ABSTRACT
The majority of transcriptionally active RNA derived from the mammalian genome does not code for protein. Long noncoding RNA (lncRNA) is the most abundant form of noncoding RNA found in the brain and is involved in many aspects of cellular metabolism. Beyond their fundamental role in the nucleus as decoys for RNA-binding proteins associated with alternative splicing or as guides for the epigenetic regulation of protein-coding gene expression, recent findings indicate that activity-induced lncRNAs also regulate neural plasticity. In this review, we discuss how lncRNAs may exert molecular control over brain function beyond their known roles in the nucleus. We propose that subcellular localization is a critical feature of experience-dependent lncRNA activity in the brain, and that lncRNA-mediated control over RNA metabolism at the synapse serves to regulate local mRNA stability and translation, thereby influencing neuronal function, learning and memory.
Introduction
The mammalian transcriptome consists of a complex repertoire of RNA species. It is estimated that 98% of all RNAs transcribed from the mammalian genome are noncoding RNAs (ncRNAs)[Citation1], which are classified based on their size, function and subcellular location [Citation2]. Long noncoding RNAs (lncRNA), in particular, have emerged as crucial players in the regulation of gene expression. LncRNA, by definition, is any RNA that is longer than 200 nucleotides and does not have any protein-coding potential. LncRNAs were first identified in the early 1990s3-[Citation3]; however, due to technical limitations, their function could not be determined and the relevance of intragenic RNAs remained hypothetical [Citation4]. After the FANTOM project was launched in the early 2000s, many more ncRNAs were identified and about one-third of cDNAs were annotated as lncRNAs [Citation5–7]. Shortly after, a role for lncRNAs in directing chromatin modifiers and the regulation of gene expression in the nucleus was reported [Citation7–10], firmly establishing lncRNAs as key players in cellular metabolism.
LncRNAs display a high degree of developmental and cell-type specificity, with approximately 40% being enriched in the mammalian brain [Citation11,Citation12]. The nuclear-enriched lncRNA Gm12371 has been shown to influence hippocampal dendritic morphology and synaptic plasticity [Citation13], and the nuclear antisense lncRNA AtLAS regulates synapsin II polyadenylation and AMPA receptor trafficking [Citation4]. These lncRNAs are expressed in a cell-type- and spatiotemporal-specific manner [Citation14] and are therefore uniquely positioned to mediate rapid responses to environmental stimuli and potentially cognition [Citation15]. In addition, many lncRNAs have been implicated in neurological disorders [Citation16,Citation17], and their role in neural development and in the ageing brain has also recently been investigated [Citation18–21]. In this review, we highlight what is currently known about lncRNAs in the brain and discuss their potential role in regulating cognitive processes, as well as their involvement in neurological disorders. We address current technologies that will advance our understanding of lncRNA in the brain and propose that alternative splicing and subcellular localization are critically important features of experience-dependent lncRNA activity in neurons. Moreover, we suggest that lncRNA-mediated control over biomolecular condensates in the form of RNA reservoirs at the synapse serve to regulate local mRNA stability and translation, which may be critical for neural plasticity, learning, and memory.
LncRNA biogenesis
The biogenesis of lncRNA is very similar to that of protein-coding RNA. Although a subset of lncRNAs are RNA polymerase III-dependent [Citation22–24], most lncRNAs are transcribed by RNA polymerase II in the opposite direction with overlapping coding exons (antisense), in the opposite direction without overlapping exons (divergent), from within introns (intronic), or between two coding genes (intergenic) [Citation25]. In addition, lncRNAs can arise through alternative splicing of the host gene, and can themselves serve as a host for other ncRNAs [Citation2,Citation26–28]. Most lncRNAs possess a 5ʹ cap and a 3ʹ poly-A tail [Citation10,Citation29–31]; although lncRNAs without a poly-A tail have also been identified [Citation30,Citation31]. Because lncRNAs share many features with coding transcripts, it is not surprising that they are also subject to alternative splicing and degradation, and in some cases, can even undergo translation [Citation32]. Similar to coding transcripts, such alternative splicing of lncRNAs could account for their cell type-specific expression as well as their subcellular localization, splice variants facilitating their interaction with a diverse array of RNA-binding proteins that are necessary for spatially and temporally resolved gene expression [Citation33]. The initial definition of lncRNA included the inability to encode peptides. However, there is evidence that brain-specific lncRNAs such as BC1 or BC200 associate with ribosomes, suggesting the potential for translation [Citation34]. Recent studies have shown that ribosomes bind to lncRNAs [Citation35,Citation36] and that lncRNAs often contain open reading frames (ORFs) [Citation37,Citation38]. Indeed, in addition to circular RNAs [Citation39,Citation40], several lncRNAs yield functional micropeptides [Citation41–45]. Nevertheless, the occupancy of ribosomes on lncRNAs does not necessarily imply the capacity to encode functional peptides [Citation46]. It is possible that these lncRNAs merely serve as guides or scaffolds for ribosomes [Citation47,Citation48]. Future research using more sensitive mass spectrometry analysis will help to delineate translating lncRNAs from the overall pool. Despite their coding potential, lncRNAs may still act independently of their peptide products via their conserved sequence modules [Citation49,Citation50].
LncRNAs show little sequence conservation
Although lncRNAs possess similar properties to coding RNAs, their sequence conservation and function remain the subject of intense debate. The ENCODE project showed that the vast majority of the transcripts arise from noncoding DNA regions with little sequence constrain, and concluded that lncRNAs are not under strong negative selection [Citation51–53]. Indeed, lncRNAs display regions that are rapidly evolving [Citation54]. Due to the lack of sequence constraint and imperfect fidelity of RNA polymerase II, proponents of the ‘transcriptional noise’ theory argue that transcripts derived from noncoding regions are merely by-products of transcriptional activity within a given locus [Citation55]. The lack of primary sequence conservation and lower levels of expression seem to support this hypothesis. However, promoters, splice sites and exons of lncRNAs display functional conservation and are enriched in chromatin factors [Citation12,Citation29,Citation56–59]. Moreover, lncRNAs are remarkably tissue-specific [Citation60–65], and lncRNA variants and single nucleotide polymorphisms (SNPs) are also associated with disease phenotype [Citation16,Citation66–70]. For example, an SNP within the antisense lncRNA, DAOA-AS1, associates with bipolar disorder [Citation71]. Therefore, a lack of primary sequence conservation does not necessarily indicate a lack of function [Citation72,Citation73].
LncRNAs are abundantly expressed in the brain
Half of all known lncRNAs are expressed in the brain [Citation11,Citation12], and some have been shown to be involved in neuronal development, neurogenesis and in the regulating cellular metabolism in the ageing brain [Citation19,Citation20,Citation36,Citation74–76]. LncRNAs exhibit cell-type and subregion-specific patterns of expression in the brain and are induced in an activity-dependent manner [Citation11,Citation13,Citation18]. Moreover, lncRNAs are universally alternatively spliced, and with advances in sequencing technology, a myriad of novel isoforms, including those associated with neuropsychiatric disorders and ageing, are rapidly emerging [Citation74,Citation77,Citation78]. Targeted enrichment and long-read sequencing have identified thousands of novel brain-specific lncRNA isoforms [Citation77,Citation78], including neuroligin-1 and regulating synaptic membrane exocytosis protein 2 (Rims2), both of which are directly involved in synaptic plasticity and memory [Citation78–80]. Interestingly, several novel exons in the intron of Rims2 give rise to alternative transcripts with unknown function [Citation78]. These studies illustrate the complexity of lncRNAome and highlight the need for targeted functional characterization of specific isoforms within different cellular contexts in both the healthy and diseased brain.
Brain-specific lncRNA activity may be critical for learning and memory
With respect to brain function, neuroscientists have historically focused on stimulus-induced nascent coding transcripts and protein synthesis [Citation81,Citation82], with the putative function of lncRNAs in cognition being completely overlooked. In 2015, the PsychENCODE project initiated a comprehensive study of lncRNAs in three neuropsychiatric diseases [Citation83] and found the expression of some lncRNAs in different brain regions associated with synaptic transmission and memory [Citation84–88]. More recently, these studies have been extended with the observation that LoNA-deficient mice display enhanced long-term potentiation (LTP) and long-term memory [Citation84]. In addition, knockout of the dendritically localized lncRNA BC1 also impairs learning and memory [Citation88]. Furthermore, the lncRNA Neat1 regulates gene expression by coordinating the deposition of repressive chromatin modifications, the dysregulation of which is associated with age-related memory impairment [Citation89]. In addition, the dysregulation of a novel Gas5 isoform has been shown influence drug-associated learning and memory [Citation90]. With respect to neurological and neurodegenerative disorders characterized by cognitive impairment, altered expression of lncRNAs has now been associated with schizophrenia [Citation21,Citation91–95], Autism [Citation96,Citation97], Parkinson’s disease (PD) [Citation98,Citation99], Alzheimer’s disease (AD) [Citation100,Citation101], Huntington’s disease (HD) [Citation102], intellectual disability [Citation103,Citation104], neurodevelopmental disorders [Citation105] and even personality disorder [Citation106].
Taken together, these studies clearly implicate lncRNA activity in the regulation of memory processes in the healthy and diseased brain and, given the extraordinary breadth and complexity of lncRNA function, the challenge now is to elucidate the context- and cell-type-specific roles of individual lncRNA variants across many learning conditions.
Subcellular compartmentalization of lncRNAs
lncRNAs share many common RNA-binding proteins (RBPs) with other RNAs with interactions being highly dependent on their localization in space and time [Citation107–109]. Indeed, lncRNAs can be trafficked to different subcellular locations within a cell [Citation30] and the trafficking of lncRNA cargo to distal compartments from the nucleus may involve the kinesin motor and its regulating partners [Citation110–112]. This capacity is dynamic and species-specific [Citation113], with the dynamic localization of lncRNAs being structure- and state-dependent [Citation114–118].
LncRNAs as guides, scaffolds and decoys in the nucleus
Nuclear lncRNAs can serve as guides to recruit transcription factors and epigenetic regulators for either initiation or inhibition of gene expression (). Guide lncRNAs accumulate at the site of transcription, acting in cis (close to neighbouring genes), trans (distantly located genes), or in some cases, both. Cis-regulating natural antisense transcripts (NATs) regulate gene expression of their sense genes by recruiting factors that activate or repress transcription. For example, the antisense lncRNA Gdnfos regulates Gdnf isoform expression and is dysregulated in the temporal gyrus of AD and HD patients [Citation119]. In AD, Bace1-AS lncRNA is upregulated, and its expression correlates with Bace1 gene expression and correlates with amyloid deposition [Citation120]. An intronic cis lncRNA transcript, Nat51A, regulates alternative splicing, and dysregulation of this lncRNA greatly increases amyloid deposition in AD patients [Citation22]. Conversely, the NAT Bdnf-AS represses brain-derived neurotrophic factor expression in the hippocampus by recruiting repressive chromatin modifier to the locus [Citation121]. In addition to the brain, this mechanism also seems to be common in cancer [Citation122,Citation123]. Finally, in contrast to NATs, sense lncRNAs, such as utNgn1, can activate their proximal genes through a chromatin looping mechanism to regulate brain development [Citation124].
Figure 1. lncRNA functions in the nucleus. In the nucleus, lncRNA interacts with mRNA and RNA-binding proteins (RBPs) to induce formation of membraneless condensates (top panel). lncRNA can act as guide to recruit DNA-binding proteins, such as transcription factors and chromatin modifiers, to the DNA to initiate transcription (A). lncRNA can also act as a scaffold or guide to bring two or more proteins (or DNA regions) into close proximity to maintain chromatin structure (B). lncRNA may also act as decoy to sequester DNA-binding proteins, such as RNA polymerases and chromatin modifiers, from interacting with DNA, and hence, leading to transcription inhibition (C). During active transcription, lncRNA can interact with RNA polymerases and chromatin modifiers at sites of transcription to induce formation of chromatin condensates (D)
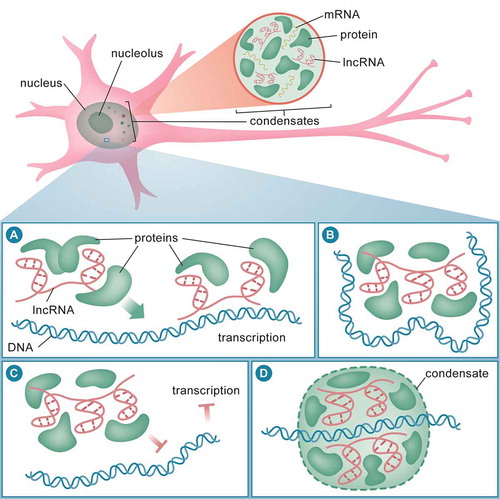
The trans mechanism of guide lncRNAs is equally important for the simultaneous activation of a plethora of genes. During neurogenesis, the lncRNAs Tuna, Paupar and Rmst can modulate the expression of distal genes that regulate pluripotency. These lncRNAs recruit repressors to the promoter of pluripotent genes to inhibit transcription, and as a result, neuronal differentiation ensues [Citation125–127]. Furthermore, the lncRNA Malat1 regulates synaptic density by recruiting splicing factors in trans to facilitate gene expression of its target transcripts [Citation128], and Neat1 can repress global gene expression via recruitment of chromatin factors to c-Fos promoter during memory formation [Citation89]. Guide lncRNAs can also regulate gene expression through both a cis and trans mechanism [Citation129]. For example, the lncRNA Evf2 can recruit transcription factors to regulate Dlx5/6 and Gad1 gene expression in the GABAergic circuitry in cis and trans, respectively [Citation130,Citation131]. In dopaminergic neurons, antisense Uchl1 lncRNA plays a dual role: increased Uchl1 expression in the nucleus in cis, and shuttle to the cytoplasm to regulate Uchl1 translation in trans [Citation116].
Scaffold lncRNAs serve as adaptors for forming discrete protein complexes and may or may not involve chromatin (), and many lncRNAs carry out this important functional role [Citation129,Citation132–135]. A classic example is the X-chromosome interacting lncRNA, Firre. It acts as a scaffold for nuclear-matrix factor and chromosomes in neural-crest cells in the developing brain [Citation136]. A recent study has also identified neuroLNC, a conserved neuron-specific nuclear lncRNA that regulates synaptic vesicle release, which serves as a scaffold for the TAR DNA-binding protein – 43 (TDP-43), where it stabilizes transcripts that are involved in synaptic transmission [Citation137].
Decoy lncRNAs prevent proteins from accessing the chromatin or DNA (). An excellent example of a decoy function is the Gas5 lncRNA [Citation86]. Using its conserved glucocorticoid receptor (GR) motif, Gas5 binds to and prevents the GR from interacting with DNA, thereby repressing transcription of GR-targeted genes [Citation138]. In addition, the Pnky lncRNA regulates neurogenesis in the postnatal brain by interacting with splicing factors [Citation139]. NATs can also act as decoys to repress the transcription of their proximal sense genes. For example, the lncRNA Lrp1-AS sequesters Hmgb2 proteins from activation Lrp1 transcription, and Lrp1-As expression is elevated in AD patients [Citation140]. The lncRNA gomafu is associated with distinct nuclear bodies [Citation141], and one of the primary functions of this lncRNA appears to be the sequestering of splicing factors to regulate transcription and splicing in neuronal cells in a context-dependent manner [Citation142].
It is also common for small RNA genes to be encoded within lncRNA loci. For example, Gas5 can host several small nucleolar RNAs (snoRNAs) within its introns [Citation143]. The presence of these snoRNAs can then regulate the maturation of the Gas5 host lncRNA [Citation144]. Because snoRNAs reside in lncRNAs, they are often categorized as sno-lncRNAs. In some cases, sno-lncRNAs regulate alternative splicing in the nucleus [Citation27]. In contrast, host lncRNAs can function independent of their resident. Rmst, the host of miR-1251 and miR-135-a2, provides one example [Citation126]. Knockdown experiments have shown that depleting the Rmst host gene has no effect on the expression of miR-1251 and miR-135-a2 during neurogenesis [Citation126]. Future studies investigating alternative splicing of lncRNAs will no doubt reveal the existence of complex repertoire of isoforms with discrete functions in the brain.
LncRNAs as critical components of biomolecular condensates in the nucleus
Once lncRNAs come in close proximity with their lncRNA binding proteins (LBPs) they can oligomerise and recruit additional proteins. Using their low complexity prion-like domain, RNA-protein complexes form membraneless liquid-liquid phase-separated (LLPS) biomolecular condensates (). In the nucleus, these entities include paraspeckles, nuclear speckles, Cajal bodies, promyelocytic leukaemia (PML) bodies or simply nuclear bodies, which are characterized by the type of RNA present within them. The first lncRNA observed in the phase-separated compartment was Xist, which coats the inactivated X chromosome to form distinct architecture in the nucleus [Citation145]. The lncRNA Neat1 is an essential component of paraspeckles and has a tendency to relocate between different compartments in response to neuronal stimuli [Citation117,Citation146]. In the motor neurons of amyotrophic lateral sclerosis (ALS) patients, the Neat1 isoform, Neat1_2, binds to the RNA-binding proteins Fused in Sarcoma (FUS) and TDP-43 and assembles them into paraspeckles [Citation114,Citation115]. Additional components of the paraspeckle have also started to emerge [Citation147–150]. Malat1, for example, is a lncRNA that has been implicated in brain function and can also form a distinct nuclear speckle. The formation of this condensate correlates with the recruitment of factors during active transcription and the splicing of genes involves in synaptic formation and maintenance [Citation128,Citation151]. Taken together, these observations clearly suggest that LLPS involves the activity of lncRNAs, which lends to their complex, context-specific functions, and may therefore play an important role in normal cellular processes and in disease pathogenesis.
LncRNAs as guides, scaffolds and decoys along the axon and at the synapse
The transport of RNA along the axon or dendrites to the synapse serves to localize critically important RNAs to the synapse for local translation that is necessary for plasticity [Citation110,Citation152]. Although direct evidence of the involvement of lncRNAs in axonal cargo transport has yet to be demonstrated, it is possible that some localized granules can host lncRNAs. The axonally localized fragile X mental retardation protein (FMRP) binds and indirectly regulates lncRNA Tug1 expression in the regulation of axonal development [Citation153]. In addition, a subset of NATs associate with kinesin motors in response to stimuli [Citation112] and these kinesin motors are capable of transporting RNA granules and splicing ribonucleoprotein complexes (RNPs) along the axon [Citation111,Citation112,Citation154]. The association of NATs with kinesin motors therefore further indicates that they are actively transported to the synapse.
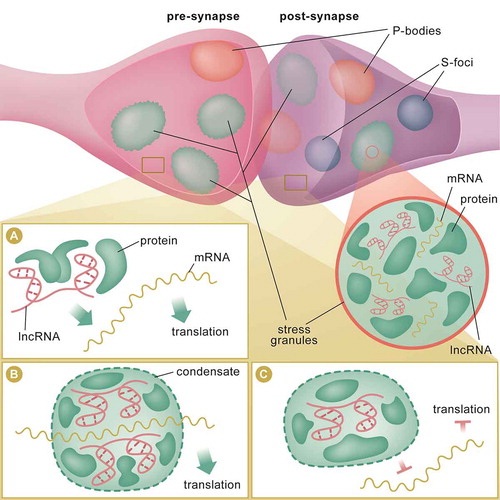
Recent studies have also identified a population of lncRNAs that accumulate in the synaptic compartment during ageing[Citation20]. BC1 was the first guide lncRNA discovered to modulate the translation of its target transcript at the synapse [Citation34,Citation155–158] (). BC1 mediates translation repression by bridging the repressor FMRP and its target mRNAs [Citation155–158]. In addition, NATs Bace1-AS and Uchl1-AS guide their sense transcripts to the synapse for protein synthesis [Citation116,Citation120,Citation159,Citation160] and guide lncRNAs in the cytoplasm have been shown to employ a similar mechanism [Citation161,Citation162]. This form of tuning and regulation by synaptic guide lncRNAs may be crucial for synaptic activity. For example, BC1 can bind to FMRP as a guide; it can also act as a bridge for FMRP and its target molecules [Citation161]. In the synapse, BC1 can also bind to translation initiation factor (eIF4A) and polyA binding protein (PABP) and precludes them from interacting with target mRNAs to initiate translation () [Citation163,Citation164]. It has also been proposed that Meg3 functions as a miRNA decoy in the synaptic compartment to mediate AMPA receptor loading at the synapse [Citation165]. Acting along similar lines, LncND reversibly sequesters miR-143-3p to regulate Notch signalling. The maintenance of the neuronal progenitor pool by this lncRNA–miRNA interaction is crucial for the expansion of the cerebral cortex in humans [Citation166]. In addition, the lncRNA NORAD acts as decoy for dendritic-localized PUMILIO to prevent them from repressing translation [Citation167,Citation168]. Despite these interesting threads of evidence; however, the functional relevance of lncRNAs in axons and at the synapse is not completely understood, and future studies will likely yield new insight into the role of localized lncRNA function in the brain.
Figure 2. lncRNA mechanisms of action at the synapse. In the pre and post-synaptic compartments, lncRNAs may localize to membraneless condensates, such as stress granules, processing bodies (P-bodies) and silencing foci (S-foci) (top panel). These biomolecular structures may serve as reservoirs for RNAs and proteins. RNAs and proteins can travel in and out, or between these storage sites in response to learning-induced stimuli or stress. lncRNA may serve as guide to direct proteins, such as translation factors and helicases, to the mRNA for local translation (A). lncRNA may act as scaffold to bring 2 or more proteins (or RNAs) into close proximity to induce formation of condensates at sites of active translation (B). LncRNA-associated condensates may act as decoy to titrate away RBPs, such as translation initiation factors, from interacting with RNAs, and therefore, inhibits local translation at the synapse (C)
LncRNAs may be key functional elements associated with localized RNA granules
Sites of RNA storage within the synapse include stress granules (SG), processing bodies (P-bodies), and neuron-specific granules called silencing foci (S-foci) (). SG and P-bodies contain discrete RNPs [Citation169] whereas P-bodies consist of mostly deadenylated transcripts, RNA decapping and RNA decay factors [Citation170]. On the other hand, dendritic stress granules house distinct RNPs, including polyadenylated transcripts, translation initiation factors and small ribosomal subunits [Citation171–174]. Neuron-specific S-foci are primarily comprised of deadenylases [Citation175] and these membraneless subcellular compartments can move RNAs and proteins freely between cytosol and other granules in response to stress or stimuli [Citation176–178]. The biogenesis and movement of these condensates is dynamic [Citation179–183]. For example, P-bodies can travel along dendrites in response to stimuli [Citation184]. Dendritic stress granules with distinct ribonucleoparticles (RNPs) can contribute to translational repression, adding another layer to posttranscriptional regulation [Citation185]. lncRNAs may associate with RNPs to form granules [Citation186], as BC1 associates with PABP, translation initiation factors and other components of the ribosome at the synapse [Citation34,Citation163,Citation164] (). Indeed, these granules have been shown to play a role in learning and long-term memory [Citation187,Citation188] and, quite extraordinarily, the formation of these membraneless condensates renders RNA in a ‘quiescent’ state where, upon stimulation, they serve to silence translation and promote RNA stability [Citation186,Citation189,Citation190]. On the other hand, disassembly of SG can rapidly recover protein synthesis [Citation175,Citation191], which suggests that some of these biomolecular condensates may primarily function as centres for sorting or aggregation that promote RNA metabolism when translation is in high demand. This ‘reservoir’ hypothesis is supported by the fact that SG also stores small ribosomal subunits, N6-metheyladenosine (m6A) modified RNAs and translational initiation components [Citation118,Citation172,Citation174]. Similar to transcription-dependent condensates in the nucleus [Citation192] (), dendritic condensates may perhaps coalesce to form translation-dependent condensates at sites of translation during protein synthesis (). This alternative hypothesis is supported by the observation that transcripts containing internal ribosome entry sites (IRES) can recruit factors to promote translation [Citation193,Citation194]. Despite the existence of RNA reservoirs and translation-dependent condensates in neurons, the functional exchange of RNA between different membraneless compartments remains to be demonstrated in the brain, and the role of lncRNAs governing this process in the context of experience-induced plasticity warrants further investigation.
Current technologies
Short read sequencing of barcoded-cDNA fragments enables the detection of RNA between 45 and 400 nucleotides [Citation195]. This technology has been incredibly been useful for generating reliable assemblies of protein-coding transcriptomes. However, short read sequencing cannot accurately determine the full repertoire of transcript isoforms, which is an important issue given that lncRNAs are universally alternatively spliced. Furthermore, because lncRNAs are often expressed at very low levels, detecting them remains a significant challenge. Single-molecule, real-time (SMRT) sequencing has the ability to yield a more complete picture of lncRNA expression patterns [Citation77]. The advantage of this technology is that RNA or cDNA is sequenced in full without the need to fragment, and information about the 5ʹ and 3ʹ end of the transcript is retained. Critical features of RNA are now accessible, including the detection of their structure state and alternatively spliced isoforms. Recently, Oxford Nanopore has evolved this approach with ultra-long read sequencing, and this technology has been adapted to sequence lncRNAs [Citation78]. Using lncRNA targeted biotin-conjugated oligonucleotides, a collection of dysregulated lncRNAs in the brain of neuropsychiatric patients has been captured and sequenced [Citation78]. In addition, RNA modifications can also be accurately determined with specific base-calling algorithms [Citation196]. The downside of Nanopore technology is that it is currently prone to a relatively high error-rate [Citation197]. Nevertheless, nanopore technology is an important advance in the ability to detect alternative lncRNA sequence variants in their native state and in multiple biological systems, including neurological diseases.
The ability to manipulate lncRNA expression with spatiotemporal resolution in vivo remains challenging due to their dynamic nature. Traditionally, lncRNA knockdown has been achieved through the use of antisense oligonucleotide (ASO) [Citation198]. This approach is quite efficient in silencing gene expression in-vivo. However, it is not possible to use ASO to pinpoint specific subcellular compartments for knockdown, which is a critical issue given that lncRNAs are functionally active depending on their location in the cell. An alternative approach is the recently discovered CRISPR-cas9 knockout technology [Citation199]. CRISPR-cas9 generates mutations or deletions using guide RNAs (gRNAs) against the genomic DNA of interest. However, the knockout effect of cas9 is permanent and therefore not ideal for achieving temporal control in the brain. To circumvent this issue, a temporally controlled CRISPR-mediated gene interference system (CRISPRi) for gene inactivation in the brain has been developed [Citation200]. The CRISPRi system utilizes a fusion peptide consisting of a catalytically inactive cas9 and a repressor protein. With gRNAs, the inactive cas9-repressor complex into close proximity to the gene hence repressing its expression; however, this method is also prone to off-target effects.
More recently, an optically controlled CRISPR-cas9 system has emerged [Citation201]. This system uses a photoconvertible Dronpa domain with the Cas9 protein, which provides both spatial and temporal control. An RNA-targeted CRISPR-cas13 has also emerged as a tool specifically designed for RNA knockdown [Citation202]. This is particularly useful as introduction of the guide in a specific time window can now be achieved. The CRISPR-cas13 system has also been adapted to RNA modifications to modulate gene expression [Citation203]. Inspired by CRISPR-cas13 system, Bryan Dickinson’s group at the University of Chicago have developed a CRISPR-cas-inspired RNA targeting system (CIRTS) [Citation204]. CIRTS is an engineered synthetic protein created by fusing different effector, RNA binding, and RNA guide recognition module into one assembly. It has the advantage of being small, which dramatically improves virus packaging and high titre production. Adapting the CRISPR-cas13, the CIRTS or the photocaged-system to the brain will enable the functional characterization of these lncRNAs, in particularly in the context of experience-dependent plasticity, learning and memory.
Overexpression of a transgene in the brain often employs a classic overexpression construct with a brain-specific promoter upstream of the transgene [Citation205]. This approach requires careful consideration in choosing the gene isoform and promoter. Constitutively activating transgenes are not ideal in most cases, and an activity-dependent promoter might not be suitable in some learning contexts. Alternatively, overexpression of a gene of interest is attainable with the CRISPR-cas9 activation system (CRISPRa) [Citation206]. Using the same concept as CRISPRi, CRISPRa uses a catalytically inactive cas9 and fuses it with a transcriptional activator. With promoter-targeted gRNAs, CRISPRa can activate genes in a targeted manner; however, the CRISPRa system is also not suitable for isoform-specific activation, and off-target gene activation remains a problem.
As indicated throughout this review, to better understand the role of lncRNAs in the brain, it is important to identify their pattern of subcellular localization. The simplest way to directly visualize lncRNA is using fluorescent in-situ hybridization (FISH) [Citation117,Citation207]. Fluorophores conjugated to probes target different regions of lncRNA without the need for a secondary substrate. However, this imaging technique might not be amenable in cases where target lncRNA expression is low. Using Z-probe technology, RNAscope has been applied to the brain for visualizing lncRNA [Citation166]. Unfortunately, FISH and RNAscope are not yet amenable for live cell imaging. Molecular beacons are used for the imaging of RNA in live cells [Citation208,Citation209]; however, similar to the FISH approach, it may not be ideal for highly structured, lowly expressed lncRNAs. For live cell imaging, different fluorescent aptamers have been developed [Citation210–215]. These aptamers emit light when bound to fluorogen, thus enabling the tagging of a specific RNA of interest. If fluorescent aptamers can be a target to lncRNAs, they will represent a powerful new tool for the tracking of lncRNA dynamics in real-time.
Finally, to understand the underlying mechanism of action of lncRNAs, identifying their functional interacting partners will be necessary. A straightforward way of purifying LBPs is by tagging the lncRNA of interest with an MS2 hairpin, immunoprecipitate the RNA-LBP complex, and followed by mass spectrometry analysis [Citation216,Citation217]. Although this approach is rapid, a caveat is that optimal binding cannot be reached due to competition with endogenous lncRNAs. An alternative is to purify the RNA-LBP complex with biotin tagged DNA oligonucleotides. With this approach, the interaction between lncRNA-LBP and chromatin can also be resolved [Citation218–220]. A limitation of this approach is the amount of starting materials required for accurate detection. Future methods that consider the cell-type specific and temporal aspects of lncRNA-LBP in the brain will need to be required. Nonetheless, the current approaches are greatly improving our understanding of lncRNA interaction networks, particularly in the brain.
Final outlook
Neural plasticity requires not only precise spatiotemporal control over gene expression, but also a dynamic interaction between RNA and various proteins. Our understanding of how lncRNAs coordinate these processes is rapidly increasing. Elucidating their functional and mechanistic role in subcellular compartments will provide a better picture of how lncRNAs mediate behavioural adaptation. Central to this challenge will be a deeper understanding of how lncRNA can adopt various structure states and how this affects their ability to interact with specific protein complexes. Last but not least, more sensitive cell-type and compartment-specific proteomic approaches will enable a deeper understanding of the functional consequences of lncRNA activity in the brain. Undoubtedly, lncRNAs will soon be shown to play an important role in the transcriptional and local translational regulation underlying learning and memory.
Acknowledgments
The authors acknowledge grant support from the Brain and Behavioural Research Foundation National (NARSAD Independent Investigator Grant-TWB), the National Institutes of Health (NIH) (R21MH103812-TWB) and the National Health and Medical Research Council (GNT1145172-TWB). The authors thank Ms Rowan Tweedale for editing of the manuscript and Dr Nick Valmas for figure graphics.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kellis M, Wold B, Snyder MP, et al. Defining functional DNA elements in the human genome. Proc Natl Acad Sci U S A. 2014;111(17):6131–6138. .
- Leighton LJ, Bredy TW. Functional Interplay between small non-coding RNAs and RNA modification in the brain. Noncoding RNA. 2018;4(2):15. doi:10.3390/ncrna4020015.
- Brown CJ, Hendrich BD, Rupert JL, et al. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992;71:527–542.
- Mattick JS. Introns: evolution and function. Curr Opin Genet Dev. 1994;4:823–831.
- Kawai J, Shinagawa A, Shibata K, et al. Functional annotation of a full-length mouse cDNA collection. Nature. 2001;409:685–690.
- Okazaki Y, Furuno M, Kasukawa T, et al. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature. 2002;420:563–573.
- Katayama S, Tomaru Y, Kasukawa T, et al. Antisense transcription in the mammalian transcriptome. Science. 2005;309:1564–1566.
- Mattick JS. Non-coding RNAs: the architects of eukaryotic complexity. EMBO Rep. 2001;2:986–991.
- Kapranov P, Cheng J, Dike S, et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316(5830):1484–1488. .
- Carninci P, Kasukawa T, Katayama S, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563.
- Mercer TR, Dinger ME, Sunkin SM, et al. Specific expression of long noncoding RNAs in the mouse brain. Proc Natl Acad Sci U S A. 2008;105(2):716–721.
- Derrien T, Johnson R, Bussotti G, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22(9):1775–1789. .
- Raveendra BL, Swarnkar S, Avchalumov Y, et al. Long noncoding RNA GM12371 acts as a transcriptional regulator of synapse function. Proc Natl Acad Sci U S A. 2018;115:E10197–E205.
- Brannan CI, Dees EC, Ingram RS, et al. The product of the H19 gene may function as an RNA. Mol Cell Biol. 1990;10:28–36.
- Brockdorff N, Ashworth A, Kay GF, et al. The product of the mouse Xist gene is a 15 kb inactive X-specific transcript containing no conserved ORF and located in the nucleus. Cell. 1992;71:515–526.
- Hon CC, Ramilowski JA, Harshbarger J, et al. An atlas of human long non-coding RNAs with accurate 5ʹ ends. Nature. 2017;543:199–204.
- Ma L, Cao J, Liu L, et al. LncBook: a curated knowledgebase of human long non-coding RNAs. Nucleic Acids Res. 2019;47:2699.
- Kadakkuzha BM, Liu XA, McCrate J, et al. Transcriptome analyses of adult mouse brain reveal enrichment of lncRNAs in specific brain regions and neuronal populations. Front Cell Neurosci. 2015;9:63.
- Liu SJ, Nowakowski TJ, Pollen AA, et al. Single-cell analysis of long non-coding RNAs in the developing human neocortex. Genome Biol. 2016;17:67.
- Chen BJ, Ueberham U, Mills JD, et al. RNA sequencing reveals pronounced changes in the noncoding transcriptome of aging synaptosomes. Neurobiol Aging. 2017;56:67–77.
- Spadaro PA, Flavell CR, Widagdo J, et al. Long noncoding RNA-directed epigenetic regulation of gene expression is associated with anxiety-like behavior in mice. Biol Psychiatry. 2015;78:848–859.
- Ciarlo E, Massone S, Penna I, et al. An intronic ncRNA-dependent regulation of SORL1 expression affecting Abeta formation is upregulated in post-mortem Alzheimer’s disease brain samples. Dis Model Mech. 2013;6:424–433.
- Massone S, Ciarlo E, Vella S, et al. NDM29, a RNA polymerase III-dependent non-coding RNA, promotes amyloidogenic processing of APP and amyloid beta secretion. Biochim Biophys Acta. 2012;1823:1170–1177.
- Martignetti JA, Brosius J. BC200 RNA: a neural RNA polymerase III product encoded by a monomeric Alu element. Proc Natl Acad Sci U S A. 1993;90:11563–11567.
- Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166.
- Valluy J, Bicker S, Aksoy-Aksel A, et al. A coding-independent function of an alternative Ube3a transcript during neuronal development. Nat Neurosci. 2015;18:666–673.
- Yin QF, Yang L, Zhang Y, et al. Long noncoding RNAs with snoRNA ends. Mol Cell. 2012;48:219–230.
- Zhang XO, Yin QF, Wang HB, et al. Species-specific alternative splicing leads to unique expression of sno-lncRNAs. BMC Genomics. 2014;15:287.
- Guttman M, Amit I, Garber M, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227.
- Cheng J, Kapranov P, Drenkow J, et al. Transcriptional maps of 10 human chromosomes at 5-nucleotide resolution. Science. 2005;308:1149–1154.
- Ravasi T, Suzuki H, Pang KC, et al. Experimental validation of the regulated expression of large numbers of non-coding RNAs from the mouse genome. Genome Res. 2006;16:11–19.
- Mukherjee N, Calviello L, Hirsekorn A, et al. Integrative classification of human coding and noncoding genes through RNA metabolism profiles. Nat Struct Mol Biol. 2017;24:86–96.
- Gruber AJ, Zavolan M. Alternative cleavage and polyadenylation in health and disease. Nat Rev Genet. 2019;20:599–614.
- Tiedge H, Fremeau RT Jr., Weinstock PH, et al. Dendritic location of neural BC1 RNA. Proc Natl Acad Sci U S A. 1991;88:2093–2097.
- Ingolia NT, Lareau LF, Weissman JS. Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell. 2011;147:789–802.
- Mills JD, Ward M, Chen BJ, et al. LINC00507 Is specifically expressed in the primate cortex and has age-dependent expression patterns. J Mol Neurosci. 2016;59:431–439.
- Ji Z, Song R, Regev A, et al. Many lncRNAs, 5ʹUTRs, and pseudogenes are translated and some are likely to express functional proteins. Elife. 2015;4:e08890.
- Cabili MN, Trapnell C, Goff L, et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25:1915–1927.
- Mo D, Li X, Raabe CA, et al. A universal approach to investigate circRNA protein coding function. Sci Rep. 2019;9:11684.
- Pamudurti NR, Bartok O, Jens M, et al. Translation of CircRNAs. Mol Cell. 2017;66:9–21 e7.
- Chen J, Brunner AD, Cogan JZ, et al. Pervasive functional translation of noncanonical human open reading frames. Science. 2020;367:1140–1146.
- van Heesch S, Witte F, Schneider-Lunitz V, et al. The Translational Landscape of the Human Heart. Cell. 2019;178:242–60 e29.
- Anderson DM, Anderson KM, Chang CL, et al. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell. 2015;160:595–606.
- Matsumoto A, Pasut A, Matsumoto M, et al. mTORC1 and muscle regeneration are regulated by the LINC00961-encoded SPAR polypeptide. Nature. 2017;541:228–232.
- Nelson BR, Makarewich CA, Anderson DM, et al. A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science. 2016;351:271–275.
- Guttman M, Russell P, Ingolia NT, et al. Ribosome profiling provides evidence that large noncoding RNAs do not encode proteins. Cell. 2013;154:240–251.
- Pircher A, Bakowska-Zywicka K, Schneider L, et al. An mRNA-derived noncoding RNA targets and regulates the ribosome. Mol Cell. 2014;54:147–155.
- Carlevaro-Fita J, Rahim A, Guigo R, et al. Cytoplasmic long noncoding RNAs are frequently bound to and degraded at ribosomes in human cells. RNA. 2016;22:867–882.
- Wadler CS, Vanderpool CK. A dual function for a bacterial small RNA: sgrS performs base pairing-dependent regulation and encodes a functional polypeptide. Proc Natl Acad Sci U S A. 2007;104:20454–20459.
- Jenny A, Hachet O, Zavorszky P, et al. A translation-independent role of oskar RNA in early Drosophila oogenesis. Development. 2006;133:2827–2833.
- Consortium EP, Birney E, Stamatoyannopoulos JA, et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816.
- Rinn JL, Euskirchen G, Bertone P, et al. The transcriptional activity of human Chromosome 22. Genes Dev. 2003;17:529–540.
- Kapranov P, Cawley SE, Drenkow J, et al. Large-scale transcriptional activity in chromosomes 21 and 22. Science. 2002;296:916–919.
- Abdelmohsen K, Panda AC, Kang MJ, et al. 7SL RNA represses p53 translation by competing with HuR. Nucleic Acids Res. 2014;42:10099–10111.
- Struhl K. Transcriptional noise and the fidelity of initiation by RNA polymerase II. Nat Struct Mol Biol. 2007;14:103–105.
- Ponjavic J, Ponting CP, Lunter G. Functionality or transcriptional noise? Evidence for selection within long noncoding RNAs. Genome Res. 2007;17:556–565.
- Nitsche A, Rose D, Fasold M, et al. Comparison of splice sites reveals that long noncoding RNAs are evolutionarily well conserved. RNA. 2015;21:801–812.
- Pheasant M, Mattick JS. Raising the estimate of functional human sequences. Genome Res. 2007;17:1245–1253.
- Kutter C, Watt S, Stefflova K, et al. Rapid turnover of long noncoding RNAs and the evolution of gene expression. PLoS Genet. 2012;8:e1002841.
- Andolfatto P. Adaptive evolution of non-coding DNA in Drosophila. Nature. 2005;437:1149–1152.
- Khaitovich P, Kelso J, Franz H, et al. Functionality of intergenic transcription: an evolutionary comparison. PLoS Genet. 2006;2:e171.
- Louro R, El-Jundi T, Nakaya HI, et al. Conserved tissue expression signatures of intronic noncoding RNAs transcribed from human and mouse loci. Genomics. 2008;92:18–25.
- Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641.
- Chen J, Shishkin AA, Zhu X, et al. Evolutionary analysis across mammals reveals distinct classes of long non-coding RNAs. Genome Biol. 2016;17:19.
- Marques AC, Ponting CP. Catalogues of mammalian long noncoding RNAs: modest conservation and incompleteness. Genome Biol. 2009;10:R124.
- Consortium EP. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74.
- Korostishevsky M, Kaganovich M, Cholostoy A, et al. Is the G72/G30 locus associated with schizophrenia? Single nucleotide polymorphisms, haplotypes, and gene expression analysis. Biol Psychiatry. 2004;56:169–176.
- Guffanti G, Galea S, Yan L, et al. Genome-wide association study implicates a novel RNA gene, the lincRNA AC068718.1, as a risk factor for post-traumatic stress disorder in women. Psychoneuroendocrinology. 2013;38:3029–3038.
- Amann-Zalcenstein D, Avidan N, Kanyas K, et al. AHI1, a pivotal neurodevelopmental gene, and C6orf217 are associated with susceptibility to schizophrenia. Eur J Hum Genet. 2006;14:1111–1119.
- Brevik EJ, van Donkelaar MM, Weber H, et al. Genome-wide analyses of aggressiveness in attention-deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2016;171:733–747.
- Hattori E, Liu C, Badner JA, et al. Polymorphisms at the G72/G30 gene locus, on 13q33, are associated with bipolar disorder in two independent pedigree series. Am J Hum Genet. 2003;72:1131–1140.
- Pang KC, Frith MC, Mattick JS. Rapid evolution of noncoding RNAs: lack of conservation does not mean lack of function. Trends Genet. 2006;22:1–5.
- Mattick JS. Makunin IV. Non-coding RNA. Hum Mol Genet. 2006;15 Spec(No 1):R17–29.
- Ramos AD, Diaz A, Nellore A, et al. Integration of genome-wide approaches identifies lncRNAs of adult neural stem cells and their progeny in vivo. Cell Stem Cell. 2013;12:616–628.
- Mercer TR, Qureshi IA, Gokhan S, et al. Long noncoding RNAs in neuronal-glial fate specification and oligodendrocyte lineage maturation. BMC Neurosci. 2010;11:14.
- Modarresi F, Faghihi MA, Patel NS, et al. Knockdown of BACE1-AS nonprotein-coding transcript modulates beta-amyloid-related hippocampal neurogenesis. Int J Alzheimers Dis. 2011;2011:929042.
- Lagarde J, Uszczynska-Ratajczak B, Carbonell S, et al. High-throughput annotation of full-length long noncoding RNAs with capture long-read sequencing. Nat Genet. 2017;49:1731–1740.
- Hardwick SA, Bassett SD, Kaczorowski D, et al. Targeted, high-resolution RNA sequencing of non-coding genomic regions associated with neuropsychiatric functions. Front Genet. 2019;10:309.
- Casaletto KB, Elahi FM, Bettcher BM, et al. Neurogranin, a synaptic protein, is associated with memory independent of Alzheimer biomarkers. Neurology. 2017;89:1782–1788.
- Kaeser PS, Deng L, Fan M, et al. RIM genes differentially contribute to organizing presynaptic release sites. Proc Natl Acad Sci U S A. 2012;109:11830–11835.
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038.
- Asok A, Leroy F, Rayman JB, et al. Molecular mechanisms of the memory trace. Trends Neurosci. 2019;42:14–22.
- Gandal MJ, Zhang P, Hadjimichael E, et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science. 2018;362(6420). doi:10.1126/science.aat8127.
- Li D, Zhang J, Wang M, et al. Activity dependent LoNA regulates translation by coordinating rRNA transcription and methylation. Nat Commun. 2018;9:1726.
- Maag JL, Panja D, Sporild I, et al. Dynamic expression of long noncoding RNAs and repeat elements in synaptic plasticity. Front Neurosci. 2015;9:351.
- Meier I, Fellini L, Jakovcevski M, et al. Expression of the snoRNA host gene gas5 in the hippocampus is upregulated by age and psychogenic stress and correlates with reduced novelty-induced behavior in C57BL/6 mice. Hippocampus. 2010;20:1027–1036.
- Lewejohann L, Skryabin BV, Sachser N, et al. Role of a neuronal small non-messenger RNA: behavioural alterations in BC1 RNA-deleted mice. Behav Brain Res. 2004;154:273–289.
- Chung A, Dahan N, Alarcon JM, et al. Effects of regulatory BC1 RNA deletion on synaptic plasticity, learning, and memory. Learn Mem. 2017;24:646–649.
- Butler AA, Johnston DR, Kaur S, et al. Long noncoding RNA NEAT1 mediates neuronal histone methylation and age-related memory impairment. Sci Signal. 2019;12(588). doi:10.1126/scisignal.aaw9277.
- Xu H, Brown AN, Waddell NJ, et al. Role of long noncoding RNA gas5 in cocaine action. Biol Psychiatry. 2020;88(10):758–766. doi:10.1016/j.biopsych.2020.05.004.
- Barry G, Briggs JA, Vanichkina DP, et al. The long non-coding RNA Gomafu is acutely regulated in response to neuronal activation and involved in schizophrenia-associated alternative splicing. Mol Psychiatry. 2014;19:486–494.
- Tian T, Wei Z, Chang X, et al. The long noncoding RNA landscape in amygdala tissues from schizophrenia patients. EBioMedicine. 2018;34:171–181.
- Taylor MS, Devon RS, Millar JK, et al. Evolutionary constraints on the disrupted in schizophrenia locus. Genomics. 2003;81:67–77.
- Millar JK, Wilson-Annan JC, Anderson S, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9:1415–1423.
- Polesskaya OO, Haroutunian V, Davis KL, et al. Novel putative nonprotein-coding RNA gene from 11q14 displays decreased expression in brains of patients with schizophrenia. J Neurosci Res. 2003;74:111–122.
- Parikshak NN, Swarup V, Belgard TG, et al. Genome-wide changes in lncRNA, splicing, and regional gene expression patterns in autism. Nature. 2016;540:423–427.
- Williams JM, Beck TF, Pearson DM, et al. A 1q42 deletion involving DISC1, DISC2, and TSNAX in an autism spectrum disorder. Am J Med Genet A. 2009;149A:1758–1762.
- Mortezaei Z, Lanjanian H, Masoudi-Nejad A. Candidate novel long noncoding RNAs, MicroRNAs and putative drugs for Parkinson’s disease using a robust and efficient genome-wide association study. Genomics. 2017;109:158–164.
- Soreq L, Guffanti A, Salomonis N, et al. Long non-coding RNA and alternative splicing modulations in Parkinson’s leukocytes identified by RNA sequencing. PLoS Comput Biol. 2014;10:e1003517.
- Mus E, Hof PR, Tiedge H. Dendritic BC200 RNA in aging and in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2007;104:10679–10684.
- Magistri M, Velmeshev D, Makhmutova M, et al. Transcriptomics profiling of alzheimer’s disease reveal neurovascular defects, altered amyloid-beta homeostasis, and deregulated expression of long noncoding RNAs. J Alzheimers Dis. 2015;48:647–665.
- Chung DW, Rudnicki DD, Yu L, et al. A natural antisense transcript at the Huntington’s disease repeat locus regulates HTT expression. Hum Mol Genet. 2011;20:3467–3477.
- D’Haene E, Jacobs EZ, Volders PJ, et al. Identification of long non-coding RNAs involved in neuronal development and intellectual disability. Sci Rep. 2016;6:28396.
- Court F, Camprubi C, Garcia CV, et al. The PEG13-DMR and brain-specific enhancers dictate imprinted expression within the 8q24 intellectual disability risk locus. Epigenetics Chromatin. 2014;7(1):5. .
- Talkowski ME, Maussion G, Crapper L, et al. Disruption of a large intergenic noncoding RNA in subjects with neurodevelopmental disabilities. Am J Hum Genet. 2012;91(6):1128–1134. .
- Labonte B, Abdallah K, Maussion G, et al. Regulation of impulsive and aggressive behaviours by a novel lncRNA. Mol Psychiatry. 2020. DOI:10.1038/s41380-019-0637-4.
- Grinman E, Espadas I, Puthanveettil SV. Emerging roles for long noncoding RNAs in learning, memory and associated disorders. Neurobiol Learn Mem. 2019;163:107034.
- Lyu Y, Bai L, Qin C. Long noncoding RNAs in neurodevelopment and Parkinson’s disease. Animal Model Exp Med. 2019;2: 239–251.
- Wei CW, Luo T, Zou SS, et al. The role of long noncoding RNAs in central nervous system and neurodegenerative diseases. Front Behav Neurosci. 2018;12:175.
- Geiger JC, Lipka J, Segura I, et al. The GRIP1/14-3-3 pathway coordinates cargo trafficking and dendrite development. Dev Cell. 2014;28(4):381–393. .
- Kanai Y, Dohmae N, Hirokawa N. Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron. 2004;43(4):513–525.
- Puthanveettil SV, Antonov I, Kalachikov S, et al. A strategy to capture and characterize the synaptic transcriptome. Proc Natl Acad Sci U S A. 2013;110:7464–7469.
- Guo CJ, Ma XK, Xing YH, et al. Distinct processing of lncRNAs contributes to non-conserved functions in stem cells. Cell. 2020. DOI:10.1016/j.cell.2020.03.006.
- Nishimoto Y, Nakagawa S, Hirose T, et al. The long non-coding RNA nuclear-enriched abundant transcript 1_2 induces paraspeckle formation in the motor neuron during the early phase of amyotrophic lateral sclerosis. Mol Brain. 2013;6(1):31. .
- Shelkovnikova TA, Robinson HK, Troakes C, et al. Compromised paraspeckle formation as a pathogenic factor in FUSopathies. Hum Mol Genet. 2014;23(9):2298–2312.
- Carrieri C, Cimatti L, Biagioli M, et al. Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature. 2012;491(7424):454–457. .
- Barry G, Briggs JA, Hwang DW, et al. The long non-coding RNA NEAT1 is responsive to neuronal activity and is associated with hyperexcitability states. Sci Rep. 2017;7:40127.
- Madugalle SU, Meyer K, Wang DO, et al. RNA N(6)-methyladenosine and the regulation of RNA localization and function in the brain. Trends Neurosci. 2020. DOI:10.1016/j.tins.2020.09.005
- Airavaara M, Pletnikova O, Doyle ME, et al. Identification of novel GDNF isoforms and cis-antisense GDNFOS gene and their regulation in human middle temporal gyrus of Alzheimer disease. J Biol Chem. 2011;286:45093–45102.
- Faghihi MA, Modarresi F, Khalil AM, et al. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nat Med. 2008;14:723–730.
- Modarresi F, Faghihi MA, Lopez-Toledano MA, et al. Inhibition of natural antisense transcripts in vivo results in gene-specific transcriptional upregulation. Nat Biotechnol. 2012;30(5):453–459. .
- Postepska-Igielska A, Giwojna A, Gasri-Plotnitsky L, et al. LncRNA Khps1 regulates expression of the proto-oncogene SPHK1 via triplex-mediated changes in chromatin structure. Mol Cell. 2015;60:626–636.
- Wang X, Arai S, Song X, et al. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature. 2008;454(7200):126–130. .
- Onoguchi M, Hirabayashi Y, Koseki H, et al. A noncoding RNA regulates the neurogenin1 gene locus during mouse neocortical development. Proc Natl Acad Sci U S A. 2012;109(42):16939–16944.
- Lin N, Chang KY, Li Z, et al. An evolutionarily conserved long noncoding RNA TUNA controls pluripotency and neural lineage commitment. Mol Cell. 2014;53:1005–1019.
- Ng SY, Bogu GK, Soh BS, et al. The long noncoding RNA RMST interacts with SOX2 to regulate neurogenesis. Mol Cell. 2013;51:349–359.
- Vance KW, Sansom SN, Lee S, et al. The long non-coding RNA Paupar regulates the expression of both local and distal genes. Embo J. 2014;33(4):296–311. .
- Bernard D, Prasanth KV, Tripathi V, et al. A long nuclear-retained non-coding RNA regulates synaptogenesis by modulating gene expression. Embo J. 2010;29(18):3082–3093. .
- Rinn JL, Kertesz M, Wang JK, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129(7):1311–1323. .
- Bond AM, Vangompel MJ, Sametsky EA, et al. Balanced gene regulation by an embryonic brain ncRNA is critical for adult hippocampal GABA circuitry. Nat Neurosci. 2009;12:1020–1027.
- Feng J, Bi C, Clark BS, et al. The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes Dev. 2006;20:1470–1484.
- Kotake Y, Nakagawa T, Kitagawa K, et al. Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene. 2011;30:1956–1962.
- Pandey RR, Mondal T, Mohammad F, et al. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol Cell. 2008;32(2):232–246. .
- Tsai MC, Manor O, Wan Y, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693.
- Yap KL, Li S, Munoz-Cabello AM, et al. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol Cell. 2010;38:662–674.
- Hacisuleyman E, Goff LA, Trapnell C, et al. Topological organization of multichromosomal regions by the long intergenic noncoding RNA Firre. Nat Struct Mol Biol. 2014;21(2):198–206. .
- Keihani S, Kluever V, Mandad S, et al. The long noncoding RNA neuroLNC regulates presynaptic activity by interacting with the neurodegeneration-associated protein TDP-43. Sci Adv. 2019;5:eaay:2670.
- Kino T, Hurt DE, Ichijo T, et al. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal. 2010;3(107):ra8.
- Ramos AD, Andersen RE, Liu SJ, et al. The long noncoding RNA Pnky regulates neuronal differentiation of embryonic and postnatal neural stem cells. Cell Stem Cell. 2015;16(4):439–447. .
- Yamanaka Y, Faghihi MA, Magistri M, et al. RNA controls LRP1 Sense transcript expression through interaction with a chromatin-associated protein, HMGB2. Cell Rep. 2015;11:967–976.
- Sone M, Hayashi T, Tarui H, et al. The mRNA-like noncoding RNA Gomafu constitutes a novel nuclear domain in a subset of neurons. J Cell Sci. 2007;120:2498–2506.
- Ishizuka A, Hasegawa Y, Ishida K, et al. Formation of nuclear bodies by the lncRNA Gomafu-associating proteins Celf3 and SF1. Genes Cells. 2014;19:704–721.
- Smith CM, Steitz JA. Classification of gas5 as a multi-small-nucleolar-RNA (snoRNA) host gene and a member of the 5ʹ-terminal oligopyrimidine gene family reveals common features of snoRNA host genes. Mol Cell Biol. 1998;18:6897–6909.
- Filippova JA, Matveeva AM, Zhuravlev ES, et al. Are small nucleolar RNAs “CRISPRable”? A Report on Box C/D Small Nucleolar RNA Editing in Human Cells. Front Pharmacol. 2019;10:1246.
- Clemson CM, McNeil JA, Willard HF, et al. XIST RNA paints the inactive X chromosome at interphase: evidence for a novel RNA involved in nuclear/chromosome structure. J Cell Biol. 1996;132:259–275.
- Chen LL, Carmichael GG. Altered nuclear retention of mRNAs containing inverted repeats in human embryonic stem cells: functional role of a nuclear noncoding RNA. Mol Cell. 2009;35:467–478.
- Kawaguchi T, Tanigawa A, Naganuma T, et al. SWI/SNF chromatin-remodeling complexes function in noncoding RNA-dependent assembly of nuclear bodies. Proc Natl Acad Sci U S A. 2015;112:4304–4309.
- Naganuma T, Nakagawa S, Tanigawa A, et al. Alternative 3ʹ-end processing of long noncoding RNA initiates construction of nuclear paraspeckles. Embo J. 2012;31:4020–4034.
- Sasaki YT, Ideue T, Sano M, et al. MENepsilon/beta noncoding RNAs are essential for structural integrity of nuclear paraspeckles. Proc Natl Acad Sci U S A. 2009;106:2525–2530.
- Sunwoo H, Dinger ME, Wilusz JE, et al. MEN epsilon/beta nuclear-retained non-coding RNAs are up-regulated upon muscle differentiation and are essential components of paraspeckles. Genome Res. 2009;19:347–359.
- Tripathi V, Ellis JD, Shen Z, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–938.
- Knowles RB, Kosik KS. Neurotrophin-3 signals redistribute RNA in neurons. Proc Natl Acad Sci U S A. 1997;94:14804–14808.
- Guo Y, Chen X, Xing R, et al. Interplay between FMRP and lncRNA TUG1 regulates axonal development through mediating SnoN-Ccd1 pathway. Hum Mol Genet. 2018;27:475–485.
- Thomas-Jinu S, Gordon PM, Fielding T, et al. Non-nuclear pool of splicing factor SFPQ regulates axonal transcripts required for normal motor development. Neuron. 2017;94:931.
- Lacoux C, Di Marino D, Boyl PP, et al. BC1-FMRP interaction is modulated by 2ʹ-O-methylation: RNA-binding activity of the tudor domain and translational regulation at synapses. Nucleic Acids Res. 2012;40:4086–4096.
- Zalfa F, Giorgi M, Primerano B, et al. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112:317–327.
- Zalfa F, Adinolfi S, Napoli I, et al. Fragile X mental retardation protein (FMRP) binds specifically to the brain cytoplasmic RNAs BC1/BC200 via a novel RNA-binding motif. J Biol Chem. 2005;280:33403–33410.
- Briz V, Restivo L, Pasciuto E, et al. The non-coding RNA BC1 regulates experience-dependent structural plasticity and learning. Nat Commun. 2017;8:293.
- Cartier AE, Djakovic SN, Salehi A, et al. Regulation of synaptic structure by ubiquitin C-terminal hydrolase L1. J Neurosci. 2009;29:7857–7868.
- Lundgren JL, Ahmed S, Schedin-Weiss S, et al. ADAM10 and BACE1 are localized to synaptic vesicles. J Neurochem. 2015;135:606–615.
- Gong C, Maquat LE. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3ʹ UTRs via Alu elements. Nature. 2011;470:284–288.
- Belanger G, Stocksley MA, Vandromme M, et al. Localization of the RNA-binding proteins Staufen1 and Staufen2 at the mammalian neuromuscular junction. J Neurochem. 2003;86:669–677.
- Lin D, Pestova TV, Hellen CU, et al. Translational control by a small RNA: dendritic BC1 RNA targets the eukaryotic initiation factor 4A helicase mechanism. Mol Cell Biol. 2008;28:3008–3019.
- Muddashetty R, Khanam T, Kondrashov A, et al. Poly(A)-binding protein is associated with neuronal BC1 and BC200 ribonucleoprotein particles. J Mol Biol. 2002;321:433–445.
- Tan MC, Widagdo J, Chau YQ, et al. The activity-induced long non-coding RNA Meg3 Modulates AMPA receptor surface expression in primary cortical neurons. Front Cell Neurosci. 2017;11:124.
- Rani N, Nowakowski TJ, Zhou H, et al. A primate lncRNA mediates notch signaling during neuronal development by sequestering miRNA. Neuron. 2016;90:1174–1188.
- Lee S, Kopp F, Chang TC, et al. Noncoding RNA NORAD regulates genomic stability by sequestering PUMILIO proteins. Cell. 2016;164:69–80.
- Vessey JP, Schoderboeck L, Gingl E, et al. Mammalian Pumilio 2 regulates dendrite morphogenesis and synaptic function. Proc Natl Acad Sci U S A. 2010;107:3222–3227.
- Youn JY, Dunham WH, Hong SJ, et al. High-Density proximity mapping reveals the subcellular organization of mRNA-associated granules and bodies. Mol Cell. 2018;69:517–32 e11.
- Sheth U, Parker R. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science. 2003;300:805–808.
- Mazroui R, Huot ME, Tremblay S, et al. Trapping of messenger RNA by Fragile X Mental Retardation protein into cytoplasmic granules induces translation repression. Hum Mol Genet. 2002;11:3007–3017.
- Kimball SR, Horetsky RL, Ron D, et al. Mammalian stress granules represent sites of accumulation of stalled translation initiation complexes. Am J Physiol Cell Physiol. 2003;284:C273–84.
- Kedersha N, Chen S, Gilks N, et al. Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol Biol Cell. 2002;13:195–210.
- Grousl T, Ivanov P, Frydlova I, et al. Robust heat shock induces eIF2alpha-phosphorylation-independent assembly of stress granules containing eIF3 and 40S ribosomal subunits in budding yeast, Saccharomyces cerevisiae. J Cell Sci. 2009;122:2078–2088.
- Baez MV, Luchelli L, Maschi D, et al. Smaug1 mRNA-silencing foci respond to NMDA and modulate synapse formation. J Cell Biol. 2011;195:1141–1157.
- Kedersha N, Cho MR, Li W, et al. Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J Cell Biol. 2000;151:1257–1268.
- Kedersha N, Stoecklin G, Ayodele M, et al. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol. 2005;169:871–884.
- Wilczynska A, Aigueperse C, Kress M, et al. The translational regulator CPEB1 provides a link between dcp1 bodies and stress granules. J Cell Sci. 2005;118:981–992.
- Jain S, Wheeler JR, Walters RW, et al. ATPase-modulated stress granules contain a diverse proteome and substructure. Cell. 2016;164:487–498.
- Kim HJ, Kim NC, Wang YD, et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature. 2013;495:467–473.
- Yang W, Li D, Ru Y, et al. Foot-and-mouth disease virus 3a protein causes upregulation of autophagy-related protein LRRC25 to inhibit the G3BP1-mediated rig-like helicase-signaling pathway. J Virol. 2020;94(8). doi:10.1128/JVI.02086-19..
- Sanders DW, Kedersha N, Lee DSW, et al. Competing protein-RNA interaction networks control multiphase intracellular organization. Cell. 2020;181:306–24 e28.
- Guillen-Boixet J, Kopach A, Holehouse AS, et al. RNA-induced conformational switching and clustering of G3BP drive stress granule assembly by condensation. Cell. 2020;181:346–61 e17.
- Cougot N, Bhattacharyya SN, Tapia-Arancibia L, et al. Dendrites of mammalian neurons contain specialized P-body-like structures that respond to neuronal activation. J Neurosci. 2008;28:13793–13804.
- Vessey JP, Vaccani A, Xie Y, et al. Dendritic localization of the translational repressor Pumilio 2 and its contribution to dendritic stress granules. J Neurosci. 2006;26:6496–6508.
- Khong A, Matheny T, Jain S, et al. The stress granule transcriptome reveals principles of mRNA accumulation in stress granules. Mol Cell. 2017;68:808–20 e5.
- Solomon S, Xu Y, Wang B, et al. Distinct structural features of caprin-1 mediate its interaction with G3BP-1 and its induction of phosphorylation of eukaryotic translation initiation factor 2alpha, entry to cytoplasmic stress granules, and selective interaction with a subset of mRNAs. Mol Cell Biol. 2007;27:2324–2342.
- Nakayama K, Ohashi R, Shinoda Y, et al. RNG105/caprin1, an RNA granule protein for dendritic mRNA localization, is essential for long-term memory formation. Elife. 2017;6:e29677. doi:10.7554/eLife.29677.
- Hubstenberger A, Courel M, Benard M, et al. P-body purification reveals the condensation of repressed mRNA regulons. Mol Cell. 2017;68:144–57 e5.
- Gallouzi IE, Parker F, Chebli K, et al. A novel phosphorylation-dependent RNase activity of GAP-SH3 binding protein: a potential link between signal transduction and RNA stability. Mol Cell Biol. 1998;18:3956–3965.
- Mazroui R, Di Marco S, Kaufman RJ, et al. Inhibition of the ubiquitin-proteasome system induces stress granule formation. Mol Biol Cell. 2007;18:2603–2618.
- Cho WK, Spille JH, Hecht M, et al. Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science. 2018;361:412–415.
- Guil S, Long JC, Caceres JF. hnRNP A1 relocalization to the stress granules reflects a role in the stress response. Mol Cell Biol. 2006;26:5744–5758.
- Bonnal S, Pileur F, Orsini C, et al. Heterogeneous nuclear ribonucleoprotein A1 is a novel internal ribosome entry site trans-acting factor that modulates alternative initiation of translation of the fibroblast growth factor 2 mRNA. J Biol Chem. 2005;280:4144–4153.
- Meyer M, Stenzel U, Myles S, et al. Targeted high-throughput sequencing of tagged nucleic acid samples. Nucleic Acids Res. 2007;35:e97.
- Liu H, Begik O, Lucas MC, et al. Accurate detection of m(6)A RNA modifications in native RNA sequences. Nat Commun. 2019;10:4079.
- Soneson C, Yao Y, Bratus-Neuenschwander A, et al. A comprehensive examination of Nanopore native RNA sequencing for characterization of complex transcriptomes. Nat Commun. 2019;10:3359.
- Stein CA, Hansen JB, Lai J, et al. Efficient gene silencing by delivery of locked nucleic acid antisense oligonucleotides, unassisted by transfection reagents. Nucleic Acids Res. 2010;38:e3.
- Swiech L, Heidenreich M, Banerjee A, et al. In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat Biotechnol. 2015;33:102–106.
- Zheng Y, Shen W, Zhang J, et al. CRISPR interference-based specific and efficient gene inactivation in the brain. Nat Neurosci. 2018;21:447–454.
- Zhou L, Retailleau P, Morel M, et al. Photoswitchable fluorescent crystals obtained by the photoreversible coassembly of a nucleobase and an azobenzene intercalator. J Am Chem Soc. 2019;141:9321–9329.
- Abudayyeh OO, Gootenberg JS, Essletzbichler P, et al. RNA targeting with CRISPR-Cas13. Nature. 2017;550:280–284.
- Cox DBT, Gootenberg JS, Abudayyeh OO, et al. RNA editing with CRISPR-Cas13. Science. 2017;358:1019–1027.
- Rauch S, He E, Srienc M, et al. Guided RNA Effector Proteins Built from Human Parts. Cell. 2019;178:122–34 e12.
- Routtenberg A, Cantallops I, Zaffuto S, et al. Enhanced learning after genetic overexpression of a brain growth protein. Proc Natl Acad Sci U S A. 2000;97:7657–7662.
- Zhou H, Liu J, Zhou C, et al. In vivo simultaneous transcriptional activation of multiple genes in the brain using CRISPR-dCas9-activator transgenic mice. Nat Neurosci. 2018;21:440–446.
- Femino AM, Fay FS, Fogarty K, et al. Visualization of single RNA transcripts in situ. Science. 1998;280:585–590.
- Alami NH, Smith RB, Carrasco MA, et al. Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron. 2014;81:536–543.
- Turner-Bridger B, Jakobs M, Muresan L, et al. Single-molecule analysis of endogenous beta-actin mRNA trafficking reveals a mechanism for compartmentalized mRNA localization in axons. Proc Natl Acad Sci U S A. 2018;115:E9697–E706.
- Song W, Filonov GS, Kim H, et al. Imaging RNA polymerase III transcription using a photostable RNA-fluorophore complex. Nat Chem Biol. 2017;13:1187–1194.
- Chen X, Zhang D, Su N, et al. Visualizing RNA dynamics in live cells with bright and stable fluorescent RNAs. Nat Biotechnol. 2019;37:1287–1293.
- Warner KD, Chen MC, Song W, et al. Structural basis for activity of highly efficient RNA mimics of green fluorescent protein. Nat Struct Mol Biol. 2014;21:658–663.
- Filonov GS, Moon JD, Svensen N, et al. Broccoli: rapid selection of an RNA mimic of green fluorescent protein by fluorescence-based selection and directed evolution. J Am Chem Soc. 2014;136:16299–16308.
- Strack RL, Disney MD, Jaffrey SR. A superfolding Spinach2 reveals the dynamic nature of trinucleotide repeat-containing RNA. Nat Methods. 2013;10:1219–1224.
- Cawte AD, Unrau PJ, Rueda DS. Live cell imaging of single RNA molecules with fluorogenic Mango II arrays. Nat Commun. 2020;11:1283.
- Xing Z, Lin C, Yang L. LncRNA pulldown combined with mass spectrometry to identify the novel LncRNA-associated proteins. Methods Mol Biol. 2016;1402:1–9.
- Yoon JH, Gorospe M. Identification of mRNA-Interacting Factors by MS2-TRAP (MS2-Tagged RNA Affinity Purification). Methods Mol Biol. 2016;1421:15–22.
- Simon MD, Wang CI, Kharchenko PV, et al. The genomic binding sites of a noncoding RNA. Proc Natl Acad Sci U S A. 2011;108:20497–20502.
- Chu C, Zhang QC, da Rocha ST, et al. Systematic discovery of Xist RNA binding proteins. Cell. 2015;161:404–416.
- Engreitz JM, Pandya-Jones A, McDonel P, et al. The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science. 2013;341:1237973.
