ABSTRACT
Influenza virus infection through seasonal epidemics and occasional pandemics has been a major public health concern for decades. Incomplete protection from vaccination and increased antiviral resistance due to frequent mutations of influenza viruses have led to a continuous need for new therapeutic options. The functional significance of host protein and influenza virus interactions has been established, but relatively less is known about the interaction of host noncoding RNAs, including microRNAs and long noncoding RNAs, with influenza viruses. In this review, we summarize host noncoding RNA profiles during influenza virus infection and the regulation of influenza virus infection by host noncoding RNAs. Influenza viral non-coding RNAs are briefly discussed. Increased understanding of the molecular regulation of influenza viral replication will be beneficial in identifying potential therapeutic targets against the influenza virus.
1. Introduction
Eukaryotic cells are capable of producing several classes of long and small noncoding RNAs (ncRNAs) closely related to the evolving complexity of organisms [Citation1]. To control unwanted genetic materials and transcripts, many types of small ncRNAs have evolved in prokaryotes and eukaryotes and play important roles in gene expression. Small ncRNAs include microRNAs (miRNAs), small interfering RNAs (siRNAs), piwi-related RNAs (piRNAs), small nucleolar RNAs (snoRNAs), and vault RNAs (vtRNAs) [Citation1]. miRNAs are approximately 21 nucleotide (nt) RNA molecules that bind with the 3ʹ-untranslated region (3ʹ-UTR) of mRNA to repress its translation. More than 60% of all mRNAs was estimated to contain miRNA-binding sites in the 3ʹ-UTR region. Similar to miRNAs, endogenous siRNAs are products of double-stranded RNA (dsRNA) cleavage by the RNase III enzyme and utilize the RNA interference (RNAi) pathway. In contrast to endogenous miRNAs and siRNAs, piRNAs are not products of RNase III cleavage and are incorporated only into argonaute proteins. piRNAs are not expressed widely and are only expressed in germlines. piRNAs are important to maintain genomic stability. snoRNAs (60–300 nt) are mostly expressed and accumulate in the nucleus and are important in ribosomal RNA modification and maturation. vtRNAs (88–100 nt) are transcribed by RNA polymerase III and are components of ribonucleoprotein particles known as vaults. Human vtRNAs are known to function in apoptosis, drug resistance and viral infections [Citation2].
Long ncRNAs (lncRNAs) are defined as RNAs with lengths > 200 nt without protein-coding capacity. They can interact with DNA, RNA and protein and act as key mediators of cellular processes, including cell growth, differentiation, survival, and apoptosis; thus, they are implicated in many diseases [Citation3].
The latest version of GENCODE (v35) identified 19,954 protein-coding genes, 17,957 lncRNA genes, 7,569 small ncRNA genes, and 14,767 pseudogenes of the total number of genes (60,656) in humans. According to the mouse genomic statistics shown in GENCODE (M25), 55,401 genes were annotated, including 21,859 protein-coding genes, 13,197 lncRNAs, and 6,108 small ncRNA genes [Citation4].
Influenza is a major health burden worldwide. It is a contagious respiratory disease caused by influenza viruses. Severe cases of influenza infection can result in hospitalization and death. Understanding the host cellular factors and their interactions with influenza viruses is key to developing therapeutics for this disease.
The roles of ncRNAs in viral infection are being recognized, including the regulation of viral replication, viral persistence, host immunity, viral evasion, and cellular transformation. In this review, we summarize the interactions of influenza virus and host ncRNAs (miRNA and lncRNA) with a focus on altered ncRNA expression profiles caused by the influenza virus and the regulation of influenza infection by ncRNAs via viral proteins and host factors. Furthermore, we highlight the potential use of miRNAs and lncRNAs as diagnostic biomarkers and therapeutic targets.
2. Influenza virus infection
2.1. Overview of influenza viruses
Influenza viruses belong to the Orthomyxoviridae family and cause a respiratory disease that is commonly known as ‘the flu’. Influenza is one of the most common causes of human respiratory infections and is the cause of annual seasonal epidemics and unpredictable pandemics. In countries with temperate climates, including the United States (U.S.), influenza activity peaks in winter months and decreases in summer, whereas in tropical regions, influenza activity is more variable. In the U.S., depending on the circulating virus strains, the severity of the influenza season is highly variable, resulting in significant morbidity and mortality. Influenza infection, particularly in infants, the elderly, and people with chronic diseases, can lead to high mortality. From 2010 to 2016, approximately 8.3% of the U.S. population experienced flu-like symptoms annually, and influenza infection affects 5%-20% of the global population each year. According to the Centers for Disease Control (CDC), since 2010, estimated influenza-associated illness in the U.S. ranged between 9 million and 45 million with 12,000–61,000 deaths annually [Citation5]. Worldwide, an estimated 291,000–646,000 people succumb to this respiratory virus each year [Citation6]. In addition to causing annual seasonal epidemics, influenza can cause unpredictable pandemics. Influenza outbreaks may have occurred since ancient times, even though detailed laboratory evidence is available only for the 4 pandemics that occurred after 1917. In 1918, the first known influenza pandemic spread around the world in three consecutive waves, infecting one-third of the world population, and an estimated 50 million people succumbed to this viral infection [Citation7].
Four types of influenza viruses have been identified to date: influenza A, B, C, and D. Among these strains, influenza A and B are the main causes of epidemics in humans. Influenza C infection generally causes mild illness and is not thought to cause human influenza epidemics. Influenza D infection primarily occurs in cattle and pigs [Citation8]. In addition to major health risks, the influenza virus has and will continue to have considerable socioeconomic impacts.
Influenza A viruses (IAVs) are characterized by a segmented genome comprising of eight negative-strand RNAs that depends on RNA-dependent RNA polymerases for their replication. This eight-segmented genome encodes at least eleven viral proteins. The IAV virion consists of three integral membrane proteins, neuraminidase (NA), haemagglutinin (HA), and matrix protein 2 (M2). Currently, there are approximately 18 and 11 known variants of HA and NA, respectively. Protruding glycoprotein spikes of HA and NA project from the host cell-derived lipid membrane of the virus, and M2 ion channels traverse this lipid envelope. All these proteins cover matrix protein 1 (M1), enclosing the virion core [Citation9]. The virion core consists of the ribonucleoprotein (RNP) complex, nonstructural protein 1 (NS1), and nonstructural protein 2 (NS2; also called nuclear export protein NEP). The RNP complex is composed of trimeric acidic and basic RNA-dependent RNA polymerases (PA, PB1, and PB2) and nucleoproteins (NPs). Each segment of the influenza genome is encapsulated as an RNP molecule. IAV RNA segments 1, 2, 3, 4, 5, and 6 each encodes PB2, PB1, PA, HA, NP and NA, respectively. Segment 7 produces M1 and M2 proteins through mRNA splicing. Segment 8 encodes two viral proteins, NS1 and NS2. Besides, several auxiliary proteins are also encoded in the influenza genome, including PB1-F2 and PB1N40 (Segment 2) and PA-X (Segment 3).
Influenza viruses infect and replicate in many different species, including humans and are highly transmissible within and between species. Influenza genome replication is dependent on RNA-dependent RNA polymerases of viral origin and has a high tendency towards errors, which lead to mutations and changes in the viral genome that can create antigenic drift. Some species can be infected simultaneously with two different IAVs, with the two viruses replicating in the same host cell, which allows entire gene segments to be interchanged and packed into new virions. This process is known as genetic reassortment and enables antigenic shift. This event is frequently seen in aquatic birds, the natural reservoir for IAVs, and pigs, which are susceptible to both avian and human IAVs [Citation10].
2.2. Influenza virus life cycle
In humans, the primary site of influenza virus infection is the respiratory tract. The influenza virus depends on the host machinery for its replication. The virus life cycle can be divided into several stages, including attachment, internalization, endosomal trafficking, endosomal acidification, fusion, uncoating, nuclear import, viral replication, nuclear export, assembly, and budding [Citation10]. Influenza virus HA recognizes sialic acid receptors on the host cell surface that act as primary virus binding sites. The terminal sialic acid moiety link is fundamental to species specificity. HA from avian influenza viruses bind to the α2-3-linked sialic acid moiety, whereas HA from human-adapted viruses binds α2-6-linked sialic acid [Citation11]. In the human respiratory tract, sialic acid α-2, 6-linkages predominate in tracheal epithelial cells, where human IAVs bind and initiate virus entry. The binding of HA to a sialic acid receptor triggers endocytosis of the incoming virion via a clathrin-dependent or independent (micropinocytosis) pathway. Following entry into the cell, the virus is transported to endosomes. The activation of the M2 ion channel triggered by the low pH in endosomes results in conformational changes in HA that exposes the fusion peptide and acidification of the virion particle. The low pH inside the virus triggers the virus uncoating and the release of viral RNPs into the cytoplasm. After release into the cytoplasm, vRNPs use host nuclear import pathways to gain entry into the host cell nucleus [Citation10].
In all influenza viruses, RNA replication occurs in the nucleus. IAV RNA synthesis involves 1) transcription where vRNAs are transcribed into viral mRNAs and 2) replication where the segmented RNA genome is first transcribed into complementary RNAs (cRNAs) and then into new viral RNA (vRNAs). Viral mRNA transcription is a unique process known as ‘cap snatching’, where viral polymerases (PB2 and PA) obtain 5′ capped primers from nascent host transcripts to initiate viral mRNA synthesis via PB1 [Citation12]. During infection, mRNA transcription occurs earlier than cRNA and vRNA transcription and is much more efficient.
Influenza protein translation depends entirely on the translational machinery of the host cell. After viral mRNAs are exported from the nucleus, the envelope proteins HA, NA, and M2 are synthesized by membrane-bound endoplasmic reticulum-associated ribosomes, and other viral proteins are translated on cytoplasmic ribosomes. The envelope proteins are subsequently folded and trafficked to the Golgi apparatus. These viral proteins have apical sorting signals, which direct them to the cell membrane via the trans-Golgi network [Citation9]. vRNPs and other nonstructural proteins are delivered to the cell periphery and assembled into new virions, and then, budding is initiated. When budding is completed, HA on the virion particle binds to the cell membrane via sialic acid receptors, and the sialidase activity of NA cleaves this attachment bond and releases new virions.
Viral proteins marshal the process of the influenza virus life cycle at each step as facilitated by the host. Targeting viral proteins to disrupt their activity has proven to be an effective method of anti-influenza therapy. Strategies to limit virus entry steps are efficient for restricting virus replication. Anti-influenza drugs such as rimantadine and amantadine interfere with virus uncoating by blocking M2 channels [Citation13]. The neuraminidase inhibitor oseltamivir is another anti-influenza drug that interferes with the release of viral progeny and halts the spread of infection [Citation14]. Baloxavir marboxil, an anti-influenza drug recently approved by the FDA, inhibits viral polymerase activity and thus restricts virus replication [Citation15]. However, the influenza virus mutates frequently, and new strains of the virus are resistant to these drugs, which target influenza viral proteins. Developing novel influenza therapeutics targeting host factors that influence the pathology and progression of infection presents a promising approach to overcome the limitations of the current used anti-influenza drugs.
3. Host microRNA-influenza virus interactions
3.1. Overview of miRNAs
miRNAs comprise a class of endogenous evolutionarily conserved small noncoding RNAs of approximately 9–24 nt in length. miRNAs function as posttranscriptional regulators of gene expression by cleaving mRNA or inhibiting protein translation by binding complementary target sequences in the 3ʹ-UTR of mRNA [Citation16]. miRNAs were first discovered in the nematode Caenorhabditis elegans in 1993. A gene isolated from C. elegans, lin-4, was found to negatively regulate the LIN-14 protein level. However, the transcripts of lin-4 are not translated into a biologically active protein but contain RNA sequences complementary to a repeated sequence in the 3ʹ-UTR of lin-14 mRNA [Citation17]. This novel method of translational gene repression was first assumed to be a process exclusive to C. elegans. However, small RNA homologs to let-7 were identified in humans in 2000 [Citation18]. Subsequently, many miRNAs have been discovered in plants and animals. Some miRNAs are highly conserved across species. One such example is the miR-854 family, which is expressed in Arabidopsis thaliana (plant), C elegans (nematode), Mus musculus (rodent), and Homo sapiens (humans), and targets the 3ʹ-UTR of uridylate-binding protein 1b (UBP1b), which is involved in basal eukaryotic transcription mechanisms [Citation19].
In theory, a 22-nt single-stranded RNA with four different ribonucleotides (adenine, guanine, cytosine, and uridine) can have more than 1013 possible sequence combinations. The latest version of miRBase (v22), an online miRNA registry, documents 38,589 miRNA precursors and 48,860 mature miRNAs from 271 organisms [Citation20]. This dataset shows a one-third-fold increase in the number sequences compared to the previous release (v20) in 2013. The human genome contains 1,917 annotated hairpin precursors and 2,654 mature miRNAs [Citation20].
To ensure consistency, ease of cataloguing miRNAs and distinguishing them from other RNAs such as siRNAs, a uniform system of annotation and nomenclature has been adopted [Citation21]. According to this nomenclature, the first 3 letters identify the organism to which the miRNA belongs. For example, hsa refers to Homo sapiens, and mmu refers to Mus musculus. Numbers are assigned to experimentally confirmed miRNAs sequentially in the order of miRNA discovery. The numbers are attached to the prefix ‘miR’ followed by a hyphen (e.g., hsa-miR-206). A capitalized R denotes the mature miRNA, as in miR-206, and an uncapitalized r refers to both the miRNA gene and precursor miRNA, as in mir-206. Similar mature miRNA sequences originating from discrete genomic locations or precursor sequences are given numeric suffixes, such as hsa-miR-9-1 and hsa-miR-9-2. Mature miRNAs that have a closely related sequence and differ by only 1 or 2 nt are given a lettered suffix (e.g., hsa-miR-106a and hsa-miR-106b). There are some exceptions to this nomenclature. When a mature miRNA is predominantly generated by one precursor miRNA strand and another miRNA is generated from the opposite strand, the predominant strand is denoted with an asterisk next to the number (e. g., miR-136*). However, when the identification of the miRNA strand that is predominantly expressed is not possible, the nomenclature refers to the strand: miR-502-5p (from the 5′ strand) and miR-502-3p (from the 3′ strand) [Citation21].
The majority of miRNA transcription is initiated and carried out by RNA polymerase II and is under the control of RNA polymerase II-associated transcription factors and epigenetic regulators. In addition to RNA polymerase II, RNA polymerase III also initiates the transcription of some viral miRNAs [Citation22]. The majority of miRNAs are processed from introns, a few are processed from exons, and some have their own promoters and can thus be independently transcribed. Some families of miRNAs characterized by similar seed sequences are transcribed as clusters. In the nucleus, primary miRNAs (pri-miRNAs) are transcribed and then processed into precursor miRNAs (pre-miRNAs) by a microprocessor complex composed of a ribonuclease III enzyme, Drosha, and an RNA-binding protein DiGeorge syndrome critical region 8 (DGCR8) [Citation23]. Drosha cleaves the hairpin structure of pri-miRNA and generates pre-miRNA, which is exported into the cytoplasm by the exportin 5 (XPO5)/RanGTP complex [Citation24]. In the cytoplasm, pre-miRNA is further processed by RNase III endonuclease Dicer, which removes the terminal loop of the pre-miRNA, resulting in a mature miRNA duplex [Citation25]. Subsequently, the mature miRNA duplex is loaded onto a member of the family of proteins known as argonaute (AGO) (AGO1-4 in humans) to form an effector complex called the miRNA-induced silencing complex (miRISC) [Citation26]. The miRISC complex consists of a guide strand of mature miRNA and AGO proteins. The miRISC complex is central to miRNA-induced mRNA suppression. This miRNA biogenesis pathway, known as a canonical pathway, is the dominant pathway by which miRNAs are processed. However, a different pathway, known as a noncanonical pathway, can also process miRNAs in a Drosha/DGCR8-independent and Dicer-independent manner [Citation27].
miRNA-mRNA interactions are dynamic and dependent on many factors, such as the subcellular location of miRNAs, abundance of target mRNAs and miRNAs and the affinity of an miRNA to its target mRNA strand. In most instances, miRNAs bind the 3′-UTR of target mRNAs to induce mRNA degradation and translational repression by mRNA deadenylation and decapping [Citation27]. However, recent studies have shown that miRNAs can interact with other regions, including the 5′-UTR, coding sequences, and gene promoters [Citation28]. Moreover, miRNAs can regulate transcription or activate translation [Citation29,Citation30]. miRNAs are also found in extracellular vesicles such as exosomes and microvesicles [Citation31], which can act as messengers to mediate cell-cell communication.
3.2. Host miRNA profiles during influenza virus infection
In the past decade, miRNA research has expanded to include studies on infectious diseases, and extensive experimental evidence shows that miRNAs are key modulators of the host response to viral infections. miRNAs play a broad range of regulatory roles and affect the manifestation and pathogenesis of viral infections in a multitude of ways. These regulations include the control of the pathogenicity of individual pathogens, the efficiency of host innate and adaptive immune responses, and the magnitude and resolution of inflammatory responses [Citation32].
Differential expression profiles of host miRNAs, also called the miRNAome, have been reported in vitro and in vivo with various influenza strains. Initially, the microarray technique was extensively used to study profiles of miRNAs responding to influenza infection. Recently, high-throughput next-generation sequencing (NGS) has been more commonly used to generate highly sensitive genome-wide miRNA expression profiles. miRNA profile datasets are available for many species, including humans, aves, pigs, mice, macaques, and dogs infected with various influenza strains [Citation33–41]. The miRNAome in response to influenza infection was also obtained from several human cells, including A549 lung epithelial cells [Citation33,Citation34,Citation42–45], HEK 293 cells [Citation46], MDCK cells [Citation47], bronchial epithelial (BEAS-2B) cells [Citation45], NCI-H292 human lung mucoepidermoid carcinoma cells [Citation35], and peripheral blood mononuclear cells (PBMCs) [Citation48]. lists the miRNAs that changed during IAV infection.
Table 1. Summary of miRNAs altered during IAV infection
3.2.1. MicroRNA profiles in animal models
Many studies have examined the changes in the miRNAome during influenza infection in animals and attributed these changes to cellular responses. Using a deep sequencing approach, one study showed that the miRNAome was modulated in chicken lung and trachea infected with an avian influenza virus [Citation41]. Chicken lungs and trachea infected with H5N3 IAV, which shows poor pathogenicity, differentially expressed 73 and 36 miRNAs, respectively, compared to the uninfected samples. Lung and trachea tissues isolated from beagles infected with the H3N2 canine influenza virus showed that 34 and 45 miRNAs were differentially expressed in the lungs and trachea, respectively [Citation38]. Many of these miRNAs were suppressed in the infected tissues. A miRNA microarray study was performed with BALB/c mice infected with reconstructed 1918 H1N1 IAV (r1918) and a seasonal H1N1 virus (A/Texas/36/91) [Citation49]. A total of 130 murine miRNAs were significantly altered in response to the IAV infection in mouse lung tissues. The lethal H1N1 r1918 strain caused strong downregulation of miR-193, miR-29a, and miR-29b, while the nonlethal seasonal H1N1 IAV caused the upregulation of miR-193 and the modest downregulation of both miR-29a and miR-29b. Another microarray study found global downregulation of miRNAs largely during the recovery stage of infection in BALB/c mice infected with influenza H1N1A/PR/8 [Citation37]. Of approximately 300 miRNAs, 22 and 114 miRNAs were downregulated on 7 and 15 days postinfection, respectively.
Compared to those in mice, the influenza-related proteins in pigs, ferrets, and macaques have a higher degree of similarity to human proteins in terms of influenza viral pattern recognition, interferon (IFN) responses, and the regulation of IFN-stimulated genes. Thus, pigs, ferrets, and macaques have significant human translational value with the ability to provide vital insights into IAV infection progression, pathogenesis, and immunity. miR-15a, miR-21, miR-146, miR-206, miR-223, and miR-451 were found to be differentially expressed in the lungs of pigs infected with IAV H1N2 [Citation39]. Pulmonary alveolar macrophages isolated from control and H1N1 swine influenza A virus-infected piglets showed 70 and 16 differentially expressed miRNAs 4 and 7 days postinfection, respectively [Citation50]. Most of these miRNAs were downregulated during the acute phase (postinfection day 4) of swine influenza but returned to normal in the recovery phase (postinfection day 7). H1N1 IAV-infected human A549 cells and ferret lungs showed upregulation of miR-1290, whose basal expression was only detected in these species and not in mouse or chicken lungs [Citation51]. In macaque lungs infected with highly pathogenic H5N1 avian influenza virus, twenty-three cellular miRNAs were found to be altered, some upregulated and some downregulated [Citation36]. Collectively, these findings suggest that the miRNA signature in response to influenza virus infection is species- and influenza strain-dependent. Moreover, there is a lack of genome-wide information on the miRNA signature during responses to IAV infection by ferrets, constituting an animal model that has a higher degree of similarity to humans.
3.2.2. MicroRNA profiles in human cells
miRNA profiles in human cells in response to influenza infection reveal unique and common miRNA signatures. A majority of studies on the miRNA profiles of human cells were performed with lung epithelial cells using various strains of influenza viruses. Microarray data showed that miR-7, miR-132, miR-146a, miR-187, miR-200 c, and miR-1275 were significantly upregulated in A549 and BEAS-2B cells infected with two different strains of IAV (H1N1 A/Udorn/72 and A/WSN/33) [Citation45]. Using a microarray, Loveday et al. compared the miRNAome of A549 cells infected with a IAV virus with low pathogenicity (swine-H1N1 virus) and a strain with high pathogenicity (avian-H7N7) and found that 40 miRNAs were commonly dysregulated: 33 miRNAs were upregulated, and 7 miRNAs were downregulated [Citation34]. Terrier et al. observed that H1N1 and H3N2 virus infections of A549 cells deregulated a total of 39 and 10 miRNAs, respectively, including 5 common miRNAs (miR-21, miR-29a, miR-29b, miR-146a, and miR-452) [Citation42]. Interestingly, the majority of these miRNAs were downregulated in the studies by Terrier et al. Another study by Lim et al. also observed a higher number of downregulated miRNAs compared to upregulated miRNAs in A549 cells infected with pH1N1 for 1–2 hours [Citation52].
Makkoch and colleagues used NGS to determine the miRNA profiles of A549 cells infected with different IAV subtypes (pandemic pH1N1, H3N2, and H5N1) [Citation33]. They found that the cells infected with the IAV pandemic strain pH1N1 expressed 29 upregulated and 10 downregulated miRNAs. Infection with IAV strain H3N2 led to 20 upregulated and 7 downregulated miRNAs, while the cells infected with the zoonotic H5N1 strain exhibited 23 upregulated and 5 downregulated miRNAs. Furthermore, upregulated miR-101, miR-193b, miR-23b, and miR-30e*, which target genes involved in apoptosis, were common to all the subtypes tested. In contrast to the findings of Loveday et al., Terrier et al., and Lim et al., the number of upregulated miRNAs were higher than the number of downregulated miRNAs for all three subtypes 24 hours postinfection.
NGS data on two lineages of influenza B virus showed that five miRNAs, namely, miR-197, miR-215, miR361, miR-1841, and miR-1842, were increased in MDCK cells [Citation47]. Furthermore, the Yamagata lineage of influenza B virus infection downregulated miR-let-7 f. Another study using microarray and NGS analyses demonstrated that the infection of A549 cells with a seasonal IAV virus of human-origin, H3N2, swine-origin H1N1, or avian-origin, H3N2, exhibited 6 upregulated miRNAs and 14 downregulated miRNAs [Citation53]. This study also found that hsa-miR-937-5p was downregulated and that hsa-miR-1290 was upregulated dramatically in the three infected groups.
By comparative data analysis using 10 different studies in several species (human, mice, macaques, dogs, and pigs) and IAV strains, Makkoch and colleagues found 15 common altered miRNAs during influenza infection, including miRNAs of the let-7 family, miR-101, miR-132, miR-146, miR-574, miR-15, miR-21, miR-29, miR-30, miR-10, miR-34, miR-548, and miR-148 [Citation33]. miRNAs of the let-7 family were downregulated by influenza infection in humans, chickens, mice, and macaques [Citation34,Citation36,Citation37,Citation41,Citation45]. Multiple studies observed that miR-101 [Citation33,Citation43], miR-132 [Citation33,Citation45], miR-146 [Citation42,Citation45], and miR-574 [Citation35,Citation45] were upregulated exclusively in human cell lines (A549 and BEAS-2B cells) infected with different IAV subtypes. In contrast, miR-101 was downregulated in the lungs of macaques and mice infected with IAV [Citation36]. Other studies showed that miR-15 was upregulated during IAV infection in human A549 cells and pig lungs [Citation33,Citation39,Citation43], while the miR-21 level was increased in human respiratory epithelial cells, mouse lungs, macaque lungs, and pig lungs [Citation33,Citation35–37]. miR-26b and miR-455 were downregulated in cells infected with human-origin IAV H3N2, and miR-222 expression was upregulated in cells infected with avian-origin IAV. These studies suggest that the host miRNA signatures caused by IAV are strain-, cell- and time-dependent.
3.3. MicroRNAs as biomarkers
The development of an infectious disease diagnostic system using miRNAs as biomarkers is actively underway [Citation54]. miRNAs are present in numerous bodily fluids and are highly stable in these fluids. They have potential as minimally invasive disease markers. Blood, serum, saliva, and bronchial wash/lavage can be used as starting materials to detect differentially expressed miRNAs in response to influenza infection. One study found that six miRNAs (miR-1260, miR-26a, miR-335*, miR-576-3p, miR-628-3p, and miR-664) were consistently dysregulated in the whole blood of H1N1-infected patients, and the use of these circulating miRNAs as biomarkers has been suggested [Citation55]. Another study found 41 significantly differentially expressed miRNAs in PBMCs isolated from critically ill patients with pandemic H1N1 (2009) IAV infection [Citation48]. miR-31 and miR-29a were significantly downregulated, while miR-148a was upregulated in critically ill patients. Moreover, this study suggested that miR-31, miR-29a, and miR-148a were valuable biomarkers for differentiating critically ill patients from healthy controls.
It has been revealed that serum levels of miR-150, miR-29c, miR-145, and miR-22 were associated with pandemic H1N1/2009 severe pneumonia [Citation56]. The serum levels of miR-20a, miR-17, miR-376c, and miR-106a were significantly elevated in patients infected with highly pathogenic H7N9 avian influenza virus compared to healthy individuals [Citation57]. A similar study found that miR-197-5p, miR-320a, miR-320d, miR-320e, and miR-765 were significantly upregulated in the serum of H7N9 IAV-infected individuals compared to the levels of serum of the controls [Citation58]. H1N1-infected patients who developed IAV-induced acute respiratory distress syndrome showed significant upregulation of miR-17-5p in extracellular vesicles isolated from bronchoalveolar lavage fluid (BALF) [Citation59].
Analysis of miRNAs in throat swabs from patients infected with influenza A or B virus revealed that miR-29a-3p, miR-30c-5p, miR-34b-5p, miR-34c-3p, miR-181a-5p, miR-205-5p, and miR-449b-5p were upregulated in both the IAV- and IBV-infected individuals, and the use of these miRNAs as strategies for the early detection of viral infection was proposed [Citation60]. Lim and co-workers established a highly sensitive early detection system and suggested that two miRNAs, miR-181c-5p and miR-1254, might be used as biomarkers in respiratory epithelial cells for detecting pandemic IAV H1N1 infection [Citation52]. Collectively, these studies suggest that miRNA marker signatures identified from arrays or NGS can be used as potential biomarkers of influenza virus infection. However, these expression profiles can depend on the sample and IAV strain; thus, additional studies are needed for validating these findings.
3.4. MicroRNA regulation of influenza virus infection
The host miRNA signature can be altered during influenza infection, resulting in changes in the cellular responses to viral infection. In turn, host miRNAs regulate influenza virus replication by directly binding to the influenza genome or indirectly targeting host factors associated with viral replication. From an evolutionary point of view, it can be speculated that influenza, an obligate intracellular microorganism, can utilize cellular miRNAs for its efficient replication while the host miRNA signature undergoes changes to defend against the virus. More than decades of research have established a link between influenza, miRNAs, and host factors.
3.4.1. Regulation of the influenza virus life cycle by host miRNAs
The influenza virus needs to overcome several miRNA barriers to complete its life cycle (). Only a few studies depict the roles of miRNAs in the early steps of influenza virus entry. Archain 1 (ARCN1) is a subunit of COPI components and plays an important role in influenza virus entry [Citation61]. Hu and colleagues found that miR-33a binds to the 3ʹ-UTR of ARCN1 and impedes virus replication at the stage of virus internalization [Citation62]. Moreover, the overexpression of miR-33a weakens vRNP activity in an ARCN1-independent manner. Importantly, IAV overcomes this barrier by suppressing the endogenous levels of miR-33a. ATP6V1A encodes a vATPase important for endosomal acidification during IAV replication [Citation63]. miR-1-3p inhibits viral replication by targeting ATP6V1A and interfering with the uncoating of the vRNP complex [Citation64]. Furin-dependent proteolytic activation is critical for H5 and H7 IAV HA precursor activation and the endosomal fusion of virions. Overexpression of miR-24 mimics in A549 cells leads to the robust suppression of both furin mRNA levels and furin activity, resulting in decreased H5N1 virulence but not H1N1 virulence [Citation65]. Interestingly, H5N1 infection specifically downregulates endogenous miR-24 levels.
Figure 1. MicroRNA regulation of the influenza virus life cycle
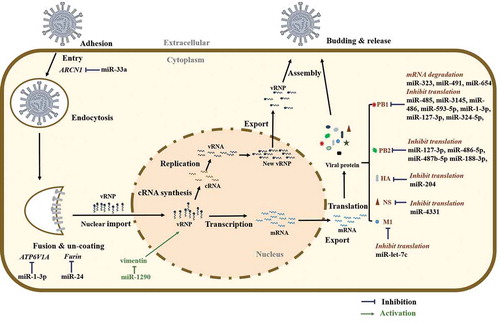
Recently, Huang and co-workers found that H1N1 IAV induces host miR-1290 expression via the extracellular signal-regulated kinase (ERK) pathway [Citation51]. The inhibition of miR-1290 suppressed IAV replication in A549 cells and a ferret model. Vimentin was identified as a target of miR-1290. They also demonstrated that vimentin interacts with viral PB2 and interferes with vRNP activity.
Similar to host mRNAs, genomic RNA of all 8 segments of influenza virus contains 5ʹ- and 3ʹ-UTRs. There are potential binding sites for miRNAs in these 3ʹ-UTRs [Citation66]. Investigation of putative miRNAs that directly target the 3ʹ-UTRs of viral sequences may reveal novel targets for therapeutics that attenuate influenza infection. Indeed, several studies have observed that many miRNAs interfere with virus replication steps at the posttranscriptional level of viral genes, particularly PB1 and PB2. Song and colleagues were the first to report that host miRNAs (miR-323, miR-491, and miR-654) can bind to the 3ʹ-UTR of viral PB1 mRNA and downregulate the PB1 protein level by mRNA degradation in MDCK cells [Citation67]. Subsequently, similar investigations have shown that human miR-485, miR-3145, miR-486, miR-593-5p, miR-1-3p, miR-127-3p, and miR-324-5p bind the 3ʹ-UTR of PB1 mRNA and thus restrict virus replication [Citation64,Citation68–71]. Human miR-127-3p, miR-486-5p, and miR-487b-5p have the potential to directly target and suppress both viral PB1 and PB2 [Citation64]. miR-188-3p directly targets viral PB2 mRNA of several IAV subtypes (H1N1, H5N6, and H7N9) and downregulates PB2 expression at both the mRNA and protein levels [Citation72]. Additionally, chicken miRNAs gga-mir-1710, gga-mir-133c, and gga-mir-146c* are predicted to target the avian H5N1 PB1 and PB1-F2 in silico [Citation73].
In addition to PB1 and PB2, miRNAs also target other influenza virus genes. Swine miR-204 and miR-4331 negatively regulate pandemic IAV strain H1N1 2009 replication by binding viral HA and NS, respectively [Citation69]. However, the virus resists this attenuation by downregulating these miRNAs. An in silico search of virus-specific host miRNAs revealed that miR-1658* is predicted to bind the NS1 gene of avian IAV [Citation74]. IAV M1 is the most abundant protein in the virion particles that constitutes the virion core and regulates vRNP export, virus assembly, and budding. Ma et al. found that miR-let-7 c reduces IAV replication by targeting the M1 gene to disrupt vRNP export, virus assembly, and budding [Citation75].
The potential of miRNA binding to the viral mRNA 3ʹ-UTR can be harnessed to develop improved vaccines against IAV. A recent investigation demonstrated that including miR-142 target sites in several RNA segments of modified H1N1 IAV inhibits the translation of viral proteins, including NP, PB1, PB2, PA and HA, with the NP-modified virus most significantly suppressing IAV replication [Citation76]. Moreover, in this study, the NP segment was modified with ubiquitously expressed (human, chicken, ferret, and canine cells) miR-21 target sites, and significant protective immunity against lethal infection of H1N1 IAV was found [Citation76].
3.4.2. Regulation of innate and adaptive immunity by host miRNAs
Host innate immunity represents the critical initial barrier that viruses must overcome to efficiently replicate and invade a new host. Upon viral infection, type I IFNs are induced via a broad spectrum of sensors called virus-associated molecular patterns. For influenza, the RNA helicase retinoic acid-inducible gene I (RIG-I) represents the major innate immune sensor [Citation77]. miRNAs have been shown to regulate the antiviral RIG-I pathway, which responds to IAV infection either directly or indirectly [Citation68,Citation78–81].
H5N1 IAV infection upregulates miR-136, which serves as a ligand to activates innate immunity by causing the accumulation of IFN-β and IL-6 [Citation79]. Antiviral defences can also be regulated via miRNA-mediated exosomal communication. Maemura and co-workers found that the ectopic expression of miR-483-3p leads to increased expression of type I IFNs and proinflammatory cytokines in response to IAV infection in mouse lung epithelial MLE-12 cells [Citation80]. This effect appeared to be mediated by two miR-483-3p targets, ring finger protein 5 (RNF5) and CD81, which are two negative regulators of RIG-I signalling. miR-483-3p is reported to be enriched in exosomes isolated in the BALF of IAV-infected mice. Therefore, exosomal transfer of miR-483-3p potentiates antiviral innate immunity. In contrast, miR-194, miR-485, and miR-340-5p downregulate RIG-I-mediated antiviral responses [Citation68,Citation78,Citation81]. miR-485 and miR-340-5p were shown to directly target RIG-I, whereas miR-194 targets fibroblast growth factor 2 (FGF2), a positive regulator of RIG-I-mediated signalling.
In addition to RIG-I signalling, miRNAs also modulate other signalling pathways to alter the antiviral state of host cells. Among the IAV-upregulated miRNAs, miR-29 suppresses DNA methyltransferase activity and induces the expression of cyclooxygenase-2 (COX2) and prostaglandin E2 (PGE2), which are critical for the accumulation of IFNλ1 in IAV-infected cells [Citation82]. miR-449b targets the histone deacetylase HDAC1, resulting in increased expression of IFN-β [Citation83]. miR-155 targets and downregulates suppressor of cytokine signalling 1 (SOCS1), a negative regulator of type I IFN signalling [Citation84]. Among the IAV-downregulated miRNAs, miR-324-5p induces type I and type III IFNs by targeting CUEDC2, the negative regulator of the JAK1-STAT3 pathway [Citation71]. miR-26a enhances type I IFN-induced ISG expression [Citation85]. miR‐650 directly targets and suppresses IFIT2 and MXA, which are important ISGs [Citation86].
Ectopic expression of miR-144 results in the suppression of the host antiviral response by targeting tumour necrosis factor receptor-associated factor 6 (TRAF6) to attenuate TRAF6-IRF7 signalling and thus to reduce type I IFNs and ISGs [Citation87]. Two similar studies have found that closely related miRNAs miR-302a and miR-302c reduce IFNβ expression by targeting interferon regulatory factor-5 (IRF-5) and NF‐κB‐inducing kinase (NIK), respectively [Citation88,Citation89].
Adaptive immunity against viral infection is initiated when dendritic cells (DCs) present viral antigens to naïve and memory T lymphocytes. Thus, DCs play important roles in host adaptive immunity through antigen presentation, recognition, and virus clearance. miR-375 is a repressor of DC maturation [Citation90]. miR-674 targets and suppresses Mbnl3 to downregulate surface markers such as CD86 and MHCII, resulting in a strong blockade of DC maturation and disruption of antigen presentation during IAV infection [Citation91]. miR-18b and miR-20a target PTEN, a kinase that regulates the pro- to pretransition during B and T cell development [Citation92]. miR-155 targets Src homology 2-containing inositol phosphatase-1 (SHIP-1) to directly downregulate T-bet, which is required for cytotoxic CD8 + T lymphocyte generation and memory cell formation [Citation93]. Collectively, these findings suggest that host miRNAs are important modulators of innate and adaptive immunity against influenza infection.
3.4.3. Regulation of inflammation by host miRNAs
Cytokines that are overproduced in response to IAV infection can spread into the systemic circulation and cause severe inflammatory symptoms such as leucopenia. The majority of patients who succumb to viral infectious diseases have excessive inflammation in their lungs, leading to injury and obstruction of the respiratory tract. miRNAs play key roles in the regulation of cytokine production and proinflammatory intracellular signalling pathways during influenza infection [Citation94].
The NF-κB pathway is involved in the production of proinflammatory genes in response to IAV infection. Deubiquitinating enzyme A20 negatively regulates NF-κB-mediated inflammation. miR-125a/b reduces A20 level, resulting in exaggerated inflammation in primary bronchial epithelial cells of patients with chronic obstructive pulmonary disease (COPD) [Citation95]. Another miRNA, miR-29c, negatively regulates NF-κB activation by acting as an RNA decoy to prevent human antigen R (HuR)-mediated A20 degradation [Citation96,Citation97].
Noncanonical activation of the NF-κB pathway depends on NF‐κB‐inducing kinase (NIK), which facilitates NF-κB nuclear translocation and the activation of downstream IFN signalling. Gui and co-workers found that miR-302c targets the 3ʹ-UTR of NIK, and thus activates NIK-mediated nuclear translocation of NF-κB and enhances IFNβ production [Citation89]. On the other hand, infecting A549 cells with H2N2 leads to the suppressed expression of miR-302c. miR-4776 facilitates IAV replication by suppressing NF-κB inhibitor β (NFKBIB). This proviral factor is downregulated by the host upon IAV infection to counteract viral replication [Citation98]. IAV induces the upregulation of miR-146a, which is associated with the suppression of NF-κB activation [Citation42].
Chen et al. observed that miR-302a targets the 3ʹ-UTR of IRF-5 and reduces IRF-5-stimulated production of TNFα, IL-8, IL-6, CCL2, and CCL5 [Citation88]. A study observed that miR-451 was strongly induced in primary splenic and lung DCs infected with influenza virus [Citation99]. This study found that miR-451 targets tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta (YWHAZ), a positive regulator of proinflammatory cytokine production. Collectively, these bodies of evidence suggest that miRNAs can exert either proinflammatory or anti-inflammatory responses to influenza virus infection. Thus, the biological functions of miRNAs should be carefully and fully examined before they are considered for development into therapeutics.
3.4.4. Regulation of other cellular signalling pathways by host miRNAs
miRNAs regulate many other signalling pathways in response to IAV infection that are important for the fate of the infection, such as apoptotic signalling, Wnt signalling, mitogen-activated protein kinase (MAPK) signalling, and TGF-β signalling [Citation35,Citation43,Citation100–102].
Apoptosis is one of the common strategies by which host cells defend against viral infection. Elimination of the virus-infected cells prevents further spread of the virus. Host cells can utilize miRNAs to regulate apoptosis. H1N1 IAV infection of A549 cells downregulates miR-34a, which reduces the protein level of proapoptotic factor Bax. IAV-mediated reduction of miR-34a and thus an elevated level of Bax lead to an increase in IAV-induced apoptosis [Citation100]. miR-29c expression is upregulated by IAV infection, and miR-29c increases IAV-induced apoptosis by targeting BCL2L2, an anti-apoptotic member of the Bcl-2 family [Citation43]. Othumpangat and colleagues found that COX6C mRNA expression was upregulated in lung epithelial cells through an IAV-mediated decrease in miR-4276 expression, resulting in an increased level of the apoptotic protein caspase-9 [Citation103]. The same group observed that IAV downregulated host miRNA-548 to promote apoptosis at an early stage of infection [Citation44].
Our group recently found that activation of the Wnt/β-catenin pathway positively regulates IAV infection [Citation104]. Wnt ligand Wnt3a activation upregulates H1N1 IAV infection in mouse lungs and epithelial E10 cells. Moreover, we observed that inhibition of Wnt/β-catenin signalling by iCRT14, a chemical inhibitor, attenuates IAV replication. Using a miRNA expression library, we identified miR-193b as a miRNA that downregulates Wnt/β-catenin signalling [Citation101]. We further showed that miR-193b targets the 3ʹ-UTR of β-catenin and suppresses Wnt signalling to control IAV infection. Moreover, miR-193b arrests the cells in G0/G1 and delays the nuclear import of vRNP in human lung epithelial cells. In vivo studies have also demonstrated that miR-193 reduces viral load in the lungs of mice.
McCaskill and co-workers found that miR-124, miR-24, and miR-744 downregulate the protein levels of MAPK-activated protein kinase 2, which is a broad-spectrum antiviral target induced by MAPK signalling [Citation102]. Furthermore, miR-141 can suppress the expression of TGF-β2, which is important to the inflammatory outcome of IAV infection [Citation35].
3.5. MicroRNAs in therapeutic applications
miRNAs control the expression of approximately 60% of the human protein-coding genes and are important in many biological processes. miRNAs are often dysregulated in disease conditions, including infectious diseases, cancers, and heart diseases. The pleiotropic nature of miRNAs makes them particularly attractive drug candidates for diseases of multifactorial origin and no current effective cure. In recent years, therapeutic miRNAs have been considered among the important candidates in the biopharmaceutical industry, thus entering into commercial space as future medicines [Citation105,Citation106]. However, miRNA clinical applications face many challenges, such as limitations in the delivery of miRNA modulators, degradation of naked particles in vivo, off-target effects of miRNA regulators, and miRNA-induced toxicity [Citation107]. Despite these challenges, miRNA-based therapeutics have evolved in recent years.
Increasing evidence shows that miRNAs might be used as potential therapeutic targets for treating influenza infection. Targeting single miRNAs by traditional antisense methods can be inadequate and laborious because of the complex nature of viral infections. One of the unique genomic features of mature miRNA function is that a family of miRNAs shares an almost identical seed sequence. Thus, the use of miRNA sponges (a single RNA sequence that consists of several tandem miRNA-binding sites present in all members) is suggested to inhibit family-wide expression [Citation108]. Using miRNA sponges to target multiple miRNAs may be valuable for treating influenza infection. In clinical settings, the delivery of miRNA mimics or antagomirs is currently under investigation. Many vectors, such as plasmids, replication-deficient viruses, and transposons, have been developed to deliver miRNA agents in vivo. Among these vectors, lentivirus vectors, adenoviral vectors, and adenovirus-associated viral (AAV) vectors are deployed widely to deliver miRNAs in vivo. Several studies have used adenovirus-expressing miRNAs to successfully deliver miRNAs into mouse lungs to study IAV infection [Citation101,Citation109]. Furthermore, nonviral transfection approaches, such as using nanoparticles and liposomes, have also attracted attention as potential miRNA delivery systems. Although delivery strategies are far from optimal, substantial progress has recently been achieved towards targeted therapy with limited off-target effects.
4. Host lncRNA-influenza virus interactions
4.1. Overview of lncRNAs
In recent years, next-generation sequencing has opened a whole new area for studying the transcriptome and has led to the discovery of a new class of lncRNAs [Citation110]. As the name suggests, these molecules are relatively long, with more than 200 nt, and lack the capacity to code proteins. LncRNAs are products of RNA polymerase II with a 5′ 7-methylguanosine cap and a 3′ poly(A) tail. Although lncRNAs exhibit less sequence conservation between different species, it is suggested that their conserved secondary structures are key to their functional roles [Citation111]. LncRNAs are predominantly located in the nucleus but can also be found in the cytoplasm [Citation112].
In general, lncRNAs can be broadly classified based on their structure, genomic location, and action. The structural classification includes linear and circular lncRNAs. Linear lncRNAs play diverse roles and regulate various cellular activities. Circular lncRNAs do not contain a 5ʹ cap or poly(A) tail, and as a result, they are much more stable than linear lncRNAs because they escape RNA decay machinery. The diversity in the structure, regulation, and function of linear lncRNAs and circular lncRNAs can be found in a recent review [Citation113].
Based on genomic location, lncRNAs can be divided into 5 groups. (1) A sense lncRNA overlaps neighbouring RNA in the positive or sense strand, (2) an antisense lncRNA overlaps RNA in the antisense strand, (3) a bidirectional lncRNA lies in the opposite stand and in the proximity of the promotor region (<1000 bp) of the overlapping protein-coding gene, (4) an intronic lncRNA is located between the introns of a protein-coding gene, and (5) an intergenic lncRNA lies between two coding genes [Citation114]
Based on their function, lncRNAs can be broadly classified as Cis-acting lncRNAs, in which a lncRNA acts at its adjacent location; trans-acting lncRNAs, which acts at a position farther from its locus; and competitive endogenous RNAs (CeRNAs), which actively compete with miRNAs for mRNA-binding sites [Citation115].
LncRNAs exhibit a diverse mode of functions under physiological and disease conditions, as these molecules can bind with various molecules, such as DNA, RNA, and protein. Although lncRNAs are thought to lack the ability to code proteins, recent studies have revealed their ability to generate functional micropeptides [Citation116–117]. LncRNAs exert their molecular action by acting as decoys, scaffolds, guides, and signals. As decoys, they bind to transcription factors, resulting in the regulation of gene transcription in either a positive or a negative manner. As scaffolds, they facilitate protein interactions and thereby regulate signalling pathways. As guides, they bind with ribonucleoprotein complexes and regulate gene transcription at the DNA level. As signals, lncRNAs can be expressed at specific cellular locations and at specific time points that represent different stages of cellular activities during physiological and pathological events [Citation3, Citation115].
Increasing evidence shows that lncRNAs regulate various biological processes in cells, such as proliferation, differentiation, apoptosis, metabolism, immunity and response to stress [Citation118]. LncRNAs exert their functions on various organs, such as those of the cardiovascular, respiratory, renal, nervous and skeletal systems. LncRNAs also play the critical roles in infectious diseases [Citation119–121].
4.2. Host lncRNA expression profiles during influenza virus infection
Recent advancements in technologies have improved the detection of lncRNAs that are differentially expressed during influenza virus infection. Various high-throughput techniques, such as genome-wide lncRNA microarrays and RNA sequencing (RNA-seq), have been employed for the successful identification of lncRNAs that are altered in various cell and animal models of influenza viral infection ().
Table 2. Differentially expressed lncRNAs involved in the regulation of influenza virus infection
Using lncRNA microarray analysis, 42 ncRNAs were found to be differentially expressed in lung epithelial A549 cells infected with WSN IAV [Citation122]. Using RNA-seq analysis, we identified 1,196 upregulated and 716 downregulated lncRNAs in A549 cells infected with the IAV/PR8 strain at an MOI of 2, and for 24 hours postinfection [Citation123]. Chai et al. compared lncRNA profiles between A549 cells and HEK293T with three IAV strains, PR8, WSN and 2009 pandemic California at an MOI of 1 and 8 hours postinfection [Citation124]. They reported the identification of 139 upregulated and 150 downregulated lncRNAs in response to these IAV strains.
In addition to cell culture systems, in vivo profiling studies have been conducted to screen for lncRNAs in mice during influenza virus infection. A study conducted by Maarouf and colleagues utilized C57BL/6 J mice infected with the WSN strain for genome-wide lncRNA profiling [Citation125]. They identified 1,864 lncRNAs that were upregulated and 703 lncRNAs that were downregulated during IAV infection. Another large-scale transcriptome profiling study using RNA-seq analysis was performed with eight mouse strains (A/J, C57BL/6 J, 129S1/SvImJ, NOD/ShiLtJ, NZO/HILt, CAST/EiJ, PWK/PhJ, and WSB/EiJ) infected with PR8 or SARS-CoV (MA15) viruses. 5,295 altered lncRNAs upon infection were identified, and the results from this study have been made available through an interactive database named MONOCLdb (MOuse NOnCode Lung database – www.monocldb.org/) [Citation126].
Neutrophils are key components in the early innate immune response of influenza virus infection. Lai et al. profiled dysregulated lncRNAs using RNA-seq analysis of IAV-infected human neutrophil samples in the acute stage of infection [Citation127]. This study identified 404 dysregulated lncRNAs, including 234 upregulated and 170 downregulated lncRNAs. It validated 26 novel lncRNAs in A549 cells infected with the IAV/Beijing/501/2009 strain and identified lncRNA AVAN (AntiVirus and Activate Neutrophil) as the most significantly upregulated lncRNA.
4.3. LncRNA regulation of influenza virus infection
The crucial roles of lncRNAs in the regulation of influenza virus infection have been explored in the past decade as a result of a significant increase in lncRNA research [Citation120,Citation121]. The functional activity of fourteen lncRNAs on influenza virus replication has been elucidated thus far (). Among those identified, most lncRNAs were found to regulate type I IFN signalling and IFN-stimulated genes (ISGs), whereas few lncRNAs have been found to interact with viral components. On the basis of their regulatory partners, we grouped and summarized the lncRNAs regulating influenza virus replication ().
Figure 2. Schematic representation of the functional roles of long noncoding RNAs involved in the regulation of influenza virus infection
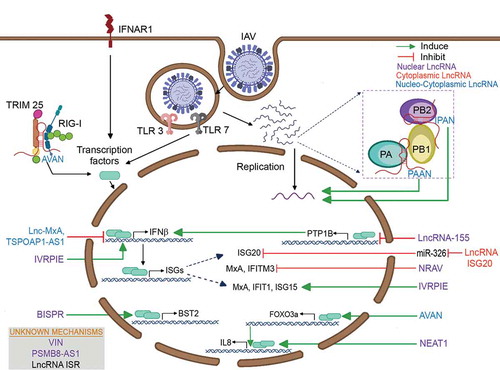
4.3.1. Modulation of IFN signalling and IFN-stimulated genes by host lncRNAs
LncRNA-155 (MIR155HG) is a host gene that encodes miR-155 and is upregulated by IAV both in vitro and in vivo [Citation125]. LncRNA-155 is predominately found in the nucleus. In vitro and in vivo RIG-I-knockout experiments revealed that lncRNA-155 is regulated by RIG‐I and TLR3‐dependent type I IFN signalling. In vitro knockdown of lncRNA-155 enhances viral replication through decreased ISG expression. Fragment deletion studies revealed that lncRNA-155 itself, not miR-155, is critical for the increased innate immune response. In vivo knockout of lncRNA-155 confirmed the impaired ISG expression and cytokine response and thus explained the increased susceptibility of lncRNA-155-knockout mice to IAV infection. Ectopic expression of lncRNA-155 inhibits PTP1B expression, which in turn increases the type I IFN response and limits IAV replication. TSPOAP1‐AS1 is a nucleocytoplasmic lncRNA induced by IAV in a dose- and time-dependent but strain-independent fashion [Citation128]. Treatment with Bay 11‐7082, an NF‐κB inhibitor, revealed that TSPOAP1‐AS1 expression is mediated by NF‐κB activation during influenza virus infection. TSPOAP1‐AS1 accumulates in the nucleus during IAV infection. Silencing of TSPOAP1‐AS1 limits IAV replication, whereas overexpressing TSPOAP1‐AS1 facilitates virus replication by negatively regulating IAV‐induced IFNβ1 levels and ISRE promoter activity.
The negative regulator of antiviral response (NRAV) is an antisense lncRNA, also known as DYNLL1-AS1. NRAV is downregulated by IAV in a time- and dose-dependent manner [Citation129]. NRAV is a nucleic-cytoplasmic lncRNA with a high percentage residing in the nucleus. The overexpression of NRAV promotes IAV replication both in vitro and in vivo. A microarray analysis of NRAV-overexpressing cells revealed the downregulation of critical ISGs, such as MxA and IFITM3. Chromatin immunoprecipitation (ChIP) assays revealed that NRAV negatively regulates these ISGs by decreasing histone 3 lysine 4 trimethylation (H3K4me3, an active mark) and increasing histone 3 lysine 27 trimethylation (H3K27me3, a repression signal) at the MxA and IFITM3 transcription start sites. Inhibiting IAV replication by promoting IFN and ISG expression (IVRPIE) is a nuclear and IAV-upregulated lncRNA. IVRPIE inhibits viral replication by positively regulating IFNβ1 and ISGs [Citation130]. ChIP assays revealed the enrichment of H3K4me3 marks at the transcription start sites of IFNβ1, IRF1, IFIT1, IFIT3, MX1, ISG15, and IFI44 L in IVRPIE-overexpressing and IAV-infected cells. RNA pull-down assays revealed that IVRPIE interacts with hnRNP U, which has been proposed to be critical for the increased expression of antiviral genes through epigenetic regulatory activity.
MxA is a well-known ISG that inhibits the PB2-NP interaction and thereby hinders the functional activity of the vRNP complex [Citation131]. Lnc-MxA is found in the locus of MxA. Lnc-MxA is an IFN-stimulated gene, and its expression is upregulated by influenza virus infection [Citation132]. Lnc-MxA is a nucleocytoplasmic lncRNA that is translocated into the nucleus during influenza infection. Lnc-MxA facilitates the replication of influenza virus by negatively regulating IFNβ induction by binding to the promoter region of IFNβ upon the formation of RNA:DNA triplexes.
ISG20 exerts its antiviral activity by inhibiting polymerase activity through its interaction with influenza nucleoproteins [Citation133]. Lnc-ISG20 is a sense lncRNA that shares the same locus as ISG20 and is upregulated during influenza virus infection [Citation124]. Similar to ISG20, Lnc-ISG20 is also an antiviral gene induced by IFNβ. Lnc-ISG20 is located in the cytoplasm. Lnc-ISG20 exerts its antiviral activity on IAV replication by acting as a ceRNA to bind miR-326 and enhance ISG20 protein expression.
RIG-I is a critical viral RNA sensor that is required for the production of type I IFNs during viral infection. Antivirus and activated neutrophil (AVAN) is found to regulate RIG-1 during IAV infection. AVAN is a lncRNA identified by the RNA-seq analysis of neutrophils from IAV-infected human patients in the acute stage of infection [Citation127]. IAV infection induces the expression of AVAN in neutrophils, monocytes and A549 cells. RNA fluorescence in situ hybridization (FISH) analysis reveals that AVAN is a nucleic-cytoplasmic lncRNA equally distributed in the nucleus and cytoplasm. The overexpression of AVAN strongly inhibits viral replication, whereas knocking it down increases viral titres. AVAN increases the expression of type I IFN genes (IFNα and IFNβ) and ISGs in IAV-infected cells. RNA pull-down assays and ubiquitination studies reveal that AVAN enhances the interaction of TRIM25 and RIG-I and increases the ubiquitination of RIG-I. In vivo studies using transgenic mice expressing human AVAN confirm that AVAN increases the survivability of IAV-infected mice compared to wild-type mice through enhanced antiviral innate immune responses via TRIM25 and RIG-I-mediated signalling. Additionally, AVAN is a bidirectional lncRNA that is located within 500 bp of FOXO3a. Chromatin isolation by RNA purification (ChIRP) analysis reveals that AVAN is bound to the promoter region of FOXO3a and positively regulates its expression, which in turn increases IL8 expression.
Two lncRNAs, lnc-Lsm3b and lnczc3h7a also regulate RIG-I signalling during vesicular stomatitis virus (VSV) infection. Lnc-Lsm3b, a cytoplasmic lncRNA is identified as a RIG-I-binding lncRNA upon RNA virus infection by UV-RIP-seq assay [Citation134]. Lnc-Lsm3b is upregulated in a time-dependent manner upon VSV and Sendai virus (SeV) infection. There is no induction of lnc-Lsm3b expression in peritoneal macrophages from VSV-infected IFNAR-deficient mice, indicating that lnc-Lsm3b is a type I IFN-induced lncRNA. Knockdown of lnc-Lsm3b using siRNA increases the production of type I IFNs and proinflammatory cytokine interleukin (IL)-6 in VSV-infected macrophages. Knockout of lnc-Lsm3b in vitro and in vivo increases type I IFN production and reduces VSV infection. A higher level of IFN-β and IL-6 in the lungs were also observed in the influenza virus (PR8)-infected lnc-Lsm3b knockout mice. RNA pull-down and individual nucleotide CLIP (iCLIP) assays show that lnc-Lsm3b competes with viral dsRNA for the binding of RIG-I’s carboxy-terminal domain (CTD) through its multibranch loop structures. Deficiency of lnc-Lsm3b significantly increases the interaction of RIG-I with TRIM25 whereas overexpression of Lnc-Lsm3b limits the TRIM25-mediated K63-linked ubiquitination.
Lnczc3h7a is a TRIM25-associated lncRNA that was identified using UV-RIP assay and further confirmed by RNA pulldown assay [Citation135]. Lnczc3h7a colocalizes with TRIM25 in the cytoplasm with or without viral infection and is induced by IFNβ1. Depletion of Lnczc3h7a decreases the levels of IFNα, IFNβ1, IFIT1, and IL6 in in macrophages infected with RNA virus (VSV and SeV). Lnczc3h7a knockout mice have a decreased type I IFN production upon VSV and IAV infection. Furthermore, Lnczc3h7a knockout mice have a higher viral load and are more susceptible to VSV infection. RNA pulldown and iCLIP assays reveal that Lnczc3h7a acts as a scaffold to enhance the interaction of TRIM25 with RIG-I and promotes K63-linked ubiquitination of RIG-I and downstream signalling.
5S ribosomal RNA pseudogene 141 (RNA5SP141) is another host ncRNA that binds to RIG-I during the DNA virus, herpes simplex virus 1 (HSV-1) infection [Citation136]. Under physiological conditions, RNA5SP141 is located in the nucleus and is translocated to the cytoplasm during HSV-1 infection. RNA5SP141 levels are increased in the RIG-I–bound fraction from HSV-1–infected cells. ATP hydrolysis assay using in vitro–transcribed RNA5SP141 reveals that RNA5SP141 is a strong agonist of RIG-I. RNA5SP141 induces the expression of IFNβ, ISGs such as ISG15 and DDX58, and the proinflammatory cytokine TNF via RIG-I. Co-immunoprecipitation assays reveal that RNA5SP141 is bound to ribosomal protein L5 (RPL5), mitochondrial ribosomal protein L18 (MRPL18) and thiosulphate-sulphur transferase (TST) that are previously known to be associated with 5S rRNA in the cytoplasm. Upon viral infection, HSV-1-mediated host translational shutoff results in the downregulation of RPL5, MRPL18, and TST levels, which allow the efficient interaction of RNA5SP141 and RIG-1. Silencing of RNA5SP141 reduces RIG-1-triggered antiviral response in cells infected with DNA viruses such as HSV-1 and Epstein-Barr virus (EBV) and also RNA viruses such as IAV.
4.3.2. Modulation of cellular targets by host lncRNAs
Nuclear enriched abundant transcript 1 (NEAT1) was the first lncRNA known to suppress influenza virus replication by regulating a cellular factor [Citation137]. As the name denotes, NEAT1 is a nuclear lncRNA that is induced through the TLR3 receptor in response to poly (I:C) treatment or influenza virus infection. NEAT1 has two isoforms, 3.7-kb NEAT1v1 and 23-kb NEAT1v2 [Citation121]. NEAT1v2 expression levels are upregulated during influenza virus infection. NEAT1v2 is involved in the formation of paraspeckles [Citation137,Citation138]. NEAT1 binds SFPQ, a paraspeckle protein that is also a repressor of IL8 transcription. During influenza infection, NEAT1 dissociates SFPQ from the promoter region of IL8, leading to the activation of IL8 transcription. The lncRNA bone marrow stromal antigen 2 or BST2 Interferon stimulated positive regulator (LncBST2/BISPR) is an IFNα2-induced gene that is upregulated upon infection with influenza virus lacking the NS1 gene but not the wild-type virus [Citation139]. Treatment with ruxolitinib (a JAK1/2 inhibitor) indicates that the expression of BISPR is STAT-dependent [Citation140]. BISPR is a nuclear lncRNA that has a bidirectional promoter. BISPR negatively regulates the expression of its neighbouring protein-coding gene, BST2/Tetherin. Tetherin is known to inhibit the progeny release of various enveloped viruses and influenza virus-like particles [Citation141]. Since the BISPR is induced earlier than BST2 upon IFN stimulation, it is speculated that BISPR acts at the promoter region of the BST2 gene, but its exact mechanism has not yet been revealed.
The LncRNA ACOD1 was identified as an IFN-independent lncRNA that was upregulated by various viruses, including IAV [Citation142]. Most mechanistic studies involve vesicular stomatitis virus (VSV). The lncRNA ACOD1 is a cytoplasmic lncRNA that directly interacts with glutamic-oxaloacetic transaminase 2 (GOT2) and promotes VSV viral replication. The mechanism through which GOT2 acts has not yet been revealed. The human ortholog of ACOD1 was found to be induced by the PR8 strain of influenza A virus, and knocking down ACOD1 expression reduced the viral titre. Although the lncRNA ACOD1 was able to reduce the viral load, the study did not verify the functional role of GOT2 in influenza virus replication. However, the mechanism of action may possibly differ as influenza virus replication occurs in the nucleus, whereas VSV replicates in the cytoplasm.
4.3.3. Modulation of viral components by host lncRNAs
Relatively few lncRNAs that directly interact with the viral components of the influenza virus have been identified [Citation143,Citation144]. A loss-of-function screening with an endoribonuclease-prepared small interfering (esiRNA) library with a total of 1,304 esiRNAs targeting human lncRNAs resulted in the identification of two lncRNAs, namely, PA-associated noncoding RNA (PAAN) and influenza virus PB1-associated noncoding RNA (IPAN), that interact with influenza viral proteins and regulate viral replication.
Both PAAN and IPAN were induced by various strains of influenza A virus in a dose- and time-dependent manner but not by IFN signalling. FISH and fractionation analyses revealed that both lncRNAs were cytoplasmic but were translocated into the nucleus during influenza virus infection [Citation143,Citation144]. Knocking down these lncRNAs resulted in the inhibition of viral RNA transcription and replication. Cross-linked RNA immunoprecipitation (CL-RIP) and RNA pulldown experiments revealed that PAAN is critical for the formation of RNA-dependent RNA polymerase (RdRp) through its interaction with the PA viral protein, whereas IPAN interacts with and stabilizes the viral PB1 protein, which is necessary for viral replication.
4.3.4. Unknown mechanisms
Virus-inducible lncRNA (VIN) was one of the earliest lncRNAs shown to be involved in IAV infection [Citation122]. VIN is upregulated by various strains of IAV and is located in the nucleus. Knockdown experiments revealed that VIN is essential for the productive replication of IAV.
Our group has identified that PSMB8-AS1 was highly induced by various strains of IAV and by IFNβ1 treatment [Citation123]. PSMB8-AS1 was found to be a nuclear lncRNA. All four isoforms of PSMB8-AS1 were upregulated by IAV infection. CRISPR interference of PSMB8-AS1 inhibited viral replication, but the exact mechanism of action is still not understood.
A lncRNA microarray analysis of influenza A/WSN/1933-infected C57BL/6 mice revealed several upregulated lncRNAs. Interferon stimulated lncRNA (LncRNA ISR) was the only lncRNA induced by both influenza virus and IFNβ treatment [Citation145]. Real-time PCR results showed that lncRNA ISR was the most highly expressed in the lungs. Silencing its expression increased viral replication, whereas overexpression diminished influenza replication. RIG-I knockout experiments and treatment with an inhibitor of NF-κB activity (BAY 11–7082) showed that lncRNA ISR expression is regulated during IAV infection through the RIG-I-dependent NF-κB signalling pathway.
5. Viral noncoding RNAs in influenza
In the last decade, much evidence has shown multiple viruses encoding their own miRNAs, known as viral miRNAs, to enable virus evasion of the host immune response [Citation146]. Most of our current understanding of viral miRNAs is based on DNA viruses that can access the nucleus and establish latent infections. DNA viruses such as herpesviruses, polyomaviruses, ascoviruses, and adenoviruses encode their own miRNAs to regulate host gene expression and thus promote the viral life cycle. For example, Kaposi’s sarcoma-associated herpesvirus encodes 12 miRNAs (miR-K1-12), of which miR-K5, miR-K9 and miR-K10a/b target Bcl-2-associated factor (BCLAF1) to increase viral replication [Citation147].
In contrast, theoretically, RNA viruses lack the ability to produce viral miRNAs because (1) most RNA viruses replicate in the cytoplasm and are thus inaccessible to the nucleus where the enzymes for miRNA biogenesis are located, (2) the RNA-based viral genome may be destroyed by ribonuclease enzyme Drosha, and (3) viral miRNA may target the viral genome itself [Citation146]. However, two groups independently reported that recombinant RNA viruses (sindbis virus and Epstein-Barr virus), which replicate in the cytoplasm, successfully produce miRNAs from a pri-miRNA substrate [Citation148,Citation149]. After this initial discovery, many other cytoplasmic RNA viruses, such as human immune deficiency virus, bovine leukaemia virus, bovine foamy virus, avian leukosis virus, West Nile virus, dengue virus and Ebola virus, have been shown to produce miRNAs [Citation150].
In contrast to that of many other RNA viruses, the influenza viral genome replicates in the nucleus and thus can utilize the nuclear miRNA processing factor Drosha. A genetically engineered pri-miRNA-containing recombinant virus is able to generate functional miRNAs without any evidence of attenuation in virus replication [Citation151]. These studies pioneered the idea that modified RNA viruses can be used as a miRNA delivery tool into cells. Influenza virus has also been shown to generate miRNA-like small RNAs by the noncanonical pathway during infection. However, these miRNA-like small RNAs lack the typical stem-loop structure of pre-miRNAs. Recently, it was reported that the H5N1 strain of the influenza virus encodes a miRNA-like small RNA, miR-HA-3p, that targets host poly(rC)-binding protein 2 (PCBP2) and plays a role in cytokine production during H5N1 infection [Citation152]. Similar miRNA-like small RNAs are also found in Ebola and HIV [Citation153,Citation154]. Collectively, these findings suggest that the influenza virus may be able to overcome replicative constraints imposed by miRNA machinery, and additional experimental evidence for influenza to produce viral miRNAs needs to be provided.
Recent studies have reported the identification of several new virus-derived RNA species that are involved in the regulation of influenza viral replication and host immune responses [Citation155–158]. These viral RNAs include small viral RNAs (svRNAs), mini viral RNAs (mvRNAs), virus-derived siRNAs (vsiRNAs) and defective interfering (DI) RNAs.
svRNAs were identified by small RNA sequencing of influenza A virus-infected lung epithelial cells [Citation155]. svRNAs are 22–27 nt in length and correspond to the 5ʹ end of viral genomic RNA of each segment. svRNAs are specific to influenza A virus and are not virus- or IFN-inducible miRNA products. Generation of svRNAs requires RNA-dependent RNA polymerase activity as well as viral proteins, NP and NS2. svRNAs directly interact with the RNA-dependent RNA polymerase complex and regulate the viral switch from transcription to replication in a segment-specific manner. The role of svRNAs in influenza virus replication is significant as anti-svRNA results in the loss of viral RNA and limits the spread of virus.
mvRNAs are short aberrant RNAs with a length of 47–125 nt. They bind RIG-I and result in the activation of RIG-I and the induction of IFNβ expression [Citation156]. mvRNAs are found to be strong agonists of the RIG-I compared to DI RNAs and full length RNA fragments. mvRNAs are produced due to erroneous polymerase activity of the influenza virus or the presence of host-specific amino acid mutations.
vsiRNAs are 21–24 nt in length with 2 nt overhangs at the 3ʹ end that are produced by host as an antiviral mechanism. Antiviral RNAi is dependent on AGO catalytic activity in an IFN-independent manner. Deep sequencing of small RNAs co-immunoprecipitated by an antibody specific to AGO from human HEK293T cells, human lung epithelial A549 cells and African green monkey epithelial Vero cells infected with PR8 lacking NS1 reveals the presence of abundant influenza vsiRNAs [Citation157]. Influenza vsiRNAs are generated from viral double-stranded RNAs by the human dicer activity and suppressed by influenza viral protein, NS1.
DI RNAs are more than 178 nt long subgenomic RNAs that were discovered during in vitro passages of influenza virus at a high multiplicity infection. DI RNAs are produced through internal deletion of viral genomic RNA [Citation158]. DI RNAs are found in human nasopharyngeal swabs from human subjects infected the influenza A (H1N1)pdm 09 virus [Citation159]. DI RNAs inhibit influenza viral replication by competing with viral ribonucleoprotein (vRNP) assembly and genome packaging. DI RNAs exhibit immunostimulatory property and activate RIG-I-mediated signalling [Citation160].
6. Conclusion
ncRNAs play important roles in influenza infection and pathogenesis. Hundreds of miRNAs and lncRNAs are dysregulated during influenza infection. Some of these ncRNAs can be antiviral or proviral factors.
More than a decade of research shows that the influenza-associated miRNA signature is virus strain-, species-, and cell-dependent. Moreover, miRNAs link host-virus interactions, which can affect many biological processes, such as innate and adaptive immunity, the cell cycle, apoptosis, and many other cellular pathways. miRNAs affect almost every critical step of antiviral immunity and thus are important in host defence against influenza infection. Interestingly, some miRNAs modulate viral gene expression directly. On the other hand, the influenza virus uses miRNAs for its efficient replication and viral evasion. Taken together, miRNAs are key regulators of host-influenza interactions.
However, many aspects of the interaction between influenza viruses, miRNAs, and hosts have not yet been fully characterized because of insufficient knowledge on miRNA-associated molecular pathways in the context of influenza infection and host immunity. Specific miRNA target identification faces greater challenges as one miRNA can target multiple mRNAs. Within a living organism, a change in one miRNA affects many molecular and cellular layers. miRNA mimics and inhibitors are extensively used to manipulate miRNA levels inside cells, and the therapeutic potential of these agents has been discussed. Several delivery systems have been tested to distribute miRNAs in vivo. However, careful manipulation needs to be established since many miRNAs are master regulators of inflammation; thus, a poor design may lead to unexpected toxicity. Importantly, based on the unique features of miRNAs, a new generation of attenuated live influenza vaccines may be developed by inserting miRNA-binding sites into the viral genome. Additionally, some miRNAs can be used as biomarkers for influenza infection, but limitations in detection levels and strain dependency need to be addressed. Collectively, influenza-associated miRNA research results have suggested that full characterization of miRNAs in the cellular environment during influenza infection is critical for understanding the diagnosis and prognosis of diseases and ultimately for developing novel antiviral therapeutics.
LncRNAs regulate influenza virus infection in many ways but mostly by altering the host immune responses, which are critical during the pathogenesis of influenza virus infection and is necessary for fighting invading pathogens. With increasing resistance to current anti-influenza drugs, there is a constant need for novel antivirals. Interest in noncoding RNAs as potential drug targets has been increasing in recent years [Citation161]. Gene-editing tools such as the CRISPR/Cas9 systems can be used to reduce the transcription of lncRNAs [Citation162]. siRNAs and shRNA can be successfully utilized to degrade lncRNAs posttranscriptionally, but their efficiency may depend on subcellular localization [Citation163]. Antisense oligonucleotides (ASOs) are becoming useful tools for targeting lncRNAs with better efficiency, particularly nuclear lncRNAs [Citation163]. Studies on small molecules specifically targeting the secondary structure of lncRNAs are still primitive but could reveal potential tools for therapeutically targeting lncRNAs [Citation164]. With increase in evidence showing the involvement of noncoding RNAs in influenza virus infection, it is crucial to identify and study the mechanisms of critical ncRNAs to potentially develop a new class of antiviral therapies against influenza virus infection in the future.
It is important to note that high-throughput studies including microarray, RNA-seq or functional screen are most often focused on one particular infectious agent such as influenza virus. Whether the identified hits apply to other viruses need to be determined. For biomarker studies, the identification of influenza virus-specific ncRNAs will aid the development of diagnostic tools. However, the identification of functional host ncRNAs that regulate the infection of various viruses is beneficial in the development of broad-spectrum antiviral drugs. Though the replication pattern of DNA and RNA viruses may differ, commonalities can be found in the mechanisms through which the host elicits immune responses to viral infection. As most host ncRNAs regulate cellular responses to viral infection in a spatiotemporal manner, future studies should adapt a holistic approach to identify universal regulatory ncRNAs.
It is also important to be aware of the emerging regulatory roles of RNA modifications such as N6-methyladenosine (m6A), 5-methylcytosine (m5C), inosine, 2′-O-methylations (Nm), pseudouridine (Ψ) in the context of viral diseases [165].
In summary, ncRNAs play an essential role in influenza viral infection. With booming research in the areas of targeting, modifications, delivery and advancements in the field of RNA therapeutics, it is clear that ncRNAs are the promising targets for the development of antiinfluenza medicines.
Acknowledgments
This work was supported by the National Institutes of Health grants HL135152 and GM103648, the Oklahoma Center for Adult Stem Cell Research-A Program of Tobacco Settlement Endowment Trust (TSET), the Oklahoma Center for the Advancement of Science and Technology (HR-20-050) and the Lundberg-Kienlen Endowment fund (to LL).
Disclosure statement
The authors have no conflicts of interest.
Additional information
Funding
References
- Hombach S, Kretz M. Non-coding RNAs: classification, biology and functioning. Adv Exp Med Biol. 2016;937(p):3–17.
- Horos R, Büscher M, Kleinendorst R, et al. The small non-coding vault rna1-1 acts as a riboregulator of autophagy. Cell. 2019;176(5):1054–1067. e12.
- Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17(1):47–62.
- Frankish A, Diekhans M, Ferreira AM, et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 2019;47(D1):D766–D773.
- Center of Disease Control. Disease burden of influenza. 2020, Apr 17; Available from: https://www.cdc.gov/flu/about/burden/index.html.
- Luliano AD, Roguski KM, Chang HH, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285–1300.
- Ziegler T, Mamahit A, Cox NJ. 65 years of influenza surveillance by a world health organization-coordinated global network. Influenza Other Respir Viruses. 2018;12(5):558–565.
- Krammer F, Smith GJD, Fouchier RAM, et al. Influenza. Nat Rev Dis Primers. 2018;4(1):3.
- Bouvier NM, Palese P. THE BIOLOGY OF INFLUENZA VIRUSES. Vaccine. 2008;26(Suppl 4):D49–D53.
- Dou D, Revol R, Östbye H, et al. Influenza A virus cell entry, replication, virion assembly and movement. Front Immunol. 2018;9:1581.
- Long JS, Mistry B, Haslam SM, et al. Host and viral determinants of influenza A virus species specificity. Nat Rev Microbiol. 2019;17(2):67–81.
- Plotch SJ, Bouloy M, Ulmanen I, et al. A unique cap(m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell. 1981;23(3):847–858.
- Jefferson T, Deeks JJ, Demicheli V, et al. Amantadine and rimantadine for preventing and treating influenza A in adults. Cochrane Database Syst Rev. 2004;(2):Cd001169.
- Dobson J, Whitley RJ, Pocock S, et al. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385(9979):1729–1737.
- Hayden FG, Sugaya N, Hirotsu N, et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med. 2018;379(10):913–923.
- Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20(1):21–37.
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854.
- Pasquinelli AE, Reinhart BJ, Slack F, et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408(6808):86–89.
- Arteaga-Vázquez M, Caballero-Pérez J, Vielle-Calzada JP. A family of microRNAs present in plants and animals. Plant Cell. 2006;18(12):3355–3369.
- Kozomara A, Birgaoanu M, Griffiths-Jones S. miRBase: from microRNA sequences to function. Nucleic Acids Res. 2018;47(D1):D155–D162.
- Ambros V, Bartel B, Bartel DP, et al. A uniform system for microRNA annotation. RNA. 2003;9:277–9.
- Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15(8):509–524.
- Denli AM, Tops BBJ, Plasterk RHA, et al. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432(7014):231–235.
- Yi R, Qin Y, Macara IG, et al. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17(24):3011–3016.
- Zhang H, Kolb FA, Jaskiewicz L, et al. Single processing center models for human dicer and bacterial rnase III. Cell. 2004;118(1):57–68.
- Hammond SM, Boettcher S, Caudy AA, et al. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001;293(5532):1146–1150.
- O'Brien J, Hayder H, Zayed Y, et al. Overview of microrna biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne). 2018;9:402.
- Xu W, San Lucas A, Wang Z, et al. Identifying microRNA targets in different gene regions. BMC Bioinformatics. 2014;7(Suppl 7):S4. 15 Suppl.
- Dharap A, Pokrzywa C, Murali S, et al. MicroRNA miR-324-3p induces promoter-mediated expression of relA gene. PLoS One. 2013;8(11):e79467.
- Rusk N. When microRNAs activate translation. Nat Methods. 2008;5(2):122–123.
- Iftikhar H, Carney GE. Evidence and potential in vivo functions for biofluid miRNAs: from expression profiling to functional testing: potential roles of extracellular miRNAs as indicators of physiological change and as agents of intercellular information exchange. Bioessays. 2016;38(4):367–378.
- Guo YE, Steitz JA. Virus meets host microrna: the destroyer, the booster, the hijacker. Mol Cell Biol. 2014;34(20):3780–3787.
- Makkoch J, Poomipak W, Saengchoowong S, et al. Human microRNAs profiling in response to influenza A viruses (subtypes pH1N1, H3N2, and H5N1). Exp Biol Med. 2016;241(4):409–420.
- Loveday EK, Svinti V, Diederich S, et al. Temporal- and strain-specific host microRNA molecular signatures associated with swine-origin H1N1 and avian-origin H7N7 influenza A virus infection. J Virol. 2012;86(11):6109–6122.
- Lam WY, Yeung AC, Ngai KL, et al. Effect of avian influenza A H5N1 infection on the expression of microRNA-141 in human respiratory epithelial cells. BMC Microbiol. 2013;13:104.
- Li Y, Li J, Belisle S, et al. Differential microRNA expression and virulence of avian, 1918 reassortant, and reconstructed 1918 influenza A viruses. Virology. 2011;421(2):105–113.
- Tan KS, Choi H, Jiang X, et al. Micro-RNAs in regenerating lungs: an integrative systems biology analysis of murine influenza pneumonia. BMC Genomics. 2014;15(1):587.
- Zhao FR, Su S, Zhou DH, et al. Comparative analysis of microRNAs from the lungs and trachea of dogs (Canis familiaris) infected with canine influenza virus. Infect Genet Evol. 2014;21:367–374.
- Skovgaard K, Cirera S, Vasby D, et al. Expression of innate immune genes, proteins and microRNAs in lung tissue of pigs infected experimentally with influenza virus (H1N2). Innate Immun. 2013;19(5):531–544.
- Gao L, Gao J, Liang Y, et al. Integration analysis of a miRNA-mRNA expression in A549 cells infected with a novel H3N2 swine influenza virus and the 2009 H1N1 pandemic influenza virus. Infect Genet Evol. 2019;74:103922.
- Wang Y, Brahmakshatriya V, Zhu H, et al. Identification of differentially expressed miRNAs in chicken lung and trachea with avian influenza virus infection by a deep sequencing approach. BMC Genomics. 2009;10:512.
- Terrier O, Textoris J, Carron C, et al. Host microRNA molecular signatures associated with human H1N1 and H3N2 influenza A viruses reveal an unanticipated antiviral activity for miR-146a. J Gen Virol. 2013;94(Pt 5):985–995.
- Guan Z, Shi N, Song Y, et al. Induction of the cellular microRNA-29c by influenza virus contributes to virus-mediated apoptosis through repression of antiapoptotic factors BCL2L2. Biochem Biophys Res Commun. 2012;425(3):662–667.
- Othumpangat S, Noti JD, Blachere FM, et al. Expression of non-structural-1A binding protein in lung epithelial cells is modulated by miRNA-548an on exposure to influenza A virus. Virology. 2013;447(1):84–94.
- Buggele WA, Johnson KE, Horvath CM. Influenza A virus infection of human respiratory cells induces primary microRNA expression. J Biol Chem. 2012;287(37):31027–31040.
- Jiao H, Zheng Z, Shuai X, et al. MicroRNA expression profiles from HEK293 cells expressing H5N1 avian influenza virus non-structural protein 1. Innate Immun. 2019;25(2):110–117.
- Saengchoowong S, Khongnomnan K, Poomipak W, et al. High-throughput microrna profiles of permissive madin-darby canine kidney cell line infected with influenza b viruses. Viruses. 2019;11:11.
- Song H, Wang Q, Guo Y, et al. Microarray analysis of microRNA expression in peripheral blood mononuclear cells of critically ill patients with influenza A (H1N1). BMC Infect Dis. 2013;13:257.
- Li Y, Chan EY, Li J, et al. MicroRNA expression and virulence in pandemic influenza virus-infected mice. J Virol. 2010;84(6):3023–3032.
- Jiang P, Zhou N, Chen X, et al. Integrative analysis of differentially expressed microRNAs of pulmonary alveolar macrophages from piglets during H1N1 swine influenza A virus infection. Sci Rep. 2015;5(1):8167.
- Huang S-Y, Huang C-H, Chen C-J, et al. novel role for mir-1290 in host species specificity of influenza a virus. Mol Ther Nucleic Acids. 2019;17:10–23.
- Lim J, Byun J, Guk K, et al. highly sensitive in vitro diagnostic system of pandemic influenza a (h1n1) virus infection with specific microrna as a biomarker. ACS Omega. 2019;4(11):14560–14568.
- Gao J, Gao L, Li R, et al. Integrated analysis of microRNA-mRNA expression in A549 cells infected with influenza A viruses (IAVs) from different host species. Virus Res. 2019;263:34–46.
- Ojha R, Nandani R, Pandey RK, et al. Emerging role of circulating microRNA in the diagnosis of human infectious diseases. J Cell Physiol. 2019;234(2):1030–1043.
- Tambyah PA, Sepramaniam S, Mohamed Ali J, et al. microRNAs in circulation are altered in response to influenza A virus infection in humans. PLoS One. 2013;8(10):e76811.
- Morán J, Ramírez-Martínez G, Jiménez-Alvarez L, et al. Circulating levels of miR-150 are associated with poorer outcomes of A/H1N1 infection. Exp Mol Pathol. 2015;99(2):253–261.
- Zhu Z, Qi Y, Ge A, et al. Comprehensive characterization of serum microRNA profile in response to the emerging avian influenza A (H7N9) virus infection in humans. Viruses. 2014;6(4):1525–1539.
- Peng F, He Ja, Loo JFC, et al. Identification of serum MicroRNAs as diagnostic biomarkers for influenza H7N9 infection. 2017;7:1–8. Virol Rep.
- Scheller N, Herold S, Kellner R, et al. Proviral micrornas detected in extracellular vesicles from bronchoalveolar lavage fluid of patients with influenza virus-induced acute respiratory distress syndrome. J Infect Dis. 2019;219(4):540–543.
- Peng F, He J, Loo JF, et al. Identification of microRNAs in throat swab as the biomarkers for diagnosis of influenza. Int J Med Sci. 2016;13(1):77–84.
- Sun E, He J, Zhuang X. Dissecting the role of COPI complexes in influenza virus infection. J Virol. 2013;87(5):2673–2685.
- Hu Y, Jiang L, Lai W, et al. MicroRNA-33a disturbs influenza A virus replication by targeting ARCN1 and inhibiting viral ribonucleoprotein activity. J Gen Virol. 2016;97(1):27–38.
- Guinea R, Carrasco L. Requirement for vacuolar proton-ATPase activity during entry of influenza virus into cells. J Virol. 1995;69(4):2306–2312.
- Peng S, Wang J, Wei S, et al. Endogenous cellular micrornas mediate antiviral defense against influenza a virus. Mol Ther Nucleic Acids. 2018;10:361–375.
- Loveday E-K, Diederich S, Pasick J, et al. Human microRNA-24 modulates highly pathogenic avian-origin H5N1 influenza A virus infection in A549 cells by targeting secretory pathway furin. J Gen Virol. 2015;96(1):30–39.
- Furuse Y, Oshitani H. Evolution of the influenza A virus untranslated regions. Infect Genet Evol. 2011;11(5):p.1150–4.
- Song L, Liu H, Gao S, et al. Cellular microRNAs inhibit replication of the H1N1 influenza A virus in infected cells. J Virol. 2010;84(17):8849–8860.
- Ingle H, Kumar S, Raut AA, et al. The microRNA miR-485 targets host and influenza virus transcripts to regulate antiviral immunity and restrict viral replication. Sci Signal. 2015;8(406):ra126.
- Zhang S, Wang R, Su H, et al. Sus scrofa miR-204 and miR-4331 negatively regulate swine h1n1/2009 influenza a virus replication by targeting viral ha and ns, respectively. Int J Mol Sci. 2017;18(4):749.
- Khongnomnan K, Makkoch J, Poomipak W, et al. Human miR-3145 inhibits influenza A viruses replication by targeting and silencing viral PB1 gene. Exp Biol Med (Maywood). 2015;240(12):1630–1639.
- Kumar A, Kumar A, Ingle H, et al. MicroRNA hsa-miR-324-5p suppresses h5n1 virus replication by targeting the viral pb1 and host CUEDC2. J Virol. 2018;92:19.
- Cui H, Zhang C, Zhao Z, et al. Identification of cellular microRNA miR-188-3p with broad-spectrum anti-influenza A virus activity. Virol J. 2020;17(1):12.
- Kumar A, Vn MA, Raut AA, et al. Identification of chicken pulmonary mirnas targeting pb1, pb1-f2, and n40 genes of highly pathogenic avian influenza virus h5n1 in silico. Bioinform Biol Insights. 2014;8:135–145.
- Asaf VN, Kumar A, Raut AA, et al. In-silico search of virus-specific host microRNAs regulating avian influenza virus NS1 expression. Theory Biosci. 2015;134(1–2):65–73.
- Ma YJ, Yang J, Fan XL, et al. Cellular microRNA let-7c inhibits M1 protein expression of the H1N1 influenza A virus in infected human lung epithelial cells. J Cell Mol Med. 2012;16(10):2539–2546.
- Waring BM, Sjaastad LE, Fiege JK, et al. MicroRNA-based attenuation of influenza virus across susceptible hosts. J Virol. 2018;92:2.
- Weber-Gerlach M, Weber F. Standing on three legs: antiviral activities of RIG-I against influenza viruses. Curr Opin Immunol. 2016;42(p):71–75.
- Wang K, Lai C, Gu H, et al. miR-194 inhibits innate antiviral immunity by targeting fgf2 in influenza h1n1 virus infection. Front Microbiol. 2017;8:2187.
- Zhao L, Zhu J, Zhou H, et al. Identification of cellular microRNA-136 as a dual regulator of RIG-I-mediated innate immunity that antagonizes H5N1 IAV replication in A549 cells. Sci Rep. 2015;5:14991.
- Maemura T, Fukuyama S, Sugita Y, et al. Lung-derived exosomal mir-483-3p regulates the innate immune response to influenza virus infection. J Infect Dis. 2018;217(9):p.1372–1382.
- Zhao L, Zhang X, Wu Z, et al. The downregulation of microrna hsa-mir-340-5p in iav-infected a549 cells suppresses viral replication by targeting RIG-I and OAS2. Mol Ther Nucleic Acids. 2019;14:509–519.
- Fang J, Hao Q, Liu L, et al. Epigenetic changes mediated by microrna mir29 activate cyclooxygenase 2 and lambda-1 interferon production during viral infection. J Virol. 2012;86(2):1010–1020.
- Buggele WA, Krause KE, Horvath CM. Small RNA profiling of influenza A virus-infected cells identifies miR-449b as a regulator of histone deacetylase 1 and interferon beta. PLoS One. 2013;8(9):e76560.
- Wang P, Hou J, Lin L, et al. Inducible microRNA-155 feedback promotes type i ifn signaling in antiviral innate immunity by targeting suppressor of cytokine signaling 1. J Immunol. 2010;185(10):6226–6233.
- Gao S, Li J, Song L, et al. Influenza A virus-induced downregulation of miR-26a contributes to reduced IFNα/β production. Virol Sin. 2017;32(4):261–270.
- Pichulik T, Khatamzas E, Liu X, et al. Pattern recognition receptor mediated downregulation of microRNA-650 fine-tunes MxA expression in dendritic cells infected with influenza A virus. Eur J Immunol. 2016;46(1):167–177.
- Rosenberger CM, Podyminogin RL, Diercks AH, et al. miR-144 attenuates the host response to influenza virus by targeting the TRAF6-IRF7 signaling axis. PLoS Pathog. 2017;13(4):e1006305.
- Chen X, Zhou L, Peng N, et al. MicroRNA-302a suppresses influenza A virus-stimulated interferon regulatory factor-5 expression and cytokine storm induction. J Biol Chem. 2017;292(52):21291–21303.
- Gui S, Chen X, Zhang M, et al. Mir-302c mediates influenza A virus-induced IFNβ expression by targeting NF-κB inducing kinase. FEBS Lett. 2015;589(24 Pt B):4112–4118.
- Lin J, Xia J, Tu CZ, et al. H9N2 avian influenza virus protein pb1 enhances the immune responses of bone marrow-derived dendritic cells by down-regulating miR375. Front Microbiol. 2017;8:287.
- Lin J, Chen YT, Xia J, et al. MiR674 inhibits the neuraminidase-stimulated immune response on dendritic cells via down-regulated Mbnl3. Oncotarget. 2016;7(31):48978–48994.
- Rivera A, Barr T, Rais M, et al. microRNAs regulate host immune response and pathogenesis during influenza infection in rhesus macaques. Viral Immunol. 2016;29(4):212–227.
- Hope JL, Stairiker CJ, Spantidea PI, et al. The transcription factor t-bet is regulated by microrna-155 in murine anti-viral cd8+ t cells via SHIP-1. Front Immunol. 2017;8:1696.
- Nejad C, Stunden HJ, Gantier MP. A guide to miRNAs in inflammation and innate immune responses. Febs J. 2018;285(20):3695–3716.
- Hsu ACY, Dua K, Starkey MR, et al. MicroRNA-125a and -b inhibit A20 and MAVS to promote inflammation and impair antiviral response in COPD. JCI Insight. 2017;2(7):e90443–e90443.
- Balkhi MY, Iwenofu OH, Bakkar N, et al. miR-29 acts as a decoy in sarcomas to protect the tumor suppressor a20 mrna from degradation by huR. Sci Signal. 2013;6(286):ra63–ra63.
- Zhang X, Dong C, Sun X, et al. Induction of the cellular miR-29c by influenza virus inhibits the innate immune response through protection of A20 mRNA. Biochem Biophys Res Commun. 2014;450(1):755–761.
- Othumpangat S, Bryan NB, Beezhold DH, et al. Upregulation of miRNA-4776 in influenza virus infected bronchial epithelial cells is associated with downregulation of nfkbib and increased viral survival. Viruses. 2017;9:5.
- Rosenberger CM, Podyminogin RL, Navarro G, et al. miR-451 regulates dendritic cell cytokine responses to influenza infection. J Immunol. 2012;189(12):5965–5975.
- Fan N, Wang J. MicroRNA 34a contributes to virus-mediated apoptosis through binding to its target gene Bax in influenza A virus infection. Biomed Pharmacother. 2016;83:1464–1470.
- Yang X, Zhao C, Bamunuarachchi G, et al. miR-193b represses influenza A virus infection by inhibiting Wnt/β-catenin signalling. Cell Microbiol. 2019;21(5):e13001.
- McCaskill JL, Ressel S, Alber A, et al. Broad-spectrum inhibition of respiratory virus infection by microrna mimics targeting p38 mapk signaling. Mol Ther Nucleic Acids. 2017;7:256–266.
- Othumpangat S, Noti JD, Beezhold DH. Lung epithelial cells resist influenza A infection by inducing the expression of cytochrome c oxidase VIc which is modulated by miRNA 4276. Virology. 2014;468-470:256–264.
- More S, Yang X, Zhu Z, et al. Regulation of influenza virus replication by Wnt/β-catenin signaling. Plos One. 2018;13(1):e0191010.
- Chakraborty C, Sharma AR, Sharma G, et al. Therapeutic miRNA and siRNA: moving from bench to clinic as next generation medicine. Mol Ther Nucleic Acids. 2017;8:132–143.
- Hanna J, Hossain GS, Kocerha J. The potential for microrna therapeutics and clinical research. Front Genet. 2019;10:478.
- Chen Y, Zhao H, Tan Z, et al. Bottleneck limitations for microRNA-based therapeutics from bench to the bedside. Pharmazie. 2015;70(3):147–154.
- Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods. 2007;4(9):721–726.
- Zhang H, Tang X, Zhu C, et al. Adenovirus-mediated artificial MicroRNAs targeting matrix or nucleoprotein genes protect mice against lethal influenza virus challenge. Gene Ther. 2015;22(8):653–662.
- Derrien T, Johnson R, Bussotti G, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22(9):1775–1789.
- Johnsson P, Lipovich L, Grander D, et al. Evolutionary conservation of long non-coding RNAs; sequence, structure, function. Biochim Biophys Acta. 2014;1840(3):1063–1071.
- Cabili MN, Dunagin MC, McClanahan PD, A, et al. Localization and abundance analysis of human lncRNAs at single-cell and single-molecule resolution. Genome Biol. 2015;16(1):1–16.
- Qin T, Li J, Zhang K-Q. Structure, regulation, and function of linear and circular long non-coding RNAs. Front Genet. 2020;11:150.
- Ma L, Bajic VB, Zhang Z. On the classification of long non-coding RNAs. RNA Biol. 2013;10(6):924–933.
- Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172(3):393–407.
- Hartford CCR, Lal A. When long noncoding becomes protein coding. Mol Cell Biol. 2020;40:6.
- Beermann J, Piccoli MT, Viereck J, et al. Non-coding rnas in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev. 2016;96(4):1297–1325.
- Shirahama S, Miki A, Kaburaki T, et al. Long non-coding rnas involved in pathogenic infection. Front Genet. 2020May;11:454.
- Landeras-Bueno S, Ortín J. Regulation of influenza virus infection by long non-coding RNAs. Virus Res. 2016;212:78–84.
- Wang J, Cen S. Roles of lncRNAs in influenza virus infection. Emerg Microbes Infect. 2020;9:1–22.
- Winterling C, Koch M, Koeppel M, et al. Evidence for a crucial role of a host non-coding RNA in influenza a virus replication. RNA Biol. 2014;11(1):66–75.
- More S, Zhu Z, Lin K, et al. Long non-coding RNA PSMB8-AS1 regulates influenza virus replication. RNA Biol. 2019;16(3):340–353.
- Chai W, Li J, Shangguan Q, et al. Lnc-ISG20 inhibits influenza a virus replication by enhancing isg20 expression. J Virol. 2018;92:16.
- Maarouf M, Chen B, Chen Y, et al. Identification of lncRNA-155 encoded by MIR155HG as a novel regulator of innate immunity against influenza A virus infection. Cell Microbiol. 2019;21(8):e13036.
- Josset L, Tchitchek N, Gralinski LE, et al. Annotation of long non-coding RNAs expressed in collaborative cross founder mice in response to respiratory virus infection reveals a new class of interferon-stimulated transcripts. RNA Biol. 2014;11(7):875–890.
- Lai C, Liu L, Liu Q, et al. Long noncoding RNA AVAN promotes antiviral innate immunity by interacting with TRIM25 and enhancing the transcription of FOXO3a. Biorxiv. 2019.
- Wang Q, Zhang D, Feng W, et al. Long noncoding RNA TSPOAP1 antisense RNA 1 negatively modulates type I IFN signaling to facilitate influenza A virus replication. J Med Virol. 2019;n/a(n/a):1–10.
- Quyang J, Zhu X, Chen Y, et al. NRAV, a long noncoding RNA, modulates antiviral responses through suppression of interferon-stimulated gene transcription. Cell Host Microbe. 2014;16(5):616–626.
- Zhao L, Xia M, Wang K, et al. A long non-coding rna ivrpie promotes host antiviral immune responses through regulating interferon β1 and isg expression. Front Microbiol. 2020;11:1–11.
- Verhelst J, Parthoens E, Schepens B, et al. Interferon-inducible protein Mx1 inhibits influenza virus by interfering with functional viral ribonucleoprotein complex assembly. J Virol. 2012;86(24):13445–13455.
- Li X, Guo G, Lu M,et al. Long noncoding rna lnc-mxa inhibits beta interferon transcription by forming rna-dna triplexes at its promoter. J Virol. 2019;93(21):e00786–19.
- Qu H, Li J, Yang L, et al. Influenza A Virus-induced expression of ISG20 inhibits viral replication by interacting with nucleoprotein. Virus Genes. 2016;52(6):759–767.
- Jiang M, Zhang S, Yang Z, et al. Self-recognition of an inducible host lncrna by rig-i feedback restricts innate immune response. Cell. 2018;173(4):906–919. e13..
- Lin H, Jiang M, Liu L, et al. The long noncoding RNA Lnczc3h7a promotes a TRIM25-mediated RIG-I antiviral innate immune response. Nat Immunol. 2019;20(7):812–823.
- Chiang JJ, Sparrer KMJ, van Gent M, et al. Viral unmasking of cellular 5S rRNA pseudogene transcripts induces RIG-I-mediated immunity. Nat Immunol. 2018;19(1):53–62.
- Imamura K, Imamachi N, Akizuki G, et al. Long noncoding rna neat1-dependent sfpq relocation from promoter region to paraspeckle mediates il8 expression upon immune stimuli. Mol Cell. 2014;53(3):393–406.
- Sasaki YTF, Ideue T, Sano M, et al. MENε/β noncoding RNAs are essential for structural integrity of nuclear paraspeckles. Proc Nat Acad Sci. 2009;106(8):2525–2530.
- Kambara H, Gunawardane L, Zebrowski E, et al. Regulation of interferon-stimulated gene BST2 by a lncRNA transcribed from a shared bidirectional promoter. Front Immunol. 2015;6(JAN):p.676.
- Barriocanal M, Carnero E, Segura V, et al. Long non-coding RNA BST2/BISPR is induced by IFN and regulates the expression of the antiviral factor tetherin. Front Immunol. 2014;5:655.
- Gnirß K, Zmora P, Blazejewska P, et al. Tetherin sensitivity of influenza a viruses is strain specific: role of hemagglutinin and neuraminidase. J Virol. 2015;89(18):9178–9188.
- Wang P, Xu J, Wang Y, et al. An interferon-independent lncRNA promotes viral replication by modulating cellular metabolism. Science. 2017;358(6366):1051–1055.
- Wang J, Wang Y, Zhou R, et al. Host long noncoding RNA lncRNA-PAAN regulates the replication of influenza A virus. Viruses. 2018;10:6.
- Wang J, Zhang Y, Li Q, et al. Influenza virus exploits an interferon-independent lncrna to preserve viral rna synthesis through stabilizing viral rna polymerase PB1. Cell Rep. 2019;27(11):3295–3304. e4.
- Pan Q, Zhao Z, Liao Y, et al. Identification of an interferon-stimulated long noncoding rna (lncrna isr) involved in regulation of Influenza A virus replication. Int J Mol Sci. 2019;20(20):5118.
- Aguado LC, tenOever B. RNA virus building blocks—miRNAs not included. PLoS Pathog. 2018;14(5):e1006963.
- Ziegelbauer JM, Sullivan CS, Ganem D. Tandem array-based expression screens identify host mRNA targets of virus-encoded microRNAs. Nat Genet. 2009;41(1):130–134.
- Shapiro JS, Varble A, Pham AM, et al. Noncanonical cytoplasmic processing of viral microRNAs. Rna. 2010;16(11):2068–2074.
- Rouha H, Thurner C, Mandl CW. Functional microRNA generated from a cytoplasmic RNA virus. Nucleic Acids Res. 2010;38(22):8328–8337.
- Li X, Zou X. An overview of RNA virus-encoded microRNAs. ExRNA. 2019;1(1):37.
- Varble A, Chua MA, Perez JT, et al. Engineered RNA viral synthesis of microRNAs. Proc Natl Acad Sci U S A. 2010;107(25):11519–11524.
- Li X, Fu Z, Liang H, et al. H5N1 influenza virus-specific miRNA-like small RNA increases cytokine production and mouse mortality via targeting poly(rC)-binding protein 2. Cell Res. 2018;28(2):157–171.
- Kaul D, Ahlawat A, Gupta SD. HIV-1 genome-encoded hiv1-mir-H1 impairs cellular responses to infection. Mol Cell Biochem. 2009;323(1–2):143–148.
- Chen Z, Liang H, Chen X, et al. An ebola virus-encoded microRNA-like fragment serves as a biomarker for early diagnosis of ebola virus disease. Cell Res. 2016;26(3):380–383.
- Perez JT, Varble A, Sachidanandam R, et al. Influenza A virus-generated small RNAs regulate the switch from transcription to replication. Proc Nat Acad Sci. 2010;107(25):11525.
- Te Velthuis AJW, Long JC, Bauer DLV, et al. Mini viral RNAs act as innate immune agonists during influenza virus infection. Nat Microbiol. 2018;3(11):1234–1242.
- Li Y, Basavappa M, Lu J, et al. Induction and suppression of antiviral RNA interference by influenza A virus in mammalian cells. Nat Microbiol. 2016;2(3):16250.
- Davis AR, Hiti AL, Nayak DP. Influenza defective interfering viral RNA is formed by internal deletion of genomic RNA. Proc Nat Acad Sci. 1980;77(1):215.
- Saira K, Lin X, DePasse JV, et al. Sequence analysis of in vivo defective interfering-like RNA of influenza A H1N1 pandemic virus. J Virol. 2013;87(14):8064–8074.
- Liu G, Lu Y, Liu Q, et aal. Inhibition of ongoing influenza a virus replication reveals different mechanisms of rig-i activation. J Virol. 2019;93(6):e02066–18.
- Matsui M, Corey DR. Non-coding RNAs as drug targets. Nat Rev Drug Discov. 2017;16(3):167–179.
- Yang J, Meng X, Pan J, et al. CRISPR/Cas9-mediated noncoding RNA editing in human cancers. RNA Biol. 2018;15(1):35–43.
- Lennox KA, Behlke MA. Cellular localization of long non-coding RNAs affects silencing by RNAi more than by antisense oligonucleotides. Nucleic Acids Res. 2015;44(2):863–877.
- Arun G, Diermeier SD, Spector DL. Therapeutic targeting of long non-coding rnas in cancer. Trends Mol Med. 2018;24(3):257–277.
- Potužník JF, Cahová H. It’s the little things (in viral RNA). mBio. 2020;11(5):e02131–20.
