ABSTRACT
5-methylcytosine (m5C) is identified as an abundant and conserved modification in various RNAs, including tRNAs, mRNAs, rRNAs, and other non-coding RNAs. The application of high-throughput sequencing and mass spectrometry allowed for the detection of m5C at a single-nucleotide resolution and at a global abundance separately; this contributes to a better understanding of m5C modification and its biological functions. m5C modification plays critical roles in diverse aspects of RNA processing, including tRNA stability, rRNA assembly, and mRNA translation. Notably, altered m5C modifications and mutated RNA m5C methyltransferases are associated with diverse pathological processes, such as nervous system disorders and cancers. This review may provide new sights of molecular mechanism and functional importance of m5C modification.
1. Introduction
More than 170 types of RNA nucleotide modifications have been documented [Citation1]. Among these modifications, m5C is an abundant and conserved post-transcriptional modification [Citation2]. With the advances in RNA m5C detection techniques, including RNA bisulphite sequencing [Citation3], immunoprecipitation-based detection methods, liquid chromatography-tandem mass spectrometry [Citation4], and third-generation sequencing [Citation5], more than 10,000 potential m5C modification sites were detected within the whole human transcriptome [Citation6]. A great number of researches have been geared towards exploring m5C modification and its distribution, regulation, and functions.
The existence of m5C was found in diverse RNA species from various organisms, including plants, animals, and microorganisms [Citation1]. tRNAs are the most enriched substrate of m5C modification and they have been most widely explored [Citation7]. The modified regions in tRNA are often at the junction of the variable loop and the T stem-loop. m5C modifications in rRNAs are mainly located in 18S rRNAs and 25S rRNAs [Citation8]. It remains less clear whether there exist m5C modifications in mRNAs and whether m5C is functional in mRNAs [Citation9]. Detected m5C sites in mRNAs are mainly located within 3ʹ untranslated region (3ʹUTR) or near translation start sites, affecting mRNA translation efficiency [Citation2]. Transcriptome-wide detection technique also uncovered m5C in 5ʹ untranslated region (5ʹUTR) and coding regions, with functions not completely defined [Citation2]. In addition, m5C modifications also occur in long non-coding RNA [Citation10], circular RNA [Citation11], small nuclear RNAs and small nucleolar RNAs [Citation12].
RNA m5C modifications are regulated by a series of proteins defined as ‘writers’, ‘readers’ and ‘erasers’. The ‘writers’ refer to RNA m5C methyltransferases (RCMTs), which consist of the NOL1/NOP2/SUN domain (NSUN) family of proteins (NSUN1 to 7 in humans) and the DNA methyltransferase (DNMT) homologue DNMT2 [Citation13,Citation14]. As ‘erasers’, ten-eleven translocation family proteins (TETs) can oxidize m5C into cytosine-5-hydroxymethylation (hm5C) in RNA [Citation15], especially in mRNA [Citation16]. At present, only two ‘readers’ have been identified. One is YBX1, recognizing m5C-modified mRNA and mediating mRNA stability [Citation17], and the other is ALYREF, recognizing methylated cytosines in GC-rich regions of mRNA and mediating mRNA nuclear export [Citation18,Citation19].
The m5C modification is crucial for several biological processes, including tRNA stability [Citation20], rRNA assembly [Citation21] and functionally stress response [Citation22,Citation23] and embryonic and organic development [Citation24]. Besides, altered m5C modifications or mutated RNA m5C methyltransferases are linked to a few pathological processes, including nervous system disorders [Citation25] and cancers [Citation26].
In this review, we outline the current detection methods of m5C in RNA, explore their advantages and disadvantages, and review the m5C distribution pattern and advances in RNA m5C methyltransferases. Additionally, we evaluate the physiological roles and pathological implications related to m5C modification.
2. Detection methods of m5C modification
2.1 RNA bisulphite sequencing (RNA-BisSeq)
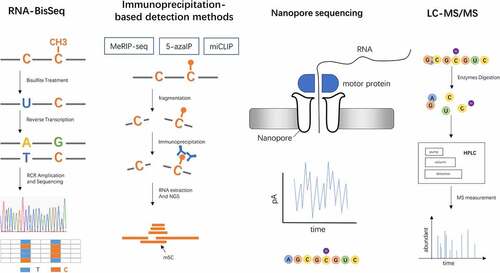
Description: Bisulphite sequencing is originally applied to detect 5mC in DNA. Currently, it has become the most used technique for sequencing RNA m5C. Bisulphite treatment converts unmethylated cytosine to uracil in single-strand RNA, whereby methylated cytosines remain unchanged. Coupling with high throughput sequencing, it maps m5C in RNA at a single-nucleotide resolution. This technique has been successfully applied to provide a global view of m5C in Drosophila tRNAs [Citation27], rRNAs, and human RNA [Citation2].
Advantages: RNA-BisSeq can provide a transcriptome-wide landscape of m5C profile with high specificity at a single-nucleotide resolution [Citation28]. Also, its protocol is relatively straightforward and mature.
Disadvantages: The efficiency of bisulphite conversion is affected by many factors including the structure of RNA, the experiment temperature, pH, and so on. RNA species of which m5C positions are well known, such as tRNAAsp(GTC) or 28S rRNA, can be used to determine the efficiency. Other cytosine modifications, such as hm5C, are also detectable leading to false-positive results. Recently reported alternative methods, such as oxBS-seq and TAB-seq, can distinguish hm5C [Citation29,Citation30]. Besides, the presence of random unconverted cytosine in the datasets would influence the analysis results. Moreover, its application requires a high amount of RNA molecules because of bisulphite treatment-induced degradation [Citation2].
2.2. Immunoprecipitation-based detection methods
2.2.1. Methylated RNA immunoprecipitation followed by sequencing (MeRIP-seq)
Description: This method utilizes RNA m5C antibodies to pull down m5C-modified RNA, available for mapping m5C sites in low-abundance RNAs. Combined with high throughput sequencing, it provides a transcriptome-wide view of the m5C profile, but not at a single-nucleotide resolution [Citation31]. Additionally, 5-methylcytosine binding domain pulldown followed by sequencing (MBD-seq) has also been applied to sequence m5C in RNA. MBD-seq captures m5C-modified RNA fragments by a protein with high affinity [Citation32].
Advantages: It avoids the interference of other RNA modifications, and provides a transcriptome-wide view of the m5C profile even in low-abundance RNAs.
Disadvantages: The specificity of this method highly relies on anti-m5C antibodies. The specificity of anti-m5C antibodies is affected by various factors. Thus, it is important to include unmodified and modified control RNA to determine the specificity of the antibody in the pulldown.
2.2.2. 5-azacytidine-mediated RNA immunoprecipitation(5-azaIP)
Description: 5-azacytidine is a cytidine analog, which is randomly incorporated into a nascent RNA transcript. At methylation sites, 5-azacytidine binds to RNA m5C methylases forming a covalent adduct, which is immunoprecipitated by enzyme-specific or tag antibodies. When RNA is released from the RNA-methylase complex, 5-aza-C ring is hydrolysed, which is read as G during sequencing [Citation33]. Therefore, it provides a transcriptome-wide landscape of m5C at a single-nucleotide resolution. This method has been adopted to reveal specific m5C sites of DNMT2 and NSUN2 in tRNA and non-coding RNA [Citation33].
Advantages: It reduces the background of non-specific RNAs due to stable covalent binding. Most importantly, it identifies enzyme-specific methylation sites at a single-nucleotide resolution.
Disadvantages: 5-azacytidine is highly toxic for cells, because it can be incorporated into DNA and RNA, altering the normal transcription. There is also concern about the incorporation efficiency of 5-azacytidine.
2.2.3. Methylation-individual nucleotide resolution crosslinking immunoprecipitation (miCLIP)
Description: MiCLIP utilizes mutated RNA m5C methyltransferases, which contain a point mutation in conserved cysteines within its catalytic domain, to effectively form irreversible enzyme-RNA catalytic intermediates. These cross-linked covalent adducts are subsequently immunoprecipitated by enzyme-specific or tag antibodies. With RNA release and high-throughput sequencing, we can identify enzyme-specific m5C sites at a single-nucleotide resolution [Citation34–36].
Advantages: It provides the same results as 5-azaIP, meanwhile avoiding 5-azacytidine toxicity.
Disadvantages: Relying on mutated enzymes, this approach requires a large amount of time and money [Citation36].
2.3. Third generation sequencing
Description: Third-generation sequencing, including SMRT sequencing and nanopore direct RNA sequencing (DRS), can also be used for single-molecule sequencing and direct RNA modification location analysis. SMRT sequencing differentiates distinct RNA modifications by monitoring HIV reverse transcriptase in real time to distinguish the different dynamic signals of normal and modified bases on the template. Nanopore DRS distinguish distinct modifications by monitoring current changes when RNA molecular traverses through nanopores [Citation37,Citation38].
Advantages: Third-generation sequencing can directly detect RNA sequences and their modifications [Citation5].
Disadvantages: This method is more costly and has a higher error rate.
2.4. Liquid chromatography-tandem mass spectrometry (LC-MS/MS)
Description: LC-MS/MS is a sensitive and quantitative method for detecting RNA modifications, m5C included. It provides a global abundance of m5C modification in samples, without specific modification sites [Citation19,Citation39,Citation40]. This method has successfully been employed to evaluate the global RNA modification extent in rRNAs [Citation6], tRNAs [Citation41], coding RNAs and non-coding RNAs [Citation42].
Advantages: This method provides a global abundance of m5C modification. Also, it can measure other RNA modifications meanwhile [Citation43,Citation44].
Disadvantages: The detection resolution of this method cannot reach a single-nucleotide level () ().
Table 1. Detection methods of RNA m5C
Figure 1. Detection methods of RNA m5C. RNA-BisSeq relied on bisulphite conversion and high-throughput sequencing. Immunoprecipitation-based detection methods are based on specific antibodies targeting m5C or enzymes, which include MeRIP-seq, 5-azaIP and miCLIP. Third generation sequencing directly detects RNA sequences and modifications. LC-MS/MS combines high pressure liquid chromatography (HPLC) separation and mass spectrometry (MS) measurement, providing a global abundance of RNA m5C modification
3. Distribution and regulation of m5C modification
m5C is introduced by RNA m5C methyltransferases, which include NSUN1 to NSUN7 and DNMT2, which are all evolutionarily conserved from archaea to eukaryotes [Citation14]. Their substrates are gradually identified, but not completely (). The NSUN family all include an S-adenosyl methionine binding-domain and two conserved catalytic cysteines in the active site [Citation45]. DNMT2 possesses a catalytic cysteine in the active site, similar with DNA methyltransferases [Citation46–48].
Table 2. RNA m5C methyltransferases and their substrates
TETs are conventionally responsible for DNA demethylation, oxidizing 5-methyl-2ʹ-deoxycytidine (5mdC) to 5-hydroxymethyl2ʹ-deoxycytidine (5hmdC), and then to 5-formyl-2ʹ-deoxycytidine (5fdC), and eventually to 5-carboxyl-2ʹ-deoxycytidine (5cadC) [Citation29,Citation49]. Recent studies have confirmed that TETs participated in the oxidation of RNA m5C into hm5C in vitro and in vivo. However, their oxidative activity in RNA is less effective than that in DNA. About 0.02% of m5C is modified to hm5C in different types of tissues, including brain, lung, and so on, and cellular RNA samples, including 293 T cells, embryonic cells and tumour cells [Citation15]. The extent of hm5C was found to be much lower than that of m5C in mammals cellular mRNAs [Citation50]. Tet1 could oxidize 5-formylcytosine (f5C) to 5-carboxycytidine (ca5C) in vitro [Citation51]. Delatte et al. provided the distribution and function of hm5C in Drosophila. They found that hm5C was preferentially present in polyadenylated RNAs, deposited by TETs [Citation16]. Functionally, TETs-mediated m5C oxidization participates in numerous physiological processes. Tet2 can increase infection-induced myelopoiesis by oxidizing m5C into hm5C in Socs3 mRNA and repressing Socs3 expression [Citation50]. Huang et al. found significantly lower RNA hm5C in human colorectal carcinoma and hepatocellular carcinoma tissues compared to tumour-adjacent normal tissues [Citation49]. This may indicate that TETs-mediated m5C oxidation may contribute to tumorigenesis.
At present, only two ‘readers’ have been discovered. ALYREF recognizes m5C modified mRNAs and mediates their nuclear-cytoplasmic shuttling [Citation18,Citation19]. YBX1 recognizes m5C modified mRNAs and mediates their stabilization [Citation17,Citation52].
3.1. m5C in tRNA
The modification m5C is highly abundant in tRNAs. Three m5C methyltransferases, DNMT2, NSUN2, and NSUN6, have been revealed to methylate cytoplasmic tRNAs [Citation53], whereas three m5C methyltransferases, NSUN2, NSUN3, and NSUN4, have been reported to modify mitochondrial tRNAs [Citation9,Citation36,Citation54].
tRNAAsp, as the most common substrate, is methylated by DNMT2 at cytosine 38 at the anticodon loop in mouse, Arabidopsis thaliana, Drosophila melanogaster [Citation7], and Dictyostelium discoideum [Citation55]. In addition to tRNAAsp(GTC), tRNAVal(AAC) and tRNAGly(GCC) are methylated by DNMT2 in Drosophila [Citation56]. Pmt1, a DNMT2 homolog in Schizosaccharomyces pombe, exerts in vitro and in vivo methylation activities on cytosine 38 of tRNAAsp and tRNAGlu [Citation57].
Substrates methylated by NSUN2 include cytosine 34 (pre-tRNALeu(CAA)), cytosine 40 (tRNAGly(GCC)), cytosines 48 and 49 (tRNAAsp(GTC), tRNAVal(AAC), and tRNAGly(GCC)), and cytosine 50 (tRNAGly(GCC)) in mice [Citation58]. In mammals, the methylated cytosines are located at positions 34, 38, 48, 49, 50, and 72 of tRNAs, and advances in detection techniques enable detection of new sites 19, 27, 40, 55, 61, 66, 70, 73, and 75 [Citation59]. Homologues of NSUN2 include Trm4p in Saccharomyces cerevisiae [Citation60], Trm4 in Saccharomyces cerevisiae, and Trm4a/Trm4b in fission yeast Schizosaccharomyces pombe [Citation61]. Besides cytoplasmic tRNAs, mitochondrial tRNAs are also substrates of NSUN2. Shinoda et al. revealed the localization of NSUN2 within mitochondria through structured illumination microscopy and revealed m5C sites 48–50 in mouse and human mitochondrial tRNAs samples. Moreover, in NSUN2 knockout (KO) mice and human cells, m5C was absent in mt-tRNAs [Citation62,Citation63].
Another research uncovered that NSUN6 methylates tRNACys(GCA) and tRNAThr(CGU/GGU/UGU) at C72 within the 3′ end of the tRNA acceptor stem in hyperthermophilic archaea [Citation64].
Haute et al. discovered for the first time that NSUN3 is an RNA methyltransferase, which methylates mitochondrial tRNAMet at cytosine 34 in the anticodon arm. NSUN3-mediated m5C modification initiates f5C biogenesis, further affecting mitochondrial proteins translation [Citation36]. Moreover, NSUN4 methylates mt-tRNAMet(CAU) at cytosine 34 [Citation9]. Shinoda et al. mapped mitochondrial tRNA m5C modifications in HEK293T cells. The methylated cytosines are located at position 48 of mt-tRNAsPhe, mt-tRNAsHis, mt-tRNAsLeu(UUR), mt-tRNAsSer(AGY), and mt-tRNAsTyr, at position 49 of mt-tRNAsGlu and mt-tRNAsSer(AGY), and at position 50 of mt-tRNAsSer(AGY) [Citation62].
3.2. m5C in rRNA
Nuclear, cytoplasmic, and mitochondrial rRNAs are all characterized by m5C modification, which mainly influences protein synthesis. In nucleolus, 25S rRNA is methylated in domain V by NSUN1(p120) and NSUN5 [Citation65]. In cytoplasm, 25S rRNA is methylated by NSUN1 [Citation66]. And in mitochondria, 12S rRNA, and 18S rRNA are methylated by NSUN4 [Citation9].
NSUN4 methylates 12S rRNA at C911 in the small ribosomal subunit and methylates 18S rRNA at C628, 631 and 632 in mitochondria [Citation67]. In yeast, Sharma et al. identified two putative methyltransferases, Rcm1 and Nop2, methylate 25S rRNA at C2278 and 2870 [Citation68]. Human proliferation-associated nucleolar antigen p120, the homologue of yeast Nop2, also displays an RNA m5C methyltransferase activity. It is also called NSUN1 in humans [Citation66]. It can restore m5C formation in domain V in endogenous yeast 25S rRNA when expressed in a Nop2-deficient yeast strain [Citation6]. NSUN5, a homologue of Rcm1, methylates 28S rRNA at C3782 in human and at C3438 in mice [Citation65].
3.3. m5C in mRNA
Transcriptome-wide mapping of m5C has been conducted in mammals, plants, bacteria, archaea, and yeast [Citation31,Citation69–71]. m5C modification in mRNA is rare or even undetectable [Citation9,Citation72,Citation73]. Advances in m5C detection techniques have promoted more extensive analysis. The present identified mRNA methyltransferase is NSUN2 [Citation2].
Through RNA-BisSeq, Squires et al. discovered 10,275 methylation sites in mRNAs and other non-coding RNAs in Hela cells, and these sites were enriched in untranslated regions or near Argonaute binding regions within mRNAs [Citation2]. Yang et al. also revealed that m5C modification was enriched in regions immediately downstream of translation initiation sites in Hela cells [Citation19]. Further, Amort et al. demonstrated the landscape of RNA m5C modifications in mouse embryonic stem cells (ESC) and brain tissues, in total poly(A) RNA and nuclear poly(A) RNA. They revealed that the m5C modification extent and distribution pattern were different in ESC and brain tissue, with a higher extent in ESC. Besides, m5C sites were mainly located within introns, CDS and 3ʹUTR in nuclear poly(A) RNA, whereas in total poly(A) RNA, they are mainly located within 3ʹUTR, CDS and around translational start sites [Citation74].
3.4. m5C in other non-coding RNAs
m5C modification also occurs in non-coding RNA [Citation2]. The main methyltransferase is NSUN2.
NSUN2 methylates non-coding vault RNA VTRNA1.1 at cytosine 69, determining the transition of VTRNA1.1 into small-vault RNAs, involved in epidermal differentiation. VTRNA1.3 was also reported as another substrate of NSUN2 [Citation75]. Moreover, microRNAs can be methylated by NSUN2, such as micro126b [Citation76]. lncRNA is also modulated by NSUN2. High-throughput sequencing has mapped the landscape of lncRNA regulated by NSUN2, whereas functional analysis has revealed some pathways in tumorigenesis. However, the mechanism via which NSUN2 modulates lncRNA and the specific modification sites are unknown [Citation77]. Amort et al. demonstrated for the first time that the two well-studied regulatory lncRNAs HOTAIR and XIST have m5C modifications and the methylated position of HOTAIR is C1683 in fragment H1 and the methylated region of XIST is A-region [Citation78].
4. Biological Functions of m5C modification
4.1. m5C Modification and RNA processing
4.1.1. tRNA metabolism
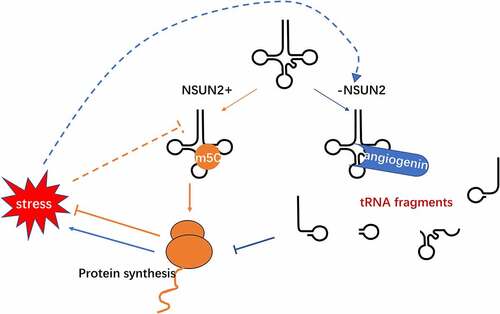
The functional characterization of DNMT2 or NSUN2‑mediated tRNA methylation in tRNA metabolism remains unclear. However, present studies have revealed that tRNA methylation can protect tRNAs against endonucleolytic cleavage, maintaining tRNA stability [Citation20]. Under stress stimuli, angiogenin is activated to cleave tRNA within anticodon loops into 5ʹ-tRNA fragments, inhibiting protein translation initiation and inducing stress granules assembly to activate stress response programme [Citation79]. Angiogenin has a higher affinity to unmethylated tRNAs, generating a larger number of angiogenin-induced tRNA fragments [Citation22,Citation23]. The released tRNA fragments repress cap-dependent translation by displacing translation initiation and elongation factors from mRNAs or by altering efficient transpeptidation, therefore leading to activated stress responses [Citation20]. The released tRNA fragments can mediate siRNA pathway downregulation, especially during the heat-shock response [Citation21]. DNMT2-mediated methylation can protect tRNAs from ribonuclease cleavage under stress conditions in Drosophila [Citation56]. Notably, global protein synthesis is reduced in the absence of NSUN2 in vitro and in vivo [Citation80].
Eukaryotic genome stability is associated with repeat regions and transposable elements. It was found that mutation of two RNA m5C methyltransferases, DNMT2 and NSUN2, reduced tRNA stability, and altered the expression of the mobile elements, mediating genome integrity in Drosophila [Citation81].
4.1.2. rRNA metabolism
The methylated cytosines in rRNA are fundamental for ribosome biogenesis, thereby influencing protein synthesis. Loss of NSUN4-mediated mitochondrial 12S rRNA methylation alters ribosome subunits assembly [Citation67]. Rcm1 and Nop2 function as above in yeast [Citation68]. NSUN5 deficiency induced decreased proliferation of mammalian cells and reduced body weight in mice, all of which were attributed to a reduction of total protein synthesis by altered ribosomes [Citation65].
4.1.3. mRNA metabolism
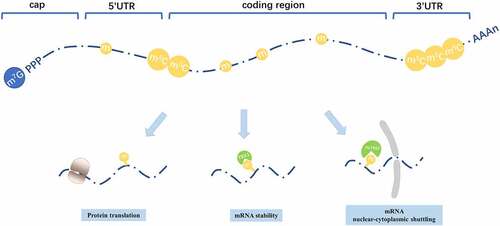
m5C modifications also participate in mRNA metabolism, including its translation, nuclear-cytoplasmic shuttling, and stability [Citation82]. However, its specific mechanism remains elusive.
m5C modifications can directly regulate mRNA translation and indirectly regulate mRNA turnover. NSUN2 methylates IL17a mRNA at cytosine 466 in the coding region, promoting IL17a translation without effects on mRNA half-life [Citation83]. NSUN2 methylates CDK1 at cytosine 1733 in the 3ʹUTR region, upregulating CDK1 translation via elevated translation initiation [Citation84]. NSUN2 methylates p27 at cytosine 64 in the 5ʹ UTR region, repressing p27 translation [Citation85]. NSUN2 methylates SHC mRNA at multiple sites across 5ʹUTR, coding region and 3ʹUTR, elevating SHC expression without effects on mRNA turnover [Citation86]. NSUN2 potentially regulates p53 expression through methylating miR-125b and mRNA 3ʹUTR region [Citation76]. m5C modification can also interact with other RNA modifications, mediating mRNA turnover and subsequent translation. Combined m5C modification by NSUN2 and m6A modification by METTL3/METT14 at the 3ʹUTR of p21 mRNA enhances p21 expression in a model of oxidative stress-induced cellular senescence. Particularly, m6A modification facilitates m5C modification, indicating that there exists an interaction between m6A and m5C [Citation39].
As to mRNA nuclear-cytoplasmic shuttling, an important discovery is an m5C reader ALYREF, which functions as an mRNA export adaptor. ALYREF-mediated nuclear-cytoplasmic shuttling of mRNA is dependent on m5C modification by NSUN2 [Citation18,Citation19]. m5C is also essential for mRNA stability. YBX1, an m5C reader, recruits ELAVL1 to stabilize HDGF mRNA, therefore promoting the pathogenesis of bladder cancer [Citation17]. In addition, m5C modifications stabilize mRNA during the maternal-to-zygotic transition, indicating m5C modifications in mRNA may participate in embryonic development. The ‘readers’ include Ybx1 and Pabpc1a in this process ()[Citation52].
Figure 2. Distribution patterns and roles of m5C in mRNA. m5C is distributed among all the regions in mRNA, mainly enriched within 3ʹUTR and around translation start sites. m5C plays various roles in mRNA metabolism, including mRNA stability and mRNA nuclear-cytoplasmic shuttling and mRNA translation
4.2. m5C Modification and stress response
RCMTs, tRNA stability, and stress response interact with one another. Notably, DNMT2-mediated methylation can protect tRNAs against ribonuclease cleavage under stress conditions in Drosophila [Citation56]. The accumulation of tRNA-derived small RNA fragments was found to activate stress response because of loss of m5C [Citation22,Citation23]. Consequently, exposure to oxidative stress lowered NSUN2 expression, reduced methylation at specific tRNA sites and resulted in lower protein synthesis. Based on these facts, NSUN2 has been regarded as a sensor of external stimuli () [Citation80]. Besides, Shanmugam et al. found that stress inhibited DNMT2 expression. The reduced DNMT2 decreased C38 methylation in tRNAAsp and diminished the translation of Asp-rich proteins. Besides, stress potentially aggravated the effects of RCMTs deletion [Citation87]. In addition to animals, m5C modifications in RNA enhance heat stress adaptation in rice. Rice with mutated osnsun2, an RNA m5C methyltransferase in rice, displayed heat-stress hypersensitivity, and impaired repair reactions [Citation88].
Figure 3. m5C in tRNA metabolism and its association with stress. Loss of NSUN2-mediated m5C in tRNA increases the angiogenin-mediated tRNAs cleavage, results in an accumulation of tRNA-derived small RNA fragments, reduces protein translation rates and thus activates stress response
NSUN5-mediated rRNA methylation has been reported to be associated with stress resistance. Lower NSUN5 could elevate stress resistance in yeast, worms, and flies along with increased lifespan. Mechanically, loss of Rcm1 disrupted the structural ribosome conformation and translational fidelity, recruited a distinct group of oxidative stress-responsive mRNAs into polysomes and increased the lifespan and stress resistance [Citation89].
4.3. m5C modification and organ development and regeneration
Cytosine methylation in RNA is fundamental for embryonic and organic development at the post-transcriptional level. Double mutants of NSUN2 and DNMT2 showed a synthetic lethal interaction in mice, which is due to impaired protein synthesis [Citation58]. DNMT2 promotes liver, brain, and retina development in zebrafish based on its methylation in cytoplasmatic tRNAs [Citation24]. DNMT2 also plays an important role in haematopoiesis through its methylation on tRNAs. Newborn DNMT2-deficient mice demonstrated delayed endochondral ossification with reduced haematopoietic stem and progenitor cell population. RNA bisulphite sequencing revealed that DNMT2 methylates tRNAAsp/Gly/Val at C38 in the bone marrow and modulates specific protein synthesis. Furthermore, DNMT2-mediated methylation on tRNAAsp has a role in the discrimination between Asp and Glu near-cognate codons, contributing to accurate polypeptide synthesis. In DNMT2 deficient cells, the lack of tRNAAsp methylation reduces the capacity of tRNAs to properly discriminate between Asp and Glu codons, leading to decreased translational fidelity, the production of unfolded proteins, and an aberrant phenotype cell population [Citation90]. DNMT2 also participates in intergenerational transmission of paternally acquired metabolic disorders through methylating sperm small non-coding RNAs in mice [Citation91]. Mutations in NSUN2 cause microcephaly and other neurological abnormalities in mice and human. In neurological abnormalities, loss of m5C results in an accumulation of 5′ tRNA fragments, which reduces protein translation rates, activates stress pathways leading to reduced cell size and increases apoptosis of cortical, hippocampal, and striatal neurons [Citation22,Citation23]. NSUN2-mediated tRNA methylation also participates in male germ cell differentiation [Citation92] and balances stem cell self-renewal and differentiation in skin [Citation93]. NSUN3-mediated mitochondrial tRNAMet methylation is essential for oxidative phosphorylation in mitochondria. However, loss of NSUN3 was revealed to influence ESC differentiation rather than its proliferation, impairing neuroectoderm developments [Citation94]. In addition to animals, m5C modification in RNA is essential for plant growth and development. David et al. discovered more than 1,000 m5C modification sites in RNA of Arabidopsis thaliana [Citation71]. Deletion of TRM4B, homologue of mammal NSUN2, resulted in loss of m5C modification and impaired root development in Arabidopsis thaliana. Besides, Yang et al. found enriched m5C modifications in mobile mRNAs in Arabidopsis thaliana, especially mobile mRNA TCTP1, facilitated their transport in distinct root cells and affected root growth [Citation95].
4.4. m5C modification and cell proliferation and senescence
Cell proliferation and senescence are regulated by several cell cycle factors. NSUN2 was reported as a downstream of MYC. Its expression was enriched in the S phase, essential for mitosis assembly [Citation96]. NSUN2 can also regulate cell proliferation and senescence through mediating p16 [Citation97], p27, p53, SHC, CDK1 mRNA methylation, and subsequent translation. NSUN2 knockdown increased p27 expression, meanwhile reduced CDK1 expression, and thus repressed cell proliferation and accelerated replicative senescence in human diploid fibroblasts [Citation85]. NSUN2-mediated SHC mRNA methylation elevated its expression and promoted human vascular endothelial cells senescence during oxidative stress or high glucose, probably by increasing ROS levels and activating p38 MAPK [Citation86]. RNA m5C modifications also mediated ageing. In human, mouse, and yeast ageing models, NSUN5 was less abundant in aged donors. Specifically, Rcm1, yeast homologue of NSUN5, mediated 28S/25S rRNA methylation, loss of which induced structural changes and altered translating ribosomes. Rcm1-deficient yeast cells showed a decrease in translational fidelity and an increase in recruitment of stress-specific mRNAs to translating ribosomes, which might explain the increased stress resistance and lifespan [Citation89].
4.5. m5C modification and nervous system disorders
Mutated NSUN2 was found in some nervous system disorders and intellectual disability, including autosomal recessive intellectual disabilities [Citation25]. Blanco et al. revealed that loss of NSUN2 induced smaller cell size in vitro and caused neuro-developmental defects in vivo due to increased tRNA fragments and higher-sensitivity to stress stimuli [Citation22]. In Dubowitz syndrome, there exists a homozygous splice mutation in NSUN2, which results in decreased m5C in tRNAAsp at C47 and C48 [Citation98].
NSUN5 is deleted in about 95% of patients with Williams-Beuren syndrome (WBS), which is characterized by cognitive disorder and hypotrophic corpus callosum. NSUN5 knockout (NSUN5-KO) mice showed deficits in spatial cognition, impaired cerebral cortex developments [Citation17], and agenesis of corpus callosum [Citation99]. Janin et al. reported DNA methylation-associated epigenetic silencing of NSUN5 as a marker of glioma with long-term survival. Loss of NSUN5 reduced methylation of 28S rRNA at C3782, resulted in impaired protein translation, and stimulated an adaptive translational program for survival against cellular stress [Citation100].
4.6. m5C modification and cancer and cancer therapies
Overexpression or higher copy number of NSUN2 is common in various human cancers, especially in oral and colorectal cancers [Citation26]. Since tRNA methylation can protect itself from rapid decay, overexpressed tRNA methyltransferases and its consistent tRNA stability are a potential mechanism for tumour development [Citation101]. NSUN2, reported as a novel downstream of MYC and named as MYC-induced SUN domain-containing protein (Misu), mediated breast cancer cell proliferation and therefore could be utilized as a potential therapy target [Citation96,Citation102]. Similar events were observed in head and neck squamous carcinoma and NSUN2 was related to patient survival [Citation103]. Overexpressed NSUN2 has also been detected in gallbladder carcinoma tissues and cells [Citation104]. Methylation of HDGF mRNA at 3ʹUTR by NSUN2 was proved to enhance its expression and promote pathogenesis in bladder cancer [Citation17]. Chen et al. provided a whole landscape of mRNA methylation in human urothelial carcinoma of the bladder and adjacent normal tissues and found hypermethylated mRNAs in cancer. Hypermethylated mRNAs were mainly enriched in cancer-related pathways [Citation17]. Elsewhere, the NSUN2lowGF-IIhigh signature increased the mortality risk in ovarian cancer [Citation19].
The cytosine analogues azacytidine and decitabine, drugs for epigenetic cancer therapy, have always been demonstrated to impede DNA methylation through covalent trapping DNMT. Moreover, azacytidines also inhibit m5C modifications in tRNAAsp at C38 potentially by inhibiting DNMT2 activity. This finding uncovered a novel mechanism of azacytidine in cancer therapy and tRNA methylation may be adopted as a biomarker of azacytidine response in patients [Citation105]. Also, there exists a correlation between T cell activation and NSUN2 mRNA level in head and neck squamous carcinoma based on TCGA database. Higher level of NSUN2 may depict positive effects in immune-checkpoint therapy [Citation106]. Moreover, NSUN2 deletion mediated lower protein translation and increased tRNAs cleavage could increase stem cell and tumour-initiating cell activity and paradoxically render them hypersensitive to stress stimuli, such as chemotherapeutic drugs therapy [Citation107]. Combined deletion of NSUN2 and METTL1, another tRNA methyltransferase, potentiated Hela cells sensitive to 5-fluorouracil (5-FU) due to decreased methylation in tRNAVal(AAC) and increased destabilization [Citation108].
5. Conclusions and perspectives
RNA modification is a new aspect of post-transcriptional gene regulation essential for RNA metabolism and participates in numerous diseases, including cancer. Although many studies have focused on RNA m5C modifications, our knowledge about it remains limited. Existing approaches have identified m5C sites in different RNA species and this modification is important for tRNA stability, mRNA translation, ribosome assembly, and other RNA metabolism processes. Altered m5C distribution and mutated RNA methyltransferases have been linked to physiological processes, including stress response, organ development, cell proliferation, senescence, and pathological processes, such as nervous system disorders and various cancers. Still, there exist many questions and challenges.
There exists a lack of consistency when mapping m5C sites. When mapping m5C sites using RNA-BisSeq, the number of putative mRNA m5C sites varies by 1,000-fold between studies [Citation69], reflecting defects of existing approaches. Also, the low amount of mRNA poses challenges. Therefore, we need a novel approach to overcome these problems with higher specificity and sensitivity. Moreover, data analysis also needs further improvement. m5C modifications are rarely found in some types of RNA including mRNA, lncRNA and so on. Thus, it is very important to develop a better data analysis method to distinguish true m5C sites form noise disturbance.
Recent studies have focused on the discovery of RNA m5C methyltransferases. YBX1 and ALYREY are the only two ‘readers’ discovered. The biological roles of m5C, such as mRNA export and tRNA metabolism, require mediation by ‘readers’. Therefore, discovering more m5C readers is essential for further elucidating its biological roles.
The biological role of m5C modification in RNA metabolism is still unclear. Recent studies only confirmed that m5C in tRNA can protect tRNA from endonucleolytic cleavage, that m5C in rRNA is essential for ribosome assembly, and that m5C in mRNA may influence mRNA stability and subsequent protein translation. However, the different functions of m5C modifications in different regions of mRNA, such as 3ʹUTR, CDS, and 5ʹUTR, are still unknown. Furthermore, RNA-BisSeq has identified thousands of m5C sites in mRNAs, whose roles remain elusive.
RNA m5C modification participates in several physiological processes and pathological diseases. However, these findings are mainly based on correlation analysis, some of which rely on in vitro researches. Therefore, more researches are required to confirm this correlation in vivo, and further mechanism is also an important part to elucidate. Besides, m5C modifications in RNA is a potential marker of drug therapies, especially nucleoside drugs. Altogether, RNA m5C modification is a potential parameter in elucidating disease development and may be utilized as a new target or marker of diseases therapies.
Acknowledgments
Author Ya-Qi Gao is responsible for collecting and arranging references, writing, and revising for this manuscript. Corresponding author Jing-Yuan Fang helps organize the structure and main contents of this manuscript. This project was supported in part by grants from the National Natural Science Foundation of China (81421001, 81830081).
Disclosure statement
The authors have declared no competing interests.
Additional information
Funding
References
- Li S, Mason CE. The pivotal regulatory landscape of RNA modifications. Annu Rev Genomics Hum Genet. 2014;15(1):127–150.
- Squires JE, Patel HR, Nousch M, et al. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 2012;40(11):5023–5033.
- Helm M, Motorin Y. Detecting RNA modifications in the epitranscriptome: predict and validate. Nat Rev Genet. 2017;18(5):275–291.
- Li S, Limbach PA. Mass spectrometry sequencing of transfer ribonucleic acids by the comparative analysis of RNA digests (CARD) approach. Analyst. 2013;138(5):1386–1394.
- Parker MT, Knop K, Sherwood AV, et al. Nanopore direct RNA sequencing maps the complexity of Arabidopsis mRNA processing and m(6)A modification. Elife. 2020;9. DOI:10.7554/eLife.49658.
- Bourgeois G, Ney M, Gaspar I, et al. Eukaryotic rRNA Modification by Yeast 5-Methylcytosine-Methyltransferases and Human Proliferation-Associated Antigen p120. PLoS One. 2015;10(7):e0133321.
- Goll MG, Kirpekar F, Maggert KA, et al. Methylation of tRNA Asp by the DNA Methyltransferase Homolog Dnmt2. Science. 2006;311(5759):395–398.
- Natchiar SK, Myasnikov AG, Kratzat H, et al. Visualization of chemical modifications in the human 80S ribosome structure. Nature. 2017;551(7681):472–477.
- Navarro IC, Tuorto F, Jordan D, et al. Translational adaptation to heat stress is mediated by RNA 5-methylcytosine in Caenorhabditis elegans. EMBO J. 2021;40(6):e105496.
- John SP, Sun J, Carlson RJ, et al. IFIT1 Exerts Opposing Regulatory Effects on the Inflammatory and Interferon Gene Programs in LPS-Activated Human Macrophages. Cell Rep. 2018;25(1):95–106 e6.
- Meng S, Zhou H, Feng Z, et al. Epigenetics in neurodevelopment: emerging Role of Circular RNA. Front Cell Neurosci. 2019;13:327.
- Stepanov G, Zhuravlev E, Shender V, et al. Nucleotide Modifications Decrease Innate Immune Response Induced by Synthetic Analogs of snRNAs and snoRNAs. Genes (Basel). 2018;9(11):531.
- Schapira M. Structural Chemistry of human RNA methyltransferases. ACS Chem Biol. 2016;11(3):575–582.
- Cheng JX, Chen L, Li Y, et al. RNA cytosine methylation and methyltransferases mediate chromatin organization and 5-azacytidine response and resistance in leukaemia. Nat Commun. 2018;9(1):1163.
- Fu L, Guerrero CR, Zhong N, et al. Tet-mediated formation of 5-hydroxymethylcytosine in RNA. J Am Chem Soc. 2014;136(33):11582–11585.
- Delatte B, Wang F, Ngoc LV, et al. RNA biochemistry. Transcriptome-wide distribution and function of RNA hydroxymethylcytosine. Science. 2016;351(6270):282–285.
- Chen X, Li A, Sun BF, et al. 5-methylcytosine promotes pathogenesis of bladder cancer through stabilizing mRNAs. Nat Cell Biol. 2019;21(8):978–990.
- Dominissini D, Rechavi G. 5-methylcytosine mediates nuclear export of mRNA. Cell Res. 2017;27(6):717–719.
- Yang X, Yang Y, Sun BF, et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res. 2017;27(5):606–625.
- Lyko F. The DNA methyltransferase family: a versatile toolkit for epigenetic regulation. Nat Rev Genet. 2018;19(2):81–92.
- Durdevic Z, Mobin MB, Hanna K, et al. The RNA methyltransferase Dnmt2 is required for efficient Dicer-2-dependent siRNA pathway activity in Drosophila. Cell Rep. 2013;4(5):931–937.
- Blanco S, Dietmann S, Flores JV, et al. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. Embo J. 2014;33(18):2020–2039.
- Flores JV, Cordero-Espinoza L, Oeztuerk-Winder F, et al. Cytosine-5 RNA methylation regulates neural stem cell differentiation and motility. Stem Cell Reports. 2017;8(1):112–124.
- Rai K, Chidester S, Zavala CV, et al. Dnmt2 functions in the cytoplasm to promote liver, brain, and retina development in zebrafish. Genes Dev. 2007;21(3):261–266.
- Khan MA, Rafiq MA, Noor A, et al. Mutation in NSUN2, which encodes an RNA methyltransferase, causes autosomal-recessive intellectual disability. Am J Hum Genet. 2012;90(5):856–863.
- Okamoto M, Hirata S, Sato S, et al. Frequent increased gene copy number and high protein expression of tRNA (cytosine-5-)-methyltransferase (NSUN2) in human cancers. DNA Cell Biol. 2012;31(5):660–671.
- Schaefer M, Pollex T, Hanna K, et al. RNA cytosine methylation analysis by bisulfite sequencing. Nucleic Acids Res. 2009;37(2):e12.
- Yang T, Low JJA, Woon ECY. A general strategy exploiting m5C duplex-remodelling effect for selective detection of RNA and DNA m5C methyltransferase activity in cells. Nucleic Acids Res. 2020;48:e5.
- Moen EL, Mariani CJ, Zullow H, et al. New themes in the biological functions of 5-methylcytosine and 5-hydroxymethylcytosine. Immunol Rev. 2015;263(1):36–49.
- Kawasaki Y, Kuroda Y, Suetake I, et al. A Novel method for the simultaneous identification of methylcytosine and hydroxymethylcytosine at a single base resolution. Nucleic Acids Res. 2017;45:e24.
- Edelheit S, Schwartz S, Mumbach MR, et al. Transcriptome-wide mapping of 5-methylcytidine RNA modifications in bacteria, archaea, and yeast reveals m5C within archaeal mRNAs. PLoS Genet. 2013;9(6):e1003602.
- Jeltsch A, Broche J, Lungu C, et al. Biotechnological applications of mbd domain proteins for DNA methylation analysis. J Mol Biol. 2019;432:1816–1823.
- Khoddami V, Cairns BR. Identification of direct targets and modified bases of RNA cytosine methyltransferases. Nat Biotechnol. 2013;31(5):458–464.
- George H, Ule J, Hussain S. Illustrating the epitranscriptome at nucleotide resolution Using methylation-iCLIP (miCLIP). Methods Mol Biol. 2017;1562:91–106.
- Hussain S, Sajini AA, Blanco S, et al. NSun2-mediated cytosine-5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell Rep. 2013;4(2):255–261.
- Van Haute L, Dietmann S, Kremer L, et al. Deficient methylation and formylation of mt-tRNA(Met) wobble cytosine in a patient carrying mutations in NSUN3. Nat Commun. 2016;7(1):12039.
- Zheng HX, Zhang XS, Sui N. Advances in the profiling of N(6)-methyladenosine (m(6)A) modifications. Biotechnol Adv. 2020;45:107656.
- Garalde DR, Snell EA, Jachimowicz D, et al. Highly parallel direct RNA sequencing on an array of nanopores. Nat Methods. 2018;15(3):201–206.
- Li Q, Li X, Tang H, et al. NSUN2-Mediated m5C Methylation and METTL3/METTL14-Mediated m6A Methylation cooperatively enhance p21 translation. J Cell Biochem. 2017;118(9):2587–2598.
- Thuring K, Schmid K, Keller P, et al. Analysis of RNA modifications by liquid chromatography-tandem mass spectrometry. Methods. 2016;107:48–56.
- Ross R, Cao X, Yu N, et al. Sequence mapping of transfer RNA chemical modifications by liquid chromatography tandem mass spectrometry. Methods. 2016;107:73–78.
- Gaston KW, Limbach PA. The identification and characterization of non-coding and coding RNAs and their modified nucleosides by mass spectrometry. RNA Biol. 2014;11(12):1568–1585.
- Zhang HY, Xiong J, Qi BL, et al. The existence of 5-hydroxymethylcytosine and 5-formylcytosine in both DNA and RNA in mammals. Chem Commun (Camb). 2016;52(4):737–740.
- Huber SM, van Delft P, Mendil L, et al. Formation and abundance of 5-hydroxymethylcytosine in RNA. Chembiochem. 2015;16(5):752–755.
- Chi L, Delgado-Olguin P. Expression of NOL1/NOP2/sun domain (Nsun) RNA methyltransferase family genes in early mouse embryogenesis. Gene Expr Patterns. 2013;13(8):319–327.
- Jurkowski TP, Meusburger M, Phalke S, et al. Human DNMT2 methylates tRNA(Asp) molecules using a DNA methyltransferase-like catalytic mechanism. Rna. 2008;14(8):1663–1670.
- Jurkowski TP, Shanmugam R, Helm M, et al. Mapping the tRNA binding site on the surface of human DNMT2 methyltransferase. Biochemistry. 2012;51(22):4438–4444.
- Schulz EC, Roth HM, Ankri S, et al. Structure analysis of Entamoeba histolytica DNMT2 (EhMeth). PLoS One. 2012;7(6):e38728.
- Huang W, Lan MD, Qi CB, et al. Formation and determination of the oxidation products of 5-methylcytosine in RNA. Chem Sci. 2016;7(8):5495–5502.
- Shen Q, Zhang Q, Shi Y, et al. Tet2 promotes pathogen infection-induced myelopoiesis through mRNA oxidation. Nature. 2018;554(7690):123–127.
- Basanta-Sanchez M, Wang R, Liu Z, et al. TET1-Mediated oxidation of 5-Formylcytosine (5fC) to 5-Carboxycytosine (5caC) in RNA. Chembiochem. 2017;18(1):72–76.
- Yang Y, Wang L, Han X, et al. RNA 5-Methylcytosine Facilitates the Maternal-to-Zygotic Transition by preventing maternal mRNA decay. Mol Cell. 2019;75(6):1188–202.e11.
- Bohnsack KE, Hobartner C, Bohnsack MT. Eukaryotic 5-methylcytosine (m(5)C) RNA Methyltransferases: mechanisms, cellular functions, and links to disease. Genes (Basel). 2019;10(2):102.
- Haag S, KE S, Ranjan N, et al. NSUN3 and ABH1 modify the wobble position of mt-tRNA Met to expand codon recognition in mitochondrial translation. EMBO J. 2016;35(19):2104–2119.
- Muller S, Windhof IM, Maximov V, et al. Target recognition, RNA methylation activity and transcriptional regulation of the Dictyostelium discoideum Dnmt2-homologue (DnmA). Nucleic Acids Res. 2013;41(18):8615–8627.
- Schaefer M, Pollex T, Hanna K, et al. RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage. Genes Dev. 2010;24(15):1590–1595.
- Becker M, Muller S, Nellen W, et al. Pmt1, a Dnmt2 homolog in Schizosaccharomyces pombe, mediates tRNA methylation in response to nutrient signaling. Nucleic Acids Res. 2012;40(22):11648–11658.
- Tuorto F, Liebers R, Musch T, et al. RNA cytosine methylation by Dnmt2 and NSun2 promotes tRNA stability and protein synthesis. Nat Struct Mol Biol. 2012;19(9):900–905.
- Auxilien S, Guerineau V, Szweykowska-Kulinska Z, et al. The human tRNA m5C methyltransferase Misu is multisite-specific. RNA Biol. 2012;9(11):1331–1338.
- Moon HJ, Redman KL. Trm4 and Nsun2 RNA:m5C Methyltransferases Form Metabolite-Dependent, Covalent Adducts with Previously Methylated RNA. Biochemistry. 2014;53(45):7132–7144.
- Muller M, Samel-Pommerencke A, Legrand C, et al. Division of labour: tRNA methylation by the NSun2 tRNA methyltransferases Trm4a and Trm4b in fission yeast. RNA Biol. 2019;16(3):249–256.
- Shinoda S, Kitagawa S, Nakagawa S, et al. Mammalian NSUN2 introduces 5-methylcytidines into mitochondrial tRNAs. Nucleic Acids Res. 2019;47(16):8734–8745.
- Van Haute L, Lee SY, McCann BJ, et al. NSUN2 introduces 5-methylcytosines in mammalian mitochondrial tRNAs. Nucleic Acids Res. 2019;47(16):8720–8733.
- Li J, Li H, Long T, et al. Archaeal NSUN6 catalyzes m5C72 modification on a wide-range of specific tRNAs. Nucleic Acids Res. 2019;47(4):2041–2055.
- Heissenberger C, Liendl L, Nagelreiter F, et al. Loss of the ribosomal RNA methyltransferase NSUN5 impairs global protein synthesis and normal growth. Nucleic Acids Res. 2019;47(22):11807–11825.
- Hussain S, Benavente SB, Nascimento E, et al. The nucleolar RNA methyltransferase Misu (NSun2) is required for mitotic spindle stability. J Cell Biol. 2009;186(1):27–40.
- Metodiev MD, Spahr H, Loguercio Polosa P, et al. NSUN4 is a dual function mitochondrial protein required for both methylation of 12S rRNA and coordination of mitoribosomal assembly. PLoS Genet. 2014;10(2):e1004110.
- Sharma S, Yang J, Watzinger P, et al. Yeast Nop2 and Rcm1 methylate C2870 and C2278 of the 25S rRNA, respectively. Nucleic Acids Res. 2013;41(19):9062–9076.
- Huang T, Chen W, Liu J, et al. Genome-wide identification of mRNA 5-methylcytosine in mammals. Nat Struct Mol Biol. 2019;26(5):380–388.
- Song J, Zhai J, Bian E, et al. Transcriptome-Wide annotation of m(5)C RNA modifications using machine learning. Front Plant Sci. 2018;9:519.
- David R, Burgess A, Parker B, et al. Transcriptome-Wide mapping of RNA 5-Methylcytosine in Arabidopsis mRNAs and noncoding RNAs. Plant Cell. 2017;29(3):445–460.
- Legrand C, Tuorto F, Hartmann M, et al. Statistically robust methylation calling for whole-transcriptome bisulfite sequencing reveals distinct methylation patterns for mouse RNAs. Genome Res. 2017;27(9):1589–1596.
- Khoddami V, Yerra A, Mosbruger TL, et al. Transcriptome-wide profiling of multiple RNA modifications simultaneously at single-base resolution. Proc Natl Acad Sci U S A. 2019;116(14):6784–6789.
- Amort T, Rieder D, Wille A, et al. Distinct 5-methylcytosine profiles in poly(A) RNA from mouse embryonic stem cells and brain. Genome Biol. 2017;18(1):1.
- Sajini AA, Choudhury NR, Wagner RE, et al. Loss of 5-methylcytosine alters the biogenesis of vault-derived small RNAs to coordinate epidermal differentiation. Nat Commun. 2019;10(1):2550.
- Yuan S, Tang H, Xing J, et al. Methylation by NSun2 represses the levels and function of microRNA 125b. Mol Cell Biol. 2014;34(19):3630–3641.
- Sun Z, Xue S, Xu H, et al. Expression profiles of long noncoding RNAs associated with the NSUN2 gene in HepG2 cells. Mol Med Rep. 2019;19:2999–3008.
- Amort T, Souliere MF, Wille A, et al. Long non-coding RNAs as targets for cytosine methylation. RNA Biol. 2013;10(6):1003–1008.
- Ivanov P, Emara MM, Villen J, et al. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol Cell. 2011;43(4):613–623.
- Gkatza NA, Castro C, Harvey RF, et al. Cytosine-5 RNA methylation links protein synthesis to cell metabolism. PLoS Biol. 2019;17(6):e3000297.
- Genenncher B, Durdevic Z, Hanna K, et al. Mutations in Cytosine-5 tRNA Methyltransferases impact mobile element expression and genome stability at specific DNA repeats. Cell Rep. 2018;22(7):1861–1874.
- Popis MC, Blanco S, Frye M. Posttranscriptional methylation of transfer and ribosomal RNA in stress response pathways, cell differentiation, and cancer. Curr Opin Oncol. 2016;28(1):65–71.
- Wang N, Tang H, Wang X, et al. Homocysteine upregulates interleukin-17A expression via NSun2-mediated RNA methylation in T lymphocytes. Biochem Biophys Res Commun. 2017;493(1):94–99.
- Xing J, Yi J, Cai X, et al. NSun2 promotes cell growth via elevating Cyclin-Dependent Kinase 1 translation. Mol Cell Biol. 2015;35(23):4043–4052.
- Tang H, Fan X, Xing J, et al. NSun2 delays replicative senescence by repressing p27 (KIP1) translation and elevating CDK1 translation. Aging (Albany NY). 2015;7(12):1143–1158.
- Cai X, Hu Y, Tang H, et al. RNA methyltransferase NSUN2 promotes stress-induced HUVEC senescence. Oncotarget. 2016;7(15):19099–19110.
- Shanmugam R, Fierer J, Kaiser S, et al. Cytosine methylation of tRNA-Asp by DNMT2 has a role in translation of proteins containing poly-Asp sequences. Cell Discov. 2015;1(1):15010.
- Tang Y, Gao CC, Gao Y, et al. OsNSUN2-Mediated 5-Methylcytosine mRNA modification enhances rice adaptation to high temperature. Dev Cell. 2020;53(3):272–86 e7.
- Schosserer M, Minois N, Angerer TB, et al. Methylation of ribosomal RNA by NSUN5 is a conserved mechanism modulating organismal lifespan. Nat Commun. 2015;6(1):6158.
- Tuorto F, Herbst F, Alerasool N, et al. The tRNA methyltransferase Dnmt2 is required for accurate polypeptide synthesis during haematopoiesis. Embo J. 2015;34(18):2350–2362.
- Zhang Y, Zhang X, Shi J, et al. Dnmt2 mediates intergenerational transmission of paternally acquired metabolic disorders through sperm small non-coding RNAs. Nat Cell Biol. 2018;20(5):535–540.
- Hussain S, Tuorto F, Menon S, et al. The mouse cytosine-5 RNA methyltransferase NSun2 is a component of the chromatoid body and required for testis differentiation. Mol Cell Biol. 2013;33(8):1561–1570.
- Blanco S, Kurowski A, Nichols J, et al. The RNA-methyltransferase Misu (NSun2) poises epidermal stem cells to differentiate. PLoS Genet. 2011;7(12):e1002403.
- Trixl L, Amort T, Wille A, et al. RNA cytosine methyltransferase Nsun3 regulates embryonic stem cell differentiation by promoting mitochondrial activity. Cell Mol Life Sci. 2018;75(8):1483–1497.
- Yang L, Perrera V, Saplaoura E, et al. m(5)C methylation guides systemic transport of messenger RNA over graft junctions in plants. Curr Biol. 2019;29(15):2465–76.e5.
- Frye M, Watt FM. The RNA methyltransferase Misu (NSun2) mediates Myc-induced proliferation and is upregulated in tumors. Curr Biol. 2006;16(10):971–981.
- Zhang X, Liu Z, Yi J, et al. The tRNA methyltransferase NSun2 stabilizes p16INK(4) mRNA by methylating the 3ʹ-untranslated region of p16. Nat Commun. 2012;3(1):712.
- Martinez FJ, Lee JH, Lee JE, et al. Whole exome sequencing identifies a splicing mutation in NSUN2 as a cause of a Dubowitz-like syndrome. J Med Genet. 2012;49(6):380–385.
- Yuan Z, Chen P, Zhang T, et al. Agenesis and Hypomyelination of corpus callosum in mice lacking Nsun5, an RNA methyltransferase. Cells. 2019;8(6):552.
- Janin M, Ortiz-Barahona V, de Moura MC, et al. Epigenetic loss of RNA-methyltransferase NSUN5 in glioma targets ribosomes to drive a stress adaptive translational program. Acta Neuropathol. 2019;138(6):1053–1074.
- Pavon-Eternod M, Gomes S, Geslain R, et al. tRNA over-expression in breast cancer and functional consequences. Nucleic Acids Res. 2009;37(21):7268–7280.
- Yi J, Gao R, Chen Y, et al. Overexpression of NSUN2 by DNA hypomethylation is associated with metastatic progression in human breast cancer. Oncotarget. 2017;8(13):20751–20765.
- Lu L, Zhu G, Zeng H, et al. High tRNA Transferase NSUN2 gene expression is associated with poor prognosis in head and neck squamous carcinoma. Cancer Invest. 2018;36(4):246–253.
- Gao Y, Wang Z, Zhu Y, et al. NOP2/Sun RNA methyltransferase 2 promotes tumor progression via its interacting partner RPL6 in gallbladder carcinoma. Cancer Sci. 2019;110(11):3510–3519.
- Schaefer M, Hagemann S, Hanna K, et al. Azacytidine inhibits RNA methylation at DNMT2 target sites in human cancer cell lines. Cancer Res. 2009;69(20):8127–8132.
- Lu L, Gaffney SG, Cannataro VL, et al. Transfer RNA methyltransferase gene NSUN2 mRNA expression modifies the effect of T cell activation score on patient survival in head and neck squamous carcinoma. Oral Oncol. 2020;101:104554.
- Blanco S, Bandiera R, Popis M, et al. Stem cell function and stress response are controlled by protein synthesis. Nature. 2016;534(7607):335–340.
- Okamoto M, Fujiwara M, Hori M, et al. tRNA modifying enzymes, NSUN2 and METTL1, determine sensitivity to 5-fluorouracil in HeLa cells. PLoS Genet. 2014;10(9):e1004639.
- Haag S, Warda AS, Kretschmer J, et al. NSUN6 is a human RNA methyltransferase that catalyzes formation of m5C72 in specific tRNAs. Rna. 2015;21(9):1532–1543.
- Long T, Li J, Li H, et al. Sequence-specific and Shape-selective RNA recognition by the Human RNA 5-Methylcytosine methyltransferase NSun6. J Biol Chem. 2016;291(46):24293–24303.
- Liu RJ, Long T, Li J, et al. Structural basis for substrate binding and catalytic mechanism of a human RNA: m5Cmethyltransferase NSun6. Nucleic Acids Res. 2017;45(11):6684–6697.
- Aguilo F, Li S, Balasubramaniyan N, et al. Deposition of 5-Methylcytosine on enhancer RNAs enables the coactivator function of PGC-1alpha. Cell Rep. 2016;14(3):479–492.
