ABSTRACT
RNA capping is a prominent RNA modification that influences RNA stability, metabolism, and function. While it was long limited to the study of the most abundant eukaryotic canonical m7G cap, the field recently went through a large paradigm shift with the discovery of non-canonical RNA capping in bacteria and ultimately all domains of life. The repertoire of non-canonical caps has expanded to encompass metabolite caps, including NAD, FAD, CoA, UDP-Glucose, and ADP-ribose, alongside alarmone dinucleoside polyphosphate caps, and methylated phosphate cap-like structures. This review offers an introduction into the field, presenting a summary of the current knowledge about non-canonical RNA caps. We highlight the often still enigmatic biological roles of the caps together with their processing enzymes, focusing on the most recent discoveries. Furthermore, we present the methods used for the detection and analysis of these non-canonical RNA caps and thus provide an introduction into this dynamic new field.
1. Introduction
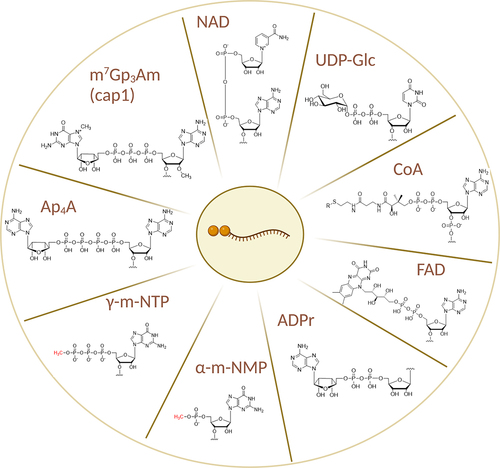
RNA modifications are present in most types of RNA, and they have a wide variety of roles from affecting RNA stability and metabolism to facilitating their cellular roles, guiding RNA-protein interactions, and more [Citation1,Citation2]. The first RNA modifications were discovered more than half a century ago, and there are more than 170 known chemical modifications of RNA to date [Citation3]. For the sake of clarity, they can be separated into three main categories based on their relative position within the RNA molecule. (i) Tail modifications are at the 3’ end of the RNA and they are usually formed through the addition of non-templated nucleotides and are a key component of quality control and RNA decay pathways [Citation4]. Although tails formed by each of the four RNA nucleotides have been observed, the most common ones are polyadenylation and polyuridylation [Citation5,Citation6]. (ii) Internal RNA modifications are contained within the transcript and regulate properties of the RNA ranging from structure and stability to binding proteins and translational fidelity [Citation7]. Internal modifications are most abundant in transfer RNAs (tRNA) and ribosomal RNAs (rRNA) [Citation8]. (iii) The 5’ ends of RNA can be modified by the addition of nonencoded nucleotides known as 5’ RNA caps (), with 7-methylguanosine (m7G) being the most abundant and most extensively studied RNA cap in eukaryotes [Citation9]. This conventional m7G cap is considered the canonical form of the RNA cap due to its prevalence and well explored role, whereas the less common and more recently discovered cofactor, metabolite and other caps are considered non-canonical.
2. Canonical capping
2.1 m7G
Historically, methylated nucleotides were considered to be unique to rRNA and tRNA, and the 5’ends of all mRNAs were thought to be triphosphorylated both in eukaryotes and prokaryotes. Then about half a century ago, the m7G cap was detected in messenger RNAs (mRNA) transcribed from the viral genomes of the cytoplasmic polyhedrosis virus and the vaccinia virus (dsRNA and DNA viruses respectively) [Citation10,Citation11]. This finding was swiftly followed by the discovery of the m7G cap in eukaryotic mRNA from HeLa cells where it was linked to the protection of the 5’end of the RNA from 5’ exonucleases [Citation12]. It was quickly ascertained that the main function of the m7G cap is twofold. First, it protects the 5’ end of the RNA from degradation by 5’ to 3’ exoribonucleases, contributing to the stability of the transcript. Second, it is required for the initiation of translation [Citation13,Citation14]. The effects of the m [Citation7]G cap on the initiation of translation were then linked to a cap binding protein, later termed the eukaryotic translation initiation factor 4E (eIF4E). Since then, it has been shown that the m7G cap is an integral component of eukaryotic translation, serving as the recognition point for the initiation of translation and regulating 5’ end interactions [Citation15]. The discovery of eIF4E started a new avenue of research into the cap through the studies of RNA cap-binding proteins [Citation16]. 5’ RNA capping, however, remained a process that was thought to be exclusive to eukaryotes.
The next 45 years shed more light on the plethora of roles of the m7G cap in mRNA. To name a few – the addition of the m7G cap to mRNA occurs co-transcriptionally during RNA polymerase II (RNA pol II) transcription, and it almost immediately regulates the ongoing process. The m7G cap recruits transcription regulators such as Mot1p that help the formation of the pre-initiation complex. This facilitates transcription initiation creating a sort of synergistic loop to promote gene expression [Citation17]. In addition, the mRNA cap facilitates the recruitment of the nuclear cap-binding complex (CBC). The protein complex specifically recognises the cap structure and, in turn, recruits other proteins to the mRNA [Citation18]. The addition of the CBC is also responsible for the recruitment of the small nuclear RNAs (snRNA), spliceosome formation, and subsequent splicing of the pre-mRNA [Citation19,Citation20]. Interestingly, even though the m7G cap is at the 5’end of the mRNA, it also plays a role in the 3’end processing of the transcript. Specifically, it seems as though the CBC stabilises the cleavage complex at the polyA site of the RNA [Citation21]. The CBC also has a role in the regulation of histone mRNAs, which are not polyadenylated and have a 3’-end stem loop instead. Upon CBC knockdown, however, the histone mRNAs start to acquire a polyA tail, suggesting that the m7G cap and the CBC are also responsible for the 3’ processing of this specific type of transcript [Citation22].
The m7G cap stabilises the RNA and protects the 5’ end from degradation by exonucleases and the removal of the cap or decapping is a key part of RNA turnover. The ability to control the decay of capped RNAs allows the cells to finetune gene expression and readily react to various stimuli [Citation23]. A number of proteins have been implicated in mRNA decapping, namely members of the Nudix family of hydrolases. These enzymes hydrolyse nucleoside diphosphates linked to the moiety x (hence the name), where x stands for any moiety [Citation24]. For instance, the human mRNA decapping enzyme 2 (hDcp2) is a Nudix protein cleaving the m7G cap from RNA [Citation25]. There is a large number of Nudix enzymes, and they are extremely versatile in their choice of substrate [Citation26]. Interestingly, Nudix enzymes have been shown to decap a number of non-canonical RNA caps as well, which will be further discussed throughout this review [Citation27,Citation28].
The m7G cap is also responsible for mRNA export from the nucleus, as the RNA export factor (REF) relies on the structure of the cap in order to bind to the transcript and transport it into the cytoplasm [Citation29]. In the cytoplasm, the m7G cap and methylations on the first two nucleotides downstream, serve as a molecular signature helping the immune system distinguish between self and foreign RNA [Citation30]. This link between the m7G cap, its methylated variants, and the innate immune response via RIG-I-like receptors is beyond the scope of this review, though it has been previously studied and described [Citation31–33]. The same is true for the other roles of the m7G cap (translation, RNA decay, etc.) on mRNA, which have also been the subject of a number of reviews [Citation34–36].
Long noncoding RNAs (lncRNA) are also RNA pol II transcripts and as such, they often have both the polyA tail and the m7G cap, which aid in nuclear export and RNA processing [Citation37]. This is even more true for long intervening ncRNAs (lincRNA), though it is important to note that lincRNAs seemingly have their own unique processing mechanisms despite their similarity to mRNA [Citation38]. MicroRNA precursors are also RNA pol II products, they are long unstructured transcripts also bearing the m7G cap [Citation39]. The m7G is again responsible for the recruitment of export proteins; after cleavage and maturation, however, the cap also plays a role during the interaction with Argonaute proteins that preferentially bind to a 5’monophosphate [Citation40]. While the effects of the m7G cap have common features in all of the aforementioned capped transcripts, the actual effects and further modifications of the cap vary greatly depending on the specific transcript, suggesting sophisticated means of regulation. Recently, it was also discovered that cells possess a cytoplasmic capping machinery, which adds another level of regulation of transcript fate [Citation41]. The uncapped transcripts can be kept in a “frozen” state and activated by recapping in order to fulfil their roles [Citation42].
2.2 TMG
The m7G cap is also a key component of the maturation of most small nuclear RNAs (snRNAs), specifically the ones that are transcribed by RNA polymerase II (Pol II) [Citation43]. These methylated transcripts are exported into the cytoplasm, where they are stabilised by the SMN complex that facilitates several maturation steps [Citation44]. These include additional methylations of the cap by the enzyme trimethylguanosine synthase 1 (TGS1) forming the 2,2,7-trimethylguanosine (TMG) cap. The TMG cap then initiates the import of the snRNA back into the nucleus by facilitating the assembly of the import complex [Citation45]. Once back in the nucleus, the TMG cap is recognised by import receptors and facilitates spliceosomal assembly [Citation46].
Several small nucleolar RNAs (snoRNA) are also capped. While the majority of snoRNAs are encoded in the introns of protein-coding genes and require splicing to be released, some have their own snRNA type genes [Citation47]. Interestingly, these capped snoRNAs – specifically the U3 and U8 RNAs are retained within the nucleus of the cell despite being capped. In fact, these snoRNAs undergo hypermethylation in the nucleoplasm before their transport to the nucleolus, where they participate in rRNA biogenesis [Citation48]. This nuclear retention is probably due to a discriminatory step during their maturation. The interaction with snoRNA binding proteins in the nucleus overrides the export signal of the cap, thus discriminating the hypermethylated transcripts from the ones being exported [Citation49].
m7G capping usually occurs co-transcriptionally during RNA synthesis by by RNA pol II transcripts [Citation50]. tRNAs are the products of RNA polymerase III (RNA pol III) and are transcribed as precursors (pre-tRNA). Pre-tRNAs are trimmed on both the 5’ and 3’ ends, nucleotides are then added to all the 3’ends as well as some 5’ends, and they are spliced and heavily modified [Citation51]. Some pre-tRNAs, however, are also m7G-capped and subsequently hypermethylated. This protects them from being degraded by 5’exonucleases and provides an alternative means of tRNA export, although the exact steps and proteins implicated in this process are yet to be discovered [Citation52].
3. Non-canonical cap-like structures
3.1 5'-γ-methyl-phosphate (γ-mNTP)
One of the earliest discovered non-canonical caps is the 5’ γ monomethyl phosphate. Unlike other caps discussed in this review, it is a simple cap-like structure without a flanking nucleotide-like moiety. Found in eukaryotic RNAs, it was initially identified on the RNA pol III transcript of U6 snRNA. It involves the addition of a methyl group to the 5’ triphosphate of uncapped RNAs [Citation53]. U3 snRNA in plants, also transcribed by RNA pol III, has the same 5'-γ-methyl-phosphate cap, as do the small noncoding RNAs B2 and 7SK [Citation54-56]. While the 7SK RNA has been shown to regulate transcription of RNA pol II through binding to the positive transcription elongation factor b (P-TEFb) while RNA B2 regulates Pol II by binding to the preinitiation complex and inhibiting it, it is unclear whether these functions are dependent on the 5'-γ-methyl-phosphate cap or not [Citation57,Citation58]. It has been shown that RNA pol III transcripts are often associated with the La protein that is necessary to stabilise them. This interaction is hindered by the 5'-γ-methyl-phosphate cap destabilising the transcript [Citation59]. It remains to be seen if this cap-like structure has any further biological implications.
3.2 5'-α –(di)methyl-phosphate (α-m-NMP)
A similar cap-like structure created by methylating or dimethylating 5' monophosphate RNA also exists on some eukaryotic RNAs. Deposited on the oxygens of the α-phosphate by an RNA methyltransferase containing the bicoid interacting 3 domain (BCDIN3D), this cap negatively regulates the maturation of micro RNAs (miRNA). The dimethylation of pre-miRNAs causes reduced processing by the enzyme Dicer leading to a lower level of mature miRNAs [Citation60]. The upregulation of BCDIN3D and consequently the dimethylation of specific miRNAs has also been implicated in breast cancer [Citation61]. The let-7 pre-miRNA is processed more effectively with the 5'-α -dimethyl-phosphate and a depletion of BCDIN3D reduced the levels of the mature let-7 miRNA. This reduction then leads to changes in the transcriptome and proteome of cancerous cells, preventing tumour formation [Citation62]. In contrast, pre-miR-145 is not processed as effectively when methylated and increased levels of mature miR-145 have been shown to suppress the tumorigenic phenotypes in breast cancer cells, suggesting a completely opposite effect that warrants further research [Citation60]. Apart from breast cancer, the upregulation of BCDIN3D and downregulation of the miRNA miR-195 − 3p was observed in cervical cancer. As such, BCDIN3D and its non-canonical capping reaction are of interest as therapeutic targets in a number of cancers [Citation63].
Interestingly, BCDIN3 also specifically recognises the 5' monophosphate of tRNAHis to monomethylate it with a higher efficiency than the aforementioned miRNAs [Citation64]. However, the levels of tRNAHis are not changed upon BCDIN knockout and the only effect seems to be a decreased affinity towards histidyl-tRNA synthetase [Citation65]. Even though BCDIN specifically recognises tRNAHis discriminating it from other tRNAs, the role of this 5'-α-methyl phosphate cap remains unknown [Citation66].
4. Non-canonical caps
The two non-canonical cap-like structures mentioned above are specific in that they only carry methyl groups on the 5' terminal phosphates. They do not have a flanking nucleotide-like moiety as is the case for the canonical m7G cap or the TMG cap. However, the m7G cap is structurally similar to nucleotide coenzymes such as nicotinamide adenine dinucleotide (NAD), flavin adenine dinucleotide (FAD), and coenzyme A (CoA). This has raised the question whether they may exert functions similar to those of 5' RNA caps. Indeed, Escherichia coli (E. Coli) RNA polymerase and T7 RNA polymerase were shown to incorporate these molecules in vitro, using them as non-canonical initiating nucleotides (NCIN) to start transcription [Citation67–69]. The first confirmation that these noncanonical caps exist in vivo came 30 years after the first in vitro experiments. Using liquid chromatography combined with mass spectrometry (LC-MS), CoA, its derivatives, and NAD were identified in small RNA fractions from two bacterial species – E. Coli and Streptomyces venezuelae [Citation70,Citation71]. Not only does this increase the chemical diversity of 5' RNA caps, but it also showed that RNA capping was not limited to eukaryotes, which was still the consensus at the time [Citation72]. However, the identity of the capped transcripts and their role within cells was still unknown.
4.1 NAD
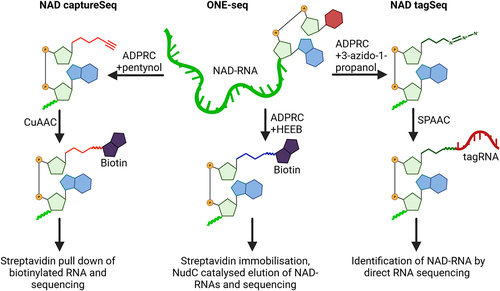
While the NAD-RNA cap was first detected using LC-MS, the first sequencing method directly showing a metabolite cap and its identity came in 2015 with the development of NAD captureSeq [Citation71,Citation73] (). Taking advantage of adenosine diphosphate-ribosyl cyclase (ADPRC) from the sea slug Aplysia californica, which specifically transglycosylates NAD with alkynyl alcohols, creating a “clickable” RNA. The enrichment of such RNAs identified them as a subset of small regulatory RNAs and mRNA-like 5'-terminal fragments in the bacterium E. coli. The decapping and degradation pathway of these RNAs was also discovered and it was shown that the NAD cap protects the transcripts from degradation. The NAD-RNA, however, can be decapped by the Nudix enzyme NudC, triggering its degradation [Citation73]. Since then, NAD captureSeq has been applied to other bacteria, archaea, and eukaryotes ranging from Saccharomyces cerevisiae to Metazoa, identifying a variety of capped transcripts [Citation74–77].
In 2018, CapZyme-seq was developed as an alternative method, using the bacterial RNA decapping enzyme NudC and the fungal decapping enzyme Rai1 to remove the metabolite RNA caps leaving behind a 5' monophosphate that can then be ligated to a barcoded RNA adapter [Citation76]. Concurrently, a sample is treated with RNA 5'-polyphosphatase (Rpp), which does the same to uncapped RNAs. The two samples are compared identifying the NAD-RNA. NudC and Rai1, however, also cleave other non-canonical caps, which may result in false positives [Citation78].
NAD tagSeq, later improved to NAD tagSeq II, uses the same principle as NAD captureSeq. The ADPRC-treated sample is clicked to a 3’ azide RNA – the “tag”. The tagged samples can then be directly sequenced by nanopore technology [Citation79] (). NAD tagSeq II replaces the copper click chemistry with the gentler strain-promoted azide-alkyne cycloaddition (SPAAC) that avoids RNA fragmentation caused by copper and allowing for a lower input of starting material [Citation80].
SPAAC-NAD-seq combines the methods mentioned above and adds a pre-treatment step with an m7G antibody which removes around 80% of the canonical cap. This is done because ADPRC exhibits some activity towards the m7G cap and thus results in false positive hits [Citation73,Citation81].
ONE-seq minimises the number of steps throughout the NAD-RNA sequencing procedure. ADPRC directly biotinylates NAD-RNAs using N-[2-(2-hydroxyethoxy) ethyl]-biotinamide (HEEB) as substrate. The biotinylated NAD-RNAs are captured by streptavidin beads, and NudC then cleaves the diphosphate bridge of the NAD cap, while potentially ADPRC ligated m7G RNAs linked through a triphosphate stay on the beads [Citation82] ().
The two newest methods are called NADcapPro and “intramolecular circularization of Noncanonical-Capped RNA” – circNC. These methods work orthogonally to eliminate the limitations of the other methods. The canonical cap is removed using an m7G decapping enzyme – the yeast scavenger mRNA decapping enzyme DcpS [Citation83]. ADPRC and SPAAC are used to biotinylate the RNAs and streptavidin beads are used for the pulldown [Citation84]. CircNC takes advantage of the NAD decapping enzyme retinoic acid induced 1 (Rai1) and circularises the leftover monophosphate RNA that is reverse transcribed and amplified [Citation84]. The detailed workings and principles of the sequencing methods are beyond the scope of this article, but they have been thoroughly analysed and explained in a recent review [Citation85].
NAD-RNA in prokaryotes
The number of identified NAD-RNAs and their roles vary greatly depending on the organisms and biological context in which they were analysed. In bacteria, the NAD cap shields the transcripts from degradation by 5' exonucleases [Citation73]. Interestingly, NADH, the reduced form of NAD, serves as an initiating substrate for the E. coli primase DnaG, suggesting a possible role in the generation of Okazaki fragments and regulation of DNA replication [Citation86]. NAD-RNA has also been shown to play a key role during the infection of E. coli by the T4 bacteriophage. During infection, the phage uses its adenosine diphosphate (ADP)-ribosyltransferase ModB to attach NAD-RNA molecules to proteins, essentially RNAylating them. The inactivation of ModB slows down the lytic cycle of the bacteriophage [Citation87]. Another bacterium, Staphylococcus aureus (S. aureus), codes for a quorum sensing regulatory RNA termed RNAIII [Citation88]. RNAIII has the 5' NAD cap in 10%-35% of its transcripts and the amount of the cap inversely correlates with the cytotoxicity of the bacteria. An increase in NAD-RNAIII causes a decrease in the expression of several toxins produced by the bacterium [Citation89]. Studies in Bacillus subtilis showed that mRNA is increasingly capped with NAD in the late exponential growth phase. Depletion of the B. subtilis deNADing enzyme BsRppH positively or negatively affected the expression of 13% genes clearly connecting NAD-RNA to gene regulation [Citation90]. NAD-RNA and its decapping machinery were also recently detected in archaea and mycobacteria, where the NAD cap probably serves as a degradation marker [Citation74,Citation91]. Both bacteria and archea also share toll-interleukin-1 receptor (TIR) domain-containing proteins that cleave the nicotinamide (NAM) moiety of NAD-RNA to start the NAD-RNA degradation process. Given the conservation of the TIR domain across species, this might present a novel conserved mechanism of deNAMing NAD-capped RNA and preparing it for further reactions [Citation92].
NAD-RNA in eukaryotes
NAD capture Seq was also applied to S. cerevisiae, which identified NAD-capped mRNAs of both cellular and mitochondrial origin, with a preference for intron-containing transcripts [Citation75]. The vast majority of NAD-RNA, however, are short 5' mRNA ends. RNA pol II uses NAD to start transcription from different promoters and this probably creates incomplete transcripts, preventing full-length RNA synthesis. It is important to note that NAD-mRNA is not translated and that there is a fast deNADing process suggesting that NAD-RNA is detrimental to yeast [Citation93]. The mitochondrial RNA polymerase, however, is much more efficient at incorporating NAD into RNA, with a reported 60% of mitochondrial RNAs being NAD-capped. This could mean that NAD-RNA serves specific functions within different cellular compartments [Citation94]. In yeast, apparently some RNAs can be NADylated post-transcriptionally, as the NAD modification appears on the 5' ends of 5'-processed snoRNAs and mRNAs. The same study also showed that NAD-RNA associates with ribosomes in mitochondria, yet again hinting at a different role for the NAD cap in different cellular compartments [Citation84].
Apart from sequencing, studies of the protein interactome of NAD-RNA provide an alternative approach to uncover the function of NAD caps. Using immobilised 3’ biotinylated NAD-RNA to perform a protein pull-down in yeast, 3 main candidate proteins were identified using mass spectrometry. This method termed 5' NAD cap RNA Affinity Purification (NcRAP) detected the cytoplasmic 5'-3' exoribonucleases Xrn1, the nuclear 5'-3' exoribonucleases Rat1, and the Rat1 interacting partner Rai1. These proteins release NAD from the RNA, returning it to the cellular pool and establishing a sort of equilibrium between NAD-RNA and NAD [Citation95]. In Arabidopsis thaliana, NAD-RNAs are also quite frequent and often transcribed from protein coding genes [Citation79]. They appear to be present in nuclear and mitochondrial transcripts, but not in those from chloroplasts. The NAD-capped transcripts were reported to be also spliced, polyadenylated, enriched within the polysomal fraction, and associated with translating ribosomes. Whether NAD-mRNAs are translated in plants, however, remains to be seen [Citation96]. NAD-RNA is also integral for the response of the plant to the hormone abscisic acid that noticeably changes the NAD-RNA transcriptome and the production of free NAD. The NAD cap destabilises the transcripts, and they are cleaved into small noncoding RNAs by the RNA-dependent RNA polymerase 6 (RDR6). This targeted degradation may be one of the factors regulating seed germination [Citation97]. Interestingly, the main RNA deNADing enzyme “decapping exoribonuclease (DXO)” is not only responsible for the decapping and subsequent degradation of NAD-RNA, but it also activates the methyltransferase AtRNMT1 that methylates the mRNA cap to convert it into m7G. This creates a direct link between non-canonical and canonical capping, hinting at the importance of NAD-RNA and its interactome [Citation98].
In mammals, NAD cap also seems to be that of a degradation marker by promoting DXO-mediated RNA decay [Citation76]. Apart from DXO, an alternative decapping pathway to DXO in human cell lines involves the cluster of differentiation 38 (CD38) [Citation99]. These glycoproteins are present on the surface of a number of immune cells and have versatile roles in cellular signalling and adhesion, often using NAD as a substrate [Citation100]. CD38 glycoproteins convert the NAD cap into an adenosine diphosphate ribose (ADPr) moiety that itself represents another type of non-canonical RNA cap [Citation101]. NAD-RNA in human cell lines also responds to the infection by the human immunodeficiency virus (HIV-1). Specific transcripts such as the snRNA U1 lose the NAD cap and generally affect the NAD-RNA status of the cell [Citation102]. Given the role of CD38 in antiviral signalling and HIV-1 infection, it is interesting to imagine NAD-RNA as a link connecting the two, however more research is necessary to test the possibility [Citation101].
It has been shown that free NAD levels in some tissues decrease with age [Citation103]. NAD-RNA levels and the identity of capped transcripts also change in mouse liver during ageing. Younger animals tended to have more NAD-RNA in genes connected to splicing and DNA repair, whereas older animals had more NAD-RNA associated with metabolic genes [Citation82]. While the datasets of NAD-RNA are growing and improving with each new sequencing method and each newly discovered decapping enzyme, a lot remains unknown about the biological function or the functional interactome of NAD-RNA [Citation27,Citation28,Citation70,Citation80,Citation82].
4.2 CoA
CoA was one of the first cofactor caps identified in RNA along with its derivate forms such as 3'-dephospho-CoA (dpCoA), succinyl- CoA, acetyl- CoA, and others in E. coli and S. venezuelae by use of LC-MS [Citation70]. The low abundance of CoA-RNA in cells makes it a difficult target for identification. Later, dpCoA-RNA was discovered and quantified also in the small RNA fraction of S. aureus. Using a modified mercury affinity electrophoresis (APM-PAGE), which retards dpCoA-RNA migration, and the E. coli enzyme phosphopantetheine adenylyltransferase (PPAT) to radioactively label the RNA, the capped RNA was detected at a concentration of 14 pmol per μg of RNA [Citation104]. The Nudix enzymes NudC and NudL are primarily responsible for the decapping of CoA-RNA in bacteria, with NudC being faster and promiscuous and NudL slower but specific for CoA-RNA [Citation105,Citation106].
In Arabidopsis thaliana, AtNUDT27 decaps CoA, the only plant Nudix enzyme as yet known to do so [Citation27]. In mammals, however, CoA-RNA is the most readily accepted substrate for the Nudix enzymes, cleaved by six different enzymes, NUDT7, NUDT8, NUDT12, NUDT15, NUDT16, and NUDT1928.
Given that dpCoA has a reactive thiol group, it is an ideal candidate for chemical conjugation using maleimides [Citation107]. This led to the development of dpCoA tagSeq, which uses a maleimide-modified RNA to “tag” the dpCoA-RNA at the 5' end with a barcode sequence. Using the dpCoA tagSeq method, the authors identified more than 40 genes that code for RNAs that are subject to dpCoA modification in mouse livers [Citation108]. It is important to note, however, that the vast majority of the identified sequences in this study were the results of false positives coming from chimeric reads between the sequences of highly abundant transcripts and the spike-in dpCoA-RNA added to the sample [Citation109]. The authors of the original study added several data processing steps in order to improve the method, leaving 7 RNAs as potential dpCoA-capped transcripts that remain to be verified [Citation110]. Unlike some of the other non-canonical caps that are only used as NCINs, dpCoA can be added as a posttranscriptional modification. The aforementioned E. Coli enzyme PPAT accepts 5' triphosphate RNA as a substrate for dpCoAylation in vitro, provided that the RNA has at least four unpaired nucleosides at the 5'end and the terminal nucleoside is an adenosine. Moreover, using model RNA sequences, PPAT was shown to add the dpCoA cap in vivo, although it is important to note that the enzyme PPAT prefers ATP as a substrate and high ATP concentrations inhibit the dpCoAylation of RNA [Citation111]. A better sequencing method and validation of the dpCoA-RNA transcripts are still required to shed more light on their identities and functions.
4.3 FAD
FAD is another adenine-containing cofactor that is accepted by RNA polymerases as an NCIN and was detected in both prokaryotic and eukaryotic RNA [Citation67,Citation112]. Like NAD-RNA, FAD-RNA is decapped by the enzymes Rai1 and DXO, in a process termed deFADing that releases the free form of FAD and leaves behind a 5'monophosphate RNA [Citation113]. FAD is also accepted by the E. Coli primase DnaG, just like NAD, further increasing the variability of primers initiating the synthesis of Okazaki fragments [Citation86].
To better understand the role of FAD-RNA, a study of its interacting proteins was conducted. Using FAD cap RNA Affinity Purification (FcRAP) based on the previously used NcRAP, the 5'-3' exoribonucleases Rat1 and XRN1 were identified along with the previously mentioned Rai1 that associates with Rat1. The bacterial 5'-3' exoribonuclease has the same deFADing activity, suggesting that it may be a conserved ability of all 5'-3' exoribonucleases [Citation114]. The spectrum of deFADing proteins was further expanded after a screen for mammalian Nudix enzymes showed that NUDT2 and NUDT16 also accept FAD-RNA as a substrate [Citation28].
While this seems to show an intricate layer of FAD-RNA decapping and regulation, the first actual biological role of a FAD-RNA came from studies on Hepatitis C virus (HCV). HCV is a positive-strand RNA virus that is under selective pressure to start its negative strand synthesis with an A residue, and it is well known that HCV requires FAD for replication in cell cultures [Citation115,Citation116]. Using the Arabidopsis thaliana Nudix pyrophosphohydrolase 23 (AtNUDX23) that specifically cleaves FAD, the authors modified CapZyme seq and showed that HCV RNA is capped with FAD in up to 75% of its transcripts. The sequencing results were also validated using LC-MS. The authors then proceeded to show that HCV replication is started using FAD as an NCIN and different viral strain transcripts were capped with different intensities. Apart from initiating viral replication, the FAD cap also protected the virus from innate immune recognition. Transfecting cells with FAD-RNA showed that the cap evades the nucleic acid sensor Retinoic acid-inducible gene I (RIG-I) and prevents the stimulation of other innate immune genes [Citation117]. It remains to be seen if other pathogens also employ this strategy of masking their transcripts with metabolite RNA caps. To date, however, this is the only shown biological role of the FAD RNA cap.
4.4 Dinucleoside polyphosphates
Dinucleoside polyphosphates (NpnNs) are another type of molecule similar to the m7G RNA cap in structure and just like NAD and the other metabolites serving as RNA caps, they are present in all cells across all domains of life [Citation118]. They are often called alarmones because their production increases under various stress conditions and their roles range from second messengers to regulators of cellular biosynthetic pathways [Citation119,Citation120].
NpnN RNA caps were discovered in the small RNA fraction of E. coli using LC-MS [Citation121]. It was also shown that various RNA polymerases accept NpnNs as NCINs and that the E. Coli enzymes RNA 5'-pyrophosphohydrolase (RppH) and bis(5'-nucleosyl)-tetraphosphatase (ApaH) function as decapping enzymes [Citation122,Citation123]. The LC-MS analysis enabled the distinction between various lengths of the polyphosphate chains of NpnNs, revealing that the caps are further regulated by methylations that protect them from cleavage by RppH. These methylations occur predominantly under stress conditions such as starvation, probably stabilising the capped transcripts, and return to normal when the stress conditions stop. Concurrently, dinucleoside tetraphosphate caps were also discovered using in vivo radioactive 32P labelling of E. coli RNAs, cap cleavage, and subsequent boronate polyacrylamide gel electrophoresis (PAGE) analysis and northern blotting. The presence of the cap in the cells also increased during disulphide stress, though the study did not explore their methylation status [Citation124]. Apart from incorporation of Np4N caps by RNA polymerase and their removal by decapping enzymes, lysyl-tRNA synthetase LysU was also identified as a capping enzyme, capable of installing the cap on 5'triphosphorylated transcripts [Citation124].
Interestingly, an NpnN with a shorter polyphosphate chain was previously discovered in E. coli. The RNA 3'-Phosphate Cyclase (RtcA) uses 5' monophosphorylated RNA as a substrate for adenylation in vitro, generating an AppN (Ap2N)-capped transcript or even an Ap2N-capped DNA. The addition of this cap is performed in a ligase-like manner leaving behind an activated molecule [Citation125]. Preadenylated RNA or DNA can then be further ligated by other ligases, even in an ATP-independent manner [Citation126]. Any other biological role of this AppN cap has not yet been discovered. A screen of Arabidopsis thaliana Nudix enzymes revealed that AtNUDT7 and AtNUDT27 both decap Ap2A-RNA, suggesting that the presence of this cap is subject to regulation [Citation27].
The most abundant NpnN, diadenosine tetraphosphate (Ap4A), was also discovered as an RNA cap in mammalian cells. Ap4A-capped RNAs were found in the fraction of RNAs longer than 200 nucleotides in two cell lines; human embryonic kidney cells (HEK293T) and rat basophilic leukaemia cells (RBL-2H3) [Citation127]. NuP1 was again used to digest the RNA and LC-MS to confirm the presence of the Ap4A RNA cap. The human nudix enzyme Nudt2 and DXO were identified as Ap4A-RNA decapping enzymes. In an attempt to elucidate the role of Ap4A-RNA, it was shown that RNAs transfected into cells are stabilised when carrying this cap. The Ap4A-RNA, however, was not translated, nor did it interact with eukaryotic translation initiation factors and, more importantly, did not elicit an immune response. Cells transfected with Ap4A-RNA failed to induce any of the innate immune genes associated with nucleic acid sensing in the cell. This suggests that the Ap4A cap is a natural component of the cellular transcriptome but it does not show if Ap4A-RNA has a specific role in mammalian cells [Citation127]. Ap4A-RNA can also be decapped by the plant enzymes AtNUDT6 and AtNUDT7, though the former preferentially cleaves the free molecule and the latter prefers the capped RNA as a substrate. The presence of Ap4A-RNA in plants, however, has not been confirmed [Citation27]. In general, NpnNs seem to provide an additional layer of transcriptional regulation, the exact mechanisms, however, are yet to be discovered.
4.5 UDP-Glucose
Nucleotide sugars were also recently discovered to function as non-canonical RNA caps. Using a transcription template under control of an acnA promoter at which UTP is utilised as an initiating nucleotide, E. coli polymerase readily accepted both uridine diphosphate glucose (UDP-Glc) and uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) [Citation128]. Both uridine diphosphate sugars are common bacterial cell wall precursors present in prokaryotic cells in high quantities [Citation129].
UDP-Glc is also the activated form of glucose used as a precursor for carbohydrate activation or for glucosyl transfer reactions in all organisms, meaning it’s also present in eukaryotic cells [Citation130]. Indeed, the UDP-Glc and UDP-GlcNAc RNA caps were also discovered in eukaryotes using the mass spectromentry-based method CapQuant [Citation112]. Quite large amounts of these caps were also detected in viral RNAs, specifically the dengue viral particles, in which UDP-GlcNAc-RNA was more abundant than in eukaryotic or prokaryotic cells. The concentration of UDP-GlcNAc-RNA in the sample was 10x higher than that of UDP-Glc-RNA [Citation115]. This could be explained by dengue virus usurping the host machinery to glycosylate its own proteins or by a general increase in UDP-GlcNAc that occurs during certain viral infections [Citation131,Citation132].
The E. Coli enzymes MurA, MurB, and MurC have been shown to modify the sugar cap by attaching a clickable moiety in vitro, presenting a potential way of pulling dow the sugar-capped RNAs. Human nudix protein NUDT5 was then identified as a UDP-GlcNAc-RNA decapping enzyme, leaving the decapped RNA ready for ligation [Citation133]. NUDT22, is another member of the Nudix hydrolase family, that has been repeatedly implicated in RNA decapping, is also known to cleave UDP-Glc into UMP and glucose 1-phosphate, making it a potential decapping enzyme for these nucleotide sugar caps, though its activity on the RNA remains to be tested [Citation134]. A specific sequencing method is needed to identify the capped transcripts and shed more light on their roles within the cell.
4.6 Adenosine diphosphate ribose
The addition of adenosine diphosphate ribose (ADPr) was long thought to be a uniquely posttranslational modification of proteins, affecting their functions and interactions. Installed by a family of enzymes called poly(ADP-ribose) polymerases (PARPs), the modification was shown to play a role in DNA repair, antiviral responses, cell cycle, and more [Citation135]. The 5' phosphorylated ends of RNA can also be modified in this manner yielding a 5'-phospho-ADPribose, introduced by 2'-phosphotransferase (Tpt1) enzymes [Citation136,Citation137]. A number of human PARPs were shown to ribosylate the phosphorylated 5' ends of RNA as well, namely PARP10, PARP11, and PARP15. The ADPr-RNA is resistant to 5' phosphatase degradation and the addition of the cap by PARP10 was shown to be reversible as several human ADP-ribosyl hydrolases decap the RNA. Interestingly, two viruses - Venezuelan equine encephalitis virus (VEEV) and severe acute respiratory syndrome coronavirus (SARS-CoV) - code for their own hydrolases capable of decapping ADPr-RNA [Citation138]. Most of the experiments, however, were done in vitro using recombinant and not full-length proteins. Thus, corresponding functional in vivo studies are required [Citation138]. Subsequent studies revealed that there is a difference in substrate specificity between TRPT1, the human homologue of Tpt1 that only modifies RNAs with purines at the 5' end and PARPs that modify purines and pyrimidines. ADPr-RNA was also detected in vivo, something that was difficult before, given the usual acidic phenol RNA extraction and the susceptibility of ADPr-RNA to acidic pH. In view of the localisation of some PARPs to stress granules, it was proposed that they can ribosylate mRNAs, which are then decapped and canonically recapped by the cytoplasmic capping machinery. However, further research is needed to verify this hypothesis [Citation139].
5. Conclusion
RNA capping is an intrinsic process that plays an integral role in post-transcriptional gene regulation and RNA metabolism. Originally thought to be limited to the canonical m7G cap and restricted only to eukaryotic RNA Pol II transcripts, the discovery of non-canonical RNA caps in various organisms has greatly expanded our understanding of RNA modifications. While the m7G cap has been meticulously studied and implicated in a wide variety of cellular processes, the roles of non-canonical RNA caps are still somewhat unknown and warrant further research in order to ascertain their functions within the cell.
The wide array of non-canonical RNA caps is chemically diverse, encompassing 5' RNA ends ranging from 5'-γ-methyl-phosphate, cofactors such as NAD, CoA, FAD, metabolites and alarmones of the dinucleoside polyphosphate family, to nucleotide sugars like UDP-glucose or ADP-ribose [Citation70,Citation121,Citation128,Citation138]. While these caps have been identified across all domains of life suggesting they are evolutionarily conserved, their significance in the regulation of biological processes has yet to be determined .
Table 1. Summary of known cap-like structures and non-canonical RNA caps.
To date, investigation of non-canonical RNA caps has implicated them in a number of cellular processes. These include RNA stability, post-transcriptional regulation, and immune evasion of pathogens. For instance, the NAD cap protects the RNA from degradation by 5' to 3’ exonucleases in bacteria but marks it for degradation in eukaryotes and has also been shown to affect the life cycles of certain pathogens [Citation87]. FAD is another example, where its presence on HCV viral transcripts facilitates their circumvention of the innate immune response [Citation120]. In fact, all of the cofactor caps were shown to interact with the cellular nucleic acid sensor RIG-I. In vitro prepared dsRNAs capped with NAD, FAD and dpCoA were purified and transfected into cells and a marked increase in antiviral signalling was observed. It is possible that the increase in signalling might have been caused by the dsRNA itself regardless of the RNA cap. This cannot be excluded because the HCV study showed the opposite results [Citation117,Citation139]. All this seems to underline the importance of non-canonical RNA capping.
Moreover, the development and improvement of innovative sequencing methods, such as NAD captureSeq or the modified CapZyme-seq have made it possible to identify and characterise non-canonically capped transcripts in a number of organisms. This has provided further insight into the mechanisms and the potential biological role of capping. Thanks to the NAD sequencing protocol, for example, we now know that the NAD cap in bacteria is mainly present on short regulatory RNAs or that there are preferred transcription start sites and consensus promoter sequences used for NAD capping [Citation73,Citation78].
Despite all these advancements in the field, many of the questions about the actual roles and functional mechanisms of non-canonical RNA caps remain unanswered. First and foremost, are they just stoichiometric incorporations of molecules readily accepted by RNA polymerases, or do they exert specific functions within the cellular transcriptome? To clarify this central question, future research should focus on trying to determine their functional interactome and the identity of the capped transcripts. To put it in a nutshell, it is imperative to develop specific sequencing methods for the different caps and identify the proteins that cap, decap, and interact with the capped RNA. More research into these caps promises to unravel the mechanisms through which they act in vivo and may potentially lead to innovative therapeutic strategies pertaining to RNA regulation and function.
Acknowledgments
We are grateful to members of the Cahova Group (Pavel Vopalensky, Ondrej Nesuta, and Maria Bianca Mititelu) for their help and advice. Figures were Created with BioRender.com. We acknowledge funding from the European Research Council Executive Agency (ERCEA) under the European Union’s Horizon Europe Framework Programme for Research and Innovation (grant agreement No. 101041374 – StressRNaction) and the Operational Programme Johannes Amos Comenius (OP JAC) project RNA for Therapy, reg. No. CZ.02.01.01/00/22_008/0004575 co-financed by the EU.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Schaefer M, Kapoor U, Jantsch MF. Understanding RNA modifications: the promises and technological bottlenecks of the ‘epitranscriptome’. Open Biol. 2017;7(5):170077.
- Lewis CJT, Pan T, Kalsotra A. RNA modifications and structures cooperate to guide RNA–protein interactions. Nat Rev Mol Cell Biol. 2017;18(3):202–210.
- Cappannini A, Ray A, Purta E, et al. MODOMICS: a database of RNA modifications and related information. 2023 update. Nucleic Acids Res. 2024;52(D1):D239–D244.
- Łabno A, Tomecki R, Dziembowski A. Cytoplasmic RNA decay pathways - enzymes and mechanisms. Biochim Biophys Acta, Mol Cell Res. 2016;1863(12):3125–3147.
- Viegas SC, Silva IJ, Apura P, et al. Surprises in the 3'-end: ‘U’ can decide too! FEBS J. 2015;282(18):3489–3499.
- Zigáčková D, Vaňáčová Š. The role of 3' end uridylation in RNA metabolism and cellular physiology. Philos Trans R Soc B. 2018;373(1762):20180171.
- Boo SH, Kim YK. The emerging role of RNA modifications in the regulation of mRNA stability. Exp Mol Med. 2020;52(3):400–408.
- Roundtree IA, Evans ME, Pan T, et al. Dynamic RNA modifications in gene expression regulation. Cell 2017;169(7):1187–1200.
- Ramanathan A, Robb GB, Chan S. H. mRNA capping: biological functions and applications. Nucleic Acids Res. 2016;44(16):7511–7526.
- Lindell TJ, Miura K. A blocked structure at the 5' terminus of mRNA from cytoplasmic polyhedrosis virus. Nature. 1975;253(5490):374–375.
- Urushibara T, Furuichi Y, Nishimura C, et al. A modified structure at the 5'-terminus of mRNA of vaccinia virus. FEBS Lett. 1975;49(3):385–389.
- Wei CM, Gershowitz A, Moss B. Methylated nucleotides block 5' terminus of HeLa cell messenger RNA. Cell. 1975;4(4):379–386.
- Muthukrishnan S, Both GW, Furuichi Y, et al. 5'-Terminal 7-methylguanosine in eukaryotic mRNA is required for translation. Nature. 1975. 1975;255(5503)33–37.
- Furuichi Y, LaFiandra A, Shatkin AJ. 5'-Terminal structure and mRNA stability. Nature 1977;266(5599):235–239.
- Gebauer F, Hentze MW. Molecular mechanisms of translational control. Nat Rev Mol Cell Biol. 2004;5(10):827–835.
- Sonenberg N, Morgan MA, Merrick WC, et al. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5'-terminal cap in mRNA. Proc Nat Acad Sci. 1978;75(10):4843–4847.
- Lahudkar S, Shukla A, Bajwa P, et al. The mRNA cap-binding complex stimulates the formation of pre-initiation complex at the promoter via its interaction with Mot1p in vivo. Nucleic Acids Res. 2011;39(6):2188–2209.
- Lewis JD, Izaurralde E. The role of the cap structure in RNA processing and nuclear export. Eur J Biochem. 1997;247(2):461–469.
- Lewis JD, Izaurralde E, Jarmolowski A, et al. A nuclear cap-binding complex facilitates association of U1 snRNP with the cap-proximal 5' splice site. Genes Dev. 1996;10(13):1683–1698.
- Pabis M, Neufeld N, Steiner MC, et al. The nuclear cap-binding complex interacts with the U4/U6·U5 tri-snRNP and promotes spliceosome assembly in mammalian cells. RNA. 2013;19(8):1054–1063.
- Flaherty SM, Fortes P, Izaurralde E, et al. Participation of the nuclear cap binding complex in pre-mRNA 3’ processing. Proc Natl Acad Sci USA. 1997;94(22):11893–11898.
- Narita T, Yung TMC, Yamamoto J, et al. NELF Interacts with CBC and Participates in 3' End Processing of Replication-Dependent Histone mRNAs. Mol Cell. 2007;26(3):349–365.
- Charenton C, Graille M. mRNA decapping: finding the right structures. Philos Trans R Soc B. 2018;373(1762):20180164.
- Mildvan AS, Xia Z, Azurmendi HF, et al. Structures and mechanisms of Nudix hydrolases. Arch Biochem Biophys. 2005;433(1):129–143.
- Wang Z, Jiao X, Carr-Schmid A, et al. The hDcp2 protein is a mammalian mRNA decapping enzyme. Proc Natl Acad Sci USA. 2002;99(20):12663–12668.
- Carreras-Puigvert J, Zitnik M, Jemth A-S, et al. A comprehensive structural, biochemical and biological profiling of the human NUDIX hydrolase family. Nat Commun. 2017;8(1):1–17.
- Mititelu MB, Hudeček O, Gozdek A, et al. Arabidopsis thaliana NudiXes have RNA-decapping activity. RSC Chem Biol. 2023;4(3):223–228.
- Sharma S, Grudzien-Nogalska E, Hamilton K, et al. Mammalian Nudix proteins cleave nucleotide metabolite caps on RNAs. Nucleic Acids Res. 2020;48(12):6788–6798.
- Nojima T, Hirose T, Kimura H, et al. The Interaction between Cap-binding Complex and RNA Export Factor Is Required for Intronless mRNA Export. J Biol Chem. 2007;282(21):15645–15651.
- Leung DW, Amarasinghe GK. When your cap matters: structural insights into self vs non-self recognition of 5' RNA by immunomodulatory host proteins. Curr Opin Struct Biol. 2016;36:133.
- Rehwinkel J, Gack MU. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat Rev Immunol. 2020;20(9):537–551.
- Drazkowska K, Tomecki R, Warminski M, et al. 2'- O -Methylation of the second transcribed nucleotide within the mRNA 5' cap impacts the protein production level in a cell-specific manner and contributes to RNA immune evasion. Nucleic Acids Res. 2022;50(16):9051–9071.
- Devarkar SC, Wang C, Miller MT, et al. Structural basis for m7G recognition and 2'-O-methyl discrimination in capped RNAs by the innate immune receptor RIG-I. Proc Natl Acad Sci USA. 2016;113(3):596–601.
- Galloway A, Cowling V. H. mRNA cap regulation in mammalian cell function and fate. Biochim Biophys Acta Gene Regul Mech. 2019;1862(3):270–279.
- Topisirovic I, Svitkin YV, Sonenberg N, et al. Cap and cap-binding proteins in the control of gene expression. Wiley Interdiscip Rev RNA. 2011;2(2):277–298.
- Hung T, Wang Y, Lin MF, et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet. 2011;43(7):621–629.
- Wu H, Yang L, Chen -L-L. The Diversity of Long Noncoding RNAs and Their Generation. Trends Genet. 2017;33(8):540–552.
- Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10(12):1957.
- Xie M, Li M, Vilborg A, et al. Mammalian 5'-capped microRNA precursors that generate a single microRNA. Cell. 2013;155(7):1568.
- Otsuka Y, Kedersha NL, Schoenberg DR. Identification of a cytoplasmic complex that adds a cap onto 5'-monophosphate RNA. Mol Cell Biol. 2009;29(8):2155–2167.
- Borden KLB, Culjkovic-Kraljacic B, Cowling VH. To cap it all off, again: dynamic capping and recapping of coding and non-coding RNAs to control transcript fate and biological activity. Cell Cycle. 2021;20(14):1347–1360.
- Mattaj IW. Cap trimethylation of U snRNA is cytoplasmic and dependent on U snRNP protein binding. Cell. 1986;46(6):905–911.
- Pánek J, Roithová A, Radivojević N, et al. The SMN complex drives structural changes in human snRNAs to enable snRNP assembly. Nat Commun 2023. 2023;14(1):1–18.
- Fischer U, Lührmann R. An essential signaling role for the m3G cap in the transport of U1 snRNP to the nucleus. Science (1979). Science (New York, N.Y.). 1990;249(4970):786–790.
- Matera AG, Wang Z. A day in the life of the spliceosome. Nat Rev Mol Cell Biol. 2014;15(2):108–121.
- Watkins NJ, Lemm I, Ingelfinger D, et al. Assembly and Maturation of the U3 snoRNP in the Nucleoplasm in a Large Dynamic Multiprotein Complex. Mol Cell. 2004;16(5):789–798.
- Terns MP, Grimm C, Lund E, et al. A common maturation pathway for small nucleolar RNAs. EMBO J. 1995;14(19):4860–4871.
- Matera AG, Terns RM, Terns MP. Non-coding RNAs: lessons from the small nuclear and small nucleolar RNAs. Nat Rev Mol Cell Biol. 2007;8(3):209–220.
- McCracken S, Fong N, Rosonina E, et al. 5'-Capping enzymes are targeted to pre-mRNA by binding to the phosphorylated carboxy-terminal domain of RNA polymerase II. Genes Dev. 1997;11(24):3306.
- Hopper AK. Transfer RNA Post-Transcriptional Processing, Turnover, and Subcellular Dynamics in the Yeast Saccharomyces cerevisiae. Genetics. 2013;194(1):43.
- Ohira T, Suzuki T. Precursors of tRNAs are stabilized by methylguanosine cap structures. Nat Chem Biol. 2016;12(8):648–655.
- Devanathan SK, Debnath TK, Xhemalçe B. Facile detection of RNA phospho-methylation in cells and tissues. Methods Enzymol. 2021;658:49–72.
- Shimba S, Buckley B, Reddy R, et al. Cap structure of U3 small nucleolar RNA in animal and plant cells is different. gamma-Monomethyl phosphate cap structure in plant RNA. J Biol Chem. 1992;267(19):13772–13777.
- Shumyatsky GP, Tillib SV, Kramerov DA. B2 RNA and 7SK RNA, RNA polymerase III transcripts, have a cap-like structure at their 5' end. Nucleic Acids Res. 1990;18(21):6347–6351.
- Jeronimo C, Forget D, Bouchard A, et al. Systematic Analysis of the Protein Interaction Network for the Human Transcription Machinery Reveals the Identity of the 7SK Capping Enzyme. Mol Cell. 2007;27(2):262–274.
- Cosgrove MS, Ding Y, Rennie WA, et al. The Bin3 RNA methyltransferase targets 7SK RNA to control transcription and translation. Wiley Interdiscip Rev RNA. 2012;3(5):633–647.
- Espinoza CA, Allen TA, Hieb AR, et al. B2 RNA binds directly to RNA polymerase II to repress transcript synthesis. Nat Struct Mol Biol. 2004;11(9):822–829.
- Bhattacharya R, Perumal K, Sinha K, et al. Methylphosphate Cap Structure in Small RNAs Reduces the Affinity of RNAs to La Protein. Gene Expr. 2002;10(5):243.
- Xhemalce B, Robson SC, Kouzarides T. Human RNA Methyltransferase BCDIN3D Regulates MicroRNA Processing. Cell. 2012;151(2):278–288.
- Yao L, Chi Y, Hu X, et al. Elevated expression of RNA methyltransferase BCDIN3D predicts poor prognosis in breast cancer. Oncotarget. 2016;7(33):53895–53902.
- Reinsborough CW, et al. BCDIN3D RNA methyltransferase stimulates Aldolase C expression and glycolysis through let-7 microRNA in breast cancer cells. Oncogene 2021. 2021;40(13):2395–2406.
- Jin M, Wang L, Zheng T, et al. MiR-195-3p inhibits cell proliferation in cervical cancer by targeting BCDIN3D. J Reprod Immunol. 2021;143:103211.
- Martinez A, Yamashita S, Nagaike T, et al. Human BCDIN3D monomethylates cytoplasmic histidine transfer RNA. Nucleic Acids Res. 2017;45(9):5423–5436.
- Tomita K, Liu Y. Human BCDIN3D is a cytoplasmic tRNAHis-specific 5'-monophosphate methyltransferase. Front Genet. 2018;9.
- Liu Y, Martinez A, Yamashita S, et al. Crystal structure of human cytoplasmic tRNAHis-specific 5'-monomethylphosphate capping enzyme. Nucleic Acids Res. 2020;48(3):1572–1582.
- Malygin AG, Shemyakin MF. Adenosine, NAD and FAD can initiate template-dependent RNA a synthesis catalyzed by Escherichia Coli RNA polymerase. FEBS Lett. 1979;102(1):51–54.
- Huang F. Efficient incorporation of CoA, NAD and FAD into RNA by in vitro transcription. Nucleic Acids Res. 2003;31(3):e8–e8.
- Bird JG, Zhang Y, Tian Y, et al. The mechanism of RNA 5' capping with NAD+, NADH and desphospho-CoA. Nature. 2016;535(7612):444–447.
- Kowtoniuk WE, Shen Y, Heemstra JM, et al. A chemical screen for biological small molecule-RNA conjugates reveals CoA-linked RNA. Proc Natl Acad Sci USA. 2009;106(19):7768–7773.
- Chen YG, Kowtoniuk WE, Agarwal I, et al. LC/MS analysis of cellular RNA reveals NAD-linked RNA. Nat Chem Biol. 2009;5(12):879–881.
- Deana A, Celesnik H, Belasco JG. The bacterial enzyme RppH triggers messenger RNA degradation by 5' pyrophosphate removal. Nature. 2008;451(7176):355–358.
- Cahová H, Winz ML, Höfer K, et al. NAD captureSeq indicates NAD as a bacterial cap for a subset of regulatory RNAs. Nature. 2014;519(7543)374–377.
- Gomes-Filho JV, Breuer R, Morales-Filloy HG, et al. Identification of NAD-RNA species and ADPR-RNA decapping in Archaea. Nat Commun. 2023;14(1):1–12.
- Walters RW, Matheny T, Mizoue LS, et al. Identification of NAD + capped mRNAs in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2017;114(3):480–485.
- Jiao X, Doamekpor SK, Bird JG, et al. 5'-end NAD+ cap in human cells promotes RNA decay through DXO-mediated deNADding. Cell. 2017;168(6):1015.
- Wang Y, Li S, Zhao Y, et al. NAD + -capped RNAs are widespread in the Arabidopsis transcriptome and can probably be translated. Proc Natl Acad Sci USA. 2019;116(24):12094–12102.
- Vvedenskaya IO, Bird JG, Zhang Y, et al. CapZyme-Seq Comprehensively Defines Promoter-Sequence Determinants for RNA 5' Capping with NAD+. Mol Cell. 2018;70(3):553–564.e9.
- Zhang H, Zhong H, Zhang S, et al. NAD tagSeq reveals that NAD + -capped RNAs are mostly produced from a large number of protein-coding genes in Arabidopsis. Proc Natl Acad Sci USA. 2019;116(24):12072–12077.
- Zhang H, Zhong H, Wang X, et al. Use of NAD tagSeq II to identify growth phase-dependent alterations in E. coli RNA NAD + capping. Proc Natl Acad Sci USA. 2021;118(14):e2026183118.
- Hu H, Flynn N, Zhang H, et al. SPAAC-NAD-seq, a sensitive and accurate method to profile NAD + -capped transcripts. Proc Natl Acad Sci USA. 2021;118(13):e2025595118.
- Niu K, Zhang J, Ge S, et al. ONE-seq: epitranscriptome and gene-specific profiling of NAD-capped RNA. Nucleic Acids Res. 2023;51(2):e12–e12.
- Wulf MG, Buswell J, Chan SH, et al. The yeast scavenger decapping enzyme DcpS and its application for in vitro RNA recapping. Scientific Rep. 2019;9(1):1–9.
- Sharma S, Yang J, Favate J, et al. NADcapPro and circNC: methods for accurate profiling of NAD and non-canonical RNA caps in eukaryotes. Commun Biol. 2023;6(1):1–14.
- Möhler M, Jäschke A. Future Perspectives for the Identification and Sequencing of Nicotinamide Adenine Dinucleotide-Capped RNAs. Acc Chem Res. 2023;56(21):3000–3009.
- Julius C, Salgado PS, Yuzenkova Y. Metabolic cofactors NADH and FAD act as non-canonical initiating substrates for a primase and affect replication primer processing in vitro. Nucleic Acids Res. 2020;48(13):7298–7306.
- Wolfram-Schauerte M, Pozhydaieva N, Grawenhoff J, et al. A viral ADP-ribosyltransferase attaches RNA chains to host proteins. Nature. 2023;620(7976):1054–1062.
- Novick RP, Ross HF, Projan SJ, et al. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12(10):3967–3975.
- Morales-Filloy HG, Zhang Y, Nübel G, et al. The 5=NAD Cap of RNAIII modulates toxin production in staphylococcus aureus isolates. J Bacteriol. 2020;202(6). doi:10.1128/JB.00591-19.
- Frindert J, Zhang Y, Nübel G, et al. Identification, Biosynthesis, and Decapping of NAD-Capped RNAs in B. subtilis. Cell Rep. 2018;24(7):1890–1901.e8.
- Ruiz-Larrabeiti O, Benoni R, Zemlianski V, et al. NAD+ capping of RNA in Archaea and Mycobacteria. bioRxiv. 2021;2021–12. doi:10.1101/2021.12.14.472595
- Wang X, et al. Toll/interleukin-1 receptor (TIR) domain-containing proteins have NAD-RNA decapping activity. Nat Commun 2024. 2024;15(1):1–14.
- Zhang Y, et al. Extensive 5'-surveillance guards against non-canonical NAD-caps of nuclear mRNAs in yeast. Nat Commun 2020. 2020;11(1):1–17.
- Bird JG, Basu U, Kuster D, et al. Highly efficient 5' capping of mitochondrial RNA with nad + and NADH by yeast and human mitochondrial RNA polymerase. Elife. 2018;7. doi:10.7554/eLife.42179.
- Sharma S, Yang J, Grudzien-Nogalska E, et al. Xrn1 is a deNADding enzyme modulating mitochondrial NAD-capped RNA. Nat Commun. 2022;13(1):1–11.
- Yu X, Willmann MR, Vandivier LE, et al. Messenger RNA 5' NAD+ Capping Is a Dynamic Regulatory Epitranscriptome Mark That Is Required for Proper Response to Abscisic Acid in Arabidopsis. Dev Cell. 2021;56(1):125–140.e6.
- Xiao C, Li K, Hua J, et al. Arabidopsis DXO1 activates RNMT1 to methylate the mRNA guanosine cap. Nat Commun. 2023;14(1):1–12.
- Abele F, Höfer K, Bernhard P, et al. A Novel NAD-RNA Decapping Pathway Discovered by Synthetic Light-Up NAD-RNAs. Biomolecules. 2020;10(4):513.
- Piedra-Quintero ZL, Wilson Z, Nava P, et al. CD38: an Immunomodulatory Molecule in Inflammation and Autoimmunity. Front Immunol. 2020;11:597959.
- Lu L, Wang J, Yang Q, et al. The role of CD38 in HIV infection. AIDS Res Ther. 2021;18(1):1–14.
- Benoni B, Potužník JF, Škríba A, et al. HIV-1 Infection Reduces NAD Capping of Host Cell snRNA and snoRNA. ACS Chem Biol. 2024;19(6):1243–1249.
- Covarrubias AJ, Perrone R, Grozio A, et al. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2020;22(2):119–141.
- Löcherer C, Bühler N, Lafrenz P, et al. Staphylococcus aureus small RNAs possess Dephospho-CoA 5'-Caps, but No CoAlation Marks. Noncoding RNA. 2022;8(4):46.
- Zhou W, Guan Z, Zhao F, et al. Structural insights into dpCoA-RNA decapping by NudC. RNA Biol. 2021;18:244.
- Spangler JR, Huang F. The E. coli NudL enzyme is a Nudix hydrolase that cleaves CoA and its derivatives. bioRxiv. 2020;2020. doi: 10.1101/2020.01.31.929182
- Ravasco JMJM, Faustino H, Trindade A, et al. Bioconjugation with Maleimides: a Useful Tool for Chemical Biology. Chem Eur J. 2019;25(1):43–59.
- Shao X, Zhang H, Zhu Z, et al. DpCoA tagSeq: barcoding dpCoA-Capped RNA for Direct Nanopore Sequencing via Maleimide-Thiol Reaction. Anal Chem. 2023;95(29):11124–11131.
- Vinther J. Comment on ‘DpCoA tagSeq: barcoding dpCoA-Capped RNA for Direct Nanopore Sequencing via Maleimide-Thiol Reaction’. Anal Chem. 2024;96(1):606–609.
- Shao X, Zhang H, Zhu Z, et al. Response to the Comment on “DpCoA tagSeq: barcoding dpCoA-Capped RNA for Direct Nanopore Sequencing via Maleimide-Thiol Reaction”. Anal Chem. 2023;96(1):610–613.
- Sapkota K, Lucas JK, Faulkner JW, et al. Post-transcriptional capping generates coenzyme A-linked RNA. RNA Biol. 2024;21(1):1–12.
- Wang J, Alvin Chew BL, Lai Y, et al. Quantifying the RNA cap epitranscriptome reveals novel caps in cellular and viral RNA. Nucleic Acids Res. 2019;47(20):e130–e130.
- Doamekpor SK, Grudzien-Nogalska E, Mlynarska-Cieslak A, et al. DXO/Rai1 enzymes remove 5'-end FAD and dephospho-CoA caps on RNAs. Nucleic Acids Res. 2020;48(11):6136–6148.
- Sharma S, Yang J, Doamekpor SK, et al. Identification of a novel deFADding activity in human, yeast and bacterial 5' to 3' exoribonucleases. Nucleic Acids Res. 2022;50(15):8807–8817.
- Cai Z, Liang TJ, Luo G. Effects of Mutations of the Initiation Nucleotides on Hepatitis C Virus RNA Replication in the Cell. J Virol. 2004;78(7):3633–3643.
- Marceau CD, Puschnik AS, Majzoub K, et al. Genetic dissection of Flaviviridae host factors through genome-scale CRISPR screens. Nature. 2016;535(7610):159–163.
- Sherwood AV, Rivera-Rangel LR, Ryberg LA, et al. Hepatitis C virus RNA is 5'-capped with flavin adenine dinucleotide. Nature. 2023;619(7971):811–818.
- McLennan AG. Dinucleoside polyphosphates—friend or foe?. Pharmacol Ther. 2000;87(2–3):73–89.
- Giammarinaro PI, Young MKM, Steinchen W, et al. Diadenosine tetraphosphate regulates biosynthesis of GTP in Bacillus subtilis. Nat Microbiol. 2022;7(9):1442–1452.
- Zegarra V, Mais C-N, Freitag J, et al. The mysterious diadenosine tetraphosphate (AP4A). MicroLife. 2023;4:1–8.
- Hudeček O, et al. Dinucleoside polyphosphates act as 5'-RNA caps in bacteria. Nat Commun. 2020;11(1):1–11.
- Benoni R, Culka M, Hudeček O, et al. Dinucleoside Polyphosphates as RNA Building Blocks with Pairing Ability in Transcription Initiation. ACS Chem Biol. 2020;15(7):1765–1772.
- Levenson-Palmer R, Luciano DJ, Vasilyev N, et al. A distinct RNA recognition mechanism governs Np 4 decapping by RppH. Proc Natl Acad Sci USA. 2022;119(6):e2117318119.
- Luciano DJ, Levenson-Palmer R, Belasco JG. Stresses that Raise Np4A Levels Induce Protective Nucleoside Tetraphosphate Capping of Bacterial RNA. Mol Cell. 2019;75(5):957–966.e8.
- Chakravarty AK, Shuman S. RNA 3'-Phosphate Cyclase (RtcA) Catalyzes Ligase-like Adenylylation of DNA and RNA 5'-Monophosphate Ends. J Biol Chem. 2011;286(6):4117–4122.
- Zhelkovsky AM, McReynolds LA. Structure-function analysis of Methanobacterium thermoautotrophicum RNA ligase - engineering a thermostable ATP independent enzyme. BMC Mol Biol. 2012;13(1):1–10.
- František Potužník J, Nešuta O, Škríba A, et al. Diadenosine Tetraphosphate (AP4A) Serves as a 5' RNA Cap in Mammalian Cells. Angew Chem. 2024;63(6):e202314951.
- Julius C, Yuzenkova Y. Bacterial RNA polymerase caps RNA with various cofactors and cell wall precursors. Nucleic Acids Res. 2017;45(14):8282–8290.
- Mirelman D. A rapid and simple procedure for the preparation of the two bacterial cell wall peptidoglycan nucleotide precursors labeled in their amino sugars. Anal Biochem. 1976;70(2):424–429.
- Roeben A, Plitzko JM, Körner R, et al. Structural basis for subunit assembly in UDP-glucose pyrophosphorylase from Saccharomyces cerevisiae. J Mol Biol. 2006;364(4):551–560.
- Idris F, Muharram SH, Diah S. Glycosylation of dengue virus glycoproteins and their interactions with carbohydrate receptors: possible targets for antiviral therapy. Arch Virol. 2016;161(7):1751.
- Hu J, Gao Q, Yang Y, et al. Hexosamine biosynthetic pathway promotes the antiviral activity of SAMHD1 by enhancing O-GlcNAc transferase-mediated protein O-GlcNAcylation. Theranostics. 2021;11(2):805.
- Ik F, Motzkus NA, Brandl L, et al. Identification and in vitro characterization of UDP-GlcNAc-RNA cap-modifying and decapping enzymes. Nucleic Acids Res. 2024;52(10):5438–5450.
- Carter M, Jemth A-S, Carreras-Puigvert J, et al. Human NUDT22 Is a UDP-Glucose/Galactose hydrolase exhibiting a unique structural fold. Structure. 2018;26(2):295–303.e6.
- Lüscher B, Bütepage M, Eckei L, et al. ADP-Ribosylation, a multifaceted posttranslational modification involved in the control of cell physiology in health and disease. Chem Rev. 2018;118(3):1092–1136.
- Munir A, Banerjee A, Shuman S. NAD+-dependent synthesis of a 5'-phospho-ADP-ribosylated RNA/DNA cap by RNA 2'-phosphotransferase Tpt1. Nucleic Acids Res. 2018;46(18):9617–9624.
- Mikolčević P, Hloušek-Kasun A, Ahel I, et al. ADP-ribosylation systems in bacteria and viruses. Comput Struct Biotechnol J. 2021;19:2366–2383.
- Munnur D, Bartlett E, Mikolčević P, et al. Reversible ADP-ribosylation of RNA. Nucleic Acids Res. 2019;47(11):5658–5669.
- Weixler L, Feijs KLH, Zaja R. ADP-ribosylation of RNA in mammalian cells is mediated by TRPT1 and multiple PARPs. Nucleic Acids Res. 2022;50(16):9426–9441.
- Singh R, Reddy M. Gamma-monomethyl phosphate: a cap structure in spliceosomal U6 small nuclear RNA. Proc Natl Acad Sci USA. 1989;86(21):8280–8283.
- Schweibenz BD, Solotchi M, Hanpude P, et al. RIG-I recognizes metabolite-capped RNAs as signaling ligands. Nucleic Acids Res. 2023;51(15):8102–8114.