ABSTRACT
Recent transcriptome-wide studies have identified a diverse pool of transfer RNA (tRNA)-derived RNAs or tRNA-derived fragments (tRFs). Some of these RNAs have been demonstrated to be functional and involved in multiple biological processes ranging from the regulation of gene expression to transgenerational epigenetic inheritance. Post-transcriptional maturation of tRNAs includes various processing events including extensive decoration by various RNA modifications, which are required for correct tRNA folding and stability. Moreover, tRNA modifications determine the pattern and specificity of tRNA cleavage. The major drawbacks of many studies in the field of tRFs are that most of them used synthetic RNAs that closely mimic endogenous tRFs in their sequence, yet lack RNA modification that is found in vivo. Here, we developed a simple method to isolate tRNA-derived stress-induced RNAs (tiRNAs), a specific subset of tRFs. Our approach is scalable, cost-effective and relies on the purification of individual tiRNAs based on a sequence-specific RNA/DNA isolation technique using DNA probes. Our method facilitates functional studies of tiRNAs by addressing how physiological RNA modifications within tRNA fragments affect their biological activities. Here, we report pilot functional studies on selected endogenous tiRNAs, namely tiRNAAla and tiRNAGly. We show that natural 5ʹ-tiRNAAla molecules assemble into G-quadruplex structures, and endogenous 5ʹ-tiRNAGly possesses translation inhibition activity.
Introduction
RNA modifications play various roles in cell physiology (reviewed in [Citation1]). They are found in both protein-coding and non-protein-coding (nc) RNAs and function in diverse aspects of RNA metabolism. Extensive RNA modifications are a characteristic feature of transfer RNA (tRNA) [Citation2], the ancient ncRNA that helps the ribosome to decode mRNA information by delivering amino acids to the growing polypeptide chain during protein synthesis. Many of tRNA modifications are evolutionarily conserved reflecting their critical roles in the tRNA folding and aiding in the codon–anticodon interaction (reviewed in [Citation3]).
Besides these mRNA translation-related processes, tRNAs are also implicated in other ‘non-canonical’ functions. One of the emerging themes in RNA biology is that tRNAs are the rich source of small ncRNAs called tRNA fragments (tRFs) (reviewed in [Citation4–Citation6]). tRNA modifications play important roles in the production of tRFs by regulating the efficiency of tRNA cleavage, where they can both inhibit and promote tRNA cleavage by specific ribonucleases (RNases) (reviewed in [Citation7]). In addition, modifications in the tRFs can also determine sites of cleavage and the specificity of RNases towards particular individual tRNAs.
Specific RNases target the anticodon loop of tRNAs [Citation8–Citation12], the region of the molecule rich in a diverse set of chemical modifications [Citation13]. One of such endoribonucleases is angiogenin (ANG), secreted RNase that is a member of the RNase A superfamily (reviewed in [Citation14,Citation15]). Under optimal conditions, ANG is localized to the nucleus where it promotes the synthesis of ribosomal RNAs or resides in the cytoplasm in the complex with protein RNH1, which inhibits its ribonuclease activity. However, under various stress conditions such as oxidative stress or nutrient starvation, ANG becomes activated by dissociation from RNH1 or by translocation from the nucleus into the cytoplasm [Citation16]. In the cytosol, ANG target anticodon loops of mature tRNAs for cleavage-producinga specific class of tRFs named tRNA-derived stress-induced RNAs (tiRNAs) [Citation8].
ANG-mediated tRNA cleavage results in the production of two subclasses of tiRNAs, which basically represent 5ʹ- and 3ʹ- halves of tRNAs [Citation17] (or 5ʹ- and 3ʹ-tiRNAs [Citation8], respectively). Several biological activities are prescribed to tiRNAs. Both 5ʹ- and 3ʹ-tiRNAs are implicated in cell survival under stress, cytochrome C, thus preventing stress-induced apoptosis [Citation18]. Specific 5ʹ-tiRNAs, derived from tRNAAla and tRNACys, inhibit translation of mRNA reporters in vitro and translation in vivo in transfection studies [Citation19]. In cells, 5ʹ-tiRNAAla/Cys-mediated inhibition of protein synthesis results in the formation of stress granules (SGs) [Citation19,Citation20], prosurvival RNA granules that help cells to cope with stress [Citation21–Citation23]. It is important to note that these experiments were done using synthetic tiRNA molecules. Nonetheless, transfection experiments using isolated from cells bulk endogenous 5ʹ-tiRNAs also promoted translation arrest and SG formation [Citation8,Citation19,Citation20].
As an average eukaryotic tRNA contains several RNA modifications, and their frequency and distribution vary in tRNA species [Citation24], it is obvious that tiRNAs themselves may also contain modified nucleotides. A recent study suggested that the presence of pseudouridine in a specific position of tRF regulates its activity [Citation25], thus reemphasizing the importance of studying tRFs in their natural context. As for tiRNAs, whether they are depleted or enriched of specific modifications and whether the absence or the presence of such modifications differentially contribute to the biological activities of a specific tiRNA is an open question.
While it is possible to isolate a bulk of fully processed natural tiRNAs from cells, isolation of the individual endogenous tiRNAs is technically challenging. Here, we provide a proof-of-principle study for the efficient isolation of natural individual tiRNAs. Our approach is based on the capture of endogenous tiRNAs using DNA oligo probes complementary to target tiRNAs. Using this method, we were able to isolate both 5ʹ- and 3ʹ-tiRNAs and demonstrate that endogenous tiRNAs indeed contain internal modifications.
Finally, we have performed initial functional analyses on the selected endogenous tiRNAs. We show that similarly to its synthetic counterpart, natural 5ʹ-tiRNAAla assembles into G-quadruplex structures. We also demonstrate that endogenous 5ʹ-tiRNAGly possesses translation inhibition activity, which is greater than synthetic 5ʹ-tiRNAGly. We further discuss the advantages and limitations of using both synthetic and natural tiRNAs for their structure-functional studies.
Results
The Suzuki lab has previously developed efficient methods for the isolation of individual RNAs, including tRNAs [Citation26,Citation27]. In this approach, solid-phase DNA probes that are complementary to target RNAs are employed for sequence-specific DNA oligo/target RNA capture. We developed our method of endogenous tiRNA isolation considering the following differences of tiRNAs with the tRNAs. First, tiRNAs are derived from their host tRNAs, thus having identical/near-identical sequences with them. Second, tiRNAs are typically 50–100 times less abundant than the tRNAs from which they are derived [Citation8,Citation28]. Third, although tiRNAs are identical to the part of their host tRNAs in sequence, their secondary structure is different from the rigid L-shaped structure of tRNAs. Fourth, it would be great to have a possibility of co-purification of host tRNAs and tiRNAs derived from them for the direct comparison of their chemical modification profiles (e.g. by mass spectrometry methods).
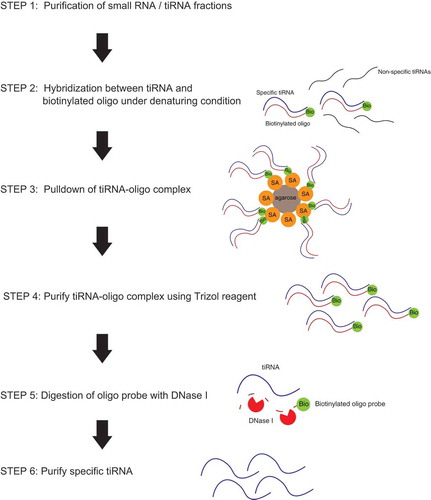
The general workflow to purify the large amounts of tiRNAs with or without the host tRNAs is demonstrated in . We start with total RNA isolation using standard methods, followed by fractionation (to separate small (up to 100–150 nt) RNAs from other RNAs; this fraction includes both tRNAs and tiRNAs) or by gel purification of tiRNA fraction only. On the next step, we hybridize selected fractions with 3ʹ-biotinylated DNA oligos complementary to the target tiRNAs under semi-denaturating conditions and pulldown DNA/RNA complexes using streptavidin agarose beads. After extensive washing to remove unbound and non-target RNAs, we digest DNA oligo probes by treatment with DNaseI to release bound RNAs and purify them using standard Trizol-based extraction.
As a proof of principle, we aimed to purify the following RNAs: (1) 5ʹ-tiRNAGly-GCC that has been reported in a number of studies as one of the most abundant tRNA fragment [Citation29–Citation32]; (2) 5ʹ-tiRNAAla-AGC, which is within least abundant tiRNAs but has been shown to be bioactive in different settings [Citation19,Citation33,Citation34]; (3) 3ʹ-tiRNAGly-GCC, which has been identified in RNA-seq experiments [Citation35,Citation36]; and (4) corresponding mature tRNAGly-GCC and tRNAAla-AGC.
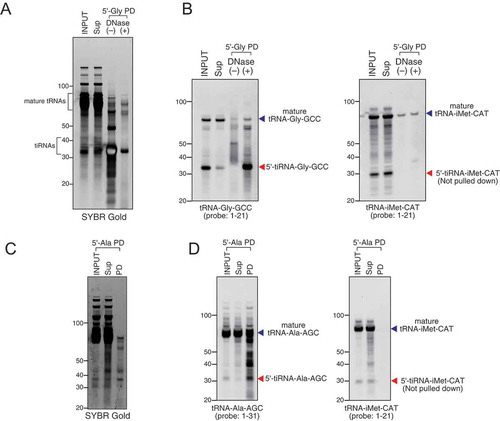
Treatment of cells with sodium arsenite or recombinant ANG is a powerful and well-established way to induce production of tiRNAs [Citation8]. We treated osteosarcoma U2OS cells with recombinant ANG and purified total cellular RNAs using standard RNA extraction methods. We designed 21–27 nt DNA capture probes (Table S1) complementary to tiRNAs and used them to pulldown and purify target tiRNAs. As demonstrated in , B, DNA capture probe against 5ʹ-tiRNAGly-GCC efficiently pulldowns both tiRNA and mature tRNA from the RNA fraction enriched in small RNAs (INPUT, SYBR GOLD staining). It is important to note that DNaseI treatment is a necessary step that eliminates potential contamination of RNA with DNA probes. Northern blotting () using a probe against tRNAGly-GCC confirms the specificity of pulldown and demonstrates that both mature tRNA and target tiRNA can be efficiently captured by the same DNA capture probe. Similar results were obtained using a capture probe against 5ʹ-tiRNAAla-AGC (, ). The specificity of pulldowns is confirmed by Northern blotting against tiRNAiMet-CAT (, , right panels). Our results demonstrate that we can efficiently co-purify tiRNAs and tRNAs from which they are derived.
Figure 2. Efficiency and specificity of oligo probe-mediated pulldown. Small RNA fraction from total RNA was used for pulldown. (A–B) Pulldown of 5ʹ-tiRNA-Gly-GCC (5ʹ-Gly) using purified small RNA fraction. (A) SYBR Gold staining and (B) Northern blotting for 5ʹ-tiRNA-Gly-GCC and 5ʹ-tiRNA-iMet-CAT. (C–D) Pulldown of 5ʹ-tiRNA-Ala-AGC (5ʹ-Ala) using purified small RNA fraction. (C) SYBR Gold staining and (D) Northern blotting for 5ʹ-tiRNA-Ala-AGC and 5ʹ-tiRNA-iMet-CAT. Sup: supernatant fraction, PD: pulldown fraction.
Next, we optimized our method to purify tiRNA and tRNA fraction separately. To purify fraction of tiRNAs, total RNA from U2OS cells is separated on TBE-UREA gels and fraction of tiRNAs is gel-purified (). DNA capture probes are then used to isolate specific tiRNAs, e.g. 5ʹ-tiRNAGly-GCC and 5ʹ-tiRNAAla-AGC (–, respectively), and 5ʹ-tiRNAHIs-Gtg and 5ʹ-tiRNALys-CTT (Fig. S1A, B). It should be noted that purified fractions of 5ʹ-tiRNAHIs-Gtg and 5ʹ-tiRNALys-CTT both demonstrate some cross-reactivity to the Northern blotting probes against tRNAGly-GCC but not to probes against tRNAiMet-CAT (Fig. S1B).
Figure 3. Endogenous tiRNA pulldown using gel-purified tiRNA fraction. (A) tiRNA fraction was gel-excised and purified from total RNA derived from angiogenin (ANG)-treated U2OS cells. (B–C) Pulldown of 5ʹ-tiRNA-Gly-GCC (5ʹ-Gly). (B) SYBR Gold staining and (C) Northern blotting for 5ʹ-tiRNA-Gly-GCC and 5ʹ-tiRNA-iMet-CAT. Red arrowhead indicates purified 5ʹ-Gly. (D–E) Pulldown of 5ʹ-tiRNA-Ala-AGC (5ʹ-Ala). (D) SYBR Gold staining and (E) Northern blotting for 5ʹ-tiRNA-Ala-AGC and 5ʹ-tiRNA-iMet-CAT. Red arrowheads indicate purified 5ʹ-Ala. (F–H) Purified endogenous tiRNA has post-transcriptional modification. Endogeno3 USDʹ-tiRNA-Gly-GCC (3ʹ-Gly) was pulled down and compared with synthetic 3ʹ-Gly (Synth. 3ʹ-Gly). (F) SYBR Gold staining, (G) Northern blotting for 3ʹ-tiRNA-Gly-GCC and (H) Immuno-Northern Blotting (INB) for 1-methyladenosine (m1A) modification. Red arrowhead indicates the band for 3ʹ-Gly. The position of m1A in 3ʹ-Gly is indicated as green circle. Sup: supernatant fraction, PD: pulldown fraction, Synth: synthetic.
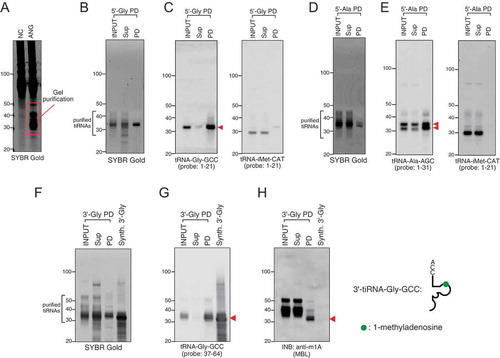
Similarly, our approach allows purification of 3ʹ-tiRNAs, e.g. 3ʹ-tiRNAGly-GCC (, ). Importantly, immuno-Northern blotting (INB) against m1A modification (using two independent m1A antibodies), which is occupying position 58 on tRNAGly-GCC [Citation37], demonstrated that 3ʹ-tiRNAGly-GCC also possesses this modification (synthetic 3ʹ-tiRNAGly-GCC was used as a control for the specificity) ( and Fig. S2A-C).
As tRNA cleavage is regulated by RNA modifications [Citation38–Citation40], it is also important to purify specific tRNAs that are the source for tiRNAs. To purify mature tRNAs, a similar approach was used where total tRNA fraction was first purified (), and then used for capture by specific DNA oligos (, ). We demonstrate that both tRNAGly-GCC and tRNAAla-AGC (, ) can be efficiently purified using our method. Apart from Northern blotting approaches, INB has been used to demonstrate the specificity of pulldowns. According to Modomics database [Citation37], both tRNAGly-GCC and tRNAAla-AGC contain pseudouridine modifications. In contrast, only tRNAAla-AGC contains internal 7-methylguanosine (m7G) modification. Indeed, INBi against these modifications showed that only tRNAAla-AGC possesses m7G modifications (anti-m7G), while both tiRNAs contain pseudouridine (anti-pseudouridine) ().
Figure 4. Endogenous mature tRNAs can be also purified. Mature tRNA-Ala-AGC (tRNA-Ala) and tRNA-Gly-GCC (tRNA-Gly) were pulled down using gel-purified mature tRNA fraction. (A) SYBR Gold staining and (B) Northern blotting for tRNA-Ala-AGC, tRNA-Gly-GCC, tRNA-His-GTG and tRNA-iMet-CAT. (C) INB for 7-methylguanosine (m7G) and pseudouridine modifications. In pulldown (PD) fraction, bands indicated by red arrowheads show the purified specific tRNAs. m7G and pseudouridine modifications are indicated as red circle and blue circle, respectively. Sup: supernatant fraction, PD: pulldown fraction.
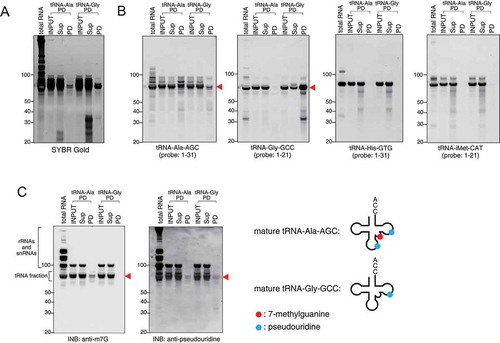
We have previously shown that selected synthetic tiRNA species promote translation inhibition in transfected cells and in in vitro assays [Citation19,Citation20]. Some of them, such as 5ʹ-tiRNAAla, require assembly of specific tetrameric G-quadruplex (G4) structures for their bioactivity [Citation33,Citation34]. Our analysis of purified natural 5ʹ-tiRNAAla-AGC (, ) shows that endogenous 5ʹ-tiRNAAla-AGC also assembles G4 structures as judged by stability and mobility in the native gels (). Importantly, these G4 structures are only assembled under G4-permissive conditions (in the presence of K+ ions) and disassembled under G4-non-permissive conditions (Li+ ions). We noted that G4 5ʹ-tiRNAAla-AGC has slightly different mobility in the gel compared to synthetic 5ʹ-tiRNAAla-AGC (, ), possibly due to the presence of internal RNA modifications (). Nonetheless, our results suggest that endogenous 5ʹ-tiRNAAla is capable of assembling G4 structures similar to synthetic counterpart.
Figure 5. Structural and functional analysis using endogenous tiRNAs. (A, B) Endogenous 5ʹ-tiRNA-Ala (5ʹ-Ala) can form G-quadruplex (G4). (A) Synthetic and endogenous 5ʹ-Ala were equilibrated in 100 mM KCl or LiCl salts. RNAs were analysed on native gels and post-stained with SYBR Gold. (B) After SYBR Gold staining, RNAs were subjected to Northern blotting for tRNA-Ala. (C) Endogenous 5ʹ-tiRNA-Gly (5ʹ-Gly) inhibits translation of mRNA reporters in vitro. Means and standard deviation were obtained from three independent experiments. *p < 0.05 vs control RNA (Ctrl RNA) and **p < 0.01 vs Ctrl RNA.
In our previous studies, we showed [Citation19] that synthetic tRNAGly-GCC possesses moderate translation inhibition activities in in vitro translation assays using rabbit reticulocyte lysates (RRL). Here, we compared the translation inhibition capacity of endogenous 5ʹ-tiRNAGly-GCC to its synthetic variant (). Synthetic 5ʹ-tiRNAAla, which strongly inhibit translation of reporter mRNAs in vitro, was used as a positive control (). Endogenous 5ʹ-tiRNAGly-GCC, as well as its synthetic counterpart, significantly reduces the translation of luciferase mRNA reporter in RRL-based translation assays. Moreover, the inhibitory activity of endogenous 5ʹ-tiRNAGly-GCC is even greater than that of the synthetic variant, possibly due to the presence of RNA modifications.
Discussion
RNA modifications have been reported in different RNA molecules. In some of these molecules such as rRNAs or tRNAs, specific modifications play key roles in their folding and functions [Citation1]. Although in vitro transcription systems or chemical synthesis of specific RNAs is commonly used to dissect different structural or functional aspects of their biology, it is desirable to obtain naturally processed RNA molecules from cells. Such isolation from cells is a challenging procedure, and in many cases, researchers have no other choice than ignoring the fact that synthetic RNA molecules have significantly different chemical compositions than natural ones.
While initially met with scepticism, the field of tRNA-derived fragments is gaining significant attention during recent years [Citation4–Citation6]. An increasing number of reports prescribe diverse biological functions to tRFs (reviewed in [Citation7,Citation41]), although most of the data are obtained with synthetic mimics of tRFs. Information about the sequence, length and relative copy number of tRFs (including tiRNAs) can be obtained from multiple RNA sequencing datasets [Citation42,Citation43]. However, since tRNAs are heavily modified, RNA-seq of tRNAs and tRFs can create bias in the analysis and interpretation of the data [Citation44,Citation45]. Moreover, such analysis can offer only limited information on the modification pattern of specific individual tRFs. Thus, we are in a critical need to develop approaches that will allow purification of endogenous tRFs from cells.
One of the best understood tRNA cleavage pathways is the biogenesis of tRFs under stress [Citation8,Citation10,Citation17]. In this pathway, ribonuclease ANG targets anticodon loops of mature cytoplasmic tRNAs to produce tiRNAs [Citation8]. In turn, tiRNAs contribute to different aspects of RNA metabolism aiming at stress adaptation and cell survival [Citation8,Citation18–Citation20,Citation46]. Some of these aspects can be reproduced by synthetic tiRNAs, e.g. transfection of both endogenous 5ʹ-tiRNAs and synthetic tiRNAs promotes the formation of SGs and inhibition of cellular translation [Citation19,Citation20]. However, exact molecular mechanisms of translation inhibition were obtained using exclusively synthetic tiRNA mimics. Whether endogenous cellular tiRNAs possess similar activities is an open question.
Here, we demonstrated that purification of individual endogenous tiRNAs is possible. Production of tiRNAs is well established and relies on the administration of stresses (such as sodium arsenite [Citation8]) or manipulations with ANG (over-expression [Citation17] or treatment with recombinant protein [Citation8]), which allows a simple way to scale up a number of cells for tiRNA isolation. We established purification approach that allows isolation of tiRNAs, which carry post-transcriptional modifications. In turn, isolated tiRNAs can be used in other studies such as analysis of their modification pattern by mass spectrometry. We showed that our protocol can be used to purify both abundant and less abundant tiRNA species. Moreover, we demonstrated that purification of 3ʹ-tiRNAs, which are commonly missed in RNA-seq studies, is also possible. Our protocol also allows co-purification of tiRNAs with their host tRNAs, or individual endogenous tRNAs.
We have also demonstrated that selected synthetic and endogenous tiRNAs behave similarly. We show that endogenous 5ʹ-tiRNAAla-AGC also assemble G4 structures similar to its synthetic variant (, ). This is important since synthetic 5ʹ-tiRNAAla-AGC inhibits translation, promotes SG formation and cytoprotection in G4-dependent manner. Similarly, our in vitro translation assays show that synthetic and endogenous 5ʹ-tRNAsGly-GCC similarly inhibit translation of mRNA reporter, although the endogenous variant has slightly enhanced yet statistically significant inhibitory activity than synthetic one (, and Table S2).
It should be noted that the purification of endogenous tiRNAs has some disadvantages. It is likely that due to the high sequence similarity, selected tiRNA species are copurified (e.g. tiRNAs derived from different tRNA isoacceptors and isodecoders). Thus, the use of synthetic tiRNAs that have precise composition and length can be more beneficial for structural and functional analyses of tiRNAs, where the contribution of specific nucleotides or modifications can be estimated. In the same time, due to the limitations in the synthesis of some RNA modifications found in tRNAs, the use of endogenous tiRNAs is also important. Moreover, purification of endogenous tRNAs and tiRNAs can be used for their sequencing and analysis by mass spectrometry, which will allow to discover principles of tRNA cleavage regulation by RNA modifications (discussed in [Citation7]).
Altogether, we presented the biochemical framework for systematic purification of tiRNAs that can be used for other applications and addressing unresolved questions in tRNA biology.
Material and methods
Cell culture and treatment
U2OS cells were cultured at 37ºC in a CO2 incubator in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% foetal bovine serum (Sigma-Aldrich) and 1% of penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO). For tiRNA generation, U2OS cells were incubated with DMEM supplemented with 0.5 µg/ml recombinant human ANG (R&D Systems, Minneapolis, MN) for 1 h. After washing with Hank’s balanced salt solution twice, total RNA was extracted with Trizol reagent (ThermoFisher Scientific, Waltham, MA).
Generation of synthetic tiRNAs
All synthetic tiRNAs and control RNA (Ctrl RNA) used in this study were synthesized and purified by Integrated DNA Technology Inc. (Coralville, IA)
Ctrl RNA: 5ʹ-Phospho-UGAAGGGUUUUUUGUGUCUCUAUUUCCUUC-3ʹ
5ʹ-Ala: 5ʹ-Phospho-GGGGGUGUAGCUCAGUGGUAGAGCGCGUGC-3ʹ
5ʹ-Gly: 5ʹ-Phospho-GCCGUGAUCGUAUAGUGGUUAGUACUCUGCG-3ʹ
3ʹ-Gly-GCC: 5ʹ-CACGCGGGAGGCCCGGGUUCGAUUCCCGGCCCAUGCACCA-3ʹ
Purification of specific RNA fractions
Small RNA (<200 nt) fraction was purified using RNA Clean & Concentrator kit (Zymo Research, Irvine, CA) according to the manufacturer’s instructions.
For purification of tiRNA/tRNA fraction, total RNA was electrophoresed on 15% Urea-TBE polyacrylamide gel (ThermoFisher Scientific). The target fraction was excised under UV light after staining with SYBR Gold (ThermoFisher Scientific). The excised gel fractions were crushed and soaked in elution buffer (20 mM Tris–HCl (pH 7.4), 250 mM sodium acetate, 1 mM EDTA and 0.25% SDS), incubated at 65ºC for 15 min, frozen at −80ºC for 15 min, and then incubated again at 65ºC for 15 min. After centrifugation, the supernatant was filtered through Zymo-Spin IIIC column. The flowthrough was mixed with an equal volume of isopropanol (Sigma-Aldrich). The precipitated RNA was recovered using Direct-zol RNA Miniprep kit (Zymo Research) according to the manufacturer’s instructions.
Pulldown of specific tiRNA/tRNA
Biotinylated oligonucleotide DNA probes were synthesized by Integrated DNA Technology Inc. . The sequences of the probes are shown in Table S1.
Purified RNA fraction and 500 pmol of biotinylated oligo probe were denatured by heating at 90ºC for 5 min, and then hybridized for 1 h by incubating in 950 µl of TBS containing 0.05% NP-40 and 200 U RNasin (Promega, Madison, WI) with gentle mixing. After hybridization, the solution was mixed with 50 µl of pre-washed streptavidin agarose resin (ThermoFisher Scientific) and further incubated at room temperature for 1 h with gentle mixing. After three times wash with wash buffer (TBS containing 0.05% NP-40), 500 µl of Trizol reagent was added into the resin, mixed with 100 µl of chloroform, and then centrifuged at 12,000 g for 15 min at 4ºC. Captured oligo/RNA hybrid was isolated from the aqueous phase using Direct-zol RNA Miniprep kit according to the manufacturer’s instructions. Residual DNA oligo probe was digested by 10 U DNaseI (New England Biolabs) supplemented with 80 U RNasin in 50 µl reaction volume at 37ºC for 3 h. After DNase treatment, 300 µl of Trizol reagent was added; then RNA was isolated using Direct-zol RNA Microprep kit (Zymo Research).
Northern blotting
RNA samples were run on 15% TBE-urea polyacrylamide gels (ThermoFisher Scientific) and transferred to positively charged nylon membranes (Roche, Basel, Switzerland). The membranes were cross-linked by UV irradiation. After cross-linking, the membranes were hybridized overnight at 40°C with digoxigenin (DIG)-labelled DNA probes in DIG Easy Hyb solution (Roche). After low stringency washes (washing twice with 2× SSC/0.1% SDS at room temperature) and high stringency wash (washing once with 1× SSC/0.1% SDS at 40ºC), the membranes were blocked in blocking reagent (Roche) for 30 min at room temperature, probed with alkaline phosphatase-labelled anti-DIG antibody (Roche) for 30 min, and washed with 1x TBS-T. Signals were visualized with CDP-Star ready-to-use (Roche) and detected using ChemiDoc imaging system (BioRad) according to the manufacturer’s instructions. Oligonucleotide probes were synthesized by IDT. DIG-labelled probes were prepared using the DIG Oligonucleotide tailing kit (2nd generation; Roche) according to the manufacturer’s instructions. The sequences of the probes are shown in Table S1.
Immuno-Northern blotting
Post-transcriptional modifications on purified tRNAs/tiRNAs were detected using INB method as previously reported [Citation47,Citation48] with slight modification. Briefly, after Northern blotting, the DIG-labelled probes were stripped off by incubating the membrane twice with 0.1x SSC/0.5% SDS at 85ºC for 15 min. The membranes were blocked in blocking reagent (Roche) for 30 min and washed with 1x TBS-T, then incubated overnight at 4ºC with modification-specific antibody diluted at 1:500 with blocking reagent. After washing with TBS-T, the membrane was incubated with Peroxidase AffiniPure Goat Anti-Mouse IgG (H+L) (Jackson ImmunoResearch, 1:5000 dilution in TBS-T) at room temperature for 1 h. After washing with TBS-T, signals were visualized with SuperSignal West Pico PLUS (ThermoFisher Scientific) and detected using ChemiDoc imaging system (BioRad) according to the manufacturer’s instructions. The modification-specific antibodies used in this study were as below, anti-1-methyladenosine (m1A) antibody (D345-3, MBL), anti-1-methyladenosine (m1A) antibody (ab208196, abcam), anti-pseudouridine antibody (D347-3, MBL) and anti-m3 G-cap, m7G-cap antibody (MABE419, Sigma-Aldrich).
Positions of post-transcriptional modifications in tRNAs were obtained from Modomics database (http://modomics.genesilico.pl/) [Citation37].
G-quadruplex formation of endogenous 5ʹ-tiRNA-Ala
Synthetic or endogenous 5ʹ-tiRNA-Ala was diluted to 10 μM in 100 mM KCl or LiCl solution, heated to 95°C for 10 min and allowed to cool gradually to room temperature. After salt equilibration, 20 pmol of synthetic 5ʹ-tiRNA-Ala and 40 pmol of endogenous 5ʹ-tiRNA-Ala were run through a 20% TBE gel (ThermoFisher Scientific) and post-stained with SYBR Gold. After SYBR Gold staining, RNAs were subjected to Northern blotting as described in ‘Northern blotting’ section. The membrane was probed with the same probe as that used for pulldown of 5ʹ-tiRNA-Ala. Hybridized probe was detected using HRP-conjugated streptavidin (Jackson ImmunoResearch, 1:10,000 dilution).
In vitro translation of mRNA reporters in Rabbit Reticulocyte Lysates (RRLs) and statistical analysis
A Flexi Rabbit Reticulocyte Lysate System (Promega) was used for the in vitro analysis of mRNA translation in RRLs as previously described [Citation19]. One hundred picomoles of control RNA (Ctrl RNA), synthetic tiRNAs, endogenous 5ʹ-tiRNA-Gly or a mixture of 5ʹ/3ʹ-tiRNAs were added to translation reactions. Data were shown as mean ± SD. Tukey–Kramer test was performed for multiple comparisons.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download Zip (656.6 KB)Acknowledgement
We thank Victoria Ivanova for assistance with preliminary experiments, and Anderson and Ivanov lab members for helpful critiques.
Supplementary material
Supplementary material for this article can be accessed here.
Additional information
Funding
References
- Nachtergaele S, He C. The emerging biology of RNA post-transcriptional modifications. RNA Biol. 2017;14:156–163.
- Cantara WA, Crain PF, Rozenski J, et al. The RNA modification database, RNAMDB: 2011 update. Nucleic Acids Res. 2011;39:D195–201.
- Lorenz C, Lunse CE, Morl M. tRNA modifications: impact on structure and thermal adaptation. Biomolecules. 2017;7:35.
- Kumar P, Kuscu C, Dutta A. Biogenesis and function of transfer RNA-related fragments (tRFs). Trends Biochem Sci. 2016;41:679–689.
- Keam SP, Hutvagner G. tRNA-derived fragments (tRFs): emerging new roles for an ancient RNA in the regulation of gene expression. Life (Basel). 2015;5:1638–1651.
- Anderson P, Ivanov P. tRNA fragments in human health and disease. FEBS Lett. 2014;588:4297–4304.
- Lyons SM, Fay MM, Ivanov P. The role of RNA modifications in the regulation of tRNA cleavage. FEBS Lett. 2018;592:2828–2844.
- Yamasaki S, Ivanov P, Hu GF, et al. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol. 2009;185:35–42.
- Tomita K, Ogawa T, Uozumi T, et al. A cytotoxic ribonuclease which specifically cleaves four isoaccepting arginine tRNAs at their anticodon loops. Proc Natl Acad Sci U S A. 2000;97:8278–8283.
- Thompson DM, Lu C, Green PJ, et al. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA. 2008;14:2095–2103.
- Ogawa T, Tomita K, Ueda T, et al. A cytotoxic ribonuclease targeting specific transfer RNA anticodons. Science. 1999;283:2097–2100.
- Donovan J, Rath S, Kolet-Mandrikov D, et al. Rapid RNase L-driven arrest of protein synthesis in the dsRNA response without degradation of translation machinery. RNA. 2017;23:1660–1671.
- Machnicka MA, Olchowik A, Grosjean H, et al. Distribution and frequencies of post-transcriptional modifications in tRNAs. RNA Biol. 2014;11:1619–1629.
- Sheng J, Xu Z. Three decades of research on angiogenin: a review and perspective. Acta Biochim Biophys Sin (Shanghai). 2016;48:399–410.
- Lyons SM, Fay MM, Akiyama Y, et al. RNA biology of angiogenin: current state and perspectives. RNA Biol. 2017;14:171–178.
- Pizzo E, Sarcinelli C, Sheng J, et al. Ribonuclease/angiogenin inhibitor 1 regulates stress-induced subcellular localization of angiogenin to control growth and survival. J Cell Sci. 2013;126:4308–4319.
- Fu H, Feng J, Liu Q, et al. Stress induces tRNA cleavage by angiogenin in mammalian cells. FEBS Lett. 2009;583:437–442.
- Saikia M, Jobava R, Parisien M, et al. Angiogenin-cleaved tRNA halves interact with cytochrome c, protecting cells from apoptosis during osmotic stress. Mol Cell Biol. 2014;34:2450–2463.
- Ivanov P, Emara MM, Villen J, et al. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol Cell. 2011;43:613–623.
- Emara MM, Ivanov P, Hickman T, et al. Angiogenin-induced tRNA-derived stress-induced RNAs promote stress-induced stress granule assembly. J Biol Chem. 2010;285:10959–10968.
- Panas MD, Ivanov P, Anderson P. Mechanistic insights into mammalian stress granule dynamics. J Cell Biol. 2016;215:313–323.
- Kedersha N, Ivanov P, Anderson P. Stress granules and cell signaling: more than just a passing phase? Trends Biochem Sci. 2013;38:494–506.
- Ivanov P, Kedersha N, Anderson P. Stress granules and processing bodies in translational control. Cold Spring Harb Perspect Biol. 2019;11:a032813.
- Boccaletto P, Machnicka MA, Purta E, et al. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res. 2018;46:D303–D7.
- Guzzi N, Ciesla M, Ngoc PCT, et al. Pseudouridylation of tRNA-derived fragments steers translational control in stem cells. Cell. 2018;173:1204–16 e26.
- Suzuki T, Suzuki T. Chaplet column chromatography: isolation of a large set of individual RNAs in a single step. Methods Enzymol. 2007;425:231–239.
- Miyauchi K, Ohara T, Suzuki T. Automated parallel isolation of multiple species of non-coding RNAs by the reciprocal circulating chromatography method. Nucleic Acids Res. 2007;35:e24.
- Saikia M, Krokowski D, Guan BJ, et al. Genome-wide identification and quantitative analysis of cleaved tRNA fragments induced by cellular stress. J Biol Chem. 2012;287:42708–42725.
- Tosar JP, Gambaro F, Sanguinetti J, et al. Assessment of small RNA sorting into different extracellular fractions revealed by high-throughput sequencing of breast cell lines. Nucleic Acids Res. 2015;43:5601–5616.
- Torres AG, Reina O, Stephan-Otto Attolini C, et al. Differential expression of human tRNA genes drives the abundance of tRNA-derived fragments. Proc Natl Acad Sci U S A. 2019;116:8451–8456.
- Sharma U, Conine CC, Shea JM, et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science. 2016;351:391–396.
- Dhahbi JM, Spindler SR, Atamna H, et al. 5ʹ tRNA halves are present as abundant complexes in serum, concentrated in blood cells, and modulated by aging and calorie restriction. BMC Genomics. 2013;14:298.
- Lyons SM, Gudanis D, Coyne SM, et al. Identification of functional tetramolecular RNA G-quadruplexes derived from transfer RNAs. Nat Commun. 2017;8:1127.
- Ivanov P, O’Day E, Emara MM, et al. G-quadruplex structures contribute to the neuroprotective effects of angiogenin-induced tRNA fragments. Proc Natl Acad Sci U S A. 2014;111:18201–18206.
- Su Z, Kuscu C, Malik A, et al. Angiogenin generates specific stress-induced tRNA halves and is not involved in tRF-3-mediated gene silencing. J Biol Chem. 2019;294:16930–16941.
- Bakowska-Zywicka K, Mleczko AM, Kasprzyk M, et al. The widespread occurrence of tRNA-derived fragments in Saccharomyces cerevisiae. FEBS Open Bio. 2016;6:1186–1200.
- Dunin-Horkawicz S, Czerwoniec A, Gajda MJ, et al. MODOMICS: a database of RNA modification pathways. Nucleic Acids Res. 2006;34:D145–9.
- Tuorto F, Liebers R, Musch T, et al. RNA cytosine methylation by Dnmt2 and NSun2 promotes tRNA stability and protein synthesis. Nat Struct Mol Biol. 2012;19:900–905.
- Schaefer M, Pollex T, Hanna K, et al. RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage. Genes Dev. 2010;24:1590–1595.
- Blanco S, Dietmann S, Flores JV, et al. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. Embo J. 2014;33:2020–2039.
- Sun C, Fu Z, Wang S, et al. Roles of tRNA-derived fragments in human cancers. Cancer Lett. 2018;414:16–25.
- Pliatsika V, Loher P, Magee R, et al. MINTbase v2.0: a comprehensive database for tRNA-derived fragments that includes nuclear and mitochondrial fragments from all the cancer genome atlas projects. Nucleic Acids Res. 2018;46:D152–D9.
- Kumar P, Mudunuri SB, Anaya J, et al. tRFdb: a database for transfer RNA fragments. Nucleic Acids Res. 2015;43:D141–5.
- Zheng G, Qin Y, Clark WC, et al. Efficient and quantitative high-throughput tRNA sequencing. Nat Methods. 2015;12:835–837.
- Cozen AE, Quartley E, Holmes AD, et al. ARM-seq: alkB-facilitated RNA methylation sequencing reveals a complex landscape of modified tRNA fragments. Nat Methods. 2015;12:879–884.
- Sobala A, Hutvagner G. Small RNAs derived from the 5ʹ end of tRNA can inhibit protein translation in human cells. RNA Biol. 2013;10:553–563.
- Mishima E, Jinno D, Akiyama Y, et al. Immuno-northern blotting: detection of RNA modifications by using antibodies against modified nucleosides. PloS One. 2015;10:e0143756.
- Mishima E, Abe T. Immuno-northern blotting: detection of modified RNA using gel separation and antibodies to modified nucleosides. Methods Mol Biol. 2019;1870:179–187.