ABSTRACT
Small RNAs (sRNAs) are short non-coding regulatory RNA sequences that silence the complementary expressive transcripts through an endogenous RNA mediated interference mechanism (RNAi). These sRNAs typically move through plasmodesmata and phloem in plants to support disease resistance, and also through septal pores and vesicles in fungi to act as effector of pathogenicity. Notably, recent reports have shown the occurrence of a bidirectional trafficking of these sRNAs between the host plants and the attacking fungal phytopathogen which have significant implication in the nature of the infection. While the trans-species sRNAs from the pathogen can silence the host mRNAs and inhibit the host immunity genes, the sRNA modules from the host plants can silence the mRNA in the pathogen by impeding the expression of the pathogenicity-related genes. In the present review, we discuss the current state of sRNA trafficking between the plant and the pathogen with special emphasis on the mechanism of cross-kingdom communication which could contribute to the development of pathogen and pest control in future agriculture.
Introduction
Eukaryotic small RNAs (sRNAs) are 20–30 nucleotides short functional regulatory RNA molecules that are implicated in growth, development, metabolism, genome integrity, and plant–pathogen interaction [Citation1]. sRNAs are widely spread in eukaryotes and regulate the gene by transcriptional gene silencing or post-transcriptional gene silencing, through the creation of mRNA break, target inhibition, and/or DNA methylation [Citation2,Citation3]. Among other, the major functions of plant small RNAs are to inhibit infections of pathogens and pests by inducing RNA interference (RNAi). RNA interference is a conserved, negative regulatory mechanism in eukaryotes and plays a pivotal role in growth, development, and host–pathogen interaction. The sRNAs loaded with the endonuclease or DICER-like protein bind to AGO and degrade the passenger strand while the guide RNA binds to the RNA-induced silencing complex (RISC) to target and silence the complementary mRNA sequence to control the expression of genes at the transcription and/or post-transcription level [Citation4]. Previously, it was believed that miRNA is non-existent in fungi. However, accumulated evidences have shown that RNAi mechanisms have a conserved, defensive, and regulatory role in maintaining genome integrity in fungi. The advent of high throughput sequencing in various ascomycetes and basidiomycetes, has led to the identification of miRNA-like RNAs (miLRNAs) similar to those found in plants and animals [Citation5–7]. After a successful fungal infection, the defence machinery of the plants is hijacked by the pathogen using these sRNAs [Citation8].
Plant-derived sRNAs can be transported to fungus as naked sRNAs, or bound to RNA-binding proteins (RBP), or enclosed inside the extracellular vesicle [Citation9]. Plant sRNAs often target the silencing of fungal genes encoding for virulence, toxin production or pathogenesis. Contrary to this, fungi can also transport sRNAs to plants for silencing the defence mechanism. These sRNAs can move through vesicles or by binding with RNA binding protein or in naked form to induce pathogenesis [Citation9]. While the extracellular vesicle (EV)-mediated sRNA transport has been reported in Botrytis cineria [Citation10], other studies have demonstrated that the majority of exRNAs are not bound to EVs [Citation11]. The analysis of the apoplastic wash fluid (AWF) purified from Arabidopsis leaves have shown that the extra-cellular naked sRNAs located outside the vesicles contribute to host-induced gene silencing [Citation11]. Based on this, the pathogen-derived sRNAs could be divided into two types: − 1) endogenous sRNAs that help in activating the virulence gene during pathogenesis, and 2) sRNAs that transport into the host cell and silence the plant immunity genes thereby acting like a sRNA effector [Citation12]. This deployment of sRNA between plants and fungi through plasma membrane for silencing the virulence gene(s) in the pathogen or the defence responsive gene(s) in plants by RNAi approach is known as cross-kingdom communication. The movement of cell non-autonomous sRNAs between two plant cells is a short-range communication that occurs through the desmotubule of plasmodesmata [Citation12]. However, the long-distance movement of sRNAs from top of the shoot to the bottom of the root is mediated by a phloem sieve tube [Citation13]. Mobile miRNAs have shown clear characteristics of a signalling molecule for plant development and stress response as evidenced from the cellular transportation of miR399 during phosphate starvation in plants [Citation14]. Alternatively, some plants can release packaged sRNAs outside the cells with the EV to be absorbed by a fungal pathogen [Citation15]. Such EV mediated cross-kingdom targeting mechanism has been detected in many plants including tomato [Citation16], and sunflower [Citation17]. Interestingly, these EV-coated sRNAs are transported into pathogenic fungi to inhibit the pathogenic responsive genes in Gossypium hirsutum and Cuscuta campestris [Citation18,Citation19]. On the other hand, a wide range of pathogenic fungi such as Botrytis cinerea [Citation20,Citation21], Verticillium dahlia [Citation22], Fusarium graminearum [Citation23], Hyaloperonospora arabidopsidis [Citation24], Blumeria graminis f. sp. tritici [Citation25] and Fusarium oxysporum f sp. lycopersici [Citation26] transport sRNAs to plants and silence the resistance mechanism. Therefore, it is essential to understand the mechanism of sRNA movement across the plant and pathogen to exploit them for agricultural benefits.
In the recent time, several RNAi-based disease prevention mechanisms have been recommended for crop protection. This includes host-induced gene silencing (HIGS) [Citation27], spray-induced gene silencing (SIGS) and nanocarrier-based spray-induced gene silencing (nSIGS), which are considered eco-friendly, stable, efficient, and sustainable strategies for protection against plant infection. In HIGS, dsRNAs and siRNAs are generated from the transgenic plant, which act upon the eukaryotic pathogen or pest. Likewise, in SIGS and nSIGS, the silencing effect is realized through exogenous spray of dsRNAs and siRNAs on the topical part of leaves infected with the pathogen [Citation28]. Also, the CRISPR/Cas9 system has emerged as one of the most novel approach for silencing of host genes and/or the pathogenic genes to regulate the host–pathogen interaction [Citation29,Citation30]. In this review, we have made a comprehensive discussion about the mechanisms of sRNA communication between plants and phytopathogenic fungi with special emphasis on EV mediated transport of sRNAs. We have also discussed about the utilization of various RNAi based approaches and their utilization in the development of crop protection strategies.
Cross kingdom communication of sRNAs between fungi and plants
Plants and pathogens interact by secreting a variety of virulence factors known as microbe associated molecular patterns (MAMPs). These MAMPs are recognized by the MAMP receptors on plant cell surfaces, thereby inducing a defence response. However, many pathogens also secrete effector proteins into host cells that intercept defence signalling and induce pathogenesis [Citation31]. These effectors are either apoplastic in nature and interact with the cell surface receptors or cytoplasmic in nature and are translocated inside the plant cell [Citation32]. More than 80 effector proteins have been cloned and characterized from major crop-infecting fungi, the majority of which are encoded by avirulence (Avr) genes [Citation33]. Recent research is beginning to reveal that fungal phytopathogens also secrete RNAs into their interacting host. This deployment of small RNA among two different interacting organisms, such as during the host–pathogen interaction, is termed as cross-kingdom sRNA interference (ckRNAi) [Citation34]. ckRNAi was first reported when the black mould fungus Botrytis cinerea sRNA was detected in the target host plants Arabidopsis thaliana and Solanum lycopersicum [Citation20]. Bc-sRNAs inhibited the AGO1 protein expression and silenced the plant immunity by creating a favourable condition for successful fungal colonization and the development of disease symptoms. These sRNAs therefore could act like a class of pathogen effectors that could regulate the host immunity by silencing the defence responsive genes in plants. In another study, Arabidopsis cells were found to secrete extracellular vesicles to transfer sRNAs into Botrytis cinerea that induced silencing of fungal genes critical to pathogenicity [Citation10]. sRNA transfer from the pathogen to the host has also been reported in animal host–parasite interactions. For example, sRNA from the fungal pathogen Beauveria bassiana was transported into mosquitoes, resulting in the silencing of toll receptor spatzle protein 4 [Citation35]. Asiatic rice stem borer, Chilo suppressalis, when fed with insect-specific miRNAs, miR-14 declined the expression of a host-specific spook (spo) and ecdysone receptor (EcR) gene, leading to large-scale mortality and developmental defects [Citation36]. Thus, the mechanism of cross-kingdom RNAi mediated by pathogen derived sRNA not only plays a major role in the control of pathogenesis but also has a significant contribution in identifying disease-causing genes that could be used in the development of novel crop protection strategies [Citation37].
Plant sRNAs and their targets in fungi
Plants not only communicate endogenously through intracellular signalling pathways but also connect externally using various secretory molecules, hormones, volatile organic compounds, and sRNAs [Citation38,Citation39]. Plant-derived sRNAs play a major role in defence response mechanisms against various pathogenic organisms including fungi, water mould, and parasitic plants by inducing gene silencing mechanisms [Citation40]. Verticillium dahlia is a soil-borne, hemibitrophic fungal pathogen responsible for devastating wilt diseases in many plants. sRNA sequencing of V. dahlia recovered from the infected cotton plants has revealed the presence of two cotton miRNAs, namely Ga-miR166 and Ga-miR159, in the pathogen [Citation18] (). These miRNAs are responsible for inhibiting the virulence of V. dahlia by silencing the Ca2+ -dependent cysteine protease calpain (clp-1) and isotrichodermin C-15 hydroxylase (HiC-15) genes. C-15 hydroxylase, which produces trichothecene metabolites, and clp-1 are both involved in fungal phytopathogenicity [Citation18]. Arabidopsis thaliana also exports sRNA to the necrotrophic fungus Botrytis cinerea and inhibits defence genes. Among the 17 Arabidopsis tetraspanin genes (TET1-TET17), TET-8 and TET-9 have shown increased expression following B. cinerea infection [Citation41]. Cai et al. [Citation10] reported that host sRNAs released into the apoplastic space are encapsulated in the TET-8 associated exosomes and transferred into Botrytis cinerea to inhibit the pathogenicity [Citation10]. Arabidopsis also deploys endogenous transcript-derived siRNAs into Phytophthora capsici oomycetes to silence the virulence-related genes in the phytopathogen [Citation42]. Inhibition of fungal pathogenesis has also been achieved through the spraying of double-stranded-noncoding small RNA (ds-sRNA) on the infected plant parts. For instance, small RNA-induced gene silencing (SIGS) has been performed by spraying a long noncoding dsRNA (791 nt CYP3-dsRNA) in barley infected with Fusarium graminaream. The dsRNA targets the fungal ergosterol gene, such as cytochrome P450 lanosterol C-14α-demethylases, causing inhibition of the fungal growth [Citation28]. Overall, these evidences indicate that plant sRNAs are presumably translocated into fungal phytopathogens to inhibit the expression of virulence factors in the pathogen and augment the host defence response.
Table 1. Plant sRNAs as regulator of fungal pathogenicity.
Fungal sRNAs and cross-kingdom RNAi
Recently, a wide range of pathogenic sRNAs have been reported to get translocated into host plants, causing loss of function of the host resistance through cross-species RNAi (). For instance, the necrotrophic fungal pathogen B. cinerea translocates sRNAs (Bc-sRNAs) to the target host plant and hijacks the defence and immune-responsive genes of the plant by suppressing RNAi machinery [Citation20]. Bc-sRNA effectors detected in Arabidopsis thaliana and Solanum lycopersicum are Bc-siR3.1, Bc-siR3.2, Bc-siR5, and Bc-siR3.7, all of which are 21 nt in length and have a significantly higher count in planta as compared to cultured fungal biomass [Citation20]. In Arabidopsis, Bc-siR3.2 targeted the genes encoding mitogen-activated protein kinase-1(MPK-1) and mitogen-activated protein kinase-2 (MPK-2), Bc-siR3.1 targeted the oxidative stress-related gene encoding peroxiredoxin (PRXIIF), and Bc-siR5 targeted the gene encoding a cell wall-associated kinase (CAW). Similarly, Bc-siR37 targeted as many as 15 immunity-specific host genes encoding WRKY7 transcription factor, protein kinases, FE12, PMR6 and RING-finger proteins [Citation21]. Likewise, Bc-siR3.2 in S. lycopersicum also targeted the MPK signalling pathways, with the primary target being, Sly-MPKKKK4 gene [Citation21]. All these genes targeted by the fungal sRNAs are involved in defence signalling and host metabolism [Citation20]. Thus, the translocated fungal sRNAs create a suitable environment for the growth of pathogens by silencing the host immune-responsive and growth-specific transcription factor genes. Notably, the pathogenic nature of the Bc-siR3.1, Bc-siR3.2, and Bc-siR5 depended on the function of the DCL protein of B. cinerea and they suppressed the cell’s RNAi mechanism by binding to the AGO protein of the host [Citation21]. Interestingly, all the Bc-sRNAs originated from the long terminal repeat (LTR) transposon region [Citation21]. LTRs are rapidly evolving groups of transposable elements and would be required for the co-evolution of RNAi mechanism [Citation43] as required for host–pathogen interaction. In another study, V. dahlia sRNAs (Vd-sRNAs) were also reported to silence defence responsive genes in Arabidopsis by binding with the host AGO1 protein [Citation44]. In a corroborative study, sRNA profiling detected a virulent specific precursor miRNA like RNA1 (VdMILR1) gene within the region of 2,083,943–2083870 of chromosome 8 of V. dahliae genome [Citation22]. The matured VdmiLR1 was synthesized through a DCL and AGO protein independent pathway and targeted a hypothetical virulence protein coding gene, VdHy1 through increased H3K9 methylation at the 3’UTR region. Thus, the epigenetic regulation of the fungal virulence factor VdHy1 by VdmiLR1 suggests a crucial role for sRNAs in the regulation of pathogenicity that can have a significant impact on pest management. In yet another recent study, sRNAs from the oomycete Hyaloperonospora arabidopsidis employ the host plant’s Argonaute (AGO)/RNA-induced silencing complex for virulence [Citation24]. A genome-wide sRNA profiling revealed 133 unique sRNAs (Hpa-sRNAs) from H. arabidopsidis, 33 of which have their target sites in the Arabidopsis genome. Two AtAGO1 enriched sRNA candidates, Hpa-sRNA2 and Hpa-sRNA90 were predicted to target the WITH NO LYSINE (K) KINASE (AtWNK)2 and APOPLASTIC, ENHANCED DISEASE SUSCEPTIBILITY1-DEPENDENT (AtAED3) genes, respectively. While AtAED3 is implicated in systemic immunity, AtWNK2 has been reported to be involved in flowering and the abiotic stress response [Citation24]. This suggests that HpasRNAs exhibit cross-kingdom RNAi (ck-RNAi) by silencing plant genes that contribute to host immunity.
Table 2. Pathogenic sRNAs impairing host immune responses through cross-kingdom RNA interference.
sRNA communication has also been reported from agriculturally significant phytopathogens infecting cereal crops. Puccinia striiformis f. sp. tritici (Pst), one of the most destructive pathogens of wheat. In planta sRNA profiling have detected a novel microRNA like RNA termed Pst-milRNA1 which targeted the wheat pathogenesis-related 2 (PR2) gene [Citation21]. Pst-milR1 is 20 bp in length, starts with uridine, and specifically targets the poly-adenylated (Poly-A) region of the beta-1,3 glucanase, a PR2 gene. Co-transformation and silencing of Pst-milR1 resulted in increased resistance, while the PR2 knock-down plants demonstrated increased susceptibility to Pst in wheat. The biotrophic fungi Blumeria graminis f. sp. hordei (Bgh) and Blumeria graminis f. sp. tritici (Bgt) cause powdery mildew disease in barley and wheat, respectively, with an evolved RNAi machinery that expresses during infection [Citation25]. A genome-wide sRNA profiling has revealed that 6 candidate sRNAs from Bgt and 15 from Bgh have solely predicted exclusive plant target genes and induced RNAi by potentially affecting various catabolic and plant transport systems during infection [Citation25]. Likewise, the Fusarium head blight caused by Fusarium graminearum is another devastating disease of wheat that causes significant yield losses. sRNA profiling of the fungal phytopathogen has reported 264 pathogenic sRNAs that have predicted targets in the wheat genome [Citation23]. Among others, Fg-sRNA1 specifically targeted a resistance related gene encoding Chitin Elicitor Binding Protein (TaCEBiP). Western blotting and fluorescence observation have shown that Fg-sRNA1 suppressed the accumulation of TaCEBiP in vitro and enhanced the invasion of F. graminearum strain PH-1. This suggests that pathogenic sRNAs like Pst-milR1 and Fg-sRNA1 are important pathogenicity factor that act as effectors to suppress host immunity by silencing the resistance-related genes in wheat.
Sclerotinia sclerotiorum is an ascomycete fungus with a wide host range of more than 600 plant species. Derbyshire et al. [Citation45] reported that S. sclerotiorum produces nearly 374 highly abundant sRNAs during infection of two of its prominent hosts, Arabidopsis thaliana and Phaseolus vulgaris. Most of these pathogenic sRNAs were predicted to target the host genes with functional domains associated with plant immunity and quantitative disease resistance (QDR). The silencing of two of the Arabidopsis target genes, AtSERK2 and AtSNAK2 resulted in enhanced susceptibility to S. sclerotiorum than the wild-type plants. While At-SERK2 belongs to the brassinosteroid, PAMP, and DAMP signalling pathways, AtSNAK2 is implicated in carbon signalling and systemic resistance [Citation45]. This suggests that the cross-kingdom movement of S. sclerotiorum sRNA contributes to the silencing of immune components in host plants. In a similar fashion, Phytophthora infestans AGO1 associated sRNAs also exhibit cross-kingdom translocation during potato leaf infection [Citation46]. Extensive target analysis of potato with pathogen derived sRNAs identified 648 sequences encoding for resistance (R) proteins. A single pathogen-specific miRNA (miR8788) was reported to target a potato alpha/beta hydrolase-type encoding gene (StABH1) encoding a membrane protein. The overexpression of StABH1 in potato inhibited the infection, while the miR8788 knock out pathogenic strain showed growth of the pathogen in potato [Citation46]. This suggests that the sRNAs encoded by P. infestans affect potato mRNA through cross-kingdom RNAi, further expanding our knowledge of the multifaceted strategies employed by this pathogen to facilitate infection.
sRNA communication has also been reported in fungal pathogens infecting fruits and vegetables. Citrus blue mould, caused by Penicillium italicum (Pit) is one of the most devastating pathogens of post-harvest citrus fruits, resulting in losses up of to 80%. The silencing of Pit-DCL1 and Pit-DCL2 has shown a variable response to disease development [Citation47]. The Pit-DCL2 RNAi transformants showed impaired pathogenicity as compared to Pit-DCL1 RNAi lines or the wild-type strain. Interestingly, sRNA profiling detected 12 novel milRNAs, 4 of which have predicted targets encoding innate-immunity or biotic stress-related proteins. More precisely, Pit-novel 7 milRNA targeted the AP2/B3 like transcription factor associated with plant immunity. This indicates that sRNA trafficking from P. italicum to citrus fruits was important in the molecular virulence mechanism during the P. italicum-citrus interaction. Likewise, Valsa mali, the necrotrophic ascomycete fungus that causes apple valsa canker disease have demonstrated adaptive regulation of virulence genes by milRNAs [Citation48]. Vm-milR16 was reported as a significant contributor to pathogenicity by adaptively regulating the expression of sucrose non-fermenting 1 (VmSNF1), 4,5-DOPA dioxygenase extradiol (VmDODA), and a hypothetical (VmHy1) protein-coding gene. However, the cross-specific movement of Vm-milR16 or its expression in planta is yet to be established. Recent evidence also indicates that milRNAs from Fusarium oxysporum f. sp. lycopersici (Fol) interfere with tomato resistance during infection [Citation26]. sRNA profiling revealed that Fol-milRNA1 is exported into tomato plants during infection, and its overexpression in planta led to enhanced virulence. Fol-milR1 targeted the tomato calcineurin-B like (CBL) protein kinase and inhibited its expression at the post-transcriptional level. Further, Fol-milR1 interfered with the host immunity machinery by binding to tomato AGO 4a. Taken together, all these studies clearly suggests that pathogenic sRNAs, are an important type of pathogenicity factor that contributes to impairing host immune responses through cross-kingdom RNA interference (ck-RNAi).
sRNAs transport between plant and fungi through extracellular vesicles
Extracellular vesicles are membrane-bound vesicular compartments released by cells to the extracellular location to facilitate cell-to-cell transport of proteins, lipids and macromolecules, cellular invasion, and trafficking with the interacting organisms [Citation49]. In animals, the EVs are classified as exosomes derived from the multivesicular bodies (MVBs) and the microvesicles derived from the plasma membrane that are involved in cell-to-cell communication [Citation50]. EVs are uniquely positioned as vectors for cross-kingdom RNAi dissemination by protecting them from enzymatic degradation and providing opportunities for cell targeting [Citation51]. Accumulated evidence and previously cited studies suggest that plant cells as well as fungi secrete different classes of EVs that have a significant role in sRNA trafficking [Citation10]. In mammals, MVBs are enriched with tetraspanin (TET) proteins on their membrane, which are commonly used as biomarkers. During plant–fungus interaction, both plants and fungi traffic sRNA across each other for silencing the target genes through EVs [Citation52]. When a plant deploys sRNAs to the fungus and inhibits their gene expression by targeting the pathogenesis and virulence-related genes, it is termed as HIGS, on the contrary when a fungus deploys small RNAs to the plant’s target genes and inhibits the gene expression is known as pathogen-induced gene silencing (PIGS) [Citation53]. Interestingly, Arabidopsis has 17 members of the TET family proteins, two of which, TET8 and TET9, are induced upon B. cineria infection [Citation10]. TET8 associated EVs in Arabidopsis are derived from MVBs and contain plant endogenous sRNAs that are taken up by B. cinerea. These exosomes deliver the plant-specific sRNAs into the fungal cell to suppress the infection by silencing the fungal pathogenicity-related genes [Citation10]. During pathogenic infections, EVs are secreted from the Golgi apparatus and supplemented with proteins, lipids, coding RNAs, noncoding RNAs, and other constituents [Citation54] (). Likewise, the EVs are also used by Arabidopsis for the transport of secondary phasiRNAs into Phytopthora capsici to silence the target genes in the pathogen [Citation40]. In a previous study, exosomes in the sunflower seedling were reported to transport sRNA to the fungal pathogen Sclerotinia sclerotium, leading to suppression of its virulence properties [Citation17]. More recently, tomato exosomes significantly inhibited the germination of spores at the infection site after treatment with multiple fungal phytopathogens, including B cinerea, Alternaria alternata, and Fusarium oxysporum [Citation16].
Figure 1. Cross kingdom RNA interference in plant–fungus interactions. Plant derived small RNAs (sRnas) can be packaged by Golgi and is efficiently absorbed by fungal cell which inhibits germination of spore and development of mycelia by cleaving the pathogenicity target gene. Fungal pathogen also deploy group of sRnas to plant to silences the host resistance gene. Plant use extracellular vesicle to transport small RNA into the fungal cell for inhibiting virulence related genes. The mechanism of transport for fungal sRnas is unclear.
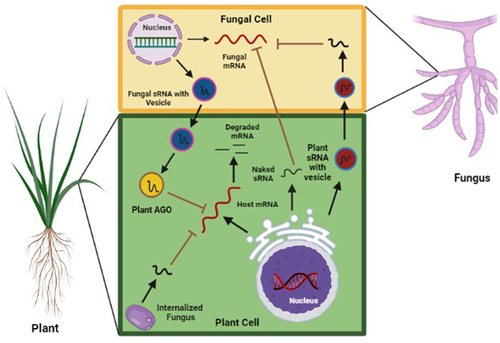
In addition to exosomes, the PENETRATION (PEN)1-associated EVs that contain several stress-related proteins have also been purified from the apoplast wash fluid of Arabidopsis leaves, suggesting that they could be associated with sRNA transport [Citation55]. Baldrich et al. [Citation56] reported the presence of tiny RNAs of 10 to 17 nt length in PEN-associated EVs, but their role in plant–pathogen interaction could not be ascertained. Arabidopsis PEN1 and PEN3 associated EVs were reported from the extracellular surrounding of the haustoria of the mildew fungus, Golovinomyces orontii, suggesting that they contribute to the defence response presumably through the transport of tiny RNAs [Citation57]. Over and above these, a third type of plant EV derived from a double membrane-bound excocyst-positive organelle (EXPO) has been reported from the apoplastic region of the Arabidopsis cells [Citation58]. However, their role in sRNA delivery or ck-RNAi is not clear. Nevertheless, the plants have adapted an EV-mediated ck-RNAi as part of an evolutionary defence mechanism for interacting with the fungal phytopathogens.
Secretion of EVs by fungi was reported in the 1960s. Hyphae of true fungi (Eumycota) secreted vesicles, termed lomasomes, that looked and behaved a lot like MVBs [Citation59]. As in plants, the EV formation in fungi is also regulated by the endosomal sorting complex required for transport (ESCRT) [Citation60]. These fungal MVBs were reported to fuse with the plasma membrane and release their content into the cell wall [Citation61]. Analysis of Aspergillus fumigatus protoplasts has recently shown that specific EVs are generated through budding in the plasma membrane similar to the microvesicles [Citation62]. An unconventional protein secretion pathway using EVs has also been reported in Phytophthora infestans, and in the rice blast fungus Magnaporthe oryzae to deliver effectors into the cytoplasm of their hosts [Citation62]. As a large number of pathogenic sRNAs have been localized in plant cytoplasm, it is most likely possible that fungal EVs would be the carriers of the sRNAs to facilitate ck-RNAi in plants. However, additional studies are needed to fully interpret the mechanism of EV-mediated ck-RNAi during host–pathogen interaction.
sRNAs transportation outside the extracellular vesicles
The experimental research discussed in the previous section suggests that the apoplastic sRNAs either get encapsulated in the EVs or tightly bound to the RNA binding proteins to avoid degradation [Citation63]. However, the removal of EVs from the AWF does not always deplete the concentration of sRNAs, suggesting that EVs are not the primary location of these siRNAs and miRNAs [Citation56]. This contradictory representation has been recently addressed through a seminal experiment by the Roger Innes group [Citation11]. EVs collected from the AWF of Arabidopsis leaves, when treated with protease and RNAse A, eliminated the majority of sRNAs of size classes 21, 22, and 24 nt. Besides, the long non-coding RNAs (lnc-RNAs), including the circular RNAs (circRNAs) also form part of the extracellular RNAs enriched with the post-transcriptional modification of N6-methyadenine sites, meaning that they are stabilized by binding with glycin rich RNA binding proteins or the Argonaute protein [Citation11]. While the lncRNAs have been implicated in multiple biological processes, including gene expression and stress response, their role in cell-to cell communication in the apoplast region is yet to be investigated. In animal system, the circRNAs have been reported from EV preparations recovered from cell culture media, suggesting that the excreted circRNAs could contribute to cellular communications [Citation64]. Taken together, it is very alluring to speculate that the lncRNAs, including the circRNAs could be responsible for pathogenic sRNA sequestration in the apoplast before they are target delivered inside the host cell [Citation11,Citation65]. In other words, the extracellular RNAs located outside the EVs could also be responsible for HIGS. However, the exact nature of the non-EV sRNA transportation requires further investigation.
Cross-kingdom sRNA communication for future agriculture
Phytopathogenic fungi are the major cause of yield losses in agriculturally important crops and affect global food security. Therefore, it is imperative to use all the alternative approaches, including the usage of sRNAs, to develop high-yielding resistant crop varieties for balanced nutrition and food security of an ever-increasing world population [Citation66]. Both plants and animals require innovative and durable antimicrobial drugs, biodegradable fungicides, and insecticides to control pathogen infection. sRNA can transport between host and pathogen through cellular boundaries and therefore could be directly used for crop improvement. ck-RNAi-based direct application strategies, including HIGS, spray-induced gene silencing (SIGS), and virus-induced gene silencing (VIGS) have been recently recommended for crop protection [Citation67].
Host induced gene silencing
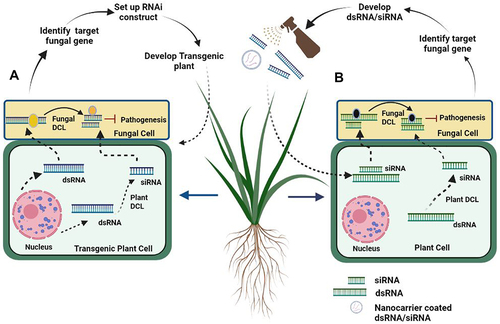
HIGS is a promising RNAi-based technology in which genetically engineered plants express dsRNAs or sRNAs that target and silence virulence-related genes through RNAi to control plant diseases () [Citation68]. HIGS has proven as an efficient tool to combat various fungal phytopathogens in a wide range of plants through in planta expression of the RNAi construct. The fungal pathogens Fusarium graminarium and Fusarium culmorum are the major causative agents of Fusarium head blight in wheat and barley. CYP3RNA, a long dsRNA from transgenic Arabidopsis and barley, was used to target the genes involved in the ergosterol biosynthesis pathway, including CYP51A, CYP51B, and CYP51C, in the pathogen [Citation69]. Suppression of fungal growth was reported after in vitro feeding of CYP3-dsRNA in both the host plants. Likewise, an sRNA mediated HIGS approach was used to target three virulence-related genes; PtMAPK1 (MAP kinase), PtCYC1 (cyclophilin), and PtCNB1 (calcineurin B) from the wheat rust pathogen, Puccinia triticina [Citation70]. Transgenic wheat lines with the hairpin RNAi demonstrated broad-spectrum resistance against not only P. triticina but also against two other forma specialis Puccinia striiformis f. sp. tritici, Puccinia graminis f. sp. tritici. HIGS-induced expression of a sRNA-based RNAi construct targeting the Aspergillus flavus alpha-amylase gene Amy1 has resulted in decreased fungal colonization and aflatoxin accumulation in maize kernels [Citation71]. In rice, in vivo HIGS techniques have been used to exhibit improved resistance to 11 strains of the rice blast fungus Magnaporthe grisea through the in planta expression of RNA hairpins targeting the pathogen-specific bZIP transcription factor MoAP1 [Citation72]. More recently, HIGS has been used to target two ergosterol biosynthetic genes, ERG6 and ERG11, from Fusarium oxysporum (Foc), which causes Panama disease in bananas [Citation73]. Transgenic bananas expressing the ERG6-RNAi and the ERG11-RNAi demonstrated enhanced resistance to the Foc TR4 strain. In yet another study, a bean pod mottle virus-based HIGS strategy was used to suppress the expression of at least three virulent-related genes from the Asian soybean rust (ASR) pathogen, Phakopsora pachyrhizi, leading to a significant reduction in ASR symptom development in soybean leaves [Citation74]. All these studies suggest that sRNA-derived HIGS is an effective strategy of RNAi for reducing fungal infection in agriculturally important plants. Nevertheless, it needs to be practiced with caution. The success of HIGS depends on a greater quantity of siRNA transport between the two organisms. As such, HIGS is not an efficient approach against necrotrophic fungi as they absorb nutrients from a dead host, which could not supply sufficient siRNAs [Citation65]. Also, HIGS-induced silencing of individual genes is inadequate to control the disease due to functional redundancy and partial deactivation of target proteins. Moreover, functional genomics has also shown that siRNAs often silence off-target genes [Citation65]. The silencing of target genes in unintended organisms, saturation of RNAi machinery, and undesirable immune stimulation also contribute to the off-target effect of HIGS [Citation75]. In addition, HIGS also faces technical difficulties with respect to certain tissues (fruits and roots) or poor siRNA uptake in case of soil-borne fungal pathogens [Citation76]. Moreover, HIGS also involves high costs and requires regulatory clearances for the commercialization of transgenic lines. In fact, transgenic crops are currently not acceptable in major parts of the world, rendering them unusable for at least the next few years. In the near future, there will be a need for an upgrade of HIGS strategy with regard to target selection, the development of efficient transformation constructs, and stable transgenic systems to realize its full potential in crop protection.
Figure 2. Strategies to prevent fungal infection by Host induced Gene Silencing (HIGS) and Spray Induced Gene Silencing (SIGS) A) HIGS pathway: Transgenic plant after producing dsRNA, undergo cleavage by using plant Dicer like (DCL), converting to small interfering RNA (siRNA). Both dsRNA and siRNA move to pathogen cell through plasma membrane silences virulence mRNA (target gene). B) SIGS Pathway: After construction of dsRNA/siRNA, are sprayed onto topical part of plant, which are directly taken by plant cell, by using plant Dicer like protein (DCL), dsRNA converted to siRNA. Both dsRNA and siRNA move towards fungal cell by plasma membrane and target fungal virulence mRNA.
Spray Induced Gene Silencing (SIGS)
Similar to the environmental RNAi exhibited by some invertebrate systems, external long dsRNA as well as short sRNAs are taken up by fungal cells that induce silencing of fungal genes in a sequence specific manner [Citation44]. This has resulted in the development of spray-induced gene silencing (SIGS), a non-transgenic RNAi approach [Citation10,Citation44]. This method involved the control of fungal growth and associated infection through the spray application of dsRNAs or sRNAs targeting fungal virulence-related genes (). Gray mould disease caused by the necrotrophic fungal pathogen B. cinerea affects the vegetables, fruits, flowers, and leaves of several plants. The SIGS-based application of dsRNA and sRNA suppresses the grey mould disease in all types of plant tissues by silencing the B. cinerea DCL1 and DCL2 genes [Citation44]. In another recent study, exogenous applications of dsRNA in grape bunches significantly reduced the virulence of B. cinerea by silencing three prominent virulence-related genes-BcCYP51, Bcchs1, and BcEF2 [Citation77]. The direct high pressure spraying of dsRNA, sRNA, and siRNA on the leaves resulted in reduced lesion size after infection. SIGS also reduced post-harvest losses of vegetables (tomato, onion lettuce), fruits (grape, strawberry, apple), and flowers (rose) caused by B. cinerea infections, establishing a new class of synthetic fungicides [Citation78]. Similarly, a preventive spray of CYP3-dsRNA on the leaf surface can regulate Fusarium head blight in barley and wheat by silencing the ergosterol biosynthetic genes [Citation28]. What more, the foliar spray of dsRNA or siRNA has efficiently suppressed the infection by several fungal phytopathogens, including B. cinerea, F. graminearum, S. sclerotiorum, Rhizoctonia solani, Zymoseptoria tritici, Aspergillus niger and Verticillium dahlia [Citation79]. All these reports suggest that SIGS mediated by dsRNA or sRNA is an effective and sustainable strategy for pre- and postharvest protection of plants against fungal infection. However, the commercial application of SIGS faces several hurdles, such as premature silencing of RNAi on the plant surface, large scale dsRNA production, and suitable RNA stabilizing agents for usage of SIGS in field conditions [Citation80]. Therefore, the effectiveness of SIGS largely depends on the RNA uptake efficiency of the pathogen, and it is therefore important to address methods to overcome such a barrier.
RNAi embedded nanoparticles
RNA, being highly unstable under extracellular conditions, is often encapsulated with artificial vesicles, lipidosomes, or nanocarriers to increase the efficiency of RNAi molecules [Citation81]. Micro- or nano encapsulation provides a safe and stable environment for the RNAi method during the subsequent release of dsRNA or sRNA. Nanocarriers act as excellent vehicles for the transport of nucleic acid due to their extremely small size, which facilitates easy mobility across the cell wall and plasma membrane. An ideal nanocarrier should be biodegradable, require fewer organic compounds, biocompatible, non-toxic, and cost-effective. The exogenous spray of dsRNA and nanocarrier conjugates on the topical part of plants, could activate the RNAi in pathogens. The spray application of dsRNA conjugated with layered double hydroxide (LDH) nanoparticles increased the efficacy of antiviral protection in Pisum sativum and Nicotiana benthamiana [Citation82]. In a similar study, Gurusamy and colleagues reported that dsRNA conjugated with chitosan nanoparticles (CNP) remained protected from degradation by endonucleases and resulted in significant knockdown of endogenous genes of the pathogen [Citation83]. The CNP-dsRNA complexes were also found intact for up to 5 days on leaf surfaces and resulted in 100% mortality of infecting pests through silencing of Helicoverpa armigera virulence genes [Citation84]. In a recent development, Escherichia coli-derived anucleated minicells were used for dsRNA encapsulation in SIGS [Citation85]. The minicell-encapsulated dsRNA (ME-dsRNA) was shielded from RNAse degradation and remained stable on strawberry surfaces. Further, the ME-dsRNA selectively knocked down the pathogen-specific chitin synthase class III (Chs3a, Chs3b) and DICER-like proteins (DCL1 and DCL2) genes of Botryotinia fuckeliana leading to inhibition of fungal growth in strawberry [Citation85]. What’s more, a wide range of nanocarriers, including DNA nanostructure, carbon dots, gold nanoclusters, single-walled carbon nanotubes, and cell-penetrating peptide (CPP) have been developed in recent times that can be improvised for the encapsulation of RNAi molecules for specifically targeting fungal phytopathogens [Citation81]. Hence, the accurate path for the uptake and release of externally applied dsRNA or siRNAs during plant-fungal interactions demands more study for practical application under field conditions.
Conclusion and future prospects
The RNA-mediated cross-kingdom communication has been recently established in diverse plant systems, confirming that movable RNA is a key regulatory molecule governing host-fungal pathogen interactions. Fungal pathogens deliver sRNAs into plants to inhibit the host immunity, while the plant sRNAs are transported into the interacting pathogen to suppress their virulence. Evidence indicates that EVs are the carriers of sRNAs across the plant host to pathogens and vice versa. While a plethora of plant EVs have been employed in sRNA transport to fungi, the details mechanisms for fungal EV biogenesis, cellular secretion, loading of RNA into the EV, and biological functions are still unknown. Understanding the different classes of EVs and their ability for encapsulation of sRNA in fungi would be crucial for ck-RNAi. Contrary to this, the recent discovery that the majority of sRNAs in the apoplast reside outside the EVs has further contradicted the actual mechanism of sRNA translocation across the species. Further, the presence of RNA-binding proteins like AGO and GRP bound with the lncRNAs in the apoplast indicates their possible function in the secretion and/or stabilization of sRNAs. It will be worth investigating the role of these lncRNAs, especially the circRNAs, in cell-to-cell communication or immune responses through sRNA trafficking. Nevertheless, the current knowledge of ck-RNAi and fungal RNA has allowed for the development of disease control strategies like HIGS and SIGS in several agriculturally important pathosystems. However, HIGS and SIGS also suffer from technical difficulties in terms of RNA uptake, mechanisms of RNA translocation by pathogens, and off-target activities. The recent innovation in novel RNA delivery methods, including nanocarriers and artificial vesicles, has tremendously improved the stability, efficacy, and scalability of ck-RNAi under field conditions. It is possible that future agriculture will concentrate around the application of environmentally friendly RNA-based fungicides, and cross-kingdom communication of sRNA will form an important research direction for controlling eukaryotic pests and pathogens.
Author contributions
RM and RKJ conceived and designed the manuscript. BM performed the literature search and wrote the manuscript. RM critically reviewed the manuscript. RKJ provided overall supervision, inputs on data presentation and prepared the final draft. All authors read and approved the final manuscript.
Declaration
This research did not involve the use of any animal or human data or tissue
Acknowledgments
Research work in the laboratory of RKJ is supported by grant from Dept. of Biotechnology (grant no. BT/PR23412/BPA/118/284/2017) Govt. of India. BM is thankful to Odisha State Higher Education Council, Higher Education Department, Govt. of Odisha, India for providing financial support in form of OURIIP research fellowship (grant no. 436/83/OSHEC dt. 29.06.21)
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Funding
References
- Summanwar A, Basu U, Rahman H, et al. Non-coding RNAs as emerging targets for crop improvement. Plant Sci. 2020;297:110521.
- Zhou X, Cui J, Meng J, et al. Interactions and links among the noncoding RNAs in plants under stresses. Theor Appl Genet. 2020;133(12):3235–3248.
- Zhang S, Dou Y, Li S, et al. DAWDLE interacts with DICER-LIKE proteins to mediate small RNA biogenesis. Plant Physiol. 2018;177(3):1142–1151.
- Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10(2):94–108.
- Kang K, Zhong J, Jiang L, et al. Identification of microRNA-Like RNAs in the filamentous fungus Trichoderma reesei by solexa sequencing. PLoS ONE. 2013;8(10):e76288.
- Lau SK, Chow WN, Wong AY, et al. Identification of microRNA-like RNAs in mycelial and yeast phases of the thermal dimorphic fungus Penicillium marneffei. PLoS Neg Trop Dis. 2013;7(8):e2398.
- Zhou Q, Wang Z, Zhang J, et al. Genome-wide identification and profiling of microRNA-like RNAs from Metarhizium anisopliae during development. Fungal Biol. 2012;116(11):1156–1162.
- Mathur M, Nair A, Kadoo N. Plant-pathogen interactions: MicroRNA-mediated trans-kingdom gene regulation in fungi and their host plants. Genomics. 2020;112(5):3021–3035.
- Wang M, Dean RA. Movement of small RNAs in and between plants and fungi. Mol Plant Pathol. 2020;21(4):589–601.
- Cai Q, Qiao L, Wang M, et al. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science. 2019;3601:1126–1129. 10.1126/science.aar4142
- Zand Karimi H, Baldrich P, Rutter BD, et al. Arabidopsis apoplastic fluid contains sRNA- and circular RNA–protein complexes that are located outside extracellular vesicles. Plant Cell. 2022;34(5):1863–1881.
- Wang M, Weiberg A, Jin H. Pathogen small RNAs: a new class of effectors for pathogen attacks. Mol Plant Pathol. 2015;16(3):219.
- Tamiru M, Hardcastle TJ, Lewsey MG. Regulation of genome‐wide DNA methylation by mobile small RNAs. New Phytol. 2018;217(2):540–546.
- Kehr J, Morris RJ, Kragler F. Long-distance transported RNAs: from identity to function. Ann Rev Plan Biol. 2022;73(1):457–474.
- Huang CY, Wang H, Hu P, et al. Small RNAs–big players in plant-microbe interactions. Cell Host Microbe. 2019;26(2):173–182.
- De Palma M, Ambrosone A, Leone A, et al. Plant roots release small extracellular vesicles with antifungal activity. Plants. 2020;9(12):1777.
- Regente M, Pinedo M, San Clemente H, et al. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J Expt Bot. 2017;68(20):5485–5495.
- Zhang T, Zhao YL, Zhao JH, et al. Cotton plants export microRnas to inhibit virulence gene expression in a fungal pathogen. Nat Plants. 2016;2(10):1–6.
- Shahid S, Kim G, Johnson NR, et al. MicroRNAs from the parasitic plant Cuscuta campestris target host messenger RNAs. Nature. 2018;553(7686):82–85.
- Weiberg A, Wang M, Lin FM, et al. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science. 2013;342(6154):118–123.
- Wang M, Weiberg A, Dellota JE, et al. Botrytis small RNA Bc-siR37 suppresses plant defense genes by cross-kingdom RNAi. RNA Biol. 2017;14(4):421–428.
- Jin Y, Zhao JH, Zhao P, et al. A fungal milRNA mediates epigenetic repression of a virulence gene in Verticillium dahliae. Philos Transe R Soc B Biol Sci. 2019;374(1767):20180309.
- Jian J, Liang X. One small RNA of Fusarium graminearum targets and silences CEBiP gene in common wheat. Microorganisms. 2019;7(10):425.
- Dunker F, Trutzenberg A, Rothenpieler JS, et al. Oomycete small RNAs bind to the plant RNA-induced silencing complex for virulence. Elife. 2020;9:e56096.
- Kusch S, Frantzeskakis L, Thieron H, et al. Small RNAs from cereal powdery mildew pathogens may target host plant genes. Fungal Biol. 2018;122(11):1050–1063.
- Ji HM, Mao HY, Li SJ, et al. Fol‐milr1, a pathogenicity factor of Fusarium oxysporum, confers tomato wilt disease resistance by impairing host immune responses. New Phytol. 2021;232(2):705–718.
- Zotti M, Dos Santos EA, Cagliari D, et al. RNA interference technology in crop protection against arthropod pests, pathogens and nematodes. Pest Manage Sci. 2018;74(6):1239–1250.
- Koch A, Biedenkopf D, Furch A, et al. An RNAi-based control of Fusarium graminearum infections through spraying of long dsRnas involves a plant passage and is controlled by the fungal silencing machinery. PLOS Pathogens. 2016;12(10):e1005901.
- Mishra R, Joshi RK, Zhao K. Genome editing in rice: recent advances, challenges, and future implications. Front Plant Sci. 2018;9:1361.
- Mishra R, Joshi RK, Zhao K. Base editing in crops: current advances, limitations and future implications. Plant Biotechnol J. 2019;18(1):20–31.
- Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444(7117):323–329.
- Park CH, Chen S, Shirsekar G, et al. The Magnaporthe oryzae effector AvrPiz-t targets the RING E3 ubiquitin ligase APIP6 to suppress pathogen-associated molecular pattern–triggered immunity in rice. Plant Cell. 2012;24(11):4748–4762. DOI:10.1105/tpc.112.105429
- Selin C, de Kievit TR, Belmonte MF, et al. Elucidating the role of effectors in plant-fungal interactions: progress and Challenges. Front Microbiol. 2016;7:600.
- Hua C, Zhao JH, Guo HS. Trans-kingdom RNA silencing in plant–fungal pathogen interactions. Mol Plant. 2020;11(2):235–244.
- Cui C, Wang Y, Liu J, et al. A fungal pathogen deploys a small silencing RNA that attenuates mosquito immunity and facilitates infection. Nat Commun. 2019;10(1):1–10.
- He K, Xiao H, Sun Y, et al. Transgenic microRNA‐14 rice shows high resistance to rice stem borer. Plant Biotechol J. 2019;17(2):461–471.
- Zeng J, Gupta VK, Jiang Y, et al. Cross-kingdom small RNAs among animals, plants and microbes. Cells. 2019;8(4):371.
- Brosnan CA, Voinnet O. Cell-to-cell and long-distance siRNA movement in plants: mechanisms and biological implications. Curr Opin Plant Biol. 2011;14(5):580–587.
- Bouwmeester H, Schuurink RC, Bleeker PM, et al. The role of volatiles in plant communication. Plant J. 2019;100(5):892–907.
- Koch A, Kogel KH. New wind in the sails: improving the agronomic value of crop plants through RNAi‐mediated gene silencing. Plant Biotechnol J. 2014;12(7):821–831.
- Boavida LC, Qin P, Broz M, et al. Arabidopsis tetraspanins are confined to discrete expression domains and cell types in reproductive tissues and form homo-and heterodimers when expressed in yeast. Plant Physiol. 2013;163(2):696–712.
- Hou Y, Zhai YI, Feng LI, et al. A Phytophthora effector suppresses trans-kingdom RNAi to promote disease susceptibility. Cell Host Microbe. 2019;25(1):153–165.
- Pourrajab F, Hekmatimoghaddam S. Transposable elements, contributors in the evolution of organisms (from an arms race to a source of raw materials). Heliyon. 2021;7:e06029.
- Wang M, Weiberg A, Lin FM, et al. Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat Plants. 2016;2(10):1–10.
- Derbyshire M, Mbengue M, Barascud M, et al. Small RNAs from the plant pathogenic fungus Sclerotinia sclerotiorum highlight host candidate genes associated with quantitative disease resistance. Molecular Plant Pathol. 2019;20(9):1279–1297.
- Hu X, Hoden KP, Liao Z, et al. Phytophthora infestans Ago1-bound miRNA promotes potato late blight disease. New Phytol. 2022;233(1):443–457.
- Yin C, Zhu H, Jiang Y, et al. Silencing dicer-like genes reduces virulence and sRNA generation in Penicillium italicum, the cause of citrus blue mold. Cells. 2020;9(2):363.
- Xu M, Guo Y, Tian R, et al. Adaptive regulation of virulence genes by microRna‐like RNAs in Valsa mali. New Phytol. 2020;227(3):899–913.
- Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Ann Rev Cell Dev Biol. 2014;30(1):255–289.
- Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRnas and microRnas is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659.
- Stotz H, Brotherton D, Inal J. Communication is key: extracellular vesicles as mediators of infection and defence during host–microbe interactions in animals and plants. FEMS Microbiol Rev. 2021;46(1):fuab044.
- Kwon S, Tisserant C, Tulinski M, et al. Inside-out: from endosomes to extracellular vesicles in fungal RNA transport. Fungal Biol Rev. 2020;34(2):89–99.
- Rajam MV, Chauhan S. Host-induced gene silencing (HIGS): an emerging strategy for the control of fungal plant diseases. In: Sarmah BK Borah BK, editors. Genome engineering for crop improvement. Concepts and strategies in plant sciences. Cham: Springer; 2021. DOI:10.1007/978-3-030-63372-1_4
- Rutter BD, Innes RW. Extracellular vesicles as key mediators of plant–microbe interactions. Curr Opin Plant Biol. 2018;44:16–22.
- Rutter BD, Innes RW. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 2017;173(1):728–741.
- Baldrich P, Rutter BD, Karimi HZ, et al. Plant extracellular vesicles contain diverse small RNA species and are enriched in 10-to 17-nucleotide “tiny. RNAs The Plant Cell. 2019;31(2):315–324.
- Micali CO, Neumann U, Grunewald D, et al. Biogenesis of a specialized plant–fungal interface during host cell internalization of Golovinomyces orontii haustoria. Cellular Microbiol. 2011;13(2):210–226.
- Wang J, Ding Y, Wang J, et al. EXPO, an exocyst-positive organelle distinct from multivesicular endosomes and autophagosomes, mediates cytosol to cell wall exocytosis in Arabidopsis and tobacco cells. Plant Cell. 2010;22(12):4009–4030.
- Moore RT, McAlear JH. Fine structure of Mycota. 5. Lomasomes—Previously uncharacterized hyphal structures. Mycologia. 1961;53(2):194–200.
- Zhao K, Bleackley M, Chisanga D, et al. Extracellular vesicles secreted by Saccharomyces cerevisiae are involved in cell wall remodelling. Commun Biol. 2019;2(1):305. DOI:10.1038/s42003-019-0538-8
- Rizzo J, Chaze T, Miranda K, et al. Characterization of extracellular vesicles produced by Aspergillus fumigatus protoplasts. mSphere. 2020;5(4). DOI:10.1128/mSphere.00476-20
- Giraldo MC, Dagdas YF, Gupta YK, et al. Two distinct secretion systems facilitate tissue invasion by the rice blast fungus Magnaporthe oryzae. Nat Commun. 2013;4(1):1–12.
- Nasfi S, Kogel KH. Packaged or unpackaged: appearance and transport of extracellular noncoding RNAs in the plant apoplast. ExRna. 2020;4:13.
- Lasda E, Parker R. Circular RNAs co-precipitate with extracellular vesicles: a possible mechanism for circRNA clearance. PLoS ONE. 2016;11(2):e0148407.
- Qi T, Guo J, Peng H, et al. Host-induced gene silencing: a powerful strategy to control diseases of wheat and barley. Int J Mol Sci. 2019;20(1):206.
- Nunes CC, Dean RA. Host‐induced gene silencing: a tool for understanding fungal host interaction and for developing novel disease control strategies. Mol Plant Pathol. 2012;13(5):519–529.
- Sang H, Kim JI. Advanced strategies to control plant pathogenic fungi by host-induced gene silencing (HIGS) and spray-induced gene silencing (SIGS). Plant Biotechnol Rep. 2020;14(1):1–8.
- Niu D, Hamby R, Sanchez JN, et al. Rnas—a new frontier in crop protection. Cur Opin Biotechnol. 2021;70:204–212.
- Koch A, Kumar N, Weber L, et al. Host-induced gene silencing of cytochrome P450 lanosterol C14α-demethylase–encoding genes confers strong resistance to Fusarium species. Proc Natl Acad Sci. 2013;110(48):19324–19329.
- Panwar V, Jordan M, McCallum B, et al. Host‐induced silencing of essential genes in Puccinia triticina through transgenic expression of RNAi sequences reduces severity of leaf rust infection in wheat. Plant Biotechnol J. 2018;16(5):1013–1023.
- Gilbert MK, Majumdar R, Rajasekaran K, et al. RNA interference-based silencing of the alpha-amylase (amy1) gene in Aspergillus flavus decreases fungal growth and aflatoxin production in maize kernels. Planta. 2018;247(6):1465–1473.
- Guo XY, Li Y, Fan J, et al. Host-induced gene silencing of MoAP1 confers broad-spectrum resistance to Magnaporthe oryzae. Front Plant Sci. 2019;10:433.
- Dou T, Shao X, Hu C, et al. Host‐induced gene silencing of Foc TR4 ERG6/11 genes exhibits superior resistance to Fusarium wilt of banana. Plant Biotech J. 2020;18(1):11.
- Hu D, Chen ZY, Zhang C, et al. Reduction of Phakopsora pachyrhizi infection on soybean through host- and spray-induced gene silencing. Mol Plant Pathol. 2020;21(6):794–807.
- Lundgren JG, Duan JJ. Rnai-based insecticidal crops: potential effects on nontarget species. BioScience. 2013;63(8):657–665.
- Yin C, Hulbert S. Host Induced Gene Silencing (HIGS), a promising strategy for developing disease resistant crops. Omics Int. 2015;4(03):1–2.
- Nerva L, Sandrini M, Gambino G, et al. Double-stranded RNAs (dsRnas) as a sustainable tool against gray mold (Botrytis cinerea) in grapevine: effectiveness of different application methods in an open-air environment. Biomolecules. 2020;10(2):200.
- McLoughlin AG, Wytinck N, Walker PL, et al. Identification and application of exogenous dsRNA confers plant protection against Sclerotinia sclerotiorum and Botrytis cinerea. Sci Rep. 2018;8(1):1–14.
- Qiao L, Lan C, Capriotti L, et al. Spray‐induced gene silencing for disease control is dependent on the efficiency of pathogen RNA uptake. Plant Biotechnol J. 2021;19(9):1756–1768.
- Hoang BTL, Fletcher SJ, Brosnan CA, et al. Rnai as a foliar spray: efficiency and challenges to field applications. Int J Mol Sci. 2022;23(12):6639.
- Tatiparti K, Sau S, Kashaw SK, et al. siRNA delivery strategies: a comprehensive review of recent developments. Nanomaterials. 2017;7(4):77.
- Mitter N, Worrall EA, Robinson KE, et al. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat Plants. 2017;3(2):1–10.
- Gurusamy D, Mogilicherla K, Palli SR. Chitosan nanoparticles help double‐stranded RNA escape from endosomes and improve RNA interference in the fall armyworm, Spodoptera frugiperda. Arch Insect Biochem Physiol. 2020;104(4):e21677.
- Kolge H, Kadam K, Galande S, et al. New frontiers in pest control: chitosan nanoparticles-shielded dsRNA as an effective topical RNAi spray for gram podborer biocontrol. ACS Applied Bio Mat. 2021;4(6):5145–5157.
- Islam MT, Davis Z, Chen L, et al. Minicell‐based fungal RNAi delivery for sustainable crop protection. Microbial Biotechnol. 2021;14(4):1847–1856.
