B member 1 (ABCB1). As UCA1 was able to sponge miR-129, ABCB1 could not be deactivated and caused continuing resistance against PTX [Citation101].
In a meta-analysis, UCA1 was evaluated for its potential as a biomarker for a number of cancer types. It was noticed that upregulation of UCA1 was associated with significantly shorter OS and progression-free survival (PFS) in patients with colorectal, ovarian, and gastric cancers as well as NSCLC [Citation102].
GAS5
GAS5 was initially identified by Schneider et al. through the process of cDNA cloning of upregulated genes in the cells demonstrating growth arrest [Citation103]. The lncRNA is poorly conserved, is approximately 0.7-kb in length, and is encoded by the intergenic GAS5 gene at the region of 1q25.1.10 [Citation104]. Earlier reports said that GAS5 could be a tumour suppressor since lower expression of the lncRNA correlated positively with greater tumour size, poor prognosis, and clinical stage in cancer types including ovarian cancer [Citation105,Citation106].
Gao and his team reported that GAS5 expression was more exiguous in epithelial ovarian carcinoma than in normal ovarian epithelium tissue and that low values of GAS5 were associated with metastasis to the lymph nodes in addition to advanced stage according to the International Federation of Gynaecology and Obstetrics (FIGO). The team also found out that after GAS5 transfection into epithelial ovarian cancer (EOC) cells, a mitochondrial apoptotic pathway was induced as the expression levels of Bax, Bcl-2-antagonist/killer (Bak), and active caspase 9/3 were significantly upregulated [Citation107]. In another report, GAS5 was determined to be significantly downregulated in EOC tissues when compared to normal ovary tissues. It was also observed in the same study that overexpression of the lncRNA-minimized cisplatin resistance both in vitro and in vivo [Citation108]. Taken together, GAS5 may therefore be considered a potent candidate as a guardian molecule preventing cancer initiation in ovary and many other tissues.
Homeobox protein D cluster antisense RNA 1 (HOXD-AS1)
HOXD-AS1 is yet another significant lncRNA that is transcribed in an antisense direction from HOXD cluster on chr2q31.2 in humans and is conserved in terms of evolution [Citation109]. Similar to other lncRNAs with oncogenic properties, HOXD-AS1 is upregulated in many tumours [Citation110,Citation111].
A noteworthy study revealed that HOXD-AS1 activated frizzled class receptor 4 (FZD4), a receptor for Wnt proteins, by directly binding to miR-608 in CAOV-3, SKOV-3, and OVCAR-3 cells as well as the samples obtained from ovarian cancer patients [Citation112]. Likewise, Zhang et al. reported that HOXD-AS1 targeted another important miRNA – miR-133a-3p – as predicted by the bioinformatics tool DIANA. HOXD-AS1 could thereby initiate the Wnt pathway cascade and prevent tumour cell death [Citation113].
In an in vitro and patient-based analysis, it was observed that elevated levels of HOXD-AS1 was negatively correlated with PFS and OS of EOC patients. Knockdown of the lncRNA resulted in reduction of migration, invasion and EMT capacity of EOC cells in vitro [Citation114].
Sprouty RTK signalling antagonist (SPRY4-IT1)
SPRY4 is an associate of SPRY family comprised of four genes (SPRY1-4), which take part in modulation of receptor tyrosine kinases (RTKs). The signalling pathways that are regulated by RTKs, namely, mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK)/Akt, are responsible for homoeostasis of development, differentiation, and cell proliferation [Citation115].
SPRY4-IT1 was initially identified in melanoma cells as originating from one intron of the protein-coding SPRY4 gene [Citation116]. It is located on the 5q31.3 chromosome site, which is 708 bp in length and the secondary structure of SPRY4_IT1 predicted to include long hairpin structures. The intronic position of the lncRNA within the SPRY4 gene suggests that SPRY4-IT1 may also participate in the MAPK signalling pathway and therefore in tumour cell reproduction, invasion, and metastasis [Citation117].
Li and his team reported overexpression of SPRY4-IT1 in cancerous tissues and cell lines of ovarian cancer, compared with expression levels in adjacent noncancerous ovarian tissues and in the healthy ovarian cell line IOSE. In addition, stimulation of SPRY4-IT1 was recognized to significantly contribute to histological grade, lymph node metastasis, FIGO stage, OS, and PFS in ovarian cancer [Citation118].
Interestingly, Yu et al. showed in both patient samples and in SKOV3 and HO8910 ovarian cancer cell lines that upregulation of SPRY4-IT1 hindered proliferation, cell cycle progression, and colony formation. Hence, researchers concluded that SPRY4-IT1 could be an anti-proliferative component and that downregulation of SPRY4-IT1 may result in progression of ovarian cancer [Citation119]. Further analyses are therefore needed to determine the mechanism of action of the lncRNA and whether it acts as a tumour suppressor or an oncogene in ovarian tissue.
MEG3
MEG3 is situated on chr14q32 in humans, and its expression is inhibited in various cancer cells as it behaves as a tumour suppressor [Citation120–Citation122]. In a previous report, Wang and colleagues suggested that both MEG3 and PTEN were inhibited in ovarian cancer and that MEG3 could stimulate PTEN upregulation. Overexpression of MEG3 promoted apoptosis and prevented invasion/migration, and in vivo experiments confirmed that MEG3 upregulation hindered tumour growth by targeting PTEN [Citation123].
Sheng and his team reported that MEG3 expression was not detected in more than 70% of EOC samples as it was inhibited in SKOV3, OVCAR3, HP8910, and ES-2 cells. It was argued that this suppression was based on hypermethylation of the promoter region of MEG3 in >60% of EOC tissue samples and in all of the ovarian cancer cells tested. In contrast, the lncRNA was shown to be expressed in many normal tissues in humans in which the promoter site did not demonstrate hypermethylation [Citation124]. Moreover, when ovarian cancer cell line OVCAR3 was treated with demethylating agent 5-aza-2-deoxycytidine, cell proliferation was inhibited significantly. These findings suggested that downregulation of MEG3 could be in part a consequence of promoter CpG hypermethylation [Citation125].
A comprehensive analysis of more than 700 ovarian cancer molecular profiles revealed that along with dynamin 3 opposite strand (DNM3OS) and myocardial infarction associated transcript (MIAT), MEG3 was also associated with EMT in ovarian cancer. Predictions pointed out that the lncRNA regulated several protein coding genes in EMT pathway. Genome-wide mapping of MEG3 binding sites showed that 73% of genes in the EMT pathway were able to bind MEG3, which is yet another indicator that MEG3 possibly regulates EMT in ovarian cancer [Citation126].
lncRNA-based diagnostics and therapeutics
lncRNAs as therapeutic targets in cancer
lncRNAs can be targeted by post-transcriptional RNA degradation, genome-editing, loss-of-function resulting from hindering the interaction of lncRNA with other components, or disturbance of the secondary structure of lncRNA. For the first approach, RNA-mediated interference (RNAi) or DNA antisense oligonucleotide (ASO) techniques can be used since they are both mRNA inhibitors. RNAi is a double-stranded structure which requires processing by several enzymes before breaking down the mRNA, while ASO is a single chain oligonucleotide that binds a Watson-Crick interaction to mRNA [Citation127]. Cheng et al. showed that siRNA targeting of lncRNA AB073614 resulted in potent obstruction of cell proliferation, invasion and activation of apoptosis in HO-8910 and OVCAR3 ovarian cancer cell lines and in vivo studies supported these data [Citation128]. On the other hand, Gordon and his team used modified ASO techniques to block the expression of MALAT-1 in anoikis-resistant ovarian cancer cell lines [Citation129].
Rupaimoole et al. reported a major impact of lncRNA ceruloplasmin (NRCP) by its being an intermediate molecule for the binding of RNA polymerase II to signal transducer and activator of transcription 1 (STAT1) and indirectly promoting glycolysis and thereby, proliferation of various ovarian cancer cell lines as well as tumour growth in an orthotopic mouse model of ovarian cancer [Citation130]. Overall, identification and adoption of different approaches to target lncRNAs may be improved and used in clinic in the future.
lncRNA-based cancer immunotherapy
An exhausted or inactive immune system plays a major part in cancer; hence, immunotherapy is one of the channels of interest for targeting cancer cells [Citation131]. Mounting evidence suggests that lncRNAs regulate the immune system in diverse ways, including mediating antigen release, immune cell activation, differentiation and/or migration, T-cell infiltration, and recognition of cancer cells [Citation132].
Guo et al. examined expression levels of lncRNAs and mRNAs in 399 ovarian cancer patients and established a co-expression network. They discovered that both RP11-284N8.3.1 and AC104699.1.1, by activating immune system response, were significantly associated with patient survival and disease stage. These two lncRNAs were co-expressed with 56 protein-coding genes, the majority of which belong to immune response elements that regulate activation and differentiation of T cells and lymphocytes [Citation133]. Hereby, enlightening the role of lncRNAs in immune system regulation in various diseases including cancer by advanced experimental and bioinformatics approaches including capture hybridization analysis of RNA targets (CHART) which analyzes genome-wide binding profile of chromatin-associated RNA molecules, chromatin isolation by RNA purification (ChiRP) and RNA antisense purification (RAP) – specific techniques to designate genomic binding regions of lncRNAs according to the affinity capture of the RNA to the chromatin by tiling antisense-oligos – may be critical in providing novel targets of therapy [Citation134–Citation136].
lncRNAs as cancer diagnostic and prognostic markers
Major genomic projects, including The Cancer Genome Atlas (TCGA), have shown that many mutations and alterations in copy number, which increase the incidence of cancer, are observed in non-coding regions of DNA. Since many lncRNAs are involved in tumour cell proliferation, invasion, and metastasis and directly behave as tumour suppressors or oncogenes, they have become a centre of attention in recent years, given their potential to be prognostic/diagnostic markers [Citation137].
In their report, Guo et al. noted that an integrated miRNA-lncRNA signature, which includes two lncRNAs and two miRNAs, could be used to sort ovarian cancer patients according to poor or good prognosis. They explained that patients with wild-type BRCA1/2 showed significant discrepancy in prognostic outcome according to a linear combination of expression levels of lncRNAs LINC01234 and CCDC144NL-AS1 as well as miRNAs miR-637 and miR-129-5p. The upregulation of the two lncRNAs and miR-129-5p were witnessed to cause poor prognosis, whereas miR-637 was associated with longer OS [Citation138].
An integrated competing endogenous RNA network analysis was implemented by using TCGA and GSE17260 with the purpose of identifying other possible RNA signatures for prognosis of recurrent ovarian cancer. Three lncRNAs (WT1-AS, NBR2, and ZNF883) were found to be highly influential on recurrent ovarian cancer. WT1-AS-hsa-miR-375-RBPMS and WT1-AS-hsa-miR-27b-TP53 were noted as important ceRNA networks in recurrence of ovarian cancer [Citation139].
A meta-analysis of the relationship between lncRNAs and ovarian cancer evaluated three databases (Cochrane Central Register of Controlled Trials, PubMed, and Embase). When 15 studies featuring 1333 ovarian cancer patients were analysed, 11 distinct lncRNAs, including HOTAIR, MALAT-1, UCA-1, and TC010441, seemed to be significantly overexpressed. In contrast, neuroblastoma associated transcript 1 (NBAT-1) was suppressed in patients, negatively affecting prognosis [Citation140].
Since the lncRNAs are specific for each kind of tumour origin, such as the lymph or blood system, circulating lncRNAs can be deceiving regarding diagnosis and/or prognosis. Quite a few lncRNAs exist in circulating tumour cells or exosomes, and being fairly preserved, they have the potential to alter the recipient microenvironment either in close range or from afar. Recently, researchers have focused on communication of exosomes secreted from malignant tumour cells and lncRNAs. The most conspicuous lncRNA biomarker is prostate cancer associated 3 (PCA3), which may be present in urine samples of patients with prostate cancer since this lncRNA is more specific than the commonly known prostate cancer biomarker prostate-specific antigen (PSA). Measurement of PCA3 level as a biomarker is the first lncRNA-based test approved by the U.S. Food and Drug Administration (FDA) [Citation141].
From the standpoint of ovarian cancer, Qui et al. provided data that suggested that MALAT-1 in EOC can be transferred via exosomes to human umbilical vein endothelial cells (HUVECs) and further stimulate angiogenesis through VEGF-A, VEGF-D, C-X-C motif chemokine 5 (CXCL5), phosphatidylinositol glycan (PIGF), IL-8 and angiogenin, and many other genes [Citation142].
Wu and colleagues isolated tumour-associated macrophages (TAMs) in the ascites of EOC patients and co-cultured them with HUVECs. They observed that the migration ability of HUVECs was significantly diminished compared with cells that were not incorporated with exosomes. It has been found that the exosomes targeted the miR-146b-5p/TRAF6/NF-κB/MMP2 pathway to reduce endothelial cell migration, and this was at least partially due to two particular lncRNAs [Citation143]. Performing liquid biopsy, which involves extraction of tumour-derived components such as circulating tumour cells, circulating tumour DNA, and exosomes from peripheral blood and subsequent evaluation by genomics and proteomics, maybe a beneficial approach to identifying such lncRNAs, which demonstrates a contingency being biomarkers in cancer patients [Citation144]. This procedure might be a potent ancillary tool for diagnosis, prognosis, and treatment since it ensures that crucial information will be obtained [Citation145]. However, certain aspects such as low levels of tumour-derived components in samples, less specificity, and difficulty in standardization and validation should be considered thoroughly [Citation146]. In this context, it is essential to support the output by sophisticated comparative computational analyses, next-generation sequencing, as well as tumour biopsy [Citation147,Citation148].
Conclusions
Ovarian cancer is the most common gynaecological malignancy in women. For ovarian cancer, platinum- and taxane-based chemotherapy remains the primary treatment option, although typically resistance against the chemotherapeutic agents or relapse occur. New therapies like Bevacizumab and PARP inhibitors are promising for some patients.
LncRNAs have now been recognized to play important roles in physiological and malignant conditions. Their altered expression has been associated with various diseases including ovarian cancer. Currently, there is no lncRNA-based therapy available which is approved by the FDA. Although there are challenges, a number of strategies may be applied such as small molecules, RNAi and ASO. A key element is the selectivity of the lncRNA targeted for therapy in a way that off-site effects are avoided.
There are several advantages of lncRNA-based approach for therapy. One of them is that targeting a single lncRNA might lead to regulation of more than one biological pathway. Likewise, by targeting a specific lncRNA, expression of certain proteins which are considered non-targetable can be manipulated. Besides, the poor specificity of small molecules can be overcome by lncRNA targeting. Disease-specific targeting is possible with this approach since most of the lncRNAs are located in the same loci with the genes relevant to incidence of diseases.
Most likely, lncRNA-based therapy should be coupled with chemotherapeutic agents as a combination therapy to alleviate toxicity and acquired drug resistance which is a major issue in ovarian cancer. In addition to gymnotic delivery (naked delivery of the nucleic acid) of the lncRNAs, delivery methods including lipid formulations might be constructed for the lncRNA based drugs to eliminate off-target effects.
In terms of diagnosis/prognosis, evaluation of lncRNA levels is highly promising, since numerous lncRNAs are substantially specific, they can preserve their stability in body fluids including whole blood, serum, urine and saliva, they can be detected easily by employing common quantification techniques such as microarray hybridization, RNA-sequencing and qRT-PCR.
In conclusion, the potential use of lncRNAs as diagnostic or predictive tools is evident however large cohorts should be studied to ascertain the value of the target. Nonetheless, the rapidly increasing understanding of lncRNA biology will lead to valuable applications to prolong PFS and OS in ovarian cancer.
Author contributions
Selin Oncul drafted, written, and reviewed this article. Paola Amero, Cristian Rodriguez-Aguayo, George A. Calin and Anil K. Sood helped review this article, and Gabriel Lopez-Berestein helped draft, write, review, and supervise the writing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–296.
- Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2(4):482–490.
- Hunn J, Rodriguez GC. Ovarian cancer: etiology, risk factors, and epidemiology. Clin Obstet Gynecol. 2012;55(1):3–23.
- Gong -T-T, Wu Q-J, Vogtmann E, et al. Age at menarche and risk of ovarian cancer: a meta-analysis of epidemiological studies. Int J Cancer. 2013;132(12):2894–2900.
- Luan -N-N, Wu Q-J, Gong -T-T, et al. Breastfeeding and ovarian cancer risk: a meta-analysis of epidemiologic studies. Am J Clin Nutr. 2013;98(4):1020–1031.
- Kolahdooz F, Ibiebele TI, van der Pols JC, et al. Dietary patterns and ovarian cancer risk. Am J Clin Nutr. 2008;89(1):297–304.
- Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and body size: individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. PLoS Med. 2012;9(4):e1001200.
- Faber MT, Kjær SK, Dehlendorff C, et al. Cigarette smoking and risk of ovarian cancer: a pooled analysis of 21 case-control studies. Cancer Causes Control. 2013;24(5):989–1004.
- Klymenko Y, Nephew KP. Epigenetic crosstalk between the tumor microenvironment and ovarian cancer cells: a therapeutic road less traveled. Cancers (Basel). 2018;10(9):295.
- Praestegaard C, Kjaer SK, Nielsen TS, et al. The association between socioeconomic status and tumour stage at diagnosis of ovarian cancer: a pooled analysis of 18 case-control studies. Cancer Epidemiol. 2016;41:71–79.
- Darcy KM, Birrer MJ. Translational research in the gynecologic oncology group: evaluation of ovarian cancer markers, profiles, and novel therapies. Gynecol Oncol. 2010;117(3):429–439.
- Lander ES. Initial impact of the sequencing of the human genome. Nature. 2011;470:187.
- Consortium EP. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74.
- Marchese FP, Raimondi I, Huarte M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017;18(1):206.
- Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921.
- Ransohoff JD, Wei Y, Khavari PA. The functions and unique features of long intergenic non-coding RNA. Nat Rev Mol Cell Biol. 2018;19(3):143–157.
- Ma L, Bajic VB, Zhang Z. On the classification of long non-coding RNAs. RNA Biol. 2013;10(6):925–933.
- Liu X, Lu Y, Zhu J, et al. A long noncoding RNA, Antisense IL-7, promotes inflammatory gene transcription through facilitating histone acetylation and switch/sucrose nonfermentable chromatin remodeling. J Iimmunol. 2019;203(6):1548–59. (Baltimore, Md: 1950).
- Ritter N, Ali T, Kopitchinski N, et al. The lncRNA Locus handsdown regulates cardiac gene programs and is essential for early mouse development. Dev Cell. 2019;50(5):644–57.e8.
- Li P, He J, Yang Z, et al. ZNNT1 long noncoding RNA induces autophagy to inhibit tumorigenesis of uveal melanoma by regulating key autophagy gene expression. Autophagy. 2019:1–14.
- Cairns J, Ingle JN, Kalari KR, et al. The lncRNA MIR2052HG regulates ERalpha levels and aromatase inhibitor resistance through LMTK3 by recruiting EGR1. BCR. 2019;21(1):47.
- Gao Y, Li X, Zhi H, et al. Comprehensive characterization of somatic mutations impacting lncRNA expression for pan-cancer. Mol Ther Nucleic Acids. 2019;18:66–79.
- Yan H, Li H, Silva MA, et al. LncRNA FLVCR1-AS1 mediates miR-513/YAP1 signaling to promote cell progression, migration, invasion and EMT process in ovarian cancer. J Exp Clin Cancer Res. 2019;38(1):356.
- Guo Q, Cheng Y, Liang T, et al. Comprehensive analysis of lncRNA-mRNA co-expression patterns identifies immune-associated lncRNA biomarkers in ovarian cancer malignant progression. Sci Rep. 2015;5:17683.
- Li Z, Niu H, Qin Q, et al. lncRNA UCA1 mediates resistance to cisplatin by regulating the miR-143/FOSL2-signaling pathway in ovarian cancer. Mol Ther Nucleic Acids. 2019;17:92–101.
- Du Mee DJM, Ivanov M, Parker JP, et al. Efficient termination of nuclear lncRNA transcription promotes mitochondrial genome maintenance. eLife. 2018;7:e31989.
- Martignetti JA, Brosius J. BC200 RNA: a neural RNA polymerase III product encoded by a monomeric Alu element. Proc Natl Acad Sci U S A. 1993;90(24):11563–11567.
- Guttman M, Amit I, Garber M, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223.
- Deveson IW, Brunck ME, Blackburn J, et al. Universal alternative splicing of noncoding exons. Cell Syst. 2018;6(2):245–55.e5.
- Shukla CJ, McCorkindale AL, Gerhardinger C, et al. High-throughput identification of RNA nuclear enrichment sequences. Embo J. 2018;37(6):e98452.
- Gudenas BL, Wang L. Prediction of lncRNA subcellular localization with deep learning from sequence features. Sci Rep. 2018;8(1):16385.
- Novikova IV, Hennelly SP, Sanbonmatsu KY. Structural architecture of the human long non-coding RNA, steroid receptor RNA activator. Nucleic Acids Res. 2012;40(11):5034–5051.
- Wilusz JE, JnBaptiste CK, Lu LY, et al. A triple helix stabilizes the 3ʹ ends of long noncoding RNAs that lack poly(A) tails. Genes Dev. 2012;26(21):2392–2407.
- Fabbri M, Girnita L, Varani G, et al. Decrypting noncoding RNA interactions, structures, and functional networks. Genome Res. 2019;29(9):1377–1388.
- Liu Z, Chen Z, Fan R, et al. Over-expressed long noncoding RNA HOXA11-AS promotes cell cycle progression and metastasis in gastric cancer. Mol Cancer. 2017;16(1):82.
- Sun M, Nie F, Wang Y, et al. LncRNA HOXA11-AS promotes proliferation and invasion of gastric cancer by scaffolding the chromatin modification factors PRC2, LSD1, and DNMT1. Cancer Res. 2016;76(21):6299.
- Li X, Chen N, Zhou L, et al. Genome-wide target interactome profiling reveals a novel EEF1A1 epigenetic pathway for oncogenic lncRNA MALAT1 in breast cancer. Am J Cancer Res. 2019;9(4):714–729.
- Hutchinson JN, Ensminger AW, Clemson CM, et al. A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genomics. 2007;8:39.
- Krchňáková Z, Thakur PK, Krausová M, et al. Splicing of long non-coding RNAs primarily depends on polypyrimidine tract and 5′ splice-site sequences due to weak interactions with SR proteins. Nucleic Acids Res. 2018;47(2):911–928.
- Raveendra BL, Swarnkar S, Avchalumov Y, et al. Long noncoding RNA GM12371 acts as a transcriptional regulator of synapse function. Proc Nat Acad Sci. 2018;115(43):E10197.
- Zhu Y, Rowley MJ, Böhmdorfer G, et al. A SWI/SNF chromatin-remodeling complex acts in noncoding RNA-mediated transcriptional silencing. Mol Cell. 2013;49(2):298–309.
- Wu XS, Wang F, Li HF, et al. LncRNA-PAGBC acts as a microRNA sponge and promotes gallbladder tumorigenesis. EMBO Rep. 2017;18(10):1837–1853.
- Chen J, Liu L, Wei G, et al. The long noncoding RNA ASNR regulates degradation of Bcl-2 mRNA through its interaction with AUF1. Sci Rep. 2016;6:32189.
- Ribeiro DM, Zanzoni A, Cipriano A, et al. Protein complex scaffolding predicted as a prevalent function of long non-coding RNAs. Nucleic Acids Res. 2018;46(2):917–928.
- Wang H, Fu Z, Dai C, et al. LncRNAs expression profiling in normal ovary, benign ovarian cyst and malignant epithelial ovarian cancer. Sci Rep. 2016;6:38983.
- Nakagawa S, Shimada M, Yanaka K, et al. The lncRNA Neat1 is required for corpus luteum formation and the establishment of pregnancy in a subpopulation of mice. Development. 2014;141(23):4618–4627.
- Xu X-F, Li J, Cao Y-X, et al. Differential expression of long noncoding RNAs in human cumulus cells related to embryo developmental potential: a microarray analysis. Reprod Sci. 2015;22(6):672–678.
- Cuomo D, Porreca I, Ceccarelli M, et al. Transcriptional landscape of mouse-aged ovaries reveals a unique set of non-coding RNAs associated with physiological and environmental ovarian dysfunctions. Cell Death Discov. 2018;4(1):112.
- Li L, Ren F, Qi C, et al. Intermittent hypoxia promotes melanoma lung metastasis via oxidative stress and inflammation responses in a mouse model of obstructive sleep apnea. Respir Res. 2018;19(1):28.
- Giatromanolaki A, Koukourakis MI, Sivridis E, et al. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001;85(6):881–890.
- Selvendiran K, Bratasz A, Kuppusamy ML, et al. Hypoxia induces chemoresistance in ovarian cancer cells by activation of signal transducer and activator of transcription 3. Int J Cancer. 2009;125(9):2198–2204.
- Ferdin J, Nishida N, Wu X, et al. HINCUTs in cancer: hypoxia-induced noncoding ultraconserved transcripts. Cell Death Differ. 2013;20(12):1675–1687.
- Fish JE, Matouk CC, Yeboah E, et al. Hypoxia-inducible expression of a natural cis-antisense transcript inhibits endothelial nitric-oxide synthase. J Biol Chem. 2007;282(21):15652–15666.
- Dong J, Xu J, Wang X, et al. Influence of the interaction between long noncoding RNAs and hypoxia on tumorigenesis. Tumour Biol. 2016;37(2):1379–1385.
- Zhou C, Ye L, Jiang C, et al. Long noncoding RNA HOTAIR, a hypoxia-inducible factor-1alpha activated driver of malignancy, enhances hypoxic cancer cell proliferation, migration, and invasion in non-small cell lung cancer. Tumour Biol. 2015;36(12):9179–9188.
- Wang Y, Huang Y, Liu H, et al. Long noncoding RNA CDKN2B-AS1 interacts with miR-411–3p to regulate ovarian cancer in vitro and in vivo through HIF-1a/VEGF/P38 pathway. Biochem Biophys Res Commun. 2019;514(1):44–50.
- Zhang H, Yao B, Tang S, et al. LINK-A long non-coding RNA (lncRNA) participates in metastasis of ovarian carcinoma and upregulates hypoxia-inducible factor 1 (HIF1α). Med Sci Monit. 2019;25:2221–2227.
- Kim A, Im M, Yim N-H, et al. Reduction of metastatic and angiogenic potency of malignant cancer by Eupatorium fortunei via suppression of MMP-9 activity and VEGF production. Sci Rep. 2014;4:6994.
- Yim GW, Kim HJ, Kim LK, et al. Long Non-coding RNA HOXA11 antisense promotes cell proliferation and invasion and predicts patient prognosis in serous ovarian cancer. Cancer Res Treat. 2017;49(3):656–668.
- Wang J, Xu W, He Y, et al. LncRNA MEG3 impacts proliferation, invasion, and migration of ovarian cancer cells through regulating PTEN. Inflammation Res. 2018;67(11):927–936.
- Ling H, Spizzo R, Atlasi Y, et al. CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic progression and chromosomal instability in colon cancer. Genome Res. 2013;23(9):1446–1461.
- Huang S, Qing C, Huang Z, et al. The long non-coding RNA CCAT2 is up-regulated in ovarian cancer and associated with poor prognosis. Diagn Pathol. 2016;11(1):49.
- Chen YG, Satpathy AT, Chang HY. Gene regulation in the immune system by long noncoding RNAs. Nat Immunol. 2017;18(9):962–972.
- Wang X, Hu Y, Cui J, et al. Coordinated targeting of MMP-2/MMP-9 by miR-296-3p/FOXCUT exerts tumor-suppressing effects in choroidal malignant melanoma. Mol Cell Biochem. 2018;445(1–2):25–33.
- Jiang M, Zhang S, Yang Z, et al. Self-recognition of an inducible host lncRNA by RIG-I feedback restricts innate immune response. Cell. 2018;173(4):906–19.e13.
- Li J, Yang C, Li Y, et al. LncRNA GAS5 suppresses ovarian cancer by inducing inflammasome formation. Biosci Rep. 2018;38(2):BSR20171150.
- Zhao L, Ji G, Le X, et al. Long Noncoding RNA LINC00092 acts in cancer-associated fibroblasts to drive glycolysis and progression of ovarian cancer. Cancer Res. 2017;77(6):1369–1382.
- Court F, Baniol M, Hagege H, et al. Long-range chromatin interactions at the mouse Igf2/H19 locus reveal a novel paternally expressed long non-coding RNA. Nucleic Acids Res. 2011;39(14):5893–5906.
- Thorvaldsen JL, Duran KL, Bartolomei MS. Deletion of the H19 differentially methylated domain results in loss of imprinted expression of H19 and Igf2. Genes Dev. 1998;12(23):3693–3702.
- Adriaenssens E, Lottin S, Dugimont T, et al. Steroid hormones modulate H19 gene expression in both mammary gland and uterus. Oncogene. 1999;18(31):4460–4473.
- Li J, Huang Y, Deng X, et al. Long noncoding RNA H19 promotes transforming growth factor-beta-induced epithelial-mesenchymal transition by acting as a competing endogenous RNA of miR-370-3p in ovarian cancer cells. Onco Targets Ther. 2018;11:427–440.
- Ohtsuka M, Ling H, Ivan C, et al. H19 Noncoding RNA, an independent prognostic factor, regulates essential Rb-E2F and CDK8-beta-catenin signaling in colorectal cancer. EBioMedicine. 2016;13:113–124.
- Zhu Z, Song L, He J, et al. Ectopic expressed long non-coding RNA H19 contributes to malignant cell behavior of ovarian cancer. Int J Clin Exp Pathol. 2015;8(9):10082–10091.
- Lavie O, Edelman D, Levy T, et al. A phase 1/2a, dose-escalation, safety, pharmacokinetic, and preliminary efficacy study of intraperitoneal administration of BC-819 (H19-DTA) in subjects with recurrent ovarian/peritoneal cancer. Arch Gynecol Obstet. 2017;295(3):751–761.
- Rinn JL, Kertesz M, Wang JK, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129(7):1311–1323.
- Liu XH, Sun M, Nie FQ, et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13:92.
- Li P, Zhang X, Wang L, et al. lncRNA HOTAIR contributes to 5FU resistance through suppressing miR-218 and activating NF-kappaB/TS signaling in colorectal cancer. Mol Ther Nucleic Acids. 2017;8:356–369.
- Yu Y, Zhang X, Tian H, et al. Knockdown of long non-coding RNA HOTAIR increases cisplatin sensitivity in ovarian cancer by inhibiting cisplatin-induced autophagy. J Buon. 2018;23(5):1396–1401.
- Ozes AR, Miller DF, Ozes ON, et al. NF-kappaB-HOTAIR axis links DNA damage response, chemoresistance and cellular senescence in ovarian cancer. Oncogene. 2016;35(41):5350–5361.
- Li J, Yang S, Su N, et al. Overexpression of long non-coding RNA HOTAIR leads to chemoresistance by activating the Wnt/beta-catenin pathway in human ovarian cancer. Tumour Biol. 2016;37(2):2057–2065.
- Santiago L, Daniels G, Wang D, et al. Wnt signaling pathway protein LEF1 in cancer, as a biomarker for prognosis and a target for treatment. Am J Cancer Res. 2017;7(6):1389–1406.
- Ji P, Diederichs S, Wang W, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22(39):8031–8041.
- Guo C, Wang X, Chen LP, et al. Long non-coding RNA MALAT1 regulates ovarian cancer cell proliferation, migration and apoptosis through Wnt/beta-catenin signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22(12):3703–3712.
- Zhuang M, Zhao S, Jiang Z, et al. MALAT1 sponges miR-106b-5p to promote the invasion and metastasis of colorectal cancer via SLAIN2 enhanced microtubules mobility. EBioMedicine. 2019;41:286–298.
- Tao F, Tian X, Ruan S, et al. miR-211 sponges lncRNA MALAT1 to suppress tumor growth and progression through inhibiting PHF19 in ovarian carcinoma. Faseb J. 2018;32(11);fj201800495RR.
- Zhao K, Yang Y, Zhang G, et al. Regulation of the Mdm2-p53 pathway by the ubiquitin E3 ligase MARCH7. EMBO Rep. 2018;19(2):305–319.
- Gao W, Thompson L, Zhou Q, et al. Treg versus Th17 lymphocyte lineages are cross-regulated by LIF versus IL-6. Cell Cycle (Georgetown, Tex). 2009;8(9):1444–1450.
- Hu J, Zhang L, Mei Z, et al. Interaction of E3 Ubiquitin Ligase MARCH7 with long noncoding RNA MALAT1 and autophagy-related protein ATG7 promotes autophagy and invasion in ovarian cancer. Cell Physiol Biochem. 2018;47(2):654–666.
- Shi D, Zhang Y, Lu R, et al. The long non-coding RNA MALAT1 interacted with miR-218 modulates choriocarcinoma growth by targeting Fbxw8. Biomed Pharmacothe. 2018;97:543–550.
- Brown CJ, Ballabio A, Rupert JL, et al. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991;349(6304):38–44.
- Kawakami T, Zhang C, Taniguchi T, et al. Characterization of loss-of-inactive X in Klinefelter syndrome and female-derived cancer cells. Oncogene. 2004;23(36):6163–6169.
- Wang C, Qi S, Xie C, et al. Upregulation of long non-coding RNA XIST has anticancer effects on epithelial ovarian cancer cells through inverse downregulation of hsa-miR-214-3p. J Gynecol Oncol. 2018;29(6):e99.
- Huang KC, Rao PH, Lau CC, et al. Relationship of XIST expression and responses of ovarian cancer to chemotherapy. Mol Cancer Ther. 2002;1(10):769–776.
- Zeidler R, Joos S, Delecluse HJ, et al. Breakpoints of Burkitt’s lymphoma t(8;22) translocations map within a distance of 300 kb downstream of MYC. Genes Chromosomes Cancer. 1994;9(4):282–287.
- Zou MF, Ling J, Wu QY, et al. Long non-coding RNA PVT1 functions as an oncogene in ovarian cancer via upregulating SOX2. Eur Rev Med Pharmacol Sci. 2018;22(21):7183–7188.
- Shen SN, Li K, Liu Y, et al. Down-regulation of long noncoding RNA PVT1 inhibits esophageal carcinoma cell migration and invasion and promotes cell apoptosis via microRNA-145-mediated inhibition of FSCN1. Mol Oncol. 2019;13(12):2554–73.
- Chen Y, Du H, Bao L, et al. LncRNA PVT1 promotes ovarian cancer progression by silencing miR-214. Cancer Biol Med. 2018;15(3):238–250.
- Luo J, Zhou J, Cheng Q, et al. Role of microRNA-133a in epithelial ovarian cancer pathogenesis and progression. Oncol Lett. 2014;7(4):1043–1048.
- Yang Q, Yu Y, Sun Z, et al. Long non-coding RNA PVT1 promotes cell proliferation and invasion through regulating miR-133a in ovarian cancer. Biomed Pharmacothe. 2018;106:61–67.
- Wang XS, Zhang Z, Wang HC, et al. Rapid identification of UCA1 as a very sensitive and specific unique marker for human bladder carcinoma. Clin Cancer Res off J Am Assoc Cancer Res. 2006;12(16):4851–4858.
- Wang J, Ye C, Liu J, et al. UCA1 confers paclitaxel resistance to ovarian cancer through miR-129/ABCB1 axis. Biochem Biophys Res Commun. 2018;501(4):1034–1040.
- Hong HH, Hou LK, Pan X, et al. Long non-coding RNA UCA1 is a predictive biomarker of cancer. Oncotarget. 2016;7(28):44442–44447.
- Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54(6):787–793.
- Avgeris M, Tsilimantou A, Levis PK, et al. Loss of GAS5 tumour suppressor lncRNA: an independent molecular cancer biomarker for short-term relapse and progression in bladder cancer patients. Br J Cancer. 2018;119(12):1477–1486.
- Zhao H, Yu H, Zheng J, et al. Lowly-expressed lncRNA GAS5 facilitates progression of ovarian cancer through targeting miR-196-5p and thereby regulating HOXA5. Gynecol Oncol. 2018;151(2):345–355.
- Wang X, Zhang J, Wang Y. Long noncoding RNA GAS5-AS1 suppresses growth and metastasis of cervical cancer by increasing GAS5 stability. Am J Transl Res. 2019;11(8):4909–4921.
- Gao J, Liu M, Zou Y, et al. Long non-coding RNA growth arrest-specific transcript 5 is involved in ovarian cancer cell apoptosis through the mitochondria-mediated apoptosis pathway. Oncol Rep. 2015;34(6):3212–3221.
- Long X, Song K, Hu H, et al. Long non-coding RNA GAS5 inhibits DDP-resistance and tumor progression of epithelial ovarian cancer via GAS5-E2F4-PARP1-MAPK axis. J Exp Clin Cancer Res. 2019;38(1):345.
- Yarmishyn AA, Batagov AO, Tan JZ, et al. HOXD-AS1 is a novel lncRNA encoded in HOXD cluster and a marker of neuroblastoma progression revealed via integrative analysis of noncoding transcriptome. BMC Genomics. 2014;15(Suppl 9):S7.
- Wang H, Huo X, Yang XR, et al. STAT3-mediated upregulation of lncRNA HOXD-AS1 as a ceRNA facilitates liver cancer metastasis by regulating SOX4. Mol Cancer. 2017;16(1):136.
- Xia H, Jing H, Li Y, et al. Long noncoding RNA HOXD-AS1 promotes non-small cell lung cancer migration and invasion through regulating miR-133b/MMP9 axis. Biomed Pharmacothe. 2018;106:156–162.
- Wang Y, Zhang W, Wang Y, et al. HOXD-AS1 promotes cell proliferation, migration and invasion through miR-608/FZD4 axis in ovarian cancer. Am J Cancer Res. 2018;8(1):170–182.
- Zhang Y, Dun Y, Zhou S, et al. LncRNA HOXD-AS1 promotes epithelial ovarian cancer cells proliferation and invasion by targeting miR-133a-3p and activating Wnt/beta-catenin signaling pathway. Biomed Pharmacothe. 2017;96:1216–1221.
- Dong S, Wang R, Wang H, et al. HOXD-AS1 promotes the epithelial to mesenchymal transition of ovarian cancer cells by regulating miR-186-5p and PIK3R3. J Exp Clin Cancer Res. 2019;38(1):110.
- Liu F, Yang X, Geng M, et al. Targeting ERK, an Achilles’ Heel of the MAPK pathway, in cancer therapy. Acta Pharm Sin B. 2018;8(4):552–562.
- Khaitan D, Dinger ME, Mazar J, et al. The melanoma-upregulated long noncoding RNA SPRY4-IT1 modulates apoptosis and invasion. Cancer Res. 2011;71(11):3852–3862.
- Das MK, Furu K, Evensen HF, et al. Knockdown of SPRY4 and SPRY4-IT1 inhibits cell growth and phosphorylation of Akt in human testicular germ cell tumours. Sci Rep. 2018;8(1):2462.
- Li H, Liu C, Lu Z, et al. Upregulation of the long non-coding RNA SPRY4-IT1 indicates a poor prognosis and promotes tumorigenesis in ovarian cancer. Biomed Pharmacother. 2017;88:529–534.
- Xie M, Nie F-Q, Sun M, et al. Decreased long noncoding RNA SPRY4-IT1 contributing to gastric cancer cell metastasis partly via affecting epithelial-mesenchymal transition. J Transl Med. 2015;13:250.
- Miyoshi N, Wagatsuma H, Wakana S, et al. Identification of an imprinted gene, Meg3/Gtl2 and its human homologue MEG3, first mapped on mouse distal chromosome 12 and human chromosome 14q. Genes Cells. 2000;5(3):211–220.
- Wei GH, Wang X. lncRNA MEG3 inhibit proliferation and metastasis of gastric cancer via p53 signaling pathway. Eur Rev Med Pharmacol Sci. 2017;21(17):3850–3856.
- Zhang X, Wu N, Wang J, et al. LncRNA MEG3 inhibits cell proliferation and induces apoptosis in laryngeal cancer via miR-23a/APAF-1 axis. J Cell Mol Med. 2019;23(10):6708–19.
- Wang J, Xu W, He Y, et al. LncRNA MEG3 impacts proliferation, invasion, and migration of ovarian cancer cells through regulating PTEN. Inflammation Res. 2018;67(11–12):927–936.
- Sheng X, Li J, Yang L, et al. Promoter hypermethylation influences the suppressive role of maternally expressed 3, a long non-coding RNA, in the development of epithelial ovarian cancer. Oncol Rep. 2014;32(1):277–285.
- Li J, Zhou D, Wang Z, et al. [Reversal effect of 5-aza-2-deoxycytidine on the maternally expressed gene 3 promoter hypermethylation and its inhibitory effect on the proliferation of epithelial ovarian cancer cells]. Zhonghua Zhong Liu Za Zhi. 2015;37(5):324–329.
- Mitra R, Chen X, Greenawalt EJ, et al. Decoding critical long non-coding RNA in ovarian cancer epithelial-to-mesenchymal transition. Nat Commun. 2017;8(1):1604.
- Wang J-H, Hendry BM, Sharpe CC. Silencing genes in the kidney: antisense or RNA interference? Nephrol Dial Transplant. 2008;23(7):2115–2118.
- Cheng Z, Guo J, Chen L, et al. A long noncoding RNA AB073614 promotes tumorigenesis and predicts poor prognosis in ovarian cancer. Oncotarget. 2015;6(28):25381–25389.
- Gordon MA, Babbs B, Cochrane DR, et al. The long non-coding RNA MALAT1 promotes ovarian cancer progression by regulating RBFOX2-mediated alternative splicing. Mol Carcinog. 2019;58(2):196–205.
- Rupaimoole R, Lee J, Haemmerle M, et al. Long noncoding RNA ceruloplasmin promotes cancer growth by altering glycolysis. Cell Rep. 2015;13(11):2395–2402.
- Gubin MM, Zhang X, Schuster H, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577.
- Ranzani V, Arrigoni A, Rossetti G, et al. Next-generation sequencing analysis of long noncoding RNAs in CD4+ T cell differentiation. Methods Mol Biol. 2017;1514:173–185.
- Guo Q, Cheng Y, Liang T, et al. Comprehensive analysis of lncRNA-mRNA co-expression patterns identifies immune-associated lncRNA biomarkers in ovarian cancer malignant progression. Sci Rep. 2015;5:17683.
- Simon MD. Capture hybridization analysis of RNA targets (CHART). In: Karen A, Roger B, Philip C, David DM, Erik S, and Koen V, Editors. Current protocols in molecular biology. John Wiley & Sons, Inc; 2013;101(1):21.25.1–25.16.
- Kuo YP, Ma CP, Chen HW, et al. A novel antisense RNA ASPACT confers multi-level suppression of PACT and associated signalling. RNA Biol. 2019;16(9):1263–1274.
- Atianand MK, Hu W, Satpathy AT, et al. A long noncoding RNA lincRNA-EPS acts as a transcriptional brake to restrain inflammation. Cell. 2016;165(7):1672–1685.
- Khurana E, Fu Y, Colonna V, et al. Integrative annotation of variants from 1092 humans: application to cancer genomics. Science. 2013;342(6154):1235587.
- Guo L, Peng Y, Meng Y, et al. Expression profiles analysis reveals an integrated miRNA-lncRNA signature to predict survival in ovarian cancer patients with wild-type BRCA1/2. Oncotarget. 2017;8(40):68483–68492.
- Wang X, Han L, Zhou L, et al. Prediction of candidate RNA signatures for recurrent ovarian cancer prognosis by the construction of an integrated competing endogenous RNA network. Oncol Rep. 2018;40(5):2659–2673.
- Ning L, Hu YC, Wang S, et al. Altered long noncoding RNAs and survival outcomes in ovarian cancer: A systematic review and meta-analysis (PRISMA Compliant). Medicine (Baltimore). 2018;97(32):e11481.
- Lee GL, Dobi A, Srivastava S. Prostate cancer: diagnostic performance of the PCA3 urine test. Nat Rev Urol. 2011;8(3):123–124.
- Qiu JJ, Lin XJ, Tang XY, et al. Exosomal metastasisassociated lung adenocarcinoma transcript 1 promotes angiogenesis and predicts poor prognosis in epithelial ovarian cancer. Int J Biol Sci. 2018;14(14):1960–1973.
- Wu Q, Wu X, Ying X, et al. Suppression of endothelial cell migration by tumor associated macrophage-derived exosomes is reversed by epithelial ovarian cancer exosomal lncRNA. Cancer Cell Int. 2017;17:62.
- Kolenda T, Rutkowski P, Michalak M, et al. Plasma lncRNA expression profile as a prognostic tool in BRAF-mutant metastatic melanoma patients treated with BRAF inhibitor. Oncotarget. 2019;10(39):3879–3893.
- Lee B, Mahmud I, Marchica J, et al. Integrated RNA and metabolite profiling of urine liquid biopsies for prostate cancer biomarker discovery. bioRxiv. 2019;599514.
- Palmirotta R, Lovero D, Cafforio P, et al. Liquid biopsy of cancer: a multimodal diagnostic tool in clinical oncology. Ther Adv Med Oncol. 2018;10:1758835918794630.
- Lalremmawia H, Tiwary BK. Identification of molecular biomarkers for ovarian cancer using computational approaches. Carcinogenesis. 2019;40(6):742–748.
- Shatsky R, Parker BA, Bui NQ, et al. Next-generation sequencing of tissue and circulating tumor DNA: the UC San Diego Moores Center for personalized cancer therapy experience with breast malignancies. Mol Cancer Ther. 2019;18(5):1001–1011.