ABSTRACT
The majority of long noncoding RNAs (lncRNAs) contain transposable elements (TEs). PAHAL, a nuclear-retained lncRNA that is inserted by a Gypsy retrotransposon, has been shown to be a vital regulator of phenylalanine hydroxylase (PAH) gene expression that controls dopamine biosynthesis and behavioural aggregation in the migratory locust. However, the role of the Gypsy retrotransposon in the transcriptional regulation of PAHAL remains unknown. Here, we identified a Gypsy retrotransposon (named Gypsy element) as an inverted long terminal repeat located in the 3′ end of PAHAL, representing a feature shared by many other lncRNAs in the locust genome. The embedded Gypsy element contains a RNA nuclear localization signal motif, which promotes the stable accumulation of PAHAL in the nucleus. The Gypsy element also provides high-affinity SRSF2 binding sites for PAHAL that induce the recruitment of SRSF2, resulting in the PAHAL-mediated transcriptional activation of PAH. Thus, our data demonstrate that TEs provide discrete functional domains for lncRNA organization and highlight the contribution of TEs to the regulatory significance of lncRNAs.
Introduction
Thousands of long noncoding RNAs (lncRNAs) have been extensively described in many species and act as vital and flexible cellular modulators to affect various fundamental biological processes via diverse mechanisms [Citation1–4]. Functional diversification of lncRNAs is the foundation of the RNA-based regulatory mechanisms that highlight the closer relationship between the degree of organic and behavioural complexity and the number of lncRNA species, rather than the number of protein-coding genes [Citation5]. Recent studies have reported that several lncRNAs participate in neuronal development and cognitive and behavioural regulation [Citation6–10]. During phase changes from the gregarious (G) to the solitarious (S) states in the migratory locust, the dopamine (DA) pathway in coding genes and noncoding RNAs plays a crucial role in the regulation of locust aggregative behaviour. The phenylalanine hydroxylase gene (PAH, also referred to as henna in Drosophila and Locusta) is a key gene for DA synthesis in this pathway [Citation11]. PAH transcriptional regulation is essential for the locust behavioural changes [Citation11]. The posttranscriptional modification of this gene by miRNA 133 is involved in locust behavioural phase changes [Citation12]. Recent findings demonstrate that PAHAL, a PAH lncRNA arranged in the sense orientation, is unique as a modulator of reversible locust behavioural changes; PAHAL functions by accelerating ancestral PAH gene expression, resulting in DA production in the locust brain [Citation13].
Mechanistically, PAHAL acts as a nuclear lncRNA to recruit serine/arginine-rich splicing factor 2 (SRSF2) to the PAH proximal promoter, promoting PAH transcriptional activation [Citation13]. Further analysis showed that the RNA nuclear localization signal motif (NLS) and the SRSF2 binding site are located at the 3′ terminus of PAHAL. The 3′ end sequence of PAHAL plays a vital role in the transcriptional regulation function and contains a long terminal repeat (LTR) of the Ty3/Gypsy retroelement [Citation13]. Therefore, PAHAL is a transposable element (TE)-embedded lncRNA.
Similar to proteins, the primary sequence of a lncRNA comprises ‘domains’ or discrete elements that modulate specific aspects of lncRNA activity, such as molecular interactions and subcellular localization [Citation1,Citation14–16]. TEs could be a possible source of lncRNA domains, providing a structured RNA platform and sequence features for the biogenesis and subcellular localization of the resident lncRNA, as well as the modulation of lncRNA downstream gene expression [Citation14,Citation16–20]. In particular, Ty3/Gypsy retroelements constitute a large family of LTR retrotransposons and are widely distributed in plants, fungi, and animals [Citation21]. Insertions of LTR remnants in and around the 5′ UTRs, introns and 3′ UTRs of genes may alter their structure, function, stabilization and evolution by providing new regulatory units, such as transcription factor binding sites, alternative polyadenylation sites, and alternative splicing sites [Citation22,Citation23]. A large fraction of LTRs lost their transposability during evolution through deletion of their internal coding genes [Citation24]. These LTRs, as embedded sequences, are abundant in mature lncRNAs and account for a significant portion of the total lncRNA sequence [Citation18]. Some studies have identified that LTRs are highly enriched at transcription start sites of long intergenic noncoding RNAs (lincRNAs) to promote transcription by providing transcription factor (TF) binding sites [Citation20]. For example, LTR7/HERVH elements seed NANOG, OCT4, and SOX2 binding sites to enhance lncRNA-RoR and lncRNA-ES3 transcription in human embryonic stem cells (ESCs) [Citation18]. The invSINEB2 element, as an effector domain, is embedded in the lncRNA AS Uchl1, which is known to be involved in the degeneration of dopaminergic neurons [Citation25–27]. The neurodegenerative disease-associated lncRNA Malat1 recruits hnRNPK to maintain nuclear speckles through the embedded SINEB1 [Citation7]. Moreover, the exonization of LTRs may be one of the possible ways of regulating phenotypic plasticity of the locusts [Citation28,Citation29]. While the contribution of TEs to lncRNA regulation is evident, the specific mechanism underlying TE-mediated regulation largely remains elusive.
Here, we showed that the residence of a noncanonical Gypsy element in a lncRNA is a common feature of many other lncRNAs in the locust genome. We found that the Gypsy element provides an NLS and three tandem SRSF2 high-affinity sites (exonic-splicing enhancer, i.e. ESE) for PAHAL. The Gypsy element determines nuclear retention, prolongs the half-life of PAHAL, and affects the affinity between PAHAL and SRSF2 via the NLS and ESEs, resulting in transcriptional activation of PAH. These results demonstrate that the embedded Gypsy element is a functionally important motif for PAHAL regulation. Our findings also provide a mechanistic explanation for the elaborate transcriptional regulation, in which a TE confers the resident lncRNA with the ability to serve as a protein binding motif to modulate protein activity, for transcriptional activation.
Materials and methods
Animals
The locusts were maintained strictly under standard conditions established by previous reports [Citation13,Citation30]. Briefly, approximately five hundred G locusts were reared in a large cage (40 cm × 40 cm × 40 cm). The S locusts were cultured individually in white metal boxes (10 cm × 10 cm × 25 cm) supplied with charcoal-filtered fresh air. The locust colonies were reared under a 14:10 light/dark photoperiod at 30 ± 2°C and fed fresh wheat seedlings and bran.
Cells
Drosophila S2 cells (Gibco, NY, USA; R69007) were grown in SFX-insect medium (HyClone, Logan, UT; SH30278.02) at 28°C. The SRSF2 protein-depleted mouse embryonic fibroblast line (SRSF2-MEFs) is an endogenous SRSF2-KO system of mouse cells that can turn off endogenous SRSF2 transcription upon addition of doxycycline (DOX) to the culture medium. We used SRSF2-MEFs to study the role of the embedded Gypsy element in the interaction of SRSF2 with PAHAL. SRSF2-MEFs were maintained in DMEM (Gibco, NY, USA; C11965500BT) supplemented with tet-free FBS (Clontech, CA, USA; 631,106) in standard culture incubators (37°C, humidified 5% CO2/95% air). SRSF2-MEFs were treated with 10 µg/mL DOX (Sigma, MO, USA; D9891-1 G) for 1 day to deplete endogenic SRSF2 transcription (DOX+) [Citation31].
RNA isolation and qPCR
Freshly harvested tissues were stored in liquid nitrogen before RNA preparation. Cultured cells were collected and then washed twice in PBS before RNA extraction. Total RNA was extracted according to the manufacturer’s instructions for TRIzol reagent (Invitrogen, CA, USA, 15,596,018). cDNA was synthesized with a Fastking RT Kit (With gDNase) (Tiangen, Beijing, China; KR116). qPCR was performed with Talent qPCR PreMix (SYBR Green) (Tiangen, Beijing, China; FP209) in a LightCycler 480 instrument (Roche, Mannheim, Germany). All the PCR products were verified through sequencing before qPCR. The housekeeping gene ribosomal protein 49 (RP49) was used as an internal control for gene expression normalization [Citation13,Citation30,Citation32]. Five to eight biological replicates were prepared for each treatment. All primers are listed in Supplementary Table S1.
Isolation and crowding of locusts
Standard procedures of isolation and crowding of locusts were performed as previously described with some modifications [Citation11,Citation13,Citation33]. Briefly, the locusts were separately reared from G nymphs in solitary rearing cages under standard conditions. The locusts were crowded by introducing 10 labelled S nymphs into an optic Perspex box (10 cm × 10 cm × 10 cm) that contained 20 G nymphs. After 0, 4, 8, 16 or 32 h of treatment, the locust brains were dissected and immediately put into liquid nitrogen for RNA preparation. Equal numbers of male and female insects were sampled for each biological replicate at the same developmental stage.
Northern blot analysis
Northern blot analysis was performed as previously described with slight amendment [Citation34]. A portion (25 μg) of DNase I-treated total RNA was extracted using TRIzol reagent. Denaturing formaldehyde agarose gels (1%) were used for sample RNA separation by electrophoresis. The separated RNA was transferred onto a BrightStar Plus membrane (Ambion, Vilnius, Lithuania, AM10102) by capillary action using Alkaline transfer buffer [5× SSC (Invitrogen, N.Y., USA, AM9763), 10 mM NaOH] overnight at room temperature (RT) and was immediately UV cross-linked for 300 s at 120 mJ/cm2 to reduce RNA degradation. The membrane was pre-hybridized for 1 h at 37°C in ULTRAhyb-Oligo Hybridization buffer (Invitrogen, Vilniue, Lithuania, AM8663). PAHAL-PAH RNA probe, which covered the overlapping sequence of PAHAL and PAH, was synthesized and labelled with biotin using T7 RNA Polymerase kit (Promega, WI, USA; P2075). The 3′ biotin-labelled U6 DNA probe [Citation35] was synthesized as endogenous control by Thermo Fisher (BJ, China). Then, the membrane was hybridized with PAHAL-PAH RNA probe and U6 DNA probe at 37°C overnight. After two washes using the washing buffer (2× SSC, 0.5% SDS) at 37°C, the blots were detected by Chemiluminescent Nucleic Acid Detection Module (Pierce, CA, USA; 89,880).
Reporter and expression plasmid construction
Different constructs were prepared: The full-length PAHAL¯ sequence (labelled as PAHAL¯), PAHAL with the Gypsy retroelement deleted (i.e.PAHALΔGypsy), PAHAL with the NLS deleted (PAHALΔNLS), the PAHAL¯ sequence with an artificial insertion of the Gypsy element immediately preceding the poly(A) sequence of PAHAL¯ (i.e. PAHAL¯Gypsy+), and PAHAL with the mutation of the tandem ESEs (i.e. MT-ESEs-PAHAL); they were cloned into the pcDNA3.1 (+) vector (overexpression vector for SRSF2-MEFs; Invitrogen, CA, USA; V79020) and pAc5.10/V5-His A vector (overexpression vector for Drosophila S2 cells; Invitrogen, CA, USA; V4110-20). pGL4.10-P + 5′UTR (with the PAH promoter fused to a firefly luciferase reporter), pcDNA3.1/PAHAL (PAHAL overexpression vector for SRSF2-MEFs), pAc5.10/V5-His A/PAHAL (PAHAL overexpression vector for Drosophila S2 cells), pcDNA3.1/lacz (negative control vector for SRSF2-MEFs), pAc5.10/V5-His A/lacz (negative control vector for Drosophila S2 cells), pcDNA3.1/V5-His/SRSF2 ORF (SRSF2 overexpression vector for SRSF2-MEFs) and pAc5.10/V5-His A/SRSF2 ORF (SRSF2 overexpression vector for Drosophila S2 cells) were constructed and described previously [Citation13]. These vectors were transfected into Drosophila S2 cells and SRSF2-MEFs.
RNA decay rate assay
For in vitro experiments, the PAHAL, PAHALΔGypsy, PAHAL¯Gypsy+ or PAHALΔNLS vector was transfected into SRSF2-MEFs using Lipofectamine 3000 (Invitrogen, CA, USA; L3000015). The second day after transfection, transcription was halted for 1 to 7 h or 1 to 4 h by adding 5 mg/mL actinomycin D (Act D) (Sigma, MO, USA; A4262-5 mg) to obtain a final concentration of 1 μg/mL. For in vivo experiments, Act D was dissolved at a concentration of 1 mg/mL in DMSO and then diluted to 0.4 μg/μL in PBS. The brains of G locusts were microinjected with 69 nL of this Act D solution for 1 to 4 h. Cells and locust brains were harvested in TRIzol at different time points to assess the decay rate of PAHAL, PAHAL¯, PAHALΔGypsy, PAHALΔNLS, PAHAL¯Gypsy+ or MT-ESEs-PAHAL RNA. Half-lives were calculated using one-phase exponential decay [Citation36].
Cell fractionation experiment
Nuclear fractionation experiments form brains or cells were performed as previously reported [Citation13,Citation37]. Twenty nymphal brains or 2 × 10 7cells were harvested by centrifugation and homogenized in cold lysis buffer [1× PBS supplemented with 0.2% IGEPAL CA-630 (Sigma, MO, USA; I8896-50 ml), 1× proteinase inhibitor (Pierce MA, USA; 88,266) and RNase inhibitor (Promega, WI, USA; N2111S)]. The cell residue in the homogenate was removed by centrifugation at 30 × g for 2 min at 4°C. The nuclear pellet was obtained by centrifugation at 425 × g for 15 min at 4°C. The residual nuclei were removed by centrifugation at 2000 × g for 10 min at 4°C to obtain the cytoplasmic fraction in the supernatant. The cell fractionation was stored at −80°C prior to RNA extraction and the RNA immunoprecipitation (RIP) assay.
RNA fluorescence in situ hybridization (FISH)
To determine whether the embedded Gypsy element affects the nuclear retention of PAHAL, fluorescence in situ hybridization (FISH) experiments were performed as previously described with some modifications [Citation13,Citation37]. Universal biotinylated RNA probes were designed for PAHAL, PAHALΔGypsy and PAHALΔNLS and then synthesized by using a T7 RNA Polymerase Kit (Promega, WI, USA, P2075). SRSF2-MEFs were seeded onto 6-well plates (Corning, NY, USA) and then transfected with pcDNA3.1/PAHAL, pcDNA3.1/PAHALΔGypsy and pcDNA3.1/PAHALΔNLS. The cells were harvested and fixed in 4% (wt/vol) paraformaldehyde for 1 h at RT. The fixed cells were permeabilized with PBST (0.5% Triton X-100 in 1× PBS) for 10 min at RT and then digested with 20 µg/mL proteinase K (Invitrogen, CA, USA; AM2548) at 37°C for 15 min. The cell pelleted was incubated with prehybridization buffer (Wuhan Boster, Wuhan, China; AR0152) at 37°C for 30 min. The cells were hybridized with probes (5 ng/µL) at 37°C overnight and then blocked with blocking buffer (2% BSA in 0.2× SSC) at 4°C for 20 min. Next, the cells were incubated with streptavidin–HRP (1:100) for 1 h at RT and then washed three times with PBS. The fluorescent biotin signal was detected with a TSA Fluorescein System (Perkin-Elmer, MA, USA; NEL701A001KT). The cells were centrifuged, resuspend in Antifade Mounting Medium (Wuhan Boster, Wuhan, China; AR1109), and then dropped onto slides. Images were captured with an LSM 710 confocal fluorescence microscope (Zeiss, Oberkochen, Germany) at 63× magnification. Supplementary Table S1 lists the primers used for FISH probe synthesis.
RIP assay
pcDNA3.1/V5-His/SRSF2 ORF was cotransfected with pcDNA3.1/PAHAL, pcDNA3.1/PAHALΔGypsy or pcDNA3.1/ PAHALΔNLS into SRSF2-MEFs that had been depleted of mouse endogenous SRSF2 by adding DOX for one day to test whether the embedded Gypsy element affects the binding of SRSF2 with PAHAL in vitro. After 3 days, 2 × 107 SRSF2-MEFs were harvested using a cell scraper. Nuclei were isolated for the RIP experiment in vitro. The binding affinity of SRSF2 to PAHAL¯ RNA in vivo was tested by performing the RIP assay on brain tissues. Nuclei were isolated from fifty brains for the RIP experiment in vivo.
A Magna RIP Quad RNA-Binding Protein Immunoprecipitation Kit (Millipore, CA, USA; 17–704) was used to perform the RIP assay. The nuclear pellet was lysed in ice-cold RIP lysis buffer spiked with 1× proteinase inhibitor and RNase inhibitor and stored at −80°C overnight. Magnetic beads were sensitized by preincubation with 5 µg of V5 antibody (Invitrogen, CA, USA; R96025) or normal mouse IgG (Millipore, CA, USA; CS200621) for 30 min at RT with rotation to form the bead–antibody complex. The supernatant of the lysate from the centrifugation was added to the bead–antibody complex. The mixture was coincubated overnight at 4°C with rotation to bind the candidate RNAs. Thereafter, 10 µL of the supernatant was sampled as the input. Candidate RNAs in the immunoprecipitate and input were analysed through qPCR.
RNA pulldown and Western blot analysis
RNA pulldown experiments were conducted according to the manufacturer’s recommendations for the Magnetic RNA–Protein Pull-Down Kit (Thermo Fisher Scientific, CA, USA; 20,164) with some modifications. Briefly, the DNA templates of the RNA probes for a series of ESEs with mutations in the embedded Gypsy element of PAHAL were synthesized by PolePolar Biotechnology Co., Ltd., Beijing, China. Biotinylated RNA probes were transcribed with a T7 RNA Polymerase Kit (Promega, WI, USA; P2075). In addition, endogenous SRSF2 was turned off in SRSF2-MEFs by treatment with DOX for 1 day. Subsequently, the cells were transfected with the pcDNA3.1/V5-His/SRSF2 ORF vector.
On the third day after transfection, the cells were lysed to extract total protein by using lysis buffer [T-PER Tissue Protein Extraction Reagent (Pierce, CA, USA; 78,510) containing 1× Halt Protease Inhibitor Cocktail, EDTA-free (Pierce, CA, USA; 87,785) and 1× RNase inhibitor (Promega, WI, USA; N2111S)]. The total protein was incubated with biotinylated RNA probes for 1 h at 4°C with rotation. RNA-binding proteins were analysed by Western blotting.
The proteins were isolated by a 10% NuPAGER Bis-Tris gel (Invitrogen, CA, USA; NP0315BOX) and subsequently transferred to PVDF membranes. The membranes were blocked with 5% (wt/vol) skim milk for 1 h at RT. SRSF2 was stained using a V5 tag monoclonal antibody (Invitrogen, CA, USA; R96025; 1:5,000) and a secondary antibody (Easybio, Beijing, China; BE0102-100; 1:5,000) and was detected with a SuperSignal West Femto Substrate Trial Kit (Pierce, CA, USA; 34,094).
Luciferase assay
To test whether the embedded Gypsy element affected the transcriptional activation function of PAHAL, we performed luciferase assays. Lipofectamine 3000 was used for plasmid delivery into cells that were expanded on 48-well plates (Corning, NY, US) for one night. The reporter plasmid (pGL4.10-P + 5′UTR, 10 ng) was cotransfected with 200 ng of the expression plasmid or negative control vector into cells with 5 ng of the internal control vector pRL-TK (Promega, WI, USA; E2241) to express Renilla luciferase. Both firefly and Renilla luciferase activities were measured using a dual-luciferase reporter assay system (Promega, WI, USA; E1960) at 30 h after incubation.
Bioinformatics and statistical analysis
The sequence motif of the NLS was WNNNNSNNAGCCC (W = A/T, S = G/C) [Citation38]. The sequence of the SRSF2 high-affinity ESE site was WSSNGYY (W = A/T, S = G/C Y = C/T) [Citation39,Citation40]. Data from the tissue expression experiment, mutational analysis of SRSF2 affinity and the nuclear retention analysis of PAHAL were analysed through ANOVA and then by post hoc Tukey’s b-test for multiple comparisons. Differences in gene expression and other values between treatments were analysed by using independent-sample Student’s t tests. The data are described as the mean ± SEM unless stated otherwise. SPSS 21.0 (SPSS Inc., IL, USA) was used for all statistical analyses. The locust genome data are available at the following website: http://www.locustmine.org. The sequence for PAHAL¯ has been deposited in GenBank under accession number KX962172. Numerical data that underlies graphs and sample image data have been uploaded to https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/UETCO0.
Results
Gypsy element-embedded lncRNAs are widespread in the locust genome
We previously defined a 2.6-kb lncRNA, PAHAL, which is involved in the feedback regulation of locust behavioural aggregation. PAHAL possesses a 217-nt LTR of the noncanonical Ty3/Gypsy retroelement (named Gypsy element) immediately preceding the poly(A) sequence [Citation13]. Eighty-three subfamilies of locust Ty3/Gypsy retroelements are annotated in Repbase [Citation41]. The Gypsy element contained in PAHAL belongs to the Gypsy-25 subfamily, the classic structure of which harbours an inverted pair of LTRs flanking the retrotransposon ()).
Figure 1. Contribution of embedded Gypsy elements to the diversification of lncRNAs in the locust genome. (a) Structure of a canonical Gypsy element (Gypsy-25 subfamily) in the locust genome. (b) Amounts of the embedded Gypsy elements transcribed with lncRNAs in the genome. (c) Structure and amounts of different embedded Gypsy elements present in locust lncRNAs. The structure of a Gypsy element in a lncRNA is illustrated in the left panel. The histogram in the right panel shows the number of each type of element present in lncRNAs. (d) Insertion profiles of Gypsy elements in locust lncRNAs. The location of the Gypsy element insertion in lncRNAs is illustrated in the left panel; 5′ and 3′ represent the lncRNA direction.
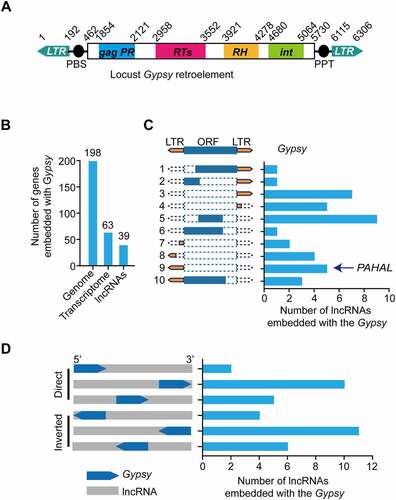
In this study, we first investigated whether Gypsy element-embedded lncRNAs are ubiquitous in the locust genome. We scanned the locust genome and transcriptomes for the Gypsy-25 retroelements and the element-embedded lncRNAs. The locust genome contained 198 copies of the elements. Among them, 62 transcripts contained at least a partial element. A total of 38 Gypsy-embedded transcripts were identified in silico as lncRNAs ()).
We annotated the structure of the elements embedded in the lncRNAs and found 10 different types of elements ()). All the Gypsy element types that we identified were noncanonical. These elements were embedded in direct or inverted directions relative to the resident lncRNA and in different lncRNA regions, such as the 5′UTR, middle region, and 3′UTR ()). In addition to PAHAL, at least four PAHAL-like lncRNAs were identified in the specified transcriptomes. Therefore, Gypsy element-embedded lncRNAs are common in the locust genome.
The presence of the embedded Gypsy element is associated with PAHAL and PAH expression
To reveal the regulatory contribution of the embedded Gypsy element to lncRNAs, we investigated the specific lncRNA PAHAL, the regulatory functions of which were well documented in our previous work [Citation13]. In addition to the PAHAL transcript from the PAH gene, we found, using 5′ and 3′ RACE, another transcript isoform of PAH, hereafter named PAHAL¯. This transcript is 2,431 nt long and has nearly the same sequence as PAHAL (from +1 nt to +2395 nt) but lacks the embedded Gypsy element and thus can act as a control for PAHAL () and Supplementary Fig. S1). Similar to PAHAL, PAHAL¯ does not possess protein-coding capacity and is a lncRNA (Supplementary Fig. S2).
Figure 2. Presence of the Gypsy element in the lncRNA PAHAL and associated gene expression changes. (a) Gene and transcript structures of locust PAH, PAHAL, and PAHAL¯. The transcriptional orientations of PAH, PAHAL and PAHAL¯ are labelled with bent arrows. The eight exons of the PAH transcript are indicated using ‘E 1–8’. The half-arrows indicate the strand-specific quantitative real-time (qPCR) primers: primers 1 and 2 for PAH, primers 3 and 4 for PAHAL, and primers 5 and 6 for PAHAL¯. The length of the entire PAH locus is drawn to scale. The black scale bar for the PAH locus represents 1 kb. The gene structure of PAHAL and PAHAL¯ is scaled up in blue. The blue scale bar for PAHAL and PAHAL¯ represents 0.1 kb. (b) Tissue expression of PAHAL and PAHAL¯ (left panel) and PAH (right panel) in locusts. qPCR was used for transcript quantification. The different letters within each gene indicate that the means are significantly different (P< 0.05). (c) The RNA levels of PAHAL, PAHAL¯ and PAH in the brains of gregarious (g) and solitarious (s) locusts. Seven biological replicates of eight brains were detected. (d) Profiles of PAHAL¯ and PAHAL expression in the brain during locust isolation and crowding. Seven to nine biological replicates of eight brains were measured. Asterisks indicate significant differences between each time point and 0 h (P < 0.05). Student’s t test: *P < 0.05; **P < 0.01; ***P < 0.001. (e) PAH and PAHAL mRNAs detected in locust brains using Northern blot analysis. The U6 snRNA was used as endogenous control.
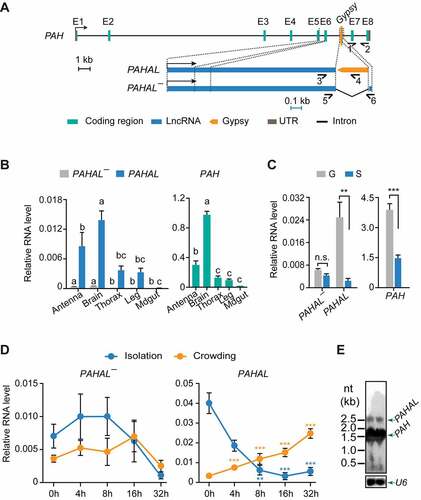
We measured the expression levels of the three transcripts of the PAH loci, that is, PAHAL, PAHAL¯, and PAH, in five tissues in fourth-instar nymphs of G locusts ()). Compared with the relatively high expression of PAHAL and PAH in the brain, the expression of PAHAL¯ was almost undetectable ()). We also analysed the expression of the three transcripts in the brains of G and S locusts since PAH and PAHAL were shown to be involved in the regulation of behavioural transition between the two phases ()). The expression of PAHAL and PAH was 10.1-fold (t test: P = 0.004, N = 7) and 2.6-fold (t test: P < 0.001, N = 7) higher, respectively, in the G locusts than in the S locusts. In contrast, PAHAL¯ presented no difference in the expression levels between the two phases (t test: P = 0.084, N = 7; )).
To examine the effect of population density on the expression of PAHAL¯ and PAHAL, we tested the time-course expression dynamics of PAHAL¯ and PAHAL transcripts in the locust brain ()). PAHAL was significantly upregulated at 8 h upon aggregation (t test: P = 0.005, N = 5) compared with the level at 0 h. The upregulation of PAHAL expression was sustained even at 32 h (t test: P < 0.0001, N = 5). In contrast, PAHAL expression was significantly downregulated at 4 h after isolation (t test: P < 0.0001, N = 4). This expression continued to decrease at 32 h after isolation (t test: P < 0.0001, N = 4). The time-course expression pattern of PAHAL is similar to that reported for PAH [Citation13]. However, the expression of PAHAL¯ was extremely low and exhibited no difference in the brains during locust aggregation and isolation. We performed Northern blot using a universal probe of the three transcripts to reveal their different transcript size and expression levels ((), two biological replicates). The expression level of PAH is extremely higher than that of PAHAL. The results imply that the embedded Gypsy element may contribute to the regulation of lncRNA expression in response to changes in population density.
The Gypsy element prolongs the half-life of PAHAL
Under the same promoter, the diverse stability of RNA may be a reason for the different abundances of PAHAL and PAHAL¯ in locust brains. Therefore, we examined whether the existence of the embedded Gypsy element influences the stability of PAHAL RNA. The secondary structures of PAHAL predicted by RNAfold showed that the Gypsy element embedded in PAHAL has the potential to fold into a stable stem-loop structure with the other part of PAHAL and decrease the minimum free energy (MFE) of PAHAL ()).
Figure 3. The Gypsy element prolongs the life-span of PAHAL. (a) The embedded Gypsy element contributes to the predicted lncRNA secondary structure. ‘MFE’ is the minimum free energy. The yellow line indicates the region of the embedded Gypsy element. (b) RNA stability of PAHAL and the Gypsy element-deleted PAHAL (i.e. PAHALΔGypsy). The SRSF2 protein-depleted mouse embryonic fibroblast line (SRSF2-MEFs) that overexpressed PAHAL or PAHALΔGypsy was treated with the transcriptional inhibitor actinomycin D (Act D) or vehicle (0.1% DMSO) for 1 to 7 h. PAHAL and PAHALΔGypsy levels were measured using RT-qPCR (N = 7). (c) Decay of PAHAL and PAHAL¯ RNA in the presence of Act D at the brain. The insets show parameters for the fitted curves using one-phase exponential decay. Eight replicates of eight brains were measured. Student’s t test: * P < 0.05; ** P < 0.01; *** P < 0.001; n.s., not significant.
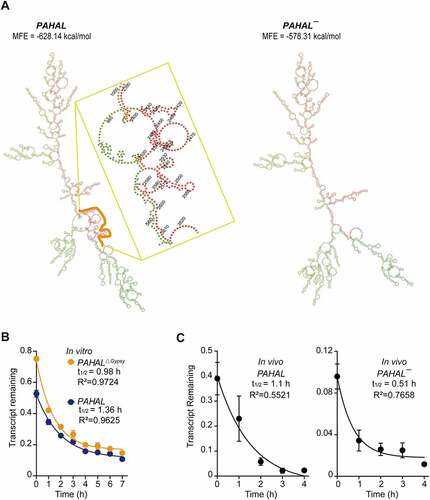
The RNA decay rate assay in the SRSF2-MEFs showed that, after transcription inhibition with actinomycin D, PAHAL with the deletion of Gypsy retroelement (labelled as PAHALΔGypsy) showed a dramatic decrease in stability, with a short half-life of 0.98 h following transcriptional inhibition, compared to the unmodified PAHAL, which displayed greater stability and longer half-life of 1.36 h ()). PAHAL RNA levels in locust brains decayed with a half-life of approximately 1.1 h. However, the RNA of PAHAL¯ lacking the embedded Gypsy element displayed a half-life of only 0.51 h under the same conditions ()). Therefore, the embedded Gypsy element helps to stabilize RNA.
The embedded Gypsy element is required for nuclear retention of PAHAL
PAHAL is known to localize primarily to the nucleus [Citation13]. Sequence analysis showed that the embedded Gypsy element contains an NLS, indicating the modulation of the subcellular localization of PAHAL ()). The nuclear fractionation experiment with the locust brains showed that 89% of PAHAL mRNA localizes in the nucleus, while only 21% of PAHAL¯ RNA retained in the nucleus, relative to nuclear RNA U6 (positive control) and cytoskeleton actin (negative control, )). Furthermore, the intracellular distribution was quantified using nuclear fractionation of SRSF2-MEFs. Unlike PAHAL, which retained 96% RNA in the nucleus relative to snRNA U2 (nuclear control) and β-actin (cytoplasmic control), PAHALΔGypsy reduced the RNA level in the nucleus to 57%. Furthermore, we deleted the NLS in PAHAL (PAHALΔNLS), which caused a reduction in RNA in the nucleus to 42% ()). A rescue experiment showed that the artificial insertion of the Gypsy element into the 3′ end of the PAHAL¯ (labelled as PAHAL¯Gypsy+) resulted in the nuclear retention of PAHAL¯Gypsy+, with up to 89% of PAHAL¯Gypsy+ retained in the nucleus ()). FISH in SRSF2-MEFs further proved that PAHALΔGypsy caused a pronounced reduction in the nuclear retention of PAHAL ()). Moreover, PAHALΔNLS also had a similar effect on nuclear retention ()).
Figure 4. The Gypsy element promotes the nuclear retention of PAHAL. (a) The red bold text represents the nuclear location signal (NLS) sequence. The conserved nucleotides are highlighted using an underscored line. The boldface with blue shading indicates the three predicted SRSF2 high-affinity sites (ESEs) in the Gypsy element embedded in PAHAL, which are labelled ESE1, ESE2 and ESE3. ‘+2441’, ‘+2496’ and ‘+2602’ represent the start sites of the three ESEs relative to the transcript start site of PAHAL. The yellow characters represent the mutant sequence of the ESEs. (b) Nuclear localization of PAHAL and PAHAL¯ in the locust brains. U6 and β-actin, stable and abundant housekeeping genes that localize to the nucleus and cytoplasm in the locust brains, respectively, were used as internal controls to test the localization of PAHAL and PAHAL¯ in the brain. Five biological replicates of twenty brains were examined for each nuclear fractionation. (c) Nucleocytoplasmic shuttling of four PAHAL RNA variants, i.e. wild-type PAHAL, PAHAL¯ with insertion of the Gypsy element into its 3′ end (labelled as PAHAL¯Gypsy+), PAHAL with Gypsy deletion (i.e. PAHALΔGypsy), and NLS-deleted PAHAL (i.e. PAHALΔNLS) in SRSF2-MEFs (N = 6). U2 and β-actin, indicate the nucleus and cytoplasm in SRSF2-MEF, respectively, were used to determine if there were specific changes in the pattern of PAHAL localization elicited by Gypsy or NLS deletion. (d) FISH detection of the subcellular localization of PAHAL, PAHALΔGypsy and PAHALΔNLS. Images are shown at 63× magnification, and scale bars represent 10 µM. The arrows indicate the subcellular location of PAHAL (in the nucleus), PAHALΔGypsy and PAHALΔNLS (in the cytoplasm). (e) The half-life of PAHAL¯Gypsy+ and PAHALΔNLS RNA in the presence of Act D at SRSF2-MEF. Six replicates were measured. Student’s t test: * P < 0.05; ** P < 0.01; *** P < 0.001; n.s., not significant.
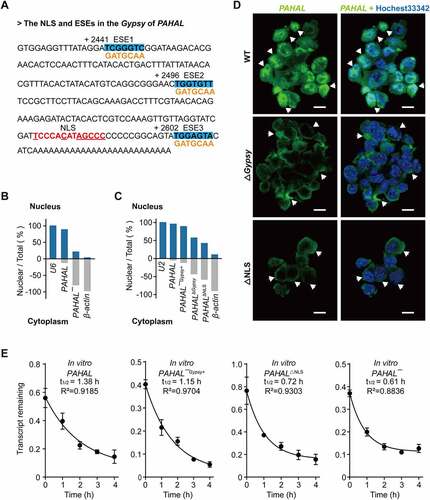
We tested the life time of PAHALΔNLS and PAHAL¯Gypsy+. The results showed that PAHAL¯Gypsy+ in the SRSF2-MEFs, upon the insertion of the Gypsy element, exhibited a dramatic increase in stability. However, the deletion of NLS speeded up the degradation of PAHALΔNLS RNA ()). Therefore, the Gypsy and NLS within the element confer nuclear retention of PAHAL and defer the degradation of PAHAL RNA in cytoplasm.
The Gypsy element affects the recruitment of SRSF2 to PAHAL
Given that the most of the 3′ terminal sequence of PAHAL is essential for PAHAL–SRSF2 tethering [Citation13], we hypothesized that the embedded Gypsy element is required for proper PAHAL binding to SRSF2. We performed in vitro RIP to investigate the change in the affinity between PAHAL and SRSF2 potentially induced by Gypsy deletion. Locust SRSF2 was co-transfected with PAHAL or PAHALΔGypsy into SRSF2-MEFs in which mouse endogenous SRSF2 was depleted by adding DOX for one day. The results showed that the rate of SRSF2 enrichment by PAHALΔGypsy decreased by 65% compared with that by PAHAL (t test: P = 0.039, N = 5; )). In vivo RIP experiment showed that PAHAL¯ that lacks the embedded Gypsy element had a SRSF2 affinity similar to that of PAHALΔGypsy (t test: P = 0.005, N = 6; )). RIP assay in SRSF2-MEFs further showed that deletion of the NLS in the Gypsy element didn’t affect the binding of SRSF2 with PAHAL ()). Analysis of the Gypsy element sequence showed that the specific element possesses three ESEs that are required for SRSF2-PAHAL binding ()). Next, we determined the specific sites in the Gypsy element involved in the interaction with SRSF2 into SRSF2-MEFs that turned off the mouse endogenous SRSF2, and then overexpressed the locust SRSF2. Mutational analysis of three ESEs within the Gypsy element revealed that the binding between PAHAL and SRSF2 was utterly disrupted by mutation of the three ESEs; in contrast, a single ESE mutation weakened the recruitment of SRSF2 (one-way ANOVA: P < 0.01, N = 3; )). Therefore, the embedded Gypsy element is necessary for PAHAL–SRSF2 binding, and the ESEs in the element may contribute to the binding.
Figure 5. The embedded Gypsy element regulates the tethering of PAHAL with SRSF2. (a) RNA immunoprecipitation (RIP) in SRSF2-MEFs verified the effect of the embedded Gypsy element on the binding of SRSF2 with PAHAL. (b) RIP was performed in locust brains to test the binding affinity of SRSF2 to PAHAL¯ RNA in vivo. Six biological replicates of fifty brains were examined. (c) Deletion of the NLS did not affect the SRSF2 binding affinity of PAHAL. (d) Mutational analysis of the SRSF2 affinity of the ESEs in the Gypsy element of PAHAL. Wild-type and mutant probes were incubated with SRSF2-MEF lysates that overexpressed the V5-tagged SRSF2 (SRSF2-V5) of locusts. MT-ESEs indicates the mutation of all three ESEs. MT-ESE1, MT-ESE2 or MT-ESE3 indicates mutated ESE1, ESE2 or ESE3, respectively. WT indicates wild type. (e) The predicted lncRNA secondary structure altered by mutation of ESEs in the Gypsy element. MT-ESEs-PAHAL means PAHAL with the mutation of ESEs in the Gypsy element. The blue shading indicates the ESEs of the embedded Gypsy element (indicated by the yellow line) in PAHAL. (f) The life time of MT-ESEs-PAHAL in the SRSF2-MEF adding Act D. (g) SRSF2 is not required for the nuclear retention of PAHAL. The following vectors were transfected into SRSF2-MEFs that had been treated using DOX for 1 day to knock out endogenous SRSF2: ‘SRSF2+’, pcDNA3.1/V5-His/SRSF2 ORF; ‘SRSF2−’, pcDNA3.1/V5-His/lacz; ‘PAHAL+’, pcDNA3.1(+)/PAHAL; ‘PAHAL−’, pcDNA 3.1(+)/lacz; ‘PAHALΔGypsy+’, pcDNA3.1(+)/PAHALΔGypsy; ‘PAHALΔGypsy−’, pcDNA 3.1(+)/lacz. Five replicates were measured. The different letters within each column indicate that the means are significantly different (P < 0.05).
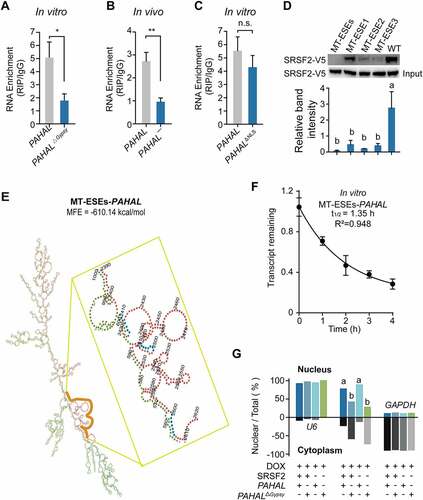
The secondary structures of PAHAL variant (i.e. MT-ESEs-PAHAL) with mutation of ESEs in the Gypsy element of PAHAL were predicted. The result showed that the mutation of ESEs altered the stable stem-loop structure of PAHAL but made no difference in the MFE ()). In vitro assayed showed that the RNA half lifetime of MT-ESEs-PAHAL in the SRSF2-MEFs is 1.35 h, similar to that of PAHAL () and )). Therefore, the mutation of the ESEs does not cause the rapid degradation of the mutant transcript.
We further examined whether SRSF2 affects the Gypsy-conferred nuclear retention of PAHAL. The nuclear fractionation experiment in SRSF2-MEFs demonstrated that the presence of SRSF2 did not affect the nuclear retention of PAHAL ()). In contrast, PAHALΔGypsy significantly increased the nuclear export of PAHAL regardless of the presence of SRSF2 ()). The results indicate that SRSF2 does not regulate the nuclear localization of PAHAL mediated by the Gypsy element.
The embedded Gypsy element is required for transcriptional regulation of PAHAL
We then evaluated whether the presence of the Gypsy element affects the regulatory function of PAHAL, because PAHAL generally promotes the transcriptional activation of the PAH promoter [Citation13]. The luciferase assay in S2 cells showed that PAHAL¯ lacking the Gypsy element had a 72% inhibitory effect on PAH promoter activity (t test: P < 0.001, N = 6; )) compared with that of PAHAL, but its effect was similar to that of lacz expression (negative control). Similarly, in S2 cells, PAHALΔGypsy did not activate the PAH promoter compared with lacz and exhibited a 66% reduction in promoter activity compared with PAHAL (t test: P < 0.001, N = 5; )). Therefore, the embedded Gypsy element is required for PAHAL-mediated PAH transcription activation.
Figure 6. The Gypsy element embedded in PAHAL is required for PAHAL-mediated transcription activation. (a) PAHAL¯ inhibits the promoter activity of PAH. ‘P + 5′UTR’ contains −1,168 to +89 bp relative to the TSS, representing the PAH promoter (‘P’: −1,168 to +1 bp) and 5′- untranslated region (‘5′UTR’: +1 to +89 bp). (b) The Gypsy element is required for the regulatory function of PAHAL. ‘Lacz’ is a frameshift mutational fragment of the lacz gene and serves as a negative control. PAHAL, PAHAL¯, PAHALΔGypsy and lacz were inserted into the pAC5.1/V5-His A vector, which is a high-level transient expression plasmid in Drosophila S2 cells. (c) The embedded Gypsy element participates in the interaction of SRSF2 and PAHAL for PAH transcription activation. SRSF2-MEFs (SRSF2-KO) were used to test the activity of the P + 5′-UTR by luciferase assays. ‘DOX +’ represents endogenic SRSF2 knockout, whereas ‘DOX – ’ or ‘Control’ indicates the normal expression of endogenic SRSF2 in SRSF2-MEFs or in S2 cells. ‘SRSF2-V5’ indicates the overexpression of the V5-tagged locust SRSF2 in S2 cells (S2, SRSF2-ov). Student’s t test: *P < 0.05; **P < 0.01; ***P < 0.001; n.s., nonsignificant. (d) The ESEs and NLS in the Gypsy of PAHAL contributes to the PAHAL-mediated transcription activation of PAH. Six replicates were measured. The different letters within each treatment indicate that the means are significantly different (P < 0.05).
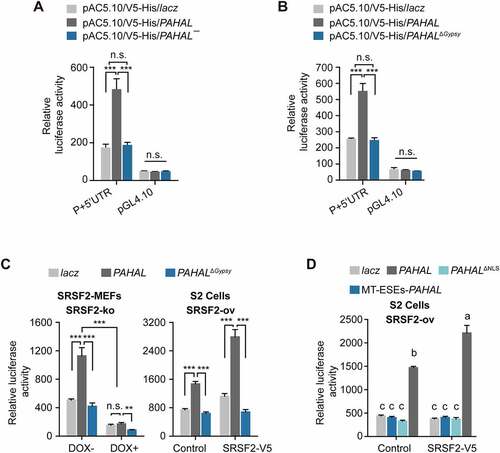
Since PAHAL acts as a nuclear lncRNA to recruit SRSF2 to the PAH proximal promoter, we explored whether the presence of the embedded Gypsy element affects the interaction between PAHAL and SRSF2 during PAHAL-mediated transcriptional activation of PAH. Mouse endogenous SRSF2 knockout by adding DOX in SRSF2-MEFs significantly reduced PAHAL-mediated transcription activity (one-way ANOVA: P < 0.001, N = 5), and deletion of the embedded Gypsy element from PAHAL further inhibited the transcription effect by approximately 50% (one-way ANOVA: P = 0.001, N = 5; ); left panel). Moreover, while PAHAL with SRSF2 overexpression in S2 cells significantly boosted PAH promoter activity (one-way ANOVA: P < 0.001, N = 5; ); right panel), deletion of the embedded Gypsy element from PAHAL absolutely abolished the effect (one-way ANOVA: P < 0.001, N = 5; ), right panel). The Gypsy element and SRSF2 exhibited a significant interaction of the regulatory effects (Mann–Whitney U test: P < 0.001, N = 5; )). Luciferase assay with mutation of the ESEs or NLS in PAHAL in S2 cells further demonstrated that the three tandem ESEs and NLS in the Gypsy of PAHAL are two elements essential for the PAHAL-mediated transcription activation of PAH (one-way ANOVA: P < 0.001, N = 6; )). Therefore, the Gypsy element is crucial for the interaction of PAHAL and SRSF2 during transcriptional activation of PAH mediated by PAHAL.
Discussion
In this study, we demonstrated the functional significance of a TE embedded in a lncRNA for the regulatory role of the lncRNA in the phase change of the migratory locust, because PAHAL is distinct as a transcriptional activator of locust behavioural plasticity, acting by accelerating ancestral PAH gene expression, resulting in DA production. PAHAL harbours a Gypsy element inserted at the 3′ end. The embedded Gypsy element is essential for PAHAL-mediated PAH transcription activation, acting by facilitating the interaction between PAHAL and SRSF2, promoting the nuclear retention of PAHAL and increasing PAHAL RNA stability. These findings highlight the contribution of TEs to the regulatory circuity of lncRNAs in locust phase changes. The Gypsy element-based PAHAL transcriptional regulation mechanism indicates the contribution of TEs to the regulatory circuity of lncRNAs in locust phase changes and the vital role of the embedded TEs in lncRNA-modulated protein activity. The embedded Gypsy element potentially provides new targets for the prevention and control of locust plagues.
The present study revealed the essential roles of embedded TEs in the mediation of the regulatory function of lncRNAs. Some lncRNAs are engaged in gene regulation, depending on their specific sequence and RNA structure [Citation42]. The Gypsy element of PAHAL is an important functional region, boosting PAH transcription activation by not only affecting the nuclear localization and life span of PAHAL but also promoting the assembly of the PAHAL-SRSF2 regulatory complex (). Importantly, these findings may not be limited to PAHAL given the presence of diverse Gypsy elements in numerous lncRNAs, particularly at the 3′ end of lncRNAs (). Thus, our results indicate that a large proportion of lncRNAs are embedded with TEs, as indicated in previous studies [Citation18,Citation43]. All major TE classes (DNA, LTR, SINE, and LINE TEs) were detected in lncRNAs in different vertebrate species [Citation18,Citation44]. The TEs embedded in these lncRNAs could supply the sequences and signals involved in the transcription and processing of lncRNAs, e.g. splicing and poly(A) sites [Citation17,Citation18,Citation45]. For example, the transcription start site of LINCROR RNA is derived from the LTR of the HERVH element [Citation20]. In healthy subjects, repeat D4Z4 expansion induces transcriptional repression of the D4Z4-derived noncoding RNA DBE-T by providing PRC2 attachment sites, preventing the development of facioscapulohumeral muscular dystrophy (FSHD) [Citation9]. The insertion of the inverted LTR of the Gypsy element in the PAH intron may provide an alternative polyadenylation processing site, which may facilitate the biogenesis of PAHAL and PAHAL¯ (). This raises the possibility that, although the types of TEs vary among species, the roles of these TEs in lncRNA biogenesis and regulation may be conserved.
Figure 7. Working model for the embedded Gypsy element-mediated lncRNA transcriptional regulation. The Gypsy element embedded in PAHAL is required for the nuclear retention and RNA stability of PAHAL. Instead, the lncRNA without the element, i.e. PAHAL¯, is exported to the cytoplasm for decay. The embedded Gypsy element boosts the recruitment of SRSF2 to form an RNA-protein complex, which is crucial for the PAHAL-mediated promoter activation of PAH. The red genomic region in the PAH locus represents the insertion site of the Gypsy element.
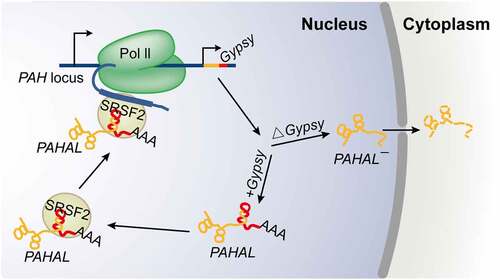
In our study, the embedded Gypsy element was necessary for the nuclear retention of PAHAL RNA in the regulation of PAH transcription. Deletion of the embedded Gypsy element or the NLS in the Gypsy element resulted in the transfer of PAHAL RNA from the nucleus to the cytoplasm (). Similar effects were reported in mice, in which the embedded invSINEB2 was required for nuclear localization of the lncRNA AS Uchl1. AS Uchl1 begins within the second intron of its target gene Uchl1 and overlaps with the first 73 nt of the mRNA [Citation46]. This result of intragenic lncRNAs appears different from the situation with lincRNAs, wherein lincRNAs without TEs are expressed at greater levels than lincRNAs with TEs [Citation20]. In many cells, mRNAs containing inverted repeated Alu elements in their 3′UTRs are inefficiently exported to the cytoplasm [Citation47]. Such mRNAs are retained in the nucleus through binding to paraspeckle-associated complexes [Citation48,Citation49].
The Gypsy-containing lncRNA also exhibited a more stable RNA structure and a longer lifetime of mRNA than the Gypsy-lacking lncRNA. The presence of the Gypsy element at the 3′ end of PAHAL may protect the lncRNA from rapid deadenylation-dependent nuclear decay by forming a triple helix RNA structure and thereby sequestering the PAHAL 3′ poly(A) tail within the internal loop [Citation2,Citation50]. Therefore, the embedded Gypsy element in PAHAL acted as a functional domain to regulate RNA export and transcription ().
The embedded Gypsy element in PAHAL recruits the bifunctional transcription/splicing factor SRSF2 by providing three conserved and tandem ESEs to activate the TF function of SRSF2 (). In contrast, the splicing factor function of SRSF2 is activated by binding with the lncRNA Malat1 [Citation51]. This suggests that although the embedded TEs are not conserved in sequence across species, they may represent conserved and discrete TF binding domains. TEs could improve the complexity of transcriptional regulation events. Embedded TEs enable a relatively small number of TFs to generate distinct combinations of TF-lncRNAs through the combined actions of lncRNAs. The tethering of hnRNPK by other lncRNAs also confirms this hypothesis. In Xist-mediated gene silencing, the B-repeat element of Xist initiates the recruitment of polycomb complexes by binding hnRNPK [Citation52]. While maintaining nuclear speckles in normal cells, hnRNPK is recruited by SINEB1 of Malat1 to improve the recruitment of nuclear speckle-localized RBPs [Citation7]. The high degree of synergy between distinct TF-lncRNA complexes is fundamental for organisms to trigger the precise spatial–temporal regulation of specific gene expression in response to a specific environmental cue. For example, the embedded invSINEB2 element of the lncRNA AS Uchl1 regulates AS Uchl1 nuclear retention and consequently inhibits AS Uchl1-enhanced translation of the sense protein-coding Uchl1 mRNA by recruiting IL enhancer-binding factor 3 (ILF3) [Citation46]. The accumulation of Alu transcripts is responsible for age-related macular degeneration by aberrant Dicer processing [Citation53]. In plants, TE-lncRNAs also play important roles in stress responses [Citation54,Citation55].
TEs could promote regulatory specificity by constructing a complex regulatory network through lncRNAs. The Gypsy element embedded in PAHAL has the potential to form a stable stem-loop structure with the other part of the lncRNA that can facilitate the recruitment of the PAHAL-protein regulatory complex to the specific DNA region. Similar effects were reported in other TE-embedded lncRNAs [Citation18,Citation56]. For example, the 7.5 tandem repeats of the A-repeats of Xist are necessary for X chromosome inactivation through the donation of loop secondary structures and even a tertiary architecture [Citation57]. TE sequences can mediate hybridization to other homologous (sense or antisense) DNA or RNA sequences, for example, through RNA-DNA triplex formation [Citation58–60]. The sense PAHAL lncRNA is expected to change orientation and form a triplex structure with the genomic DNA region where the Gypsy element is embedded [Citation61]. Complementary interactions mediated by the embedded Gypsy sequences could target PAHAL to specific PAH loci [Citation56]. PAHAL recruits SRSF2 via the Gypsy element, facilitating rapid local enrichment of SRSF2. The PAHAL-SRSF2 complex is brought with the promoter of PAH into close spatial proximity by the three-dimensional folding of chromosomes. Subsequently, the ESEs of the nascent RNA of PAH could compete for SRSF2 from the PAHAL-SRSF2 complex to activate the transcription of PAH. We thus speculated that the ESEs of the nascent RNA of PAH bind to SRSF2 with a higher affinity than the Gypsy element of PAHAL, which specifically hybridizes to PAH loci. Therefore, the TEs in lncRNAs appear to act as hubs where nucleic acids and proteins can be agglomerated and facilitate the regulatory specificity of lncRNAs. This mechanism is particularly important for the elaborate control of behavioural plasticity in response to changing environmental signals.
Author contributions
Conceptualization: B.C. and L.K.; Methodology: B.C. and X.Z.; Investigation: X.Z.; Data Analysis: B.C. and X.Z.; Writing of original draft: B.C. and X.Z.; Writing, review, and editing: B.C. and L.K.; Supervision, B.C. and L.K.
Supplemental Material
Download Zip (300.5 KB)Acknowledgments
We are grateful to Xiang-Dong Fu for providing SRSF2-MEFs.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482:339–346.
- Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17:47–62.
- Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys. 2011;508:1–12.
- Chen LL. Linking long noncoding RNA localization and function. Trends Biochem Sci. 2016;41:761–772.
- Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15:7–21.
- Bernard D, Prasanth KV, Tripathi V, et al. A long nuclear-retained non-coding RNA regulates synaptogenesis by modulating gene expression. EMBO J. 2010;29:3082–3093.
- Nguyen TM, Kabotyanski EB, Reineke LC, et al. The SINEB1 element in the long non-coding RNA MALAT 1 is necessary for TDP-43 proteostasis. Nucleic Acids Res. 2020;48:2621–2642.
- Soshnev AA, Ishimoto H, McAllister BF, et al. A conserved long noncoding RNA affects sleep behavior in Drosophila. Genetics. 2011;189:455–468.
- Cabianca DS, Casa V, Bodega B, et al. A long ncRNA links copy number variation to a polycomb/trithorax epigenetic switch in FSHD muscular dystrophy. Cell. 2012;149:819–831.
- Spadaro PA, Flavell CR, Widagdo J, et al. Long noncoding RNA-directed epigenetic regulation of gene expression is associated with anxiety-like behavior in mice. Biol Psychiatry. 2015;78:848–859.
- Ma Z, Guo W, Guo X, et al. Modulation of behavioral phase changes of the migratory locust by the catecholamine metabolic pathway. Proc Natl Acad Sci USA. 2011;108:3882–3887.
- Yang M, Wei Y, Jiang F, et al. MicroRNA-133 inhibits behavioral aggregation by controlling dopamine synthesis in locusts. Plos Genet. 2014;10:e1004206.
- Zhang X, Xu Y, Chen B, et al. Long noncoding RNA PAHAL modulates locust behavioural plasticity through the feedback regulation of dopamine biosynthesis. PLoS Genet. 2020;16:e1008771.
- Carlevaro-Fita J, Polidori T, Das M, et al. Ancient exapted transposable elements promote nuclear enrichment of human long noncoding RNAs. Genome Res. 2019;29:208–222.
- Mercer TR, Mattick JS. Structure and function of long noncoding RNAs in epigenetic regulation. Nat Struct Mol Biol. 2013;20:300–307.
- Johnson R, Guigo R. The RIDL hypothesis: transposable elements as functional domains of long noncoding RNAs. RNA. 2014;20:959–976.
- Gong C, Maquat LE. LncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3ʹUTRs via Alu elements. Nature. 2011;470:284–288.
- Kapusta A, Kronenberg Z, Lynch VJ, et al. Transposable elements are major contributors to the origin, diversification, and regulation of vertebrate long noncoding RNAs. PLoS Genet. 2013;9:e1003470.
- Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641.
- Kelley D, Rinn J. Transposable elements reveal a stem cell-specific class of long noncoding RNAs. Genome Biol. 2012;13:R107.
- Llorens C, Futami R, Covelli L, et al. The Gypsy database (GyDB) of mobile genetic elements: release 2.0. Nucleic Acids Res. 2010;39:D70–D74.
- Galindo-González L, Mhiri C, Deyholos MK, et al. LTR-retrotransposons in plants: engines of evolution. Gene. 2017;626:14–25.
- Feschotte C. Transposable elements and the evolution of regulatory networks. Nat Rev Genet. 2008;9:397–405.
- Mourier T, Willerslev E. Retrotransposons and non-protein coding RNAs. Brief Funct Genomic Proteomic. 2009;8:493–501.
- Tran HH, Dang SNA, Nguyen TT, et al. Drosophila ubiquitin C-terminal hydrolase knockdown model of Parkinson’s disease. Sci Rep. 2018;8:4468.
- Podbevšek P, Fasolo F, Bon C, et al. Structural determinants of the SINE B2 element embedded in the long non-coding RNA activator of translation AS Uchl1. Sci Rep. 2018;8:3189.
- Carrieri C, Cimatti L, Biagioli M, et al. Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature. 2012;491:454–457.
- Jiang F, Yang M, Guo W, et al. Large-scale transcriptome analysis of retroelements in the migratory locust, Locusta migratoria. Plos One. 2012;7:e40532.
- Guo W, Wang XH, Zhao DJ, et al. Molecular cloning and temporal-spatial expression of I element in gregarious and solitary locusts. J Insect Physiol. 2010;56:943–948.
- Chen B, Li SQ, Ren Q, et al. Paternal epigenetic effects of population density on locust phase-related characteristics associated with heat-shock protein expression. Mol Ecol. 2015;24:851–862.
- Lin SR, Xiao R, Sun PQ, et al. Dephosphorylation-dependent sorting of SR splicing factors during mRNP maturation. Mol Cell. 2005;20:413–425.
- Yang P, Hou L, Wang X, et al. Core transcriptional signatures of phase change in the migratory locust. Protein Cell. 2019;10:883–901.
- Guo W, Wang X, Ma Z, et al. CSP and takeout genes modulate the switch between attraction and repulsion during behavioral phase change in the migratory locust. Plos Genet. 2011;7:e1001291.
- Gonzalez I, Munita R, Agirre E, et al. A lncRNA regulates alternative splicing via establishment of a splicing-specific chromatin signature. Nat Struct Mol Biol. 2015;22:370–376.
- Wei Y. Epigenetics of Locusta migratoria: small RNAs involved in phase transitions. (Doctoral dissertation). Institute of zoology: Chinese academy of Sciences, Beijing (China), 2010.
- Beckedorff FC, Ayupe AC, Crocci-Souza R, et al. The intronic long noncoding RNA ANRASSF1 recruits PRC2 to the RASSF1A promoter, reducing the expression of RASSF1A and increasing cell proliferation. PLoS Genet. 2013;9:e1003705.
- He J, Chen Q, Wei Y, et al. MicroRNA-276 promotes egg-hatching synchrony by up-regulating brm in locusts. Proc Natl Acad Sci USA. 2016;113:584–589.
- Zhang B, Gunawardane L, Niazi F, et al. A novel RNA motif mediates the strict nuclear localization of a long noncoding RNA. Mol Cell Biol. 2014;34:2318–2329.
- Daubner GM, Cléry A, Jayne S, et al. A syn–anti conformational difference allows SRSF2 to recognize guanines and cytosines equally well. EMBO J. 2012;31:162–174.
- Ji X, Zhou Y, Pandit S, et al. SR proteins collaborate with 7SK and promoter-associated nascent RNA to release paused polymerase. Cell. 2013;153:855–868.
- Bao WD, Kojima KK, Kohany O. Repbase update, a database of repetitive elements in eukaryotic genomes. Mob DNA. 2015;6:11.
- Engreitz JM, Haines JE, Perez EM, Munson, G, Chen, J, Kane, M, McDonel, PE, Guttman, M, Lander, ES, et al. Neighborhood regulation by lncRNA promoters, transcription, and splicing. Nature. 2016;539(7629);452–455.
- Kannan S, Chernikova D, Rogozin IB, et al. Transposable element insertions in long intergenic non-coding RNA genes. Front Bioeng Biotechnol. 2015;3:71.
- Hu S, Wang X, Shan G. Insertion of an Alu element in a lncRNA leads to primate-specific modulation of alternative splicing. Nat Struct Mol Biol. 2016;23:1011–1019.
- Lee JY, Ji Z, Tian B. Phylogenetic analysis of mRNA polyadenylation sites reveals a role of transposable elements in evolution of the 3ʹ-end of genes. Nucleic Acids Res. 2008;36:5581–5590.
- Fasolo F, Patrucco L, Volpe M, et al. The RNA-binding protein ILF3 binds to transposable element sequences in SINEUP lncRNAs. FASEB J. 2019;33:13572–13589.
- Hu SB, Xiang JF, Li X, et al. Protein arginine methyltransferase CARM1 attenuates the paraspeckle-mediated nuclear retention of mRNAs containing IRAlus. Genes Dev. 2015;29:630–645.
- Chen LL, DeCerbo JN, Carmichael GG. Alu element‐mediated gene silencing. EMBO J. 2008;27:1694–1705.
- Chen LL, Carmichael GG. Altered nuclear retention of mRNAs containing inverted repeats in human embryonic stem cells: functional role of a nuclear noncoding RNA. Mol Cell. 2009;35:467–478.
- Brown JA, Bulkley D, Wang J, et al. Structural insights into the stabilization of MALAT1 noncoding RNA by a bipartite triple helix. Nat Struct Mol Biol. 2014;21:633–640.
- Tripathi V, Ellis JD, Shen Z, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–938.
- Pintacuda G, Wei G, Roustan C, et al. hnRNPK recruits PCGF3/5-PRC1 to the Xist RNA B-repeat to establish polycomb-mediated chromosomal silencing. Mol Cell. 2017;68:955–969 e910.
- Kaneko H, Dridi S, Tarallo V, et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011;471:325–330.
- Wang D, Qu Z, Yang L, et al. Transposable elements (TEs) contribute to stress-related long intergenic noncoding RNAs in plants. Plant J. 2017;90:133–146.
- Lv Y, Hu F, Zhou Y, et al. Maize transposable elements contribute to long non-coding RNAs that are regulatory hubs for abiotic stress response. BMC Genomics. 2019;20:864.
- Holdt LM, Hoffmann S, Sass K, et al. Alu elements in ANRIL non-coding RNA at chromosome 9p21 modulate atherogenic cell functions through trans-regulation of gene networks. PLoS Genet. 2013;9:296.
- Windrem MS, Osipovitch M, Liu Z, et al. Human iPSC glial mouse chimeras reveal glial contributions to schizophrenia. Cell Stem Cell. 2017;21:195–208.e196.
- Mondal T, Subhash S, Vaid R, et al. MEG3 long noncoding RNA regulates the TGF-β pathway genes through formation of RNA-DNA triplex structures. Nature Commun. 2015;6:7743.
- Cloutier SC, Wang S, Ma WK, et al. Regulated formation of lncRNA-DNA hybrids enables faster transcriptional induction and environmental adaptation. Mol Cell. 2016;61:393–404.
- Postepska-Igielska A, Giwojna A, Gasri-Plotnitsky L, et al. LncRNA Khps1 regulates expression of the proto-oncogene SPHK1 via triplex-mediated changes in chromatin structure. Mol Cell. 2015;60:626–636.
- Li Y, Syed J, Sugiyama H. RNA-DNA triplex formation by long noncoding RNAs. Cell Chem Biol. 2016;23:1325–1333.
