ABSTRACT
Long noncoding RNAs (lncRNAs) have been found to associate with all major types of malignancies and play important roles in regulating several hallmarks of cancer by interacting with proteins, DNA, and RNA. The possible functions of lncRNAs and their roles in the regulation of tumour growth will be reported and discussed in the present review. In our recent report, based on genetic mice models and a series of systematic analyses, we suggested that lncRNAs also play critical roles in the regulation of antigen presentation in tumour cells and allow tumour cells to escape immune surveillance, which further broadens the scope of understanding lncRNA functions and how they relate to cancer immunotherapy resistance.
Identifying lncRNAs in cancer
Gene alteration is considered an important cause of tumorigenesis [Citation1]. With the advancement of large-scale sequencing technology, numerous somatic mutations, copy number alterations, and cancer-related single-nucleotide polymorphisms (SNPs) have been discovered in clinical cancer patient samples [Citation2]. Interestingly, the majority of these gene alterations are in the noncoding regions of the genome [Citation3–5]. Among these non-coding genes, long non-coding RNAs (lncRNAs) are emerging as a new class of indispensable players involved in the tumorigenesis [Citation3,Citation5,Citation6]. Moreover, the dysregulation of a number of lncRNA targets has been reported to associate with the stage and prognosis of many tumor types [Citation7,Citation8] including breast cancer [Citation9,Citation10], lung cancer [Citation11,Citation12], and liver cancer [Citation13,Citation14], as well as linked to resistance against chemotherapy and targeted therapy [Citation15,Citation16]. For example, the overexpression of the lncRNAHOX transcript antisense intergenic RNA(HOTAIR) promotes the metastasis of breast cancer cells [Citation17]. The overexpression of the lncRNA Breast Cancer Anti-Oestrogen Resistance 4(BCAR4) correlates with advanced breast cancer, metastasis and anti-oestrogen resistance [Citation18]. Although much has been learned about the multiple functions of lncRNAs in tumour cell proliferation, apoptosis, migration, invasion, and maintenance of stemness during cancer development [Citation19,Citation20], little is known about their potential roles in regulating tumour immunity as tumour cell intrinsic factors that affect cancer immunotherapy.
Potential roles of LncRNAs as tumour intrinsic factors in cancer immunotherapy
In recent years, breakthroughs in the field of tumour immunotherapy have greatly improved the effectiveness of tumour treatments. Nevertheless, only a small percentage of people benefit from tumour immunotherapy, and the majority of patients develop primary or secondary resistance upon treatment [Citation21]. Although there are many studies focusing on the causes of tumour cell resistance, the detailed mechanisms of this process are still unclear. Recently, some tumour associated lncRNAs have been recognized as tumour cell intrinsic factors that mediate tumour cell escape of immune surveillance. These tumour-associated lncRNAs may play a vital role in immunotherapy resistance [Citation22,Citation23]. For example, our laboratory and other groups have demonstrated that abnormally highly expressed lncRNAs in tumour cells can assist tumour cells with completing immune escape by mediating tumour cell antigen-presenting pathways and regulating tumor cell PD-L1 expression. In our recent work, we found that high expression of long intergenic non-coding RNA for kinase activation (LINK-A) facilitates breast cancer cell escape of immune surveillance [Citation24]. Song et al. reported that HOTAIR overexpression in gastric cancer cells might also be involved in tumour cell immune escape mechanisms involving HLA-G up regulation via inhibiting miR-152 [Citation25]. The lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) has been found to positively regulate the expression of PD-L1 in diffuse large B-cell lymphoma(DLBCL) through sponging miR-195, thus promoting cancer cell migration and immune scape by regulating proliferation and apoptosis of CD8+ Tcells [Citation26]. Alternative pathways through which lncRNAs may influence immunotherapy resistance in non-tumour cells, such as immune cells, require further investigation to comprehensively illustrate the role of lncRNAs in modulating immunotherapeutic efficacy. Taken together, lncRNAs have been found to play important roles in pathways associated with immune function, providing a foundation for mechanistically exploring and establishing the significance of their expression in cancer cells.
The LncRNA LINK-A correlates with breast cancer development in a LINK-A-expressing mouse model and human breast cancer patients
Recent works from our lab further demonstrated how LINK-A functions to down regulate cancer cell antigen presentation and intrinsic tumour suppression [Citation24]. LINK-A was originally identified in the cytoplasm and shown to be highly expressed in triple-negative breast cancer(TNBC). Further studies demonstrated that LINK-A-dependent signaling pathway activation promotes breast cancer glycolysis reprogramming and tumorigenesis [Citation27]. RNA Scope analysis further supported the conclusion that higher LINK-A expression correlates with the advanced lymph node metastasis stage and shorter survival times for breast cancer patients [Citation9,Citation27]. Recently, we reported that conditional expression of LINK-A in mouse mammary glands – MMTV-Tg(LINK-A) – results in mammary gland hyperplasia by 12 weeks of age and ductal carcinoma in situ (DCIS) by 19 weeks of age. The median tumour-free survival for MMTV-Tg(LINK-A) animals is 161 days (< 6 months), and about 90% of tumour-bearing mice exhibit lung metastases. Additionally, these tumours show morphological resemblance to human breast adenocarcinoma. Genetically, MMTV-Tg(LINK-A) tumours harbour a similar mutation burden to human TNBC and exhibit non-silent somatic mutations of Trp53 and Pik3ca genes. MMTV-Tg(LINK-A) tumours also show transcriptional co-clustering with human basal-like breast cancers compared to other subtypes [Citation24]. Metabolically, MMTV-Tg(LINK-A) breast tumours model human TNBC metabolic signatures.
LINK-A serves as tumour intrinsic factor that regulates antigen presentation in breast cancer cells
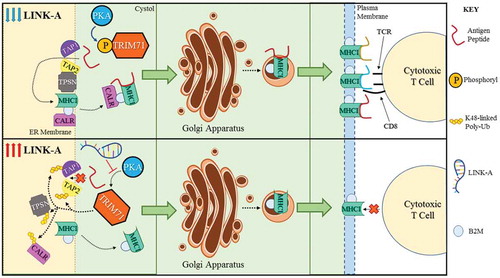
LINK-A facilitates the association between phosphatidylinositol (3,4,5)-trisphosphate (PIP3) and inhibitory G-protein coupled receptors(GPCRs) (CNR2, HRH1, ADA2A, ACM4 and OPRM), facilitating the recruitment of the Gi alpha subunit to GPCRs. The expression of LINK-A leads to reduced cyclic AMP (cAMP) levels, inactivation of cAMP-dependent protein kinase (PKA), and PKA-mediated phosphorylation of the E3 ligase TRIM71 (also known as LIN-41) at Serine 3. Consequently, TRIM71 catalyzes K48-linked polyubiquitination and proteasome-mediated degradation of Rb, p53, and peptide-loading complex (PLC) components upon LINK-A expression (). Treating MMTV-Tg(LINK-A) tumour-bearing mice with LINK-A Locked Nucleic Acids (LNAs) or Rauwolscine, an antagonist of Adrenoceptor Alpha 2A (ADA2A), led to stabilization of Rb, p53, and the PLC components and improved the infiltration and cytotoxicity of tumour-resident CD8+ Tcells in vivo. When combined with immune checkpoint blockers, the LINK-A LNAs or Rauwolscine treatments exhibit synergistic efficacy in repressing breast tumour growth. Most importantly, human breast cancer tissues with high LINK-A expression are associated with decreased activated CD8 + T cell and antigen-presenting cell (APC) tumor infilitration. LINK-A up regulation and PLC down regulation are correlated in TNBC patients whose tumours are resistant to anti-PD-1 immunotherapy. Taken together, these research findings introduce the new paradigm that specific lncRNAs act as oncogenes in the development of breast cancer that resembles human TNBC, situating lncRNAs as viable therapeutic targets. A lncRNA-directed targeted therapy using LNAs could serve as a promising strategy for improving breast tumour antigenicity and sensitizing TNBC patients to immunotherapy. Further insights into the therapeutic potential of targeting lncRNAs in TNBC might therefore yield important considerations for improving the treatment efficacy and survival outcomes of future immunotherapies.
Figure 1. Molecular mechanisms of the effect of LINK-A expression on antigen presentation machinery.
Concluding remarks and future perspectives
The discovery and functional study of lncRNAs as tumour intrinsic factors show promise for expanding our horizons on the regulatory mechanisms of cancer. Although we have realized the importance of lncRNAs in regulating tumour cell immune escape, this represents only the tip of the iceberg. How lncRNAs affect the tumour microenvironment and regulate the function of tumour immune cells still requires further research to determine clinical application prospects. Likewise, the emergence of lncRNAs as key players in the regulation of immunotherapeutic efficacy in tumour cells suggests that the study of tissue-specific lncRNAs and how they further influence immune resistance/immunotherapy may serve as an interesting direction for future research. Taken together, the expression of lncRNAs situates them as promising targets for cancer immunotherapy.
Disclosure statement
The authors have no conflicts of interest to disclose.
Additional information
Funding
References
- Sugimura T, Terada M, Yokota J, et al. Multiple genetic alterations in human carcinogenesis. Environ Health Perspect. 1992;98:5–12.
- Berger MF, Mardis ER. The emerging clinical relevance of genomics in cancer medicine. Nat Rev Clin Oncol. 2018;15:353–365.
- Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15:7–21.
- Maurano MT, Humbert R, Rynes E, et al. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337:1190–1195.
- Melton C, Reuter JA, Spacek DV, et al. Recurrent somatic mutations in regulatory regions of human cancer genomes. Nat Genet. 2015;47:710–716.
- Beroukhim R, Mermel CH, Porter D, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905.
- Jendrzejewski J, He H, Radomska HS, et al. The polymorphism rs944289 predisposes to papillary thyroid carcinoma through a large intergenic noncoding RNA gene of tumor suppressor type. Proc Natl Acad Sci U S A. 2012;109:8646–8651.
- Zhao X, Wei X, Zhao L, et al. The rs6983267 SNP and long non-coding RNA CARLo-5 are associated with endometrial carcinoma. Environ Mol Mutagen. 2016;57:508–515.
- Lin A, Hu Q, Li C, et al. The LINK-A lncRNA interacts with PtdIns(3,4,5)P3 to hyperactivate AKT and confer resistance to AKT inhibitors. Nat Cell Biol. 2017;19:238–251.
- Niu Y, Bao L, Chen Y, et al. HIF-2-induced long non-coding RNA RAB11B-AS1 promotes hypoxia-mediated angiogenesis and breast cancer metastasis. Cancer Res. 2020;80:964–975.
- Chen Z, Lei T, Chen X, et al. Long non-coding RNA in lung cancer. Clinica chimica acta. Int J Clin Chem. 2019;504:190–200.
- Loewen G, Jayawickramarajah J, Zhuo Y, et al. Functions of lncRNA HOTAIR in lung cancer. J Hematol Oncol. 2014;7:90.
- Mehra M, Chauhan R. Long noncoding RNAs as a key player in hepatocellular carcinoma. Biomark Cancer. 2017;9:1179299X17737301.
- Wang Y, Zhu P, Luo J, et al. LncRNA HAND2-AS1 promotes liver cancer stem cell self-renewal via BMP signaling. Embo J. 2019;38:e101110.
- Deng H, Zhang J, Shi J, et al. Role of long non-coding RNA in tumor drug resistance. Tumour Biol. 2016;37:11623–11631.
- Sun R, Wang R, Chang S, et al. Long non-coding RNA in drug resistance of non-small cell lung cancer: a mini review. Front Pharmacol. 2019;10:1457.
- Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076.
- Xing Z, Lin A, Li C, et al. lncRNA directs cooperative epigenetic regulation downstream of chemokine signals. Cell. 2014;159:1110–1125.
- Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152:1298–1307.
- Slack FJ, Chinnaiyan AM. The role of non-coding RNAs in oncology. Cell. 2019;179:1033–1055.
- Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–723.
- Denaro N, Merlano MC, Lo Nigro C. Long noncoding RNAs as regulators of cancer immunity. Mol Oncol. 2019;13:61–73.
- Zhou Y, Zhu Y, Xie Y, et al. The role of long non-coding RNAs in immunotherapy resistance. Front Oncol. 2019;9:1292.
- Hu Q, Ye Y, Chan LC, et al. Oncogenic lncRNA downregulates cancer cell antigen presentation and intrinsic tumor suppression. Nat Immunol. 2019;20:835–851.
- Song B, Guan Z, Liu F, et al. Long non-coding RNA HOTAIR promotes HLA-G expression via inhibiting miR-152 in gastric cancer cells. Biochem Biophys Res Commun. 2015;464:807–813.
- Wang QM, Lian GY, Song Y, et al. LncRNA MALAT1 promotes tumorigenesis and immune escape of diffuse large B cell lymphoma by sponging miR-195. Life Sci. 2019;231:116335.
- Lin A, Li C, Xing Z, et al. The LINK-A lncRNA activates normoxic HIF1alpha signalling in triple-negative breast cancer. Nat Cell Biol. 2016;18:213–224.
