ABSTRACT
Cancer is a complex process in which protein-coding and non-coding genes play essential roles. Long noncoding RNAs (lncRNAs), as a subclass of noncoding genes, are implicated in various cancer processes including growth, proliferation, metastasis, and angiogenesis. Due to presence in body fluids such as blood and urine, lncRNAs have become novel biomarkers in cancer detection, diagnosis, progression, and therapy response. Remarkably, increasing evidence has verified that lncRNAs play essential roles in chemoresistance by targeting different signalling pathways. Autophagy, a highly conserved process in response to environmental stresses such as starvation and hypoxia, plays a paradoxical role in inducing resistance or sensitivity to chemotherapy agents. In this regard, we reviewed chemoresistance, the role of lncRNAs in cancer, and the role of lncRNAs in chemoresistance by modulating autophagy.
1. Introduction
Cancer is the first or second leading cause of morbidity and mortality among noncommunicable diseases (NCDs) in the world [Citation1]. Due to the rapid growth of the population and ageing problem, cancer mortality rate is increasing [Citation2]. Although chemotherapy is one of the effective treatments in cancer, resistance to chemotherapy challenges the clinical outcome of cancer treatment and leads to cancer relapse, metastasis, and the main barrier in response to treatment [Citation3]. Therefore, understanding chemoresistance mechanisms are very crucial.
Non-coding RNAs (ncRNAs), RNA transcripts which are not translated into proteins, regulate many physiological and pathological processes and pathways and are classified into two groups according to their molecular size: small non-coding RNAs (sncRNAs), ≤200 nucleotides, and long non-coding RNAs (lncRNAs), ≥200 nucleotides [Citation4]. Various classes of ncRNAs have been identified, including microRNAs (miRNAs), PIWI–interacting RNAs (piRNAs), circular RNAs (circRNAs), small nucleolar RNA (snRNA), and lncRNAs [Citation5]. It has been demonstrated that lncRNAs are aberrantly expressed or dysfunctioned in various diseases, such as cancer. Thus, in this paper, we will discuss about roles of lncRNAs in cancer, resistance to chemotherapy, and targeting autophagy in chemoresistance.
2. Chemoresistance in cancer
Despite the great achievements in cancer therapy during the last decade, resistance of cancer cells to classical chemotherapy agents and radiotherapy continue to be a major problem in cancer treatment [Citation6]. Based on the time of resistance development, drug resistance in cancer cells can be grouped intrinsic and acquired resistance [Citation7]. Both occur due to genomic mutations in the cancer cells and/or to epigenetic alterations [Citation8]. Intrinsic resistance or innate resistance exists before administration of therapies owing to the pre-existing genetic mutations that reduce tumour cells’ responsiveness to therapeutic agents, tumour heterogeneity, and activation of pathways against anticancer drugs. On the other hand, acquired resistance refers to gradual reduction efficacy of anticancer drug during treatment which can be a result of alteration in expression levels of anticancer drug targets, activation of new proto-oncogenes, and changes in tumour microenvironment (TME) [Citation7]. Therefore, understanding the molecular mechanisms of resistance to chemotherapy helps in finding novel therapeutic strategies for cancer therapy. According to various studies, we review the molecular mechanisms of chemoresistance in cancer cells.
2.1. Drug efflux
The major reason for chemoresistance is decreased intracellular accumulation of chemotherapy agents owing to the activation of drug efflux mechanisms, including ATP binding cassette (ABC) family transporters [Citation9]. ABC family transporters are transmembrane proteins that translocate different substrates across cellular membranes coupling with ATP hydrolysis [Citation10]. There are 48 genes for ABC transporters in the human genome which are classified into seven subfamilies (ABCA-ABCG) [Citation11]. Among them, 13 transporters are highly involved in the multidrug resistance (MDR) to cancer chemotherapy, including multidrug resistance proteins (MRPs/ABCCs), P− gp (MDR1/ABCB1), and breast cancer resistance protein (BCRP/ABCG2) [Citation12].
P-glycoprotein (p-gp), a 170 kDa protein, transports a variety of substrates and is able to detoxificate cytotoxic drug via efflux in cancer cells [Citation11]. It has been demonstrated that P-gp upregulated in different kinds of cancers, including neuroblastomas, leukaemia, breast, and ovarian cancers [Citation13]. Multidrug resistance-associated protein 1 (MRP1) or ABCC1 also pumps out a broad range of anticancer drugs and its overexpression and association with resistance has been reported in different cancers, including breast, lung, and prostate cancers [Citation14–16]. Furthermore, ABCG2 is the main drug efflux transporter in breast cancer and associated with resistance to chemotherapeutic agents [Citation7]. It has been demonstrated that ABCG2 overexpressed in many other cancers, such as leukaemia and lung cancer [Citation17,Citation18].
2.2. Changes in the activity of oncogenes and tumour suppressor genes
Although oncogenes are genes with oncogenic potential, their overexpression can lead to resistance to chemotherapy and poor outcome. For example, overexpression of growth factor receptors activate phosphatidylinositol 3-kinas (PI3 K)/Akt/NF-κB axis which upregulates the transcription of anti-apoptotic genes [Citation8]. Overexpression of Akt in cisplatin or mitoxantrone-treated non-small-cell lung carcinoma (NSCLC) cells lead to delay in the activation of p53 and upregulation of anti-apoptotic Bcl-xL, resulting in chemoresistance [Citation3]. Furthermore, Sun et al. revealed that bladder cancer cells with resistance to cisplatin show rapid tumorigenesis, more aggressiveness, EMT, and drug resistance through NF-κB activation [Citation19].
Besides induction of positive signals for growth, tumour cells also are able to suppress inhibitors of proliferation. It has been reported that 53BP1 loss in colorectal cancer cells induces chemoresistance to 5-fluorouracil (5-FU) via inhibiting the ATM-CHK2-P53 pathway [Citation20]. Zhang et al. found that the accumulation of p53 mutant Arg282Trp mediates resistance to cisplatin through binding to ERP29 promoter and upregulating its expression [Citation21]. Furthermore, Lakshmanan et al. reported that MUC16 regulates TSPYL5 via the JAK2/STAT3/GR axis and induces resistance to gemcitabine and cisplatin by p53 downregulation in lung cancer cell [Citation22].
2.3. DNA repair
Many chemotherapy agents, such as cisplatin and 5-FU, kill cancer cells by interacting with DNA and inducing DNA damage. As a general response, DNA damages activate a DNA damage response (DDR) results in an interruption of DNA synthesis, an arrest of the cell cycle, and activation of repair pathways [Citation23]. These pathways have a vital role in the maintenance of cellular genomic integrity, whereas genomic instability is known as cancer hallmark [Citation24]. Thus, DDR mechanisms in affected cells with anticancer drugs may reduce the efficacy of the drugs, leading to chemoresistance [Citation25]. It has been shown that genes involved in DDR, such as FANCG, FEN1, RAAD23B, are overexpressed in human colon cancer cells resistance to 5-FU [Citation26,Citation27]. Li et al. found that the downregulation of ERCC1 or XPF, endonucleases in nucleotide excision repair system, is associated with increased sensitivity to cisplatin in both tumour tissues and cell lines [Citation28]. Furthermore, high expression of O6-alkylguanine DNA alkyltransferase (MGMT) leads to resistance to alkylating agents [Citation29]. Augustine et al. reported that resistance to temozolomide (TMZ) is associated with MGMT upregulation in melanoma [Citation30].
2.4. Apoptosis pathways
Apoptosis or programmed cell death is a complex process that affects a wide range of genes, leading to remove abnormal or unwanted cells for maintaining the stability of organism. In the apoptosis pathway, proteins play either anti-apoptosis or pro-apoptosis roles [Citation31]. Thus, resisting cell death and pro-apoptotic signals is another hallmark of cancer and most of the chemotherapy agents kill cancer cells via inducing apoptosis. Understanding the mechanisms by which cancer cells escape apoptosis is used to design novel therapeutic agents [Citation8]. For example, designing BH3 mimetics to antagonizing Bcl-2 proteins [Citation32], reactivating the p53 pathway either by activation of TRAIL death receptor [Citation33] or with inhibiting Mdm2, or with directly interacting of molecules with p53 [Citation34].
2.5. Epithelial-mesenchymal transition (EMT)
EMT is a complex and reversible process in which epithelial cells lose their characteristics and gain mesenchymal properties, leading to the cytoskeleton remodelling and initiation of tumour cells migration and metastasis [Citation35]. During the process, epithelial cells lose E-cadherin, as an epithelial adhesion molecule, and acquire mesenchymal markers such as fibronectin and/or vimentin [Citation36]. Research findings have revealed the role of EMT and EMT inducing transcriptional factors, including Snail, Twist, Slug, Zeb1, and Zeb2, in therapy resistance. For example, Zheng et al. found that EMT induces resistance to chemotherapy in pancreatic cancer. They showed that knocking out EMT transcription factor, Twist1 and Snail1 enhances sensitivity to gemcitabine in the mouse model of pancreatic adenocarcinoma [Citation37]. Kim et al. demonstrated that 5-FU-resistant colon cancer cells show morphological changes relative to parental cells, including an increase in fibronectin, Twist, Zeb1, and Zeb2 [Citation38]. Furthermore, EMT transcription factors promote chemoresistance by inducing the expression of ABC transporters [Citation39–41] and the knocking down of EMT transcription factors sensitizes cancer cells to chemotherapy agents via suppressing drug efflux [Citation42–44].
2.6. Tumour microenvironment
Tumours are complex of malignant cells and various types of cells, such as fibroblasts, immune-inflammatory cells, adipose cells, neuroendocrine cells, vascular networks, as well as extracellular matrix (ECM) which create the tumour microenvironment (TME) [Citation45]. The dynamic interaction among components of TME leads to tumour initiation, growth, migration, and development of chemoresistance [Citation46]. One of the TME factors contributes to drug resistance is physical and biochemical barriers which limit penetration of drugs into solid tumours [Citation47]. Hypoxia and limited accessibility of cells to oxygen which arises from uncontrolled growth of cancer cells leads to upregulation of hypoxia-inducible factor 1 (HIF-1) [Citation48,Citation49]. HIF-1 stimulates drug efflux by the overexpression of MDR-1 and P-gp genes [Citation12,Citation50]. Based on the ‘reversed pH gradient’ concept in which extracellular environment of cancer cells have decreased pH, base anticancer drugs show weak distribution, enabling tumour cells to avoid apoptosis and resistance to the therapy [Citation51,Citation52]. Furthermore, secreting colony-stimulating factor-1 (CSF-1) by tumour-associated macrophages (TAMs) supports the proliferation and survival of cancer cells in glioblastoma multiform [Citation53,Citation54].
2.7. Cancer stem cells
Two models have been proposed for the cancer initiation: 1) the stochastic model proposes that each cancer cell dedifferentiates into a cancer stem cell (CSC), 2) the hierarchical model suggests that CSCs are the progenitors of differentiated subpopulation of tumour cells [Citation9]. CSCs are resistance to most anticancer agents, including chemotherapy. Resistance to chemotherapy of CSCs is related to mechanisms are discussed above, such as ABC transporters, changes in DNA repair mechanisms, gaining EMT phenotype, TME obstacles, overactivation of pro-survival signals, overactivation of anti-apoptotic signals, and overinhibition of pro-apoptotic signals [Citation55,Citation56]. Drug inactivating enzymes also make the CSCs resistance to chemotherapeutic drugs. For example, platinum drugs are inactivated by the thiol glutathione [Citation57] or TMZ, paclitaxel, epirubicin, and doxorubicin (DOX) by the aldehyde dehydrogenase (ALDH) [Citation58–60]. Furthermore, since chemotherapeutic drugs target proliferative cells and CSCs are quiescent, they are resistance to chemotherapy [Citation9].
3. LncRNAs and their roles in cancer
As mRNAs, lncRNAs are transcribed by pol II, spliced, 5` capped, and 3` polyadenylated [Citation61]. They can be classified based on the genomic loci: 1) intergenic lncRNAs are located between two protein-coding genes, 2) intronic lncRNAs are located between two exons and within an intronic region, 3) antisense lncRNAs originate from transcription of complementary strands, 4) bidirectional lncRNAs stem from bidirectional transcription of protein-coding genes, and 5) enhancer RNAs (eRNAs) stem from enhancer regions [Citation62]. Furthermore, lncRNAs are classified based on their mode of action: 1) signal lncRNAs express specifically in response to different stimuli [Citation63], 2) guide lncRNAs direct enzymatically active or regulatory proteins to proper locations in the genome, 3) decoy lncRNAs act as molecular sink to limit the availability of regulatory factors and modulate gene expression, and 4) scaffold lncRNAs provide an transient assembly platform for regulatory factors and enzymatic complexes [Citation61].
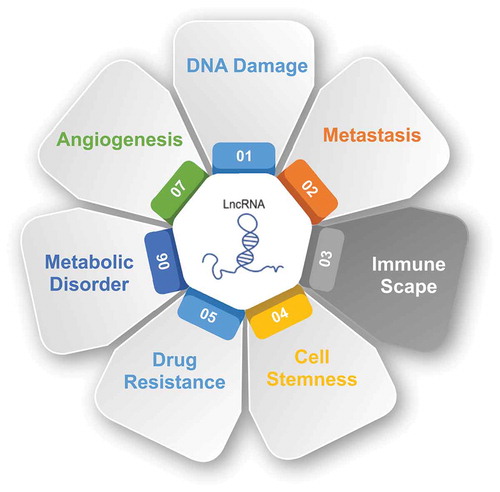
An increasing number of studies have demonstrated diverse roles of lncRNAs in cancer (). Since lncRNAs can act as oncogenes or tumour suppressor genes or both, dysfunction or aberrant expression of lncRNAs is closely associated with cancer cell proliferation, migration, invasion, apoptosis, immune escape, metabolic disorders, maintenance of stemness, and angiogenesis [Citation64,Citation65]. summarizes the roles of various lncRNAs in several cancers. Furthermore, increasing studies suggested lncRNAs as potential biomarkers in cancer detection and diagnosis [Citation66,Citation67]. Expression of many lncRNAs is more tissue-specific than mRNAs [Citation68], thus they can be used as reliable markers for tumour origin compared with mRNAs. Moreover, obtaining of lncRNAs is easy and less invasive due to presence of lncRNAs in body fluids such as plasma and urine [Citation69]. For example, the lncRNA PCA3 detection in the urine has been reported as a diagnostic biomarker in prostate cancer and the lncRNA HULC detection in the plasma makes it as a diagnostic biomarker in hepatocellular carcinoma [Citation70]. All of these features, introduce lncRNAs as superior diagnostic and therapeutic biomarkers. This vital information introduces promising targets for cancer therapy based on novel technologies including, siRNAs, ribozymes, antisense oligos, aptamers, nanobodies, CRISPR, ZNFs, TALENs, and RNA decoys [Citation71].
Table 1. The roles of lncRNAs in cancer
4. Autophagy in cancer
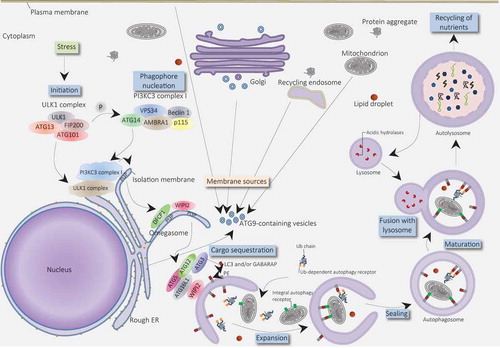
Although Christian de Duve firstly introduced the term ‘autophagy’ in 1963 [Citation86], the significance of autophagy in health and disease was highlighted when Yoshinori Ohsumi was awarded the 2016 Nobel Prize in Physiology or Medicine for his work on identification and characterization of autophagy mechanisms [Citation87]. Autophagy is a highly conserved process in response to environmental stresses such as starvation in which cells start to recycle and degrade macromolecules, including organelles, proteins, carbohydrates, and lipids as an energy supply and for synthesis of vital components [Citation88]. In this process, cellular organelles and components are engulfed by autophagosomes, double-membrane vesicles, for delivery to the lysosome which provides proteases for degradation of autophagosomes contents [Citation89]. The molecular mechanism of autophagy contains several conserved autophagy-related genes (ATGs). The formation of the autophagosome consists of three steps: initiation, nucleation, and elongation (). The initiation step begins with the activation of ULK1 (ATG1) complex (compromising ATG13, ATG101, FIP200, ULK1, and ULK2) which phosphorylates and activates class III PI3 K complex (compromising ATG14, UVRAG, VPS15, VPS34, AMBRA1, and Beclin-1) leads to generating phosphatidylinositol 3‐phosphate (PI3P) on the endoplasmic reticulum (ER) structure called the omegasome. PI3P recruits WIPI2 and DFCP1 to the omegasome for expanding and growth of the structure [Citation90]. In the next step, ATG conjugation system is required for the elongation of the omegasome. WIPI2 directly binds to ATG16L1 which recruits the ATG12~ ATG5–ATG16L1 complex that enhances the ATG3-mediated conjugation of γ-aminobutyric acid receptor-associated proteins (GABARAPs) and microtubule-associated protein light chain 3 (LC3) proteins to membrane-resident phosphatidylethanolamine (PE) [Citation91]. As a result, the omegasome expands and autophagic components are surrounded and enveloped in a double-membrane vesicle, autophagosome [Citation92]. In the final step, autophagosome fusion with lysosome is mediated by the Rab GTPases (Rab7), SNAREs (soluble N-ethylmaleimide-sensitive factor activating protein receptors), ESCRT (endosomal sorting complex required for transport), and class C Vps proteins [Citation93]. Following digestion of the autophagosome components, essential products such as amino acids and fatty acids export back into cytosol by lysosomal transporters or permeases, playing a crucial role in survival of starving cells [Citation94,Citation95].
4.1. The roles of autophagy in cancer
Autophagy plays dual roles in cancer biology and its regulation contributes to the oncogenes or tumour suppressor proteins expression, whereas the activation of oncogenes leads to the suppression of autophagy and enhancement of tumour formation, and negative regulation of tumour suppressor factors induces autophagy and suppresses cancer initiation [Citation96].
4.1.1. Autophagy and tumour promotion
Several studies demonstrated that autophagy promotes growth and survival of cancer cells [Citation97,Citation98]. Stressful conditions, such as nutrient deprivation and hypoxia, in the TME especially in the central part of solid tumours, activates autophagy to survive under these stresses by providing substrates for the production of adenosine triphosphate (ATP) [Citation96,Citation99]. Under hypoxic conditions, hypoxia-inducible factor-1 alpha (HIF-1α) induces autophagy activation and cancer progression [Citation100]. Therefore, autophagy promotes tumour cell survival via enhancing tolerance to stress and supplying nutrients to encounter the metabolic demands of tumours. The deletion of core autophagy proteins also supports tumour promotion roles of autophagy. It has been reported that autophagy suppression by deletion of Beclin-1 induces cell death [Citation101,Citation102]. Wei et al. reported that deletion of FIP200 and subsequent suppression of autophagy in mouse model of breast cancer suppresses tumour initiation and progression [Citation103]. Moreover, it has been found that ATG5 overexpressed in prostate [Citation104] and gastric [Citation105] cancers, while ATG7 overexpressed in bladder cancer [Citation106].
Several studies showed links between autophagy, metastasis, and CSCs. Cufí et al. reported that autophagy inhibition reduces the migratory and invasive capabilities in breast CSCs, leading to decreased expression of the mesenchymal marker (vimentin) and an increase of the epithelial marker (CD24) [Citation107]. In another study, Galavotti et al. found that the upregulation of DRAM1 and SQSTM1, as autophagy regulators, are correlated with the expression of mesenchymal markers in glioblastoma CSCs [Citation108].
4.1.2. Autophagy and tumour suppression
As mentioned above, autophagy plays a dual role in tumorigenesis. It has been shown that almost 50% of breast, prostate, and ovarian cancers carry one allele of Beclin-1 [Citation109–111]. Beclin-1, a key component of in the nucleation of autophagosome and a tumour suppressor, haploinsufficiency suppresses autophagy and leads to cancer progression [Citation112]. Other studies also reported downregulation of Beclin-1 in different cancers, including HCC [Citation113], osteosarcoma [Citation114], and glioblastoma [Citation115]. Mutation of another autophagic factor, UVRAG, inhibits autophagy and causes gastric and colon cancers [Citation116,Citation117]. Moreover, autophagy prevents tumorigenesis through the decreasing of reactive oxygen species (ROS) and oxidative stress [Citation96,Citation118].
Apart from the prometastatic potential of autophagy, it can also prevent the metastatic capability of tumour cells. It has been shown that autophagy adversely correlates with metastasis and EMT in pancreatic, breast, glioblastoma, and gastric CSCs [Citation119]. Catalano et al. revealed that downregulation of Beclin-1, ATG5 or ATG7 in glioblastoma cells increases migration and invasion of tumour cells and upregulates EMT regulators, suggesting that autophagy reduces EMT and metastasis in glioblastoma cells [Citation120].
4.2. Autophagy in chemoresistance
Similar to the paradoxical role of autophagy in tumorigenesis and tumour inhibition, autophagy can induce resistance or sensitivity to anticancer drugs. Thus, we delineate autophagy roles in the chemoresistance of cancer therapy.
4.2.1. Inhibition of autophagy overcomes resistance
Several studies have verified that tumour resistance to chemotherapy can be enhanced via upregulation of autophagy, thus inhibition of autophagy augments resistance to the therapeutic agents. Furthermore, elevated levels of autophagy has been reported in patients with poor prognosis. Thus, developing pharmacological components for inhibiting autophagy has attracted increasing attention. The Food and Drug Administration (FDA) approved chloroquine (CQ) and hydroxychloroquine (HSQ) as autophagy inhibitors which are combined with anticancer therapies in (pre)clinical trials. CQ and HSQ are antimalarial drugs that are concentrated in acidic lysosomes, raise the pH, inhibit lysosomal activity, and subsequently inhibit autophagy [Citation121].
A study investigated the effect of autophagy inhibition on DOX-treated triple-negative breast cancer cells. They found that combination of DOX and an inhibitor of autophagy, 3-methyladenine (3-MA), sensitized cancer cells to DOX by switching cell death type from apoptosis to necroptosis [Citation122]. The autophagy inhibitory activity of 3-MA is related to its ability in the prevention of autophagosome formation via inhibiting class III PI3 K [Citation123]. Similarly, CQ reversed resistance to DOX in DOX-resistance breast cancer cells [Citation124]. Furthermore, CQ considerably enhances tumour sensitivity to 5-FU both in vitro and in vivo [Citation125,Citation126]. Pantoprazole, a proton pump inhibitor (PPI), also inhibits autophagy by accumulation in acidic endosomes and rising their pH [Citation127]. Qian et al. showed that pantoprazole sensitized cancer cells to seven clinically-used chemotherapy by inhibition of autophagy [Citation128]. In addition to pharmaceutical components, genetically silencing of autophagy also have been proposed to increase tumour cells’ sensitivity to chemotherapy. For example, An et al. found that miR-23b-3p sensitized chemoresistance gastric cancer cells to chemotherapeutic agents such as 5-FU, vincristine, and cisplatin by targeting ATG12 and high-mobility group box 2 (HMGB2), suggesting inhibition of autophagy enhances tumour sensitivity to chemotherapy [Citation129]. Anna et al. investigated the effects of autophagy inhibition via chloroquine treatment or CRISPR/Cas9 ATG5 knockout on ovarian CSCs. They revealed that autophagy inhibition reduces resistance to carboplatin [Citation130].
4.2.2. Promotion of autophagy overcomes resistance
In contrast to a pro-survival role and ability in inhibition of apoptosis, autophagy also has a pro-death role in which excessive or uncontrolled autophagy can induce apoptosis or autophagic cell death (type II programmed cell death) [Citation123]. Based on this concept, several studies have been designed to investigate the therapeutic potential of autophagy in different cancers.
Lee et al. investigated the effect of suberoylanilide hydroxamic acid (SAHA), a histone deacetylase (HDAC) inhibitor, on tamoxifen-resistant MCF-7 (TAMR/MCF-7) cells. They found that SAHA significantly increased autophagic cell death in TAMR/MCF-7 cells and suppressed tumour growth [Citation131]. NVP-BEZ235, a dual inhibitor of phosphatidylinositol 3-kinase (PI3 K)/mammalian target of rapamycin (mTOR), suppressed cisplatin-resistant urothelial cancer cell proliferation by autophagy flux [Citation132]. Similarly, Westhoff et al. reported that NVP-BEZ235 enhanced DOX–induced apoptosis and sensitized neuroblastoma cells to DOX [Citation133]. It has been shown that RAD001, an autophagy activator, synergistically sensitized papillary thyroid cancer (PTC) cells to DOX via inducing autophagy [Citation134].
5. LncRNAs, chemoresistance, and autophagy
As mentioned above, the expression of lncRNAs is extensively altered in cancers and participate in tumorigenesis by playing an oncogenic or tumour-suppressive role. Notably, increasing evidence has demonstrated that lncRNAs also play essential roles in resistance to cancer drugs, in particular chemotherapy agents. For example, Huang et al. surveyed the contribution of lncRNAs in resistance to docetaxel in breast cancer cell lines by sequencing of the whole genome. Their analyses revealed that 50 and 22 lncRNAs consistently upregulated and downregulated, respectively, in both docetaxel-resistant cell lines. Further analyses showed that four upregulated lncRNAs located near or within the ABCB1 locus, suggesting a regulatory relationship between the four lncRNAs and the ABCB1 gene. They also reported the lncRNA EPB41L4A-AS2 as a potential biomarker for sensitivity to docetaxel [Citation135]. summarizes some dysregulated lncRNAs are involved in chemoresistance. In this section, we review and discuss how lncRNAs mediate chemoresistance in the tumour by modulating autophagy.
Table 2. LncRNAs and their function in chemoresistance
5.1. BLACAT1
LncRNA bladder cancer-associated transcript 1 (BLACAT1), located on 1q32.1 chromosome and with a length of 2616 bp, is overexpressed in many cancers which promotes tumour cells proliferation, migration, and invasion [Citation147,Citation148]. Several studies demonstrated that lncRNA BLACAT1 is involved in resistance to chemotherapy. It has been shown that BLACAT1 is upregulated in the oxaliplatin resistance of gastric cancer cells and tissue, whereas BLACAT1 knockdown suppresses tumour growth via decreasing drug resistance-related genes, including ABCB1 [Citation149]. Huang et al. revealed that lncRNA BLACAT1 promotes chemoresistance through modulating autophagy. They indicated that the expression of BLACAT1, ATG7, MRP1, LC3-II/LC3-I, and Beclin-1 was meaningfully upregulated in cisplatin-resistant NSCLC cells compared with in cisplatin-sensitive cells. They also identified miR-17 as a target of lncRNA BLACAT1 and showed that BLACAT1 activates autophagy by inhibiting miR-17. BLACAT1/miR-17/ATG7 pathway contributes in resistance to cisplatin, which provide promising target for NSCLC treatment [Citation150].
5.2. MALAT1
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), located on 11q13 chromosome and with a length of 8.5 kb, contributes to cancer cell proliferation, migration, invasion, and angiogenesis [Citation151]. Cai et al. found the significant upregulation of MALAT1 in TMZ‐resistant glioblastoma cells and MALAT1 promotes the chemoresistance by suppressing miR-101, whereas MALAT1 knockdown alleviates resistance of glioblastoma cells to TMZ through promoting apoptosis and inhibiting cell proliferation [Citation152]. It has been shown that MALAT1 is overexpressed in chemoresistance gastric cancer and involved in chemoresistance by regulating autophagy [Citation153,Citation154]. YiRen et al. found that MALAT1 improves autophagy-associated chemoresistance in gastric cancer cells. They revealed that MALAT1 knockdown effectively inhibited autophagy and sensitized gastric cancer cells to cisplatin. Mechanistically, MALAT1 sequestrate miR-23b-3p which reduces inhibitory effect of miR-23b-3p on ATG12 [Citation153]. In another study, Xi et al. indicated that lncRNA MALAT1 potentiates resistance to cisplatin by improving autophagy in cisplatin-resistance gastric cancer cells. They identified miR-30b as a direct target of MALAT1, whereas overexpression of miR-30b significantly reduced chemoresistance by inhibiting ATG5 [Citation154].
5.3. XIST
The X inactivate-specific transcript (XIST), transcribed from the inactive X chromosome, is dysregulated in glioma, HCC, colorectal cancer, gastric cancer, breast cancer, ovarian cancer, cervical cancer, nasopharyngeal carcinoma, and pancreatic cancer [Citation155]. It was proved that the dysfunctional expression of lncRNA XIST is associated with tumour stage, distant metastasis, lymph node metastasis, and overall survival [Citation156]. Zhu et al. reported that lncRNA XIST increases resistance to doxorubicin by regulating the miR-124/SGK1 axis in colorectal cancer, whereas XIST knockdown inhibits chemoresistance [Citation157]. Xiao et al. also indicated that lncRNA XIST greatly upregulated in 5-FU resistance colorectal cancer cells which XIST knockdown reversed resistance to 5-FU [Citation158]. Moreover, lncRNA XIST is able to regulate chemoresistance through modulating autophagy. It has been demonstrated that lncRNA XIST is overexpressed in NSCLC tumours and cisplatin-resistant cells. Knockdown of lncRNA XIST significantly alleviated chemoresistance in cisplatin-resistant A549 cells. Mechanistically, lncRNA XIST targets miR-17 to attenuate ATG7 expression. Thus, lncRNA XIST knockdown attenuates resistance to cisplatin via autophagy suppression [Citation159].
5.4. SNHGs
Small nucleolar RNA host genes (SNHGs) have acknowledged roles in various cancers. For example, SNHG1 is involved in proliferation and invasion of glioma cells [Citation160] or SNHG12 promotes gastric cancer progression and associated with poor prognosis of patients by targeting miR-16 [Citation161]. Recent studies indicated that lncRNA SNHGs are able to influence the response of cancer cells to chemotherapy agents. Wang et al. reported that knockdown of SNHG12 attenuates the resistance of NSCLC cells to cisplatin, paclitaxel, and gefitinib by inducing apoptosis [Citation162]. Another study demonstrated that SNHG6 increases resistance to 5-FU in colorectal cancer cells, whereas knockdown of SNHG6 inhibits tumour growth in animal model and improves 5-FU therapy. It has been shown that SNHG6 promotes resistance to 5-FU by promoting autophagy. Mechanistically, SNHG6 promotes ULK1-induced autophagy by sponging miR‑26a‑5p [Citation163]. Another member of the SNHGs family that enhances resistance through activating autophagy is SNHG14. Zhang et al. found that SNHG14 overexpression enhances resistance of pancreatic cancer cells to gemcitabine by stimulating autophagy. They revealed that SNHG14 acts as a sponge for miR-101 which could inhibit autophagy [Citation164].
5.5. HULC
Highly upregulated in liver cancer (HULC), located on 6p24.3 chromosome with a length of approximately 500 bp, dysregulated in different cancers and contributes in tumour cell proliferation, survival, growth, invasion, and angiogenesis [Citation165]. It has been reported that lncRNA HULC overexpressed in gastric cancer patients compared with healthy ones and knockdown of HULC enhances sensitivity of gastric cancer cells to chemotherapeutic agents [Citation166]. Xiong et al. found that lncRNA HULC can activate autophagy via stabilizing silent information regulator 1 (Sirt1) protein in HCC. Furthermore, they demonstrated that HULC downregulates the expression of miR-6825-5p, miR6845-5p, and miR6886-3p, leading to a higher expression of ubiquitin-specific peptidase 22 (USP22) and Sirt1. Moreover, silencing HULC using siRNAs sensitized HCC cells to oxaliplatin, 5-FU, and pirarubicin. They suggested that HULC/USP22/Sirt1/protective autophagy axis may be a potential target in developing strategy for sensitizing HCC to chemotherapy [Citation167].
5.6. CASC2
Cancer susceptibility candidate 2 (CASC2), located on 10q26 chromosome, is a tumour suppressor lncRNA which aberrantly expressed in human cancers, including gastric, colorectal, glioma, lung, and bladder cancers [Citation168]. Several studies have been proved the role of CASC2 in resistance to chemotherapy drugs. For example, it has been demonstrated that overexpression of CASC2 enhances cisplatin sensitivity in gastric cancer, whereas CASC2 knockdown increased chemoresistance [Citation169]. Similarly, upregulation of CASC2 in cisplatin-resistance HCC and oesophageal squamous cell carcinoma cells enhances cisplatin sensitivity by targeting miR-222 and miR-181a, respectively [Citation170,Citation171]. Jiang et al. found that CASC2 is downregulated in glioma. They showed that lncRNA CASC2 upregulation enhances sensitivity of glioma cells to TMZ by inhibiting autophagy. Mechanistically, CASC2 negatively regulates miR-193a-5p and its target gene, mTOR, which is an autophagy inhibitor [Citation172].
5.7. KCNQ1OT1
The KCNQ1 opposite strand/antisense transcript 1 (KCNQ1OT1), located on 11p15.5 chromosome, participates in tumorigenesis and chemoresistance of different cancers. For example, Ren et al. demonstrated that lncRNA KCNQ1OT1is highly expressed in lung adenocarcinoma and correlates with big tumour size, lymphatic metastasis, poor differentiation, and TNM stages. They reported that knockdown of KCNQ1OT1 attenuates resistance of lung adenocarcinoma to paclitaxel [Citation173]. Another study also proved that knockdown of KCNQ1OT1 enhances sensitivity of osteosarcoma cells to cisplatin [Citation174]. Li et al. found that KCNQ1OT1 enhances resistance of colon cancer to oxaliplatin by targeting autophagy. Their further analyses indicated that KCNQ1OT1 acts as a sponge for miR-34a, leading to the upregulation of ATG4B and promoting protective autophagy. Thus, chemoresistance of colon cancer cells is regulated by KCNQ1OT1/miR-34a/Atg4B axis which suggests a novel target for developing colon cancer therapeutics [Citation175].
5.8. GAS5
Growth arrest-specific 5 (GAS5) located on 1q25 chromosome, is a tumour suppressor lncRNA that downregulates in many cancers and its level of expression regulates proliferation, apoptosis, EMT, and metastasis of several cancers [Citation176]. Duo to tumour-suppressive role, GAS5 inhibits tumour progression and chemoresistance. For example, GAS5 overexpression in epithelial ovarian cancer effectively enhances the sensitivity of cells to cisplatin and increases apoptosis [Citation177]. Overexpression of GAS5 decreases tumour growth in mice, while GAS5 knockdown significantly increased cell resistance to gemcitabine and 5-FU in pancreatic cancer cells [Citation178]. Zhang et al. also reported that GAS5 could affect chemoresistance by regulating autophagy. They found that expression of GAS5 in NSCLC tissues is substantially higher than adjacent ones. Moreover, they reported that knockdown of GAS5 increases cisplatin IC50 of lung cancer cell lines, while overexpression of GAS5 decreases cisplatin IC50 of cisplatin resistance cells. They also indicated that knockdown of GAS5 in lung cancer cells and its overexpression in cisplatin resistance cells decreases and increases autophagy, respectively. Thus, GAS5 increases cisplatin sensitivity via inhibiting autophagy [Citation179].
5.9. H19
H19, located on 11p15.5 chromosome, is an oncogene lncRNA that contributes to various human cancers, including gastric, thyroid, breast, and HCC [Citation180]. LncRNA H19 is involved in proliferation, invasion, migration, and metastasis of cancer cells [Citation181,Citation182]. It has been shown that lncRNA H19 modulates the resistance of breast cancer to tamoxifen [Citation183,Citation184]. Gao et al. found that knockdown of H19 enhances the sensitivity of breast cancer cells to tamoxifen by inhibiting EMT and Wnt pathway [Citation183], while Wang et al. indicated that H19 promotes resistance to tamoxifen in breast cancer through increasing autophagy [Citation184]. Overexpression of H19 promotes tamoxifen resistance and autophagy by downregulating methylation of Beclin-1 promoter [Citation184]. Another study reported that H19 enhances the resistance of colorectal cancer to 5-FU through acting as a sponge for miR-194–5p and promoting Sirt1-mediated autophagy [Citation185].
5.10. HOTAIR
Aberrant expression of HOX transcript antisense intergenic RNA (HOTAIR), located on 12q13.13 chromosome with a length of 2158 bp, has been reported in various human cancers [Citation186,Citation187]. LncRNA HOTAIR plays important roles in tumour proliferation, progression, angiogenesis, poor prognosis, and drug resistance [Citation187]. It has been shown that HOTAIR overexpression induces chemoresistance in ovarian cancer. It is suggested that lncRNA HOTAIR enhances DDR and NF-κB pathway [Citation188]. Xiao et al. also found that knockdown of HOTAIR inhibits cell proliferation and reverses chemoresistance by targeting miR-203a-3p and Wnt/β-catenin pathway in colorectal cancer [Citation189]. Sun at al. conducted a study to reveal the effect of HOTAIR on autophagy and chemoresistance. They resulted that HOTAIR modulates resistance to cisplatin by affecting the expression of Beclin-1, MDR, and P-gp in endometrial cancer cell line [Citation190].
5.11. Other lncRNAs
In addition to mentioned lncRNAs, there are other ones mediate chemoresistance by modulating autophagy. For example, lncRNA AC023115.3 suppresses resistance to cisplatin in glioblastoma cells by inhibiting autophagy. Mechanistically, AC023115.3 reduces the inhibitory effect of miR-26a on glycogen synthase kinase 3 (GSK3) [Citation191]. GSK3 induces degradation of Mcl-1, a Bcl-2 family member, leading to a decrease in autophagy [Citation191]. Cai et al. conducted a microarray analysis to compare differential lncRNA expression in doxorubicin-resistant gallbladder cancer cells and their parental cells. They found that gallbladder cancer drug resistance-associated lncRNA1 (GBCDRlnc1) regulates chemoresistance by activating autophagy, whereas GBCDRlnc1 knockdown sensitized gallbladder cancer cells to doxorubicin via inhibiting autophagy [Citation192]. It has been shown that the expression of linc00515 and ATG14 is considerably higher in melphalan-resistant myeloma cells. Linc00515 knockdown inhibits chemoresistance and autophagy in myeloma cells. Mechanistically, linc00515 targets miR-140-5p, which attenuates inhibitory effect of miR-140-5p on ATG14 [Citation193]. Wang et al. reported that lncRNA CTA significantly downregulated in osteosarcoma tissues and its low expression markedly associated with tumour size, the advanced clinical stage, and poor prognosis. They found that overexpression of CTA sensitized osteosarcoma cells to doxorubicin via inhibiting autophagy. LncRNA CTA directly targets miR-210 [Citation194].
6. Conclusions and perspectives
LncRNAs, as a large and heterogeneous subclass of ncRNAs, play indispensable role in different aspects of tumorigenesis and are considered as novel biomarkers in cancer diagnosis and prognosis. Regarding to pivotal roles of lncRNAs in chemoresistance and modulation of autophagy, antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), and gene-editing tools including CRISPR/Cas9 to target lncRNAs should be used inside tumour cells and reverse resistance to chemotherapy agents.
Although most of the studies have discussed on the lncRNAs typically function as sponges for autophagy-related miRNAs, they also have more complex functions in the regulation of autophagy, including transcriptional regulation, histone remodelling, and protein–protein interactions. Certainly, more studies to address these functions and interactions will open new doors for finding new targets and new strategies for overcoming chemoresistance in cancer therapy. On the other hand, classifying the roles of lncRNAs based on different types of autophagy, such as mitophagy, will help to investigate their functions more specifically. Furthermore, it should also be noticed that lncRNAs exert their functions directly or indirectly through binding to single or multiple molecules. Therefore, comprehensive study and deep insight into the molecular targets of lncRNAs in autophagy is crucial for reversing chemoresistance. Moreover, there are some challenges in modulating autophagy in drug resistance which can be considered in future investigations, including creating balance between tumorigenesis and tumour suppressive roles of autophagy, selectively targeting autophagic machinery, and defining the role of cellular context in upregulation or downregulation of autophagy. However, to promote therapeutic interventions in cancer by targeting the functional relationship between lncRNAs and autophagy, further studies on pre-clinical and clinical trials are urgently required.
Disclosure of potential conflicts of interest
The authors declare that there is no conflict of interest.
Additional information
Funding
References
- Abubakar I, Tillmann T, Banerjee A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;385(9963):117–171.
- Jiang M-C, Ni -J-J, Cui W-Y, et al. Emerging roles of lncRNA in cancer and therapeutic opportunities. Am J Cancer Res. 2019;9(7):1354.
- Zheng H-C. The molecular mechanisms of chemoresistance in cancers. Oncotarget. 2017;8(35):59950.
- Iyer MK, Niknafs YS, Malik R, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47(3):199.
- Goradel NH, Mohammadi N, Haghi‐Aminjan H, et al. Regulation of tumor angiogenesis by microRNAs: state of the art. J Cell Physiol. 2019;234(2):1099–1110.
- Jo Y, Choi N, Kim K, et al. Chemoresistance of cancer cells: requirements of tumor microenvironment-mimicking in vitro models in anti-cancer drug development. Theranostics. 2018;8(19):5259.
- Wang X, Zhang H, Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019;2:141–160.
- Rebucci M, Michiels C. Molecular aspects of cancer cell resistance to chemotherapy. Biochem Pharmacol. 2013;85(9):1219–1226.
- Nunes T, Hamdan D, Leboeuf C, et al. Targeting cancer stem cells to overcome chemoresistance. Int J Mol Sci. 2018;19(12):4036.
- Sauna ZE, Kim I-W, Ambudkar SV. Genomics and the mechanism of P-glycoprotein (ABCB1). J Bioenerg Biomembr. 2007;39(5–6):481–487.
- Xue X, Liang XJ. Overcoming drug efflux-based multidrug resistance in cancer with nanotechnology. Chin J Cancer. 2012;31(2):100–109.
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48–58.
- Zheng HC. The molecular mechanisms of chemoresistance in cancers. Oncotarget. 2017;8(35):59950–59964.
- Yin J, Zhang J. Multidrug resistance-associated protein 1 (MRP1/ABCC1) polymorphism: from discovery to clinical application. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36(10):927–938.
- Munoz M, Henderson M, Haber M, et al. Role of the MRP1/ABCC1 multidrug transporter protein in cancer. IUBMB Life. 2007;59(12):752–757.
- Cho S, Lu M, He X, et al. Notch1 regulates the expression of the multidrug resistance gene ABCC1/MRP1 in cultured cancer cells. Proc Natl Acad Sci U S A. 2011;108(51):20778–20783.
- Mao Q, Unadkat JD. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport–an update. Aaps J. 2015;17(1):65–82.
- Horsey AJ, Cox MH, Sarwat S, et al. The multidrug transporter ABCG2: still more questions than answers. Biochem Soc Trans. 2016;44(3):824–830.
- Sun Y, Guan Z, Liang L, et al. NF-kappaB signaling plays irreplaceable roles in cisplatin-induced bladder cancer chemoresistance and tumor progression. Int J Oncol. 2016;48(1):225–234.
- Yao J, Huang A, Zheng X, et al. 53BP1 loss induces chemoresistance of colorectal cancer cells to 5-fluorouracil by inhibiting the ATM-CHK2-P53 pathway. J Cancer Res Clin Oncol. 2017;143(3):419–431.
- Zhang Y, Hu Y, Wang JL, et al. Proteomic identification of ERP29 as a key chemoresistant factor activated by the aggregating p53 mutant Arg282Trp. Oncogene. 2017;36(39):5473–5483.
- Lakshmanan I, Salfity S, Seshacharyulu P, et al. MUC16 regulates TSPYL5 for lung cancer cell growth and chemoresistance by suppressing p53. Clin Cancer Res. 2017;23(14):3906–3917.
- Pilie PG, Tang C, Mills GB, et al. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat Rev Clin Oncol. 2019;16(2):81–104.
- Damia G, Broggini M. Platinum resistance in ovarian cancer: role of DNA repair. Cancers (Basel). 2019;11:1.
- Helleday T, Petermann E, Lundin C, et al. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8(3):193–204.
- de Angelis PM, Fjell B, Kravik KL, et al. Molecular characterizations of derivatives of HCT116 colorectal cancer cells that are resistant to the chemotherapeutic agent 5-fluorouracil. Int J Oncol. 2004;24(5):1279–1288.
- De Angelis PM, Svendsrud DH, Kravik KL, et al. Cellular response to 5-fluorouracil (5-FU) in 5-FU-resistant colon cancer cell lines during treatment and recovery. Mol Cancer. 2006;5:20.
- Li Q, Gardner K, Zhang L, et al. Cisplatin induction of ERCC-1 mRNA expression in A2780/CP70 human ovarian cancer cells. J Biol Chem. 1998;273(36):23419–23425.
- Augustine CK, Yoo JS, Potti A, et al. Genomic and molecular profiling predicts response to temozolomide in melanoma. Clin Cancer Res. 2009;15(2):502–510.
- Drablos F, Feyzi E, Aas PA, et al. Alkylation damage in DNA and RNA–repair mechanisms and medical significance. DNA Repair (Amst). 2004;3(11):1389–1407.
- Chen L, Zeng Y, Zhou S-F. Role of apoptosis in cancer resistance to chemotherapy., Current understanding of apoptosis - programmed cell death. 2018.
- Azmi AS, Mohammad RM. Non-peptidic small molecule inhibitors against Bcl-2 for cancer therapy. J Cell Physiol. 2009;218(1):13–21.
- Dimberg LY, Anderson CK, Camidge R, et al. On the TRAIL to successful cancer therapy? Predicting and counteracting resistance against TRAIL-based therapeutics. Oncogene. 2013;32(11):1341–1350.
- Mandinova A, Lee SW. The p53 pathway as a target in cancer therapeutics: obstacles and promise. Sci Transl Med. 2011;3(64):64rv1.
- Acloque H, Adams MS, Fishwick K, et al. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119(6):1438–1449.
- Smith BN, Bhowmick NA. Role of EMT in metastasis and therapy resistance. J Clin Med. 2016;5:2.
- Zheng X, Carstens JL, Kim J, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527(7579):525–530.
- Kim AY, Kwak JH, Je NK, et al. Epithelial-mesenchymal transition is associated with acquired resistance to 5-fluorocuracil in HT-29 colon cancer cells. Toxicol Res. 2015;31(2):151–156.
- Zhu K, Chen L, Han X, et al. Short hairpin RNA targeting Twist1 suppresses cell proliferation and improves chemosensitivity to cisplatin in HeLa human cervical cancer cells. Oncol Rep. 2012;27(4):1027–1034.
- Tsou SH, Chen TM, Hsiao HT, et al. A critical dose of doxorubicin is required to alter the gene expression profiles in MCF-7 cells acquiring multidrug resistance. PLoS One. 2015;10(1):e0116747.
- Li W, Liu C, Tang Y, et al. Overexpression of Snail accelerates adriamycin induction of multidrug resistance in breast cancer cells. Asian Pac J Cancer Prev. 2011;12(10):2575–2580.
- Hamada S, Satoh K, Hirota M, et al. The homeobox gene MSX2 determines chemosensitivity of pancreatic cancer cells via the regulation of transporter gene ABCG2. J Cell Physiol. 2012;227(2):729–738.
- Lee SH, Oh SY, Do SI, et al. SOX2 regulates self-renewal and tumorigenicity of stem-like cells of head and neck squamous cell carcinoma. Br J Cancer. 2014;111(11):2122–2130.
- Hou Y, Zhu Q, Li Z, et al. The FOXM1-ABCC5 axis contributes to paclitaxel resistance in nasopharyngeal carcinoma cells. Cell Death Dis. 2017;8(3):e2659.
- Wang M, Zhao J, Zhang L, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8(5):761–773.
- Senthebane DA, Rowe A, Thomford NE, et al. The role of tumor microenvironment in chemoresistance: to survive, keep your enemies closer. Int J Mol Sci. 2017;18:7.
- Jo Y, Choi N, Kim K, et al. Chemoresistance of cancer cells: requirements of tumor microenvironment-mimicking in vitro models in anti-cancer drug development. Theranostics. 2018;8(19):5259–5275.
- Hashemi Goradel N, Ghiyami-Hour F, Jahangiri S, et al. Nanoparticles as new tools for inhibition of cancer angiogenesis. J Cell Physiol. 2018;233(4):2902–2910.
- Goradel NH, Asghari MH, Moloudizargari M, et al. Melatonin as an angiogenesis inhibitor to combat cancer: mechanistic evidence. Toxicol Appl Pharmacol. 2017;335:56–63.
- Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627.
- Webb BA, Chimenti M, Jacobson MP, et al. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11(9):671–677.
- Wojtkowiak JW, Verduzco D, Schramm KJ, et al. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol Pharm. 2011;8(6):2032–2038.
- Quail DF, Bowman RL, Akkari L, et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science. 2016;352(6288):aad3018.
- De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23(3):277–286.
- Nunes T, Hamdan D, Leboeuf C, et al. Targeting cancer stem cells to overcome chemoresistance. Int J Mol Sci. 2018;19:12.
- Zhao J. Cancer stem cells and chemoresistance: the smartest survives the raid. Pharmacol Ther. 2016;160:145–158.
- Meijer C, Mulder NH, Timmer-Bosscha H, et al. Relationship of cellular glutathione to the cytotoxicity and resistance of seven platinum compounds. Cancer Res. 1992;52(24):6885–6889.
- Schafer A, Teufel J, Ringel F, et al. Aldehyde dehydrogenase 1A1–a new mediator of resistance to temozolomide in glioblastoma. Neuro Oncol. 2012;14(12):1452–1464.
- Croker AK, Allan AL. Inhibition of aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and radiation resistance of stem-like ALDHhiCD44(+) human breast cancer cells. Breast Cancer Res Treat. 2012;133(1):75–87.
- Tanei T, Morimoto K, Shimazu K, et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res. 2009;15(12):4234–4241.
- Balas MM, Johnson AM. Exploring the mechanisms behind long noncoding RNAs and cancer. Noncoding RNA Res. 2018;3(3):108–117.
- Fernandes JCR, Acuna SM, Aoki JI, et al. Long non-coding RNAs in the regulation of gene expression: physiology and disease. Noncoding RNA. 2019;5:1.
- Ma L, Bajic VB, Zhang Z. On the classification of long non-coding RNAs. RNA Biol. 2013;10(6):925–933.
- Jiang MC, Ni JJ, Cui WY, et al. Emerging roles of lncRNA in cancer and therapeutic opportunities. Am J Cancer Res. 2019;9(7):1354–1366.
- Li J, Meng H, Bai Y, et al. Regulation of lncRNA and its role in cancer metastasis. Oncol Res. 2016;23(5):205–217.
- Wang X, Chen X, Zhang D, et al. Prognostic and clinicopathological role of long non-coding RNA taurine upregulated 1 in various human malignancies: A systemic review and meta-analysis. Tumour Biol. 2017;39(7):1010428317714361.
- Wang M, Dong X, Feng Y, et al. Prognostic role of the long non-coding RNA, SPRY4 intronic transcript 1, in patients with cancer: a meta-analysis. Oncotarget. 2017;8(20):33713–33724.
- Derrien T, Johnson R, Bussotti G, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22(9):1775–1789.
- Gu Y, Chen T, Li G, et al. LncRNAs: emerging biomarkers in gastric cancer. Future Oncol. 2015;11(17):2427–2441.
- Yarmishyn AA, Kurochkin IV. Long noncoding RNAs: a potential novel class of cancer biomarkers. Front Genet. 2015;6:145.
- Tripathi MK, Doxtater K, Keramatnia F, et al. Role of lncRNAs in ovarian cancer: defining new biomarkers for therapeutic purposes. Drug Discov Today. 2018;23(9):1635–1643.
- Jia J, Li F, Tang XS, et al. Long noncoding RNA DANCR promotes invasion of prostate cancer through epigenetically silencing expression of TIMP2/3. Oncotarget. 2016;7(25):37868–37881.
- Huang JK, Ma L, Song WH, et al. LncRNA-MALAT1 promotes angiogenesis of thyroid cancer by modulating tumor-associated macrophage FGF2 protein secretion. J Cell Biochem. 2017;118(12):4821–4830.
- Zheng A, Song X, Zhang L, et al. Long non-coding RNA LUCAT1/miR-5582-3p/TCF7L2 axis regulates breast cancer stemness via Wnt/beta-catenin pathway. J Exp Clin Cancer Res. 2019;38(1):305.
- Shang A, Wang W, Gu C, et al. Long non-coding RNA HOTTIP enhances IL-6 expression to potentiate immune escape of ovarian cancer cells by upregulating the expression of PD-L1 in neutrophils. J Exp Clin Cancer Res. 2019;38(1):411.
- Zhao J, Du P, Cui P, et al. LncRNA PVT1 promotes angiogenesis via activating the STAT3/VEGFA axis in gastric cancer. Oncogene. 2018;37(30):4094–4109.
- Li H, An J, Wu M, et al. LncRNA HOTAIR promotes human liver cancer stem cell malignant growth through downregulation of SETD2. Oncotarget. 2015;6(29):27847–27864.
- Schmidt K, Joyce CE, Buquicchio F, et al. The lncRNA SLNCR1 mediates melanoma invasion through a conserved SRA1-like region. Cell Rep. 2016;15(9):2025–2037.
- Yang B, Zhang L, Cao Y, et al. Overexpression of lncRNA IGFBP4-1 reprograms energy metabolism to promote lung cancer progression. Mol Cancer. 2017;16(1):154.
- Lu Z, Xiao Z, Liu F, et al. Long non-coding RNA HULC promotes tumor angiogenesis in liver cancer by up-regulating sphingosine kinase 1 (SPHK1). Oncotarget. 2016;7(1):241–254.
- Yang W, Xu X, Hong L, et al. Upregulation of lncRNA GAS5 inhibits the growth and metastasis of cervical cancer cells. J Cell Physiol. 2019;234(12):23571–23580.
- Wang TH, Lin YS, Chen Y, et al. Long non-coding RNA AOC4P suppresses hepatocellular carcinoma metastasis by enhancing vimentin degradation and inhibiting epithelial-mesenchymal transition. Oncotarget. 2015;6(27):23342–23357.
- Zhang CY, Yu MS, Li X, et al. Overexpression of long non-coding RNA MEG3 suppresses breast cancer cell proliferation, invasion, and angiogenesis through AKT pathway. Tumour Biol. 2017;39(6):1010428317701311.
- Gong F, Dong D, Zhang T, et al. Long non-coding RNA FENDRR attenuates the stemness of non-small cell lung cancer cells via decreasing multidrug resistance gene 1 (MDR1) expression through competitively binding with RNA binding protein HuR. Eur J Pharmacol. 2019;853:345–352.
- Kong J, Sun W, Li C, et al. Long non-coding RNA LINC01133 inhibits epithelial-mesenchymal transition and metastasis in colorectal cancer by interacting with SRSF6. Cancer Lett. 2016;380(2):476–484.
- Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8(11):931–937.
- Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17(9):528–542.
- Yin Z, Pascual C, Klionsky DJ. Autophagy: machinery and regulation. Microb Cell. 2016;3(12):588–596.
- Bento CF, Renna M, Ghislat G, et al. Mammalian autophagy: how does it work? Annu Rev Biochem. 2016;85:685–713.
- Ravikumar B, Sarkar S, Davies JE, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90(4):1383–1435.
- Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19(6):349–364.
- Aghaei M, Motallebnezhad M, Ghorghanlu S, et al. Targeting autophagy in cardiac ischemia/reperfusion injury: a novel therapeutic strategy. J Cell Physiol. 2019;234(10):16768–16778.
- Bhutia SK, Mukhopadhyay S, Sinha N, et al. Autophagy: cancer’s friend or foe? Adv Cancer Res. 2013;118:61–95.
- Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome formation in mammalian cells. Cell Struct Funct. 2002;27(6):421–429.
- Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12(Suppl 2):1542–1552.
- Yun CW, Lee SH. The roles of autophagy in cancer. Int J Mol Sci. 2018;19:11.
- Luo T, Fu J, Xu A, et al. PSMD10/gankyrin induces autophagy to promote tumor progression through cytoplasmic interaction with ATG7 and nuclear transactivation of ATG7 expression. Autophagy. 2016;12(8):1355–1371.
- Liu M, Jiang L, Fu X, et al. Cytoplasmic liver kinase B1 promotes the growth of human lung adenocarcinoma by enhancing autophagy. Cancer Sci. 2018;109(10):3055–3067.
- Santana-Codina N, Mancias JD, Kimmelman AC. The role of autophagy in cancer. Annu Rev Cancer Bio. 2017;1(1):19–39.
- Mazure NM, Pouyssegur J. Hypoxia-induced autophagy: cell death or cell survival? Curr Opin Cell Biol. 2010;22(2):177–180.
- White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009;15(17):5308–5316.
- Degenhardt K, Mathew R, Beaudoin B, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10(1):51–64.
- Wei H, Wei S, Gan B, et al. Suppression of autophagy by FIP200 deletion inhibits mammary tumorigenesis. Genes Dev. 2011;25(14):1510–1527.
- Kim MS, Song SY, Lee JY, et al. Expressional and mutational analyses of ATG5 gene in prostate cancers. Apmis. 2011;119(11):802–807.
- Ge J, Chen Z, Huang J, et al. Upregulation of autophagy-related gene-5 (ATG-5) is associated with chemoresistance in human gastric cancer. PLoS One. 2014;9(10):e110293.
- Zhu J, Li Y, Tian Z, et al. ATG7 overexpression is crucial for tumorigenic growth of bladder cancer in vitro and in vivo by targeting the ETS2/miRNA196b/FOXO1/p27 axis. Mol Ther Nucleic Acids. 2017;7:299–313.
- Cufi S, Vazquez-Martin A, Oliveras-Ferraros C, et al. Autophagy positively regulates the CD44(+) CD24(-/low) breast cancer stem-like phenotype. Cell Cycle. 2011;10(22):3871–3885.
- Galavotti S, Bartesaghi S, Faccenda D, et al. The autophagy-associated factors DRAM1 and p62 regulate cell migration and invasion in glioblastoma stem cells. Oncogene. 2013;32(6):699–712.
- Futreal PA, Soderkvist P, Marks JR, et al. Detection of frequent allelic loss on proximal chromosome 17q in sporadic breast carcinoma using microsatellite length polymorphisms. Cancer Res. 1992;52(9):2624–2627.
- Gao X, Zacharek A, Salkowski A, et al. Loss of heterozygosity of the BRCA1 and other loci on chromosome 17q in human prostate cancer. Cancer Res. 1995;55(5):1002–1005.
- Aita VM, Liang XH, Murty VV, et al. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics. 1999;59(1):59–65.
- Marinković M, Šprung M, Buljubašić M, et al. Autophagy modulation in cancer: current knowledge on action and therapy. Oxid Med Cell Longev. 2018;2018:18.
- Qiu DM, Wang GL, Chen L, et al. The expression of beclin-1, an autophagic gene, in hepatocellular carcinoma associated with clinical pathological and prognostic significance. BMC Cancer. 2014;14:327.
- Zhang Z, Shao Z, Xiong L, et al. Expression of Beclin1 in osteosarcoma and the effects of down-regulation of autophagy on the chemotherapeutic sensitivity. J Huazhong Univ Sci Technolog Med Sci. 2009;29(6):737–740.
- Huang X, Bai HM, Chen L, et al. Reduced expression of LC3B-II and Beclin 1 in glioblastoma multiforme indicates a down-regulated autophagic capacity that relates to the progression of astrocytic tumors. J Clin Neurosci. 2010;17(12):1515–1519.
- Ionov Y, Nowak N, Perucho M, et al. Manipulation of nonsense mediated decay identifies gene mutations in colon cancer cells with microsatellite instability. Oncogene. 2004;23(3):639–645.
- Kim MS, Jeong EG, Ahn CH, et al. Frameshift mutation of UVRAG, an autophagy-related gene, in gastric carcinomas with microsatellite instability. Hum Pathol. 2008;39(7):1059–1063.
- Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22(3):377–388.
- Nazio F, Bordi M, Cianfanelli V, et al. Autophagy and cancer stem cells: molecular mechanisms and therapeutic applications. Cell Death Differ. 2019;26(4):690–702.
- Catalano M, D’Alessandro G, Lepore F, et al. Autophagy induction impairs migration and invasion by reversing EMT in glioblastoma cells. Mol Oncol. 2015;9(8):1612–1625.
- Solomon VR, Lee H. Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur J Pharmacol. 2009;625(1–3):220–233.
- Aydinlik S, Erkisa M, Cevatemre B, et al. Enhanced cytotoxic activity of doxorubicin through the inhibition of autophagy in triple negative breast cancer cell line. Biochim Biophys Acta Gen Subj. 2017;1861(2):49–57.
- Taylor MA, Das BC, Ray SK. Targeting autophagy for combating chemoresistance and radioresistance in glioblastoma. Apoptosis. 2018;23(11–12):563–575.
- Guo B, Tam A, Santi SA, et al. Role of autophagy and lysosomal drug sequestration in acquired resistance to doxorubicin in MCF-7 cells. BMC Cancer. 2016;16(1):762.
- Sasaki K, Tsuno NH, Sunami E, et al. Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on colon cancer cells. BMC Cancer. 2010;10:370.
- Sasaki K, Tsuno NH, Sunami E, et al. Resistance of colon cancer to 5-fluorouracil may be overcome by combination with chloroquine, an in vivo study. Anticancer Drugs. 2012;23(7):675–682.
- Marino ML, Fais S, Djavaheri-Mergny M, et al. Proton pump inhibition induces autophagy as a survival mechanism following oxidative stress in human melanoma cells. Cell Death Dis. 2010;1:e87.
- Tan Q, Joshua AM, Wang M, et al. Up-regulation of autophagy is a mechanism of resistance to chemotherapy and can be inhibited by pantoprazole to increase drug sensitivity. Cancer Chemother Pharmacol. 2017;79(5):959–969.
- An Y, Zhang Z, Shang Y, et al. miR-23b-3p regulates the chemoresistance of gastric cancer cells by targeting ATG12 and HMGB2. Cell Death Dis. 2015;6:e1766.
- Pagotto A, Pilotto G, Mazzoldi EL, et al. Autophagy inhibition reduces chemoresistance and tumorigenic potential of human ovarian cancer stem cells. Cell Death Dis. 2017;8(7):e2943.
- Lee YJ, Won AJ, Lee J, et al. Molecular mechanism of SAHA on regulation of autophagic cell death in tamoxifen-resistant MCF-7 breast cancer cells. Int J Med Sci. 2012;9(10):881–893.
- Li JR, Cheng CL, Yang CR, et al. Dual inhibitor of phosphoinositide 3-kinase/mammalian target of rapamycin NVP-BEZ235 effectively inhibits cisplatin-resistant urothelial cancer cell growth through autophagic flux. Toxicol Lett. 2013;220(3):267–276.
- Westhoff MA, Faham N, Marx D, et al. Sequential dosing in chemosensitization: targeting the PI3K/Akt/mTOR pathway in neuroblastoma. PLoS One. 2013;8(12):e83128.
- Lin CI, Whang EE, Donner DB, et al. Autophagy induction with RAD001 enhances chemosensitivity and radiosensitivity through Met inhibition in papillary thyroid cancer. Mol Cancer Res. 2010;8(9):1217–1226.
- Huang P, Li F, Li L, et al. lncRNA profile study reveals the mRNAs and lncRNAs associated with docetaxel resistance in breast cancer cells. Sci Rep. 2018;8(1):17970.
- Fan Y, Shen B, Tan M, et al. Long non-coding RNA UCA1 increases chemoresistance of bladder cancer cells by regulating Wnt signaling. Febs J. 2014;281(7):1750–1758.
- Liao Y, Shen L, Zhao H, et al. LncRNA CASC2 interacts with miR-181a to modulate glioma growth and resistance to TMZ through PTEN pathway. J Cell Biochem. 2017;7(118):1889–1899.
- Fang Z, Chen W, Yuan Z, et al. LncRNA-MALAT1 contributes to the cisplatin-resistance of lung cancer by upregulating MRP1 and MDR1 via STAT3 activation. Biomed Pharmacother. 2018;101:536–542.
- Kun-Peng Z, Xiao-Long M, Chun-Lin Z. LncRNA FENDRR sensitizes doxorubicin-resistance of osteosarcoma cells through down-regulating ABCB1 and ABCC1. Oncotarget. 2017;8(42):71881–71893.
- An J, Lv W, Zhang Y. LncRNA NEAT1 contributes to paclitaxel resistance of ovarian cancer cells by regulating ZEB1 expression via miR-194. Onco Targets Ther. 2017;10:5377–5390.
- Yan J, Dang Y, Liu S, et al. LncRNA HOTAIR promotes cisplatin resistance in gastric cancer by targeting miR-126 to activate the PI3K/AKT/MRP1 genes. Tumour Biol. 2016;37:16345–16355.
- Feng SQ, Zhang XY, Fan HT, et al. Up-regulation of LncRNA MEG3 inhibits cell migration and invasion and enhances cisplatin chemosensitivity in bladder cancer cells. Neoplasma. 2018;65(6):925–932.
- Wu H, Zou Q, He H, et al. Long non-coding RNA PCAT6 targets miR-204 to modulate the chemoresistance of colorectal cancer cells to 5-fluorouracil-based treatment through HMGA2 signaling. Cancer Med. 2019;8(5):2484–2495.
- Li B, Xie D, Zhang H. Long non-coding RNA GHET1 contributes to chemotherapeutic resistance to Gemcitabine in bladder cancer. Cancer Chemother Pharmacol. 2019;84(1):187–194.
- Zhang S, Ma H, Zhang D, et al. LncRNA KCNQ1OT1 regulates proliferation and cisplatin resistance in tongue cancer via miR-211-5p mediated Ezrin/Fak/Src signaling. Cell Death Dis. 2018;9(7):742.
- Wang M, Hu H, Wang Y, et al. Long non-coding RNA TUG1 mediates 5-fluorouracil resistance by acting as a ceRNA of miR-197-3p in colorectal cancer. J Cancer. 2019;10(19):4603–4613.
- Lu H, Liu H, Yang X, et al. LncRNA BLACAT1 may serve as a prognostic predictor in cancer: evidence from a meta-analysis. Biomed Res Int. 2019;2019:10.
- Su J, Zhang E, Han L, et al. Long noncoding RNA BLACAT1 indicates a poor prognosis of colorectal cancer and affects cell proliferation by epigenetically silencing of p15. Cell Death Dis. 2017;8(3):e2665.
- Wu X, Zheng Y, Han B, et al. Long noncoding RNA BLACAT1 modulates ABCB1 to promote oxaliplatin resistance of gastric cancer via sponging miR-361. Biomed Pharmacother. 2018;99:832–838.
- Huang FX, Chen HJ, Zheng FX, et al. LncRNA BLACAT1 is involved in chemoresistance of nonsmall cell lung cancer cells by regulating autophagy. Int J Oncol. 2019;54(1):339–347.
- Li ZX, Zhu QN, Zhang HB, et al. MALAT1: a potential biomarker in cancer. Cancer Manag Res. 2018;10:6757–6768.
- Cai T, Liu Y, Xiao J. Long noncoding RNA MALAT1 knockdown reverses chemoresistance to temozolomide via promoting microRNA-101 in glioblastoma. Cancer Med. 2018;7(4):1404–1415.
- YiRen H, YingCong Y, Sunwu Y, et al. Long noncoding RNA MALAT1 regulates autophagy associated chemoresistance via miR-23b-3p sequestration in gastric cancer. Mol Cancer. 2017;16(1):174.
- Xi Z, Si J, Nan J. LncRNA MALAT1 potentiates autophagyassociated cisplatin resistance by regulating the microRNA30b/autophagyrelated gene 5 axis in gastric cancer. Int J Oncol. 2019;54(1):239–248.
- Zhou Q, Hu W, Zhu W, et al. Long non coding RNA XIST as a prognostic cancer marker - A meta-analysis. Clin Chim Acta. 2018;482:1–7.
- Hu S, Chang J, Li Y, et al. Long non-coding RNA XIST as a potential prognostic biomarker in human cancers: a meta-analysis. Oncotarget. 2018;9(17):13911–13919.
- Zhu J, Zhang R, Yang D, et al. Knockdown of long non-coding RNA XIST inhibited doxorubicin resistance in colorectal cancer by upregulation of miR-124 and downregulation of SGK1. Cell Physiol Biochem. 2018;51(1):113–128.
- Xiao Y, Yurievich UA, Yosypovych SV. Long noncoding RNA XIST is a prognostic factor in colorectal cancer and inhibits 5-fluorouracil-induced cell cytotoxicity through promoting thymidylate synthase expression. Oncotarget. 2017;8(47):83171–83182.
- Sun W, Zu Y, Fu X, et al. Knockdown of lncRNA-XIST enhances the chemosensitivity of NSCLC cells via suppression of autophagy. Oncol Rep. 2017;38(6):3347–3354.
- Wang Q, Li Q, Zhou P, et al. Upregulation of the long non-coding RNA SNHG1 predicts poor prognosis, promotes cell proliferation and invasion, and reduces apoptosis in glioma. Biomed Pharmacother. 2017;91:906–911.
- Zhao G, Wang S, Liang X, et al. Oncogenic role of long non-coding RNA SNHG12 in gastric cancer cells by targeting miR-16. Exp Ther Med. 2019;18(1):199–208.
- Wang P, Chen D, Ma H, et al. LncRNA SNHG12 contributes to multidrug resistance through activating the MAPK/Slug pathway by sponging miR-181a in non-small cell lung cancer. Oncotarget. 2017;8(48):84086–84101.
- Wang X, Lan Z, He J, et al. LncRNA SNHG6 promotes chemoresistance through ULK1-induced autophagy by sponging miR-26a-5p in colorectal cancer cells. Cancer Cell Int. 2019;19:234.
- Zhang X, Zhao P, Wang C, et al. SNHG14 enhances gemcitabine resistance by sponging miR-101 to stimulate cell autophagy in pancreatic cancer. Biochem Biophys Res Commun. 2019;510(4):508–514.
- Yu X, Zheng H, Chan MT, et al. HULC: an oncogenic long non-coding RNA in human cancer. J Cell Mol Med. 2017;21(2):410–417.
- Zhang Y, Song X, Wang X, et al. Silencing of LncRNA HULC enhances chemotherapy induced apoptosis in human gastric cancer. J Med Biochem. 2016;35(2):137–143.
- Xiong H, Ni Z, He J, et al. LncRNA HULC triggers autophagy via stabilizing Sirt1 and attenuates the chemosensitivity of HCC cells. Oncogene. 2017;36(25):3528–3540.
- Yu X, Zheng H, Tse G, et al. CASC2: an emerging tumour-suppressing long noncoding RNA in human cancers and melanoma. Cell Prolif. 2018;51(6):e12506.
- Li Y, Lv S, Ning H, et al. Down-regulation of CASC2 contributes to cisplatin resistance in gastric cancer by sponging miR-19a. Biomed Pharmacother. 2018;108:1775–1782.
- Zhu D, Yu Y, Qi Y, et al. Long non-coding RNA CASC2 enhances the antitumor activity of cisplatin through suppressing the Akt pathway by inhibition of miR-181a in esophageal squamous cell carcinoma cells. Front Oncol. 2019;9:350.
- Liu Z, Dang C, Xing E, et al. Overexpression of CASC2 improves cisplatin sensitivity in hepatocellular carcinoma through sponging miR-222. DNA Cell Biol. 2019;38(11):1366–1373.
- Jiang C, Shen F, Du J, et al. Upregulation of CASC2 sensitized glioma to temozolomide cytotoxicity through autophagy inhibition by sponging miR-193a-5p and regulating mTOR expression. Biomed Pharmacother. 2018;97:844–850.
- Ren K, Xu R, Huang J, et al. Knockdown of long non-coding RNA KCNQ1OT1 depressed chemoresistance to paclitaxel in lung adenocarcinoma. Cancer Chemother Pharmacol. 2017;80(2):243–250.
- Qi X, Yu XJ, Wang XM, et al. Knockdown of KCNQ1OT1 suppresses cell invasion and sensitizes osteosarcoma cells to CDDP by upregulating DNMT1-mediated Kcnq1 expression. Mol Ther Nucleic Acids. 2019;17:804–818.
- Li Y, Li C, Li D, et al. lncRNA KCNQ1OT1 enhances the chemoresistance of oxaliplatin in colon cancer by targeting the miR-34a/ATG4B pathway. Onco Targets Ther. 2019;12:2649–2660.
- Ji J, Dai X, Yeung SJ, et al. The role of long non-coding RNA GAS5 in cancers. Cancer Manag Res. 2019;11:2729–2737.
- Long X, Song K, Hu H, et al. Long non-coding RNA GAS5 inhibits DDP-resistance and tumor progression of epithelial ovarian cancer via GAS5-E2F4-PARP1-MAPK axis. J Exp Clin Cancer Res. 2019;38(1):345.
- Gao ZQ, Wang JF, Chen DH, et al. Long non-coding RNA GAS5 antagonizes the chemoresistance of pancreatic cancer cells through down-regulation of miR-181c-5p. Biomed Pharmacother. 2018;97:809–817.
- Zhang N, Yang GQ, Shao XM, et al. GAS5 modulated autophagy is a mechanism modulating cisplatin sensitivity in NSCLC cells. Eur Rev Med Pharmacol Sci. 2016;20(11):2271–2277.
- Liu Y, He A, Liu B, et al. Potential role of lncRNA H19 as a cancer biomarker in human cancers detection and diagnosis: a pooled analysis based on 1585 subjects. Biomed Res Int. 2019;2019:11.
- Yang F, Bi J, Xue X, et al. Up-regulated long non-coding RNA H19 contributes to proliferation of gastric cancer cells. Febs J. 2012;279(17):3159–3165.
- Lv M, Zhong Z, Huang M, et al. lncRNA H19 regulates epithelial-mesenchymal transition and metastasis of bladder cancer by miR-29b-3p as competing endogenous RNA. Biochim Biophys Acta Mol Cell Res. 2017;1864(10):1887–1899.
- Gao H, Hao G, Sun Y, et al. Long noncoding RNA H19 mediated the chemosensitivity of breast cancer cells via Wnt pathway and EMT process. Onco Targets Ther. 2018;11:8001–8012.
- Wang J, Xie S, Yang J, et al. The long noncoding RNA H19 promotes tamoxifen resistance in breast cancer via autophagy. J Hematol Oncol. 2019;12(1):81.
- Wang M, Han D, Yuan Z, et al. Long non-coding RNA H19 confers 5-Fu resistance in colorectal cancer by promoting SIRT1-mediated autophagy. Cell Death Dis. 2018;9(12):1149.
- Tang Q, Hann SSHOTAIR. An oncogenic long non-coding RNA in human cancer. Cell Physiol Biochem. 2018;47(3):893–913.
- Botti G, Scognamiglio G, Aquino G, et al. LncRNA HOTAIR in tumor microenvironment: what role? Int J Mol Sci. 2019;20:9.
- Ozes AR, Miller DF, Ozes ON, et al. NF-kappaB-HOTAIR axis links DNA damage response, chemoresistance and cellular senescence in ovarian cancer. Oncogene. 2016;35(41):5350–5361.
- Xiao Z, Qu Z, Chen Z, et al. LncRNA HOTAIR is a prognostic biomarker for the proliferation and chemoresistance of colorectal cancer via MiR-203a-3p-mediated wnt/ss-catenin signaling pathway. Cell Physiol Biochem. 2018;46(3):1275–1285.
- Sun MY, Zhu JY, Zhang CY, et al. Autophagy regulated by lncRNA HOTAIR contributes to the cisplatin-induced resistance in endometrial cancer cells. Biotechnol Lett. 2017;39(10):1477–1484.
- Ma B, Yuan Z, Zhang L, et al. Long non-coding RNA AC023115.3 suppresses chemoresistance of glioblastoma by reducing autophagy. Biochim Biophys Acta Mol Cell Res. 2017;1864(8):1393–1404.
- Cai Q, Wang S, Jin L, et al. Long non-coding RNA GBCDRlnc1 induces chemoresistance of gallbladder cancer cells by activating autophagy. Mol Cancer. 2019;18(1):82.
- Lu D, Yang C, Zhang Z, et al. Knockdown of Linc00515 inhibits multiple myeloma autophagy and chemoresistance by upregulating miR-140-5p and downregulating ATG14. Cell Physiol Biochem. 2018;48(6):2517–2527.
- Wang Z, Liu Z, Wu S. Long non-coding RNA CTA sensitizes osteosarcoma cells to doxorubicin through inhibition of autophagy. Oncotarget. 2017;8(19):31465–31477.