ABSTRACT
With advancing age, human muscle loses strength and function, but the molecular causes of these losses are unknown. Skeletal muscle shows an age-dependent decline in the levels of different proteins, but whether such decline is associated with reduced translation has not been studied. To address this gap of knowledge, we used the technique of ribosome profiling to study translation in muscle from middle-aged and old individuals. Using ribosome occupancy as a measure of translation status, several mRNAs showed differential translation with age. Older age was associated with lower translation of myosin and titin isoforms and more broadly with the translation of proteins involved in oxidative phosphorylation encoded by the mitochondrial genome. Based on our findings, we propose that mitochondrial proteins are less translated in old skeletal muscle.
Introduction, results, discussion
Several lines of research suggest that the regulation of protein translation is important for human longevity. Treatment with the drug rapamycin, a caloric restriction mimetic that prolongs life span in animal models, functions at least in part by reducing protein translation [Citation1,Citation2]. In C. elegans, lowering protein translation by either drug treatment or RNAi targeting ribosome components was found to enhance longevity [Citation3–6]. Similarly, in D. Melanogaster, a mutation in the translation factor 4E-BP decreases translation, reduces protein aggregation in muscle and increases longevity [Citation7]. The mechanisms whereby lowering translation promotes longevity are not understood, and it is unknown whether modifications of translation occur during human ageing.
To address this gap of knowledge, we analysed the translational profiles of skeletal muscle biopsies from three middle-aged and two old human subjects. The middle-aged subjects were 43 years old (y.o.) (Caucasian female), 46 y.o. (Caucasian male), and 41 y.o. (African American female), and the old subjects were 83 y.o. (Caucasian female) and 85 y.o. (Caucasian male). Transcript-specific translation was globally evaluated by ribosome profiling, a technique that identifies ribosome-protected mRNA fragments by RNASeq analysis. The identification of ribosome-protected mRNAs segments provides evidence that a certain mRNAs is engaged in active translation at a given point in time in a given cell or tissue. Ribosome profiling and total RNA profiling were performed side-by-side by using RNASeq analysis to estimate for each transcript the ratio of translating mRNAs with overall mRNAs present in the tissue.
Briefly, muscle biopsies were performed from vastus lateralis and each sample was split for both RNASeq and ribosome profiling (). For ribosome profiling, muscle was immersed into a polysome preparation buffer containing cycloheximide to fix ribosomes in place before digestion with RNase I. Protected ribosome footprints were then extracted in Trizol, purified using a Qiagen RNA kit, and finally isolated on a denaturing gel. In parallel, Total RNASeq libraries were digested with proteinase K, and full-length RNA was isolated using the Qiagen Fibrous RNA kit. Ribosome profiling (RiboSeq) libraries were single-end sequenced to a depth of 150 to 250 million reads, while Total RNASeq libraries were paired-end sequenced on a NextSeq to a depth of 600 to 800 million reads. All reads were then aligned to the known protein-coding gene transcriptome using Ensembl hg38.
Figure 1. Workflow of ribosome profiling experiment and ribosome occupancy analysis. (A) Bergström needle biopsies from vastus lateralis muscles were obtained from healthy adults. Samples were collected from three middle-aged and two old individuals, as shown. Ribosome profiling and Total RNA libraries (RiboSeq and RNASeq, respectively) were prepared. The bioinformatic workflow for the ribosome profiling data filtered out contaminating non-coding RNA sequences, in particular rRNAs, tRNAs, snRNAs, and snoRNAs, and then aligned the RiboSeq reads to the known transcriptome. In parallel, Total RNASeq data was aligned to the transcriptome. Finally, translational efficiency (TE) was calculated for each known transcript by finding the ratio RiboSeq/RNASeq. TE was then compared between muscle from younger and older participants. (B) Overall distributions of ribosome profiling and Total RNA sequencing data. The scatterplots show, for each known transcript, the normalized level of reads from the ribosome profiling libraries in transcripts per million (TPM) in the y-axes (RiboSeqTPM) and the RNA sequencing in the x-axes (RNASeqTPM). The y-axis represents the level of ribosome occupancy and hence translation level, while the x-axis is a representation of the steady-state levels of mRNAs in the cell (the balance between mRNA transcription and mRNA degradation). Highlighted are the transcripts whose translation was significantly different between younger and older individuals: red dots represent mitochondrial transcripts, which cluster together and were decreased with age, green dots represent mRNAs showing decreasing translation in older muscle, and blue represent mRNAs showing increased translation in older muscle. Descriptions of significantly changed transcripts are found in
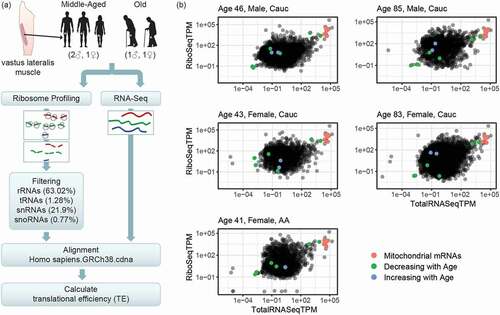
Table 1. Transcripts with translational efficiency significantly different between age groups. Negative fold changes indicate lower translation with older age and, viceversa, positive fold changes indicate higher translation with older age. Significance was tested using a Wald test with Benjamini-Hochberg adjustment
To look systematically at the global relationship between transcription and translation in our samples, we first calculated the number of reads aligning to each known human transcript (TPM, transcripts per million) for both the ribosome profiling and the Total RNA libraries. In , each dot represents normalized read counts for ribosome profiling (y-axis, RiboSeqTPM) and their relationship with normalized read counts for Total RNA (y-axis, RNASeqTPM) for each transcript isoform, and shows the extent to which RNA expression and translation are related. Looking across all transcripts and all samples, we observed a general correlation between mRNA levels and levels of ribosome occupancy, but also some transcripts which had substantially higher or lower ribosome binding than others. Importantly, we found that mitochondrial transcripts, which are translated by mitochondrial ribosomes, were abundantly recovered by our protocol (, green dots).
The ratio between ribosome profiling reads and Total RNA reads is an estimate of the probability of ribosome binding to a given transcript and represents the translational efficiency (TE) of that transcript. We asked whether there were specific transcripts whose TE was different according to age. We divided the samples into two age groups of young (ages 43, 41 and 46) and old (ages 83 and 85). Fifteen transcripts were significantly differentially translated between age groups (). Consistent with previous reports of decreased overall skeletal muscle translation with age [Citation8], we found that 13 of these 15 transcripts were less translated in muscle from older participants. Interestingly, 7 of the mRNAs displaying reduced ribosome engagement in old muscle were encoded by the mitochondrial genome (mtDNA). The mtDNA contains only 37 genes, of which 13 are protein-coding; all 13 proteins are components of oxidative phosphorylation complexes [Citation9]. Since 7 out of these 13 mRNAs appeared less translated, our data suggest that mitochondrial transcripts are poorly translated in older human skeletal muscle. The remaining six mitochondrial mRNAs also decreased in translation with age, although not an extent to reach statistical significance (Table S1). Additionally, we found that translational engagement of myosin heavy chain 4 (MYH4) and two isoforms of titin (TTN), two protein components of sarcomeres, were lower with older age. The ribosome occupancy on mRNAs encoding three transcription factors was also significantly associated with age; two of them increased with age [Poly(rC) binding protein 2 (PCBP2) and Kelch like family member 31 (KLHL31)] and one of them decreased with age [Zinc finger BED-type containing 9 (ZBED9)]. When examining the positions of the ribosome footprints on these transcripts, we do not find patterns suggesting ribosomal pausing, as the ribosomal profiling reads appear to be evenly distributed along the transcripts in both the young and old samples (Supplemental Data S1). Of the two transcripts whose ribosome occupancy increased with age, one, KLHL31 mRNA, contains an internal ribosome entry site (IRES) [Citation10], while the other, PCBP2 mRNA, encodes an IRES trans-activating factor [Citation11], suggesting cap-independent translation might increase overall. The transcripts showing increased translation with age did not share other common features such as upstream open reading frames or long untranslated regions. The mechanism of these changes will be investigated in future work.
We and other labs have documented a decline in mitochondrial proteins in skeletal muscle with age [Citation12,Citation13]. Our current study suggests that such a decline may involve a decrease in the translation of mtDNA-encoded proteins. Several studies have examined changes in the translation of mitochondrial proteins with age using isotopic labelling of protein translation [Citation14–16]. In Rooyackers et al., human subjects were given an infusion of labelled amino acid; analysis of mitochondria from muscle biopsies showed reduced incorporation of the label and hence reduced translation [Citation15]. Using a similar approach, Miller et al. fed mice with heavy-isotope water, then analysed the mitochondrial fraction for isotopic incorporation and found increased translation with age [Citation16]. However, these studies cannot differentiate the translation rates of specific mRNAs, since the label is delivered to the whole animal. To our knowledge our study is the first to examine the translation of specific mitochondrial mRNAs in ageing skeletal muscle. In another study using rat liver, Marcus et al. isolated mitochondria from liver samples and then incubated inner-membrane fractions to label new translation, finding a decrease in mitochondrial translation with age. Our ribosome profiling data from ageing human skeletal muscle are in line with this latter study, as we find specific decreases in the translational engagement of mtDNA-encoded components of the mitochondrial proteome, but no significant differences in the translation of mitochondrial proteins encoded by the nuclear DNA. We found no changes in the levels of mRNAs encoding protein components of the mitochondrial ribosome (Table S2), suggesting that the decrease in mitochondrial translation is not due to an overall decrease in the production of mitochondrial ribosomes.
Our results further suggest that mitochondrial biogenesis in skeletal muscle decreases with age at least in part through a decline in mitochondrial translation. Furthermore, it is possible that the observed age-associated changes in mitochondrial translation occur in other tissues, since mitochondrial dysfunction is widely observed across ageing tissues. The regulation of the mitochondrial translation machinery remains poorly understood. While cytosolic translation is regulated by translation activators and repressors, typically by binding to the 5ʹ untranslated regions (5ʹUTRs) of cytosolic mRNAs, mitochondrial mRNAs do not have 5ʹUTRs [Citation17], and it is thus unclear how their translation is modulated. Only one factor, translational activator of COX I (TACO1), has so far been identified as a regulator of mitochondrial mRNA translation, but its molecular mechanism of action is unknown [Citation18]. Progress towards elucidating how mitochondrial translation is regulated will help to identify the molecular factors responsible for the mitochondrial decline in ageing skeletal muscle.
Experimental procedures
Human subjects and biopsies
Study participants were from the Baltimore Longitudinal Study of Ageing, a longitudinal study of ageing supported by the National Institute on Ageing. Muscle biopsies were obtained from healthy individuals as previously reported[Citation19]. Briefly, after anaesthetization with 2% Lidocaine, a 6-mm Bergström biopsy was used to obtain 200 to 400 mg of tissue, which was then snap frozen in liquid nitrogen and stored until further processing. Before thawing, muscle tissue was pulverized into a powder using a liquid nitrogen cooled mortar.
Ribosome profiling, preparation of RNASeq libraries, and sequencing
To prepare ribosome profiling RNASeq libraries, a protocol based on Ingolia, et al. [Citation20], was used with some modifications. First, pulverized muscle powder was fully homogenized by trituration in a polysome buffer containing 20 mM Tris-Cl, pH 7.5; 150 mM NaCl; 5 mM MgCl2; 1 mM dithiothreitol; and 100 mg/ml cycloheximide (Sigma-Aldrich). The lysate was then cleared by centrifugation at 20,000 x g for 10 minutes at 4°C, then treated with 1% NP-40 (Sigma-Aldrich) and Turbo DNase (ThermoFisher) at 25 U/ml for 10 minutes on ice, and cleared again by centrifugation at 20,000 x g for 10 minutes at 4°C. The RNA concentration was then measured using Qubit High Sensitivity RNA kit according to the manufacturer’s protocol, and RNase I (ThermoFisher) was added to a concentration of 1 µg RNA:10 units RNase I. Digestion was performed at room temperature with agitation for 45 minutes. The digestion was stopped with 200 U of RNase inhibitor (SuperaseIn, ThermoFisher) and the digested sample was layered over 1 ml of 1 M sucrose cushion containing RNase inhibitor in a 13-mm x 51-mm ultracentrifugation tube (Beckman-Coulter), and ribosomes were then pelleted by ultracentrifugation in a TLA 100.3 rotor for 2 hours at 70,000 rpm at 4°C. The resulting pellet was then resuspended in Qiazol (Qiagen), purified, and separated on a 15% TBE-urea gel. Footprint size selection, dephosphorylation, and linker ligation were performed according to the Ingolia procedure with the following modifications: footprint sizes were selected between 28 and 34 nucleotides, the Universal miRNA Cloning Linker (NEB) was used, and T4 RNA Ligase 2, truncated K227Q (NEB) was used. For rRNA depletion, the RiboZero kit (Illumina) was used after linker ligation, using the manufacturer’s protocol, with 8 µl of rRNA removal solution, 18 µl of RNase-free water, and 4 µl RiboZero buffer, for a total volume per sample of 40 µl. The reaction was then purified using the RNA clean and concentrator-5 (Zymo), eluting into 10 µl water. Reverse transcription, circularization, and PCR amplification were performed according to Ingolia et al., except that CircLigase 2 was substituted and ATP omitted from the circularization reaction. Ribosome profiling libraries were sequenced using an Illumina HiSeq 2500, single-end, 75-bp reads, with a maximum depth per sample of between 150 million and 250 million reads.
To prepare Total RNASeq libraries, pulverized tissue was homogenized in a buffer containing proteinase K. Digestion was performed at 55°C for 10 minutes. Samples were then extracted using the Qiagen Fibrous Tissue kit. Total RNAs were then extracted using the Qiagen Fibrous Tissue kit. After quality checking on the Agilent Bioanalyzer, the rRNA was depleted using the RiboMinus kit according to the manufacturer’s protocols. cDNA was prepared using a NuGen Ovation RNA-Seq system V2 and fragmented by sonication using a Biorupter for library preparation. Libraries were prepared using the Illumina TruSeq ChIP library preparation kit. RNA-seq libraries were sequenced paired-end on an Illumina NextSeq, with a read depth between 600 million and 800 million reads.
Bioinformatics
Ribosome profiling libraries were processed by clipping the cloning linker sequence CTGTAGGCACCATCAAT. Ribosome profiling libraries are well known to be contaminated with rRNAs, which are part of the ribosome itself, not translated sequences. Furthermore, other noncoding RNAs, specifically tRNAs, snRNAs, snoRNAs, are also bound to the ribosome itself and should not be considered translated sequences [Citation21]. Therefore, the ribosome profiling reads were first aligned to a database of known human rRNAs, tRNAs, snRNAs and snoRNAs and any aligning reads were filtered out. The average rate of contamination across all libraries is shown in . The filtered reads from the ribosome profiling libraries (RiboSeq) as well as the Total RNA (RNASeq) reads were aligned to the Ensembl human transcriptome (Homo_sapiens.GRCh38.cdna) using Salmon [Citation22]. Finally, the translational efficiency of each transcript was determined by taking the ratio of the ribosome profiling reads to the Total RNA reads for each transcript, and the two age groups were compared using RiboRex, with transcripts [Citation23]. Transcripts were included in the analysis only if they were detected in all five samples and above a minimum TPM of 0.001 in RiboSeq. Upstream open reading frames and untranslated region lengths were determined using Ensembl Human (GRCh38.p13) genome annotations. For mitoribosome expression analysis, results of Salmon alignment were analysed for differential expression with DESeq2 [Citation24,Citation25].
Author contributions
R. Tharakan, M. Gorospe, and L. Ferrucci conceived and designed the project. R. Tharakan and Y. Piao performed ribosome profiling experiments. R. Tharakan, C. Ubaida-Mohien, and L. Ferrucci analyzed data. All authors contributed to writing of the manuscript.
Disclosure of potential conflict of interest
No potential conflict of interest was reported by the authors.
Supplemental Material
Download Zip (225 KB)Acknowledgments
This work was supported in its entirety by the National Institute on Aging Intramural Research Program, NIH. We would like to acknowledge the Baltimore Longitudinal Study of Aging subjects and team for the muscle biopsy samples. We would also like to acknowledge Nicholas Ingolia and William Wood for technical advice and assistance.
Data availability statement
Raw RNASeq datasets from all samples, both RiboSeq and TotalRNASeq, are openly available in NCBI GEO at www.ncbi.nlm.nih.gov/geo with accession number GSE152558.25
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Kaeberlein M, Kennedy BK. Ageing: A midlife longevity drug? Nature. 2009;460(7253):331–332.
- Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493(7432):338–345.
- Hansen M, Taubert S, Crawford D, et al. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6(1):95–110.
- Hamilton B, Dong Y, Shindo M, et al. A systematic RNAi screen for longevity genes in C. elegans. Genes Dev. 2005;19(13):1544–1555.
- Syntichaki P, Troulinaki K, Tavernarakis N. eIF4E function in somatic cells modulates ageing in Caenorhabditis elegans. Nature. 2007;445(7130):922–926.
- Solis GM, Kardakaris R, Valentine ER, et al. Translation attenuation by minocycline enhances longevity and proteostasis in old post-stress-responsive organisms. Elife. 2018;7. DOI:10.7554/eLife.40314.
- Demontis F, Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143(5):813–825.
- Welle S, Thornton C, Jozefowicz R, et al. Myofibrillar protein synthesis in young and old men. Am J Physiol. 1993;264(5 Pt 1):E693–698.
- Taanman JW. The mitochondrial genome: structure, transcription, translation and replication. Biochim Biophys Acta. 1999;1410(2):103–123.
- Weingarten-Gabbay S, Elias-Kirma S, Nir R, et al. Comparative genetics. Systematic discovery of cap-independent translation sequences in human and viral genomes. Science. 2016;351(6270):6270.
- Asnani M, Pestova TV, Hellen CU. PCBP2 enables the cadicivirus IRES to exploit the function of a conserved GRNA tetraloop to enhance ribosomal initiation complex formation. Nucleic Acids Res. 2016;44(20):9902–9917.
- Ubaida-Mohien C, Lyashkov A, Gonzalez-Freire M, et al. Discovery proteomics in aging human skeletal muscle finds change in spliceosome, immunity, proteostasis and mitochondria. Elife. 2019;8. DOI:10.7554/eLife.49874.
- Peterson CM, Johannsen DL, Ravussin E. Skeletal muscle mitochondria and aging: a review. J Aging Res. 2012;2012:194821.
- Marcus DL, Ibrahim NG, Freedman ML. Age-related decline in the biosynthesis of mitochondrial inner membrane proteins. Exp Gerontol. 1982;17(5):333–341.
- Rooyackers OE, Adey DB, Ades PA, et al. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A. 1996;93(26):15364–15369.
- Miller BF, Robinson MM, Bruss MD, et al. A comprehensive assessment of mitochondrial protein synthesis and cellular proliferation with age and caloric restriction. Aging Cell. 2012;11(1):150–161.
- Temperley RJ, Wydro M, Lightowlers RN, et al. Human mitochondrial mRNAs–like members of all families, similar but different. Biochim Biophys Acta. 2010;1797(6–7):1081–1085.
- Weraarpachai W, Antonicka H, Sasarman F, et al. Mutation in TACO1, encoding a translational activator of COX I, results in cytochrome c oxidase deficiency and late-onset Leigh syndrome. Nat Genet. 2009;41(7):833–837.
- Gonzalez-Freire M, Scalzo P, D’Agostino J, et al. Skeletal muscle ex vivo mitochondrial respiration parallels decline in vivo oxidative capacity, cardiorespiratory fitness, and muscle strength: the Baltimore Longitudinal Study of Aging. Aging Cell. 2018;17(2):e12725.
- Ingolia NT, Brar GA, Rouskin S, et al. The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nat Protoc. 2010;1797(6–7):1534–1550.
- McGlincy NJ, Ingolia NT. Transcriptome-wide measurement of translation by ribosome profiling. Methods. 2017;126:112–129.
- Patro R, Duggal G, Love MI, et al. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods. 2017;14(4):417–419.
- Li W, Wang W, Uren PJ, et al. Riborex: fast and flexible identification of differential translation from Ribo-seq data. Bioinformatics. 2017;33(11):1735–1737.
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550.
- Ravi Tharakan CU-M, Piao Y, Gorospe M, et al. Ribosome profiling analysis of aging human skeletal muscle. NCBI Gene Expression Omnibus. 2020.
