Abstract
Use of enzymes in cosmetic products is novel and the safety of these products is not well understood. The safety of a prototype enzyme-containing body moisturizer lotion was tested via measures of skin compatibility and potential to induce protease-specific IgE antibody in a clinical study. Female, atopic subjects (n = 1,100) used body lotion containing 100 ppm protease (Y217L BPN′) for 5 consecutive days per month, for 18 months. Regular lotion was used the remaining days of each month. Skin evaluation and skin prick tests (SPT) were conducted every 3 months. Measures of skin hydration were made in a subset of subjects at 3-month intervals: skin biopsies occurred at baseline and at the first 3-month timepoint. Serum from SPT positive subjects was tested for specific IgE in an immunoCAP assay. Clinical evaluation and histopathology showed no skin irritation and increased hydration of the skin over time. Three of 864 subjects completing the study developed IgE antibody to the enzyme: 1 subject after 6 months product use and 2 subjects after 15 months product use. A fourth subject was found with IgE antibody 3 months after study termination. None had allergic symptoms associated with product use. Intermittent exposure to a low level of protease enzyme in a body lotion led to the development of specific IgE antibody in 0.46% of subjects. While this study showed favorable skin compatibility of the protease containing lotion, the occurrence of allergic antibody to the enzyme was unacceptable for product commercialization.
INTRODUCTION
Microbial enzymes have been used safely in detergent products for nearly 30 years. With the exception of a few isolated cases of allergic reactions among consumers exposed to enzyme powders in very dusty laundry products in the late 1960s, it has become a rare event for consumers to develop allergic antibodies to enzymes, much less allergic symptoms, through the use of enzyme-containing laundry products (Belin et al., Citation1970; Zetterstrom and Wide, Citation1974; Zetterstrom, Citation1977; Sarlo et al., Citation2003; Cormier et al., Citation2004). On the other hand, the use of a Bacillus protease enzyme (Y217L BPN′) in a prototype beauty bar soap led to the development of enzyme-specific IgE antibody in 6.5% of a test population after 4 to 6 months of product use (Kelling et al., Citation1998). This experience, along with reports of allergy to papain in cosmetologists and among users of papain-containing contact lens cleaning solutions led us to conclude that the use of enzymes in personal care products must be carefully assessed before the product is commercialized (Bernstein et al., Citation1984; Fisher, Citation1985).
Protease enzymes have desquamatory effects on skin and in appropriate vehicles can improve the feel and appearance of dry skin. However, since enzymes are recognized allergens, the risk for inducing IgE allergic antibody with its attendant increased risk of allergic disease must be addressed. In order to minimize the risk of allergy, a body lotion was designed with a low level of enzyme that would be used intermittently on a monthly basis and still provide the positive skin effects. To assess the safety of this prototype lotion, a prospective safety-in-use clinical study among atopic women was conducted. The results of this clinical study are described in this paper.
METHODS
Test Subjects
Test subjects were female volunteers from the United States, Canada, France and Germany who regularly used hand and body moisturizer lotions. Individuals were excluded if they were employed by the Procter & Gamble Company (P&G), were participating in another panel, or employed in an industry that used enzymes. Subjects were eligible to enroll in the 18-month study if they were between the ages of 18 and 60, in good health with no complicating medical conditions, skin prick test (SPT) positive to 1 or more common aeroallergens, SPT (−) to the protease enzyme and had acceptable skin assessment and pulmonary function testing, as detailed next. These subjects answered a questionnaire that assessed how they used personal care and laundry products. The P&G Institutional Review Board as well as the Ethics Committees of the different testing laboratories reviewed and approved all clinical protocols. All subjects signed an informed consent. The study was conducted in accordance with Good Clinical Practices (GCP) for Trial of Medicinal Products and the ICH Guideline for GCP (CPMP/ICH/135/95).
Test Products
Enzyme containing moisturizing lotion (test lotion) and regular moisturizing lotion (maintenance lotion) were produced by P&G (Rusham Park, UK). The lotion consisted of glycerin, petrolatum, betaine, water, alcohol, Vitamin E, and other minor ingredients such as chelator, perfume, color, and so forth. Purified protease enzyme Y217L BPN′ from Bacillus amyloliquifaciens was supplied by Genencor International (Palo Alto, CA). Enzyme was formulated into the lotion at 100 ppm protein. The test lotion was supplied in a 50 ml pump pack containing 2 chambers comprising a moisturizing lotion in one compartment and the active enzyme in the second compartment. The pack was designed to dispense 0.5 mg of enzyme per day. The body moisturizing maintenance lotion was commercially available and supplied in a 150 ml tube. The stability of the enzyme in the product throughout the duration of the study was confirmed using standard activity assays (Rothgeb et al., Citation1988).
Study Design
Potential test subjects (n = 1,925) were screened for eligibility into the study. Subjects were not eligible to participate in the study if they were nursing, pregnant or planned to get pregnant, had asthma or chronic pulmonary disease, suffered from a chronic evolutive nonstabilized disease, were a known diabetic, used topical drugs on dry skin areas, took oral steroids, had a current skin infection or other overt skin disorder on the dry skin body locations (excluding acne) that would make data interpretation inconclusive or had a deformation or physical abnormality of these same locations (such as a scar, a lesion, a birthmark, or a tattoo) that could interfere with the skin grading procedures. Eligible test subjects were SPT positive to at least 1 common aeroallergen and were SPT negative to the protease enzyme at the 500 ug/ml concentration. These subjects were enrolled in the baseline visit where they were retested by SPT with 500 ug/ml and 50 ug/ml protease enzyme. Additionally, they received a urine pregnancy test, serum collection, pulmonary function test, and a skin examination. One thousand, one hundred (1,100) women were enrolled in this study with 549 from the United States and Canada, 274 from Germany and 277 from France. They were instructed to use the test lotion on dry skin areas for the first 5 days of the month and the maintenance lotion for the remainder of the month. They were required to visit the test center at least 6 times during the treatment period (18, 28-day cycles) to obtain product, be evaluated (by SPT) for IgE antibody to the enzyme, be evaluated for visual skin condition, and have an opportunity to report adverse events and changes in medications. They received a urine pregnancy test at months 12 and 18. A subset of subjects (n = 67) agreed to be assessed for skin condition by capacitance and transepidermal water loss (TEWL) at every month. Another subset (n = 58) provided a skin biopsy at baseline and at month 3. If a positive SPT to enzyme was observed, the subject was removed from the study and asked to return 7–21 days later for a retest. A positive SPT at retest confirmed the development of IgE antibody to enzyme and the subject was enrolled in a follow-up protocol that included SPT with other enzymes commonly used in laundry products, collection of serum for analysis of enzyme specific IgE by immunoCAP assay and an interview plus home visit to ascertain if the test subject had any unusual use of the test product or other enzyme-containing marketed products.
Skin Prick Tests
The enzyme SPT reagents were prepared by P&G from purified versions of the commercially used enzymes obtained from Genencor International or Novozymes, A/S (Denmark). These included the protease used in the body lotion (Y217L BPN′ from Bacillus amyloliquifaciens), protease from Bacillus licheniformis, protease from Bacillus lentus, amylase from Bacillus amyloliquifaciens and amylase from Bacillus licheniformis. Solutions of 500 μ g protein/ml and 50 μ g protein/ml were prepared using a 50% glycerin/saline solution. Test solutions were stored under refrigeration until needed. The enzymes and enzyme concentrations were identical to those used in the medical surveillance program at Procter & Gamble detergent manufacturing facilities (Schweigert et al., Citation2000). Eight aeroallergens common to North America and Europe were obtained from Hollister-Stier (Spokane, WA) and Stallergenes (Paris, France). These included: Dermatophagoides farinea, Dermatophagoides pteryonyssinus, cat dander, dog dander, Alternaria, Cladosporium, Graminae mix, and pollen (Betalucea or ragweed). These were reconstituted in 50% glycerin/saline solution according to manufacturer's instructions. The negative control was the glycerin/saline and the positive control was 2.75 mg/ml histamine phosphate in the glycerin/saline vehicle. Tests were performed using the method described by Pepys (Citation1975). A drop of the test or control material was placed on the volar surface of the forearm and a slight prick of the surface epidermis was made using a sterile, 26–27 gauge needle. The test site was observed after 15 minutes for the presence of a wheal and flare reaction. The sizes of the wheal and flare at the largest points were recorded. A test was considered positive if the site exhibited a wheal of at least 3 mm larger than the negative control, and a visible flare accompanied by a positive response to histamine. Any positive reactions to the enzymes were confirmed by repeat testing on the opposite arm.
Skin Evaluation, Capacitance, and TEWL
The skin condition of the body areas where the test product was applied was evaluated in all study subjects visually for overt redness or dryness at baseline and at months 3, 6, 9, 12, and 18 and classified as mild, moderate, or severe. Capacitance and TEWL measurements of skin hydration were taken on a subset of subjects at baseline and at months 3, 6, 9, 12, and 18 (Martinsen et al., Citation1995; Hildebrandt et al., Citation1998). A Corneometer CM820 (Courage & Khazaka) was used to measure the degree of hydration in the stratum corneum. The probe was placed on the lateral face of both lower legs and the average of duplicate readings reported. An increase in capacitance readings indicated an increase in skin hydration. An Evaporimeter EP-1 (ServoMed) was used to measure TEWL or water vapor flux from the skin surface (reported as units of gH2O/m2/h). The probe was placed on the lateral surface of both lower legs and the average of duplicate readings reported. The medial aspects of both upper arms were used for control measurements. A decrease in TEWL indicated an increase in skin hydration.
Punch Biopsy and Histopathology
A punch biopsy was aseptically collected from the skin on the right leg at baseline and at month 3 from a subset of subjects. The biopsy was cut in half with one-half placed in 10% neutral-buffered formalin and the other half immersed in isopentane chilled with dry ice (−70°C) for a few minutes before being frozen at −70°C. The formalin fixed section was stained with H&E and assessed for epidermal thickness (in microns), the number of epidermal cell layers and mitotic figures in the epidermis (in 5 randomly selected fields). The frozen section was stained with methylene blue and assessed for the number of stratum corneum layers and for spongiosis. A board-certified pathologist examined the tissues in a blind fashion.
Analysis of Sera for IgE Antibody
Enzyme-specific IgE in serum was measured with the Pharmacia UniCAP fluoroenzyme immunoassay (Uppsala, Sweden) as previously described (Kelling et al., Citation1998). Serum from occupationally exposed SPT positive individuals was used as the positive control and a nonspecific high IgE sample (500 to 2,000 IgE units/ml) was used as a negative control. Briefly, cellulose carriers coupled with enzyme protein were incubated with serum from test subjects followed by rabbit anti human IgE-β -galactosidase. Substrate (4-methylumbellifery-β-galactoside) was added and the fluorescence measured in a FluoroCount 96 (Pharmacia). Classification of RAST class was done on the basis of fluorescence values and IgE units of the manufacturer's standard, which was run in parallel with the enzyme assay.
Pulmonary Function
Forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) were measured by flow-volume spirometers on all test subjects at the baseline visit (Mallinckrodt, St. Charles, MO; Schiller Company, Hamburg, Germany, IMS Equipment, Phoenix, AZ). The best FEV1 and FEV1/FVC(%) values were chosen from a minimum of 3 valid tracings conforming to acceptability criteria established by the American Thoracic Society (Citation1995).
Habits and Practices
All subjects answered a questionnaire regarding personal cleansing habits and home cleansing habits. Information on the types of products used, frequency of use, and any potential off-use of a product was collected. All test subjects were required to record their use of the enzyme-containing lotion and maintenance lotion throughout the study by either paper diary or by a voice activated recording system.
Statistics
All data from the different test sites were pooled for analyses using the Microsoft Office WinSTAT for Excel software package. Frequency counts (% of total) were produced for all endpoints assessed in this study (e.g., % skin prick test positive, % withdrawals, etc.). Poisson regression analysis was applied to compare the rate of positive skin prick test responses in the clinical study to an expected rate in the general population.
RESULTS
Demographics of the Test Population
The age range of the test population was 18 to 60 years with a mean age of 36.9 years. The test population was split so that 49.9% (n = 549) were in North America and 50.1% (n = 551) were in Europe. The majority (92%) was Caucasian and the remaining subjects were Black (3%), Asian (2%), Hispanic (2%), or classified as “other” (1%). At least 56% of the test subjects reported using oral contraceptives; 9% reported use of topical corticosteroids, 6% reported use of oral antihistamines and 6% reported use of anti-anxiety medications. A variety of over-the-counter and prescription drugs were used by low percentages of the study population. As a group, the mean FEV1 and FEV1/FVC were within 98% of predicted values.
Development of IgE Antibody to Enzyme
shows the frequency counts and the wheal and flare data of SPT positive test subjects over the course of the 18-month study. shows the SPT data from the follow-up tests on each test subject. Subject #92167 developed IgE antibody to Y217L BPN′ by month 6. She also had IgE antibody to the unrelated subtilisin protease from Bacillus licheniformis. An extensive review of her product use history showed that she started to use enzyme-containing carpet cleaning products designed to clean pet stains 3 months into the clinical study. Since both proteases could be found in these products, her sensitization to Y217L BPN′ could not be definitively linked to the body lotion.
Number of individuals with IgE antibody to Y217L BPN′ enzyme
Follow-up SPT with Y217L BPN′ and proteases and amylases in detergent products
Subjects no. 92152 and no. 82018 developed IgE antibody to the Y217L BPN′ by month 15. Repeat skin tests showed reproducible wheal and flare to the enzyme. Both subjects were SPT negative to other enzymes commonly found in detergent products. Neither subject had a change in product use that could account for the development of IgE to Y217L BPN′. Therefore, these sensitizations were considered linked to the body lotion. None of the subjects that completed the study at month 18 were SPT positive to Y217L BPN′. Three months after the termination of this study, 49 subjects were inadvertently recruited to participate in another clinical trial. One of these subjects (no. 81103) was confirmed as SPT positive to Y217L BPN′. An intensive review of her work history and product use history did not reveal a source of exposure to this enzyme other than from the body lotion study.
Assessment of Enzyme-Specific IgE Antibody in Serum
shows the results of the serological analysis of baseline and postexposure sera from the SPT positive test subjects. None of the 4 test subjects had IgE antibody to Y217L BPN′ in their baseline serum. The postexposure serum samples from all 4 test subjects did show IgE antibody to Y217L BPN′ classified as RAST class 2. In addition, the baseline and postexposure serum samples from subject no. 92167 showed the development of IgE antibody to protease from Bacillus licheniformis (RAST class 0 to RAST class 2—data not shown).
Assessment of Skin Condition: TEWL, Capacitance, Skin Grade, and Histopathology
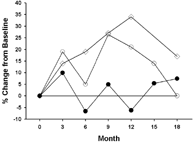
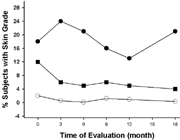
shows the percent change in TEWL readings for body lotion treated skin (lower legs) and control skin. While the TEWL measurements were slightly higher on the control skin, there were no differences between baseline measurements and postexposure measurements from the body lotion treated skin. The percent change in capacitance readings from treated skin on the lower legs showed a trend to higher capacitance readings during the treatment phase vs. baseline indicating greater hydration of the treated skin (). shows the percent of test subjects presenting with mild, moderate, or severe skin grades over the course of the study. There was a trend to see a lessening of skin grades as the study progressed. The histopathology showed no indication of inflammation of the treated skin as compared to control sites.
1 The percent change in TEWL (measured gH2O/m2/h) from enzyme treated skin sites (-◯-) and nil-enzyme treated skin sites (-•-) and the percent change in capacitance (measured in au units) from enzyme-treated skin sites (-⋄-) over time. There was less fluctuation of TEWL measurements from enzyme treated skin as compared to control skin sites. There was an increase in capacitance measurements over time.
2 The percent of test subjects with visually assessed mild (-•-), moderate (-█-), or severe (-◯-) skin grades over time. There was an increase in the percent of subjects with mild skin grades (∼ 8%) and a decrease in the percent of subjects with moderate to severe skin grades during the first 3–6 months of the study. These changes were not statistically significant.
Summary of Adverse Events and Withdrawals
A total of 237 test subjects did not complete the study. Ten subjects were withdrawn from the study due to adverse events. Three of the 10 were withdrawn due to enzyme SPT (+) responses. Two developed moderate to severe depression that was not linked to use of the study product, 2 experienced an exacerbation of dry skin, and another 2 developed mild rash that may have been exacerbated by use of lotion (nil enzyme). The tenth test subject was the victim of a homicide. Fifteen test subjects were withdrawn from the study due to pregnancy. The remaining 212 subjects were dropped due to noncompliance with the protocol or withdrawal of consent.
DISCUSSION
Intermittent exposure to a body lotion containing a low level of Bacillus protease enzyme (100 ppm) that was designed to treat dry skin led to the development of enzyme-specific IgE antibody in at least 0.46% of the study population. None of these individuals developed symptoms of type 1 allergy attributed to the enzyme-lotion product. The development of IgE antibody to enzyme in the body lotion occurred after 6 to 18 months of product use. In addition, one of these individuals developed an allergic antibody to a different Bacillus protease enzyme, presumably through use of a carpet-cleaning product designed to clean pet stains.
Protease enzymes used at appropriate levels have a desquamatory effect on the skin, eliminating the dead, dry skin flakes that accumulate on the surface of the stratum corneum. Enzyme addition to body washes or body lotions was viewed as one approach to boost the performance of such products in providing the consumer with visual and tactile improvement of their dry skin. However, enzymes are allergens and the safety of enzyme-containing personal care products must be assessed before placing them in the market. Incorporating a Bacillus protease in a beauty bar led to the development of IgE antibody in 6.5% of a study population after 4 to 6 months of use of the bar in the shower (Kelling et al., Citation1998). The average aerosol exposure to the enzyme in the shower was 10 ng/m3. While the study subjects used the product daily, they were exposed for only 2 to 3 minutes per day. This product was not subsequently commercialized.
Previous work in 2 different laboratories showed that enzyme deposited on skin can remain there for up to 24 hours and can be aerosolized in the shower or shed with the skin into bedding. A lotion containing less than 0.02% enzyme protein left a reservoir of protein on the skin that generated enzyme aerosols of 0.2 to 1 ng/m3 during showering (Johnston et al., Citation1999). A lotion containing less than 0.03% enzyme led to aerosolization of enzyme from shed skin squames during handling of bed linens (Blaikie et al., Citation1999; Pocalyko et al., Citation2002). Therefore, minimizing exposure to enzyme in a personal care product was made a primary criteria for product design. Incorporating a low level of Bacillus protease in a body lotion that would only be used 5 days of the month was viewed as a potentially safe way to deliver the desquamatory effects of the enzyme and reduce the risk of type-1 allergy. A prospective safety-in-use clinical study was conducted to confirm the risk assessment and support the safety of the product.
The skin assessments showed that the use of the body lotion did not have an adverse effect. There was a small increase in the number of women with mild skin irritation and a small decrease in cases of moderate to severe skin irritation at month 3 due to the shift of women from the moderate/severe categories to the mild category (not statistically significant). These numbers of moderate to severe irritation cases either leveled off or continued to decrease over the next 9 months, a time frame that included winter months that are typically associated with skin irritation and dry skin. TEWL and capacitance measures of skin hydration showed no loss of hydration for the enzyme-treated skin sites. The changes in TEWL for the treated skin sites remained unchanged (less than 10% up or down) during the study whereas there was greater variation in skin hydration for the control sites (5% to 26% up). The capacitance values for the treated skin sites also increased over time (up to 34%) suggesting an improvement in skin hydration. Taken together, the visual and objective scores for enzyme-treated skin showed a lessening of skin irritation and an overall improvement in skin hydration.
The test subjects did not have IgE allergic antibody to the Bacillus protease prior to being placed in the study as detected by the SPT. The highest nonirritating concentration of 500 ug protein/ml was used for the screening process but dropped to 50 ug protein/ml for on-study measures to assure consistency with the previous clinical study of the enzyme-containing bar soap. At month 6, only 1 test subject revealed a positive SPT to 50 ug/ml enzyme. She was removed from the study and enrolled in a follow-up assessment that occurred at least 4 weeks after her last exposure to body lotion. The SPT to 50 ug/ml decreased in size upon retesting but she demonstrated a positive response at 500 ug/ml. Her postexposure serum showed enzyme-specific IgE antibody in the class 2 category whereas her baseline serum tested negative for IgE antibody. The test subject was also SPT positive to a serine protease made by Bacillus licheniformis. Her baseline serum tested negative for IgE antibody to this protein but her postexposure sample was positive in the serological test. A review of her use of the body lotion did not reveal any unusual application. However, 3 months prior to her positive SPT, the test subject used spray carpet cleaners for pet stains. Analysis of these products showed that one contained very low levels of the Bacillus amyloliquifaciens protease used in the body lotion and that another product contained very high levels of a protease from Bacillus licheniformis. While her expected exposure to the Bacillus amyloliquifaciens protease from the carpet cleaner was very low (low pg/m3 range), we could not rule this out as a cause for her sensitization to the enzyme at that time. We did conclude that her IgE antibody to the B. licheniformis protease was due to the higher exposure to this enzyme in the cleaner. Therefore, the study was allowed to progress.
By month 15, 2 additional subjects developed IgE antibody to the B. amyloliqufaciens protease used in the body lotion. This was confirmed by repeat skin tests and by serology. They were SPT negative to other enzymes typically found in detergent products. A review of body lotion usage did not reveal any unusual applications and there were no changes in uses of other enzyme-containing products. The development of IgE antibody to the enzyme was attributed to exposure via the body lotion.
The study was allowed to finish and all remaining test subjects were SPT negative to the enzyme tested at 50 ug/ml. However, 3 months after the end of the study, 49 of these individuals were recruited by 1 of the test sites to participate in a different study and 1 person was found to be SPT positive to the Y217L BPN′ enzyme. This individual also had enzyme-specific IgE antibody detected by the serological test and was SPT negative to other enzymes typically used in detergent products. This individual had not developed any new habits or started using new enzyme-containing products since she was recruited into the original study. Her sensitization was attributed to the use of the enzyme-containing body lotion since this was the only identified exposure that could have led to her IgE development.
It is possible that other test subjects developed IgE antibody to the enzyme but were not detected at the final assessment due to (1) lack of sufficient sensitivity of SPT reagent at 50 ug/ml and/or (2) the IgE response was developing at month 18 and there was not enough IgE fixed to mast cells to be detected by the SPT. Since the 50 ug/ml concentration has been shown to detect IgE in clinical test subjects and is used in the occupational medical monitoring program for detergent workers, we believe the latter explanation (kinetics) is the most likely explanation. If that is the case, then it is possible that other test subjects may have been developing IgE antibody to the enzyme at the time the subjects were exiting the study. Since 1 of 49 (2%) of test subjects was found with IgE antibody to enzyme, then 2% of the remaining 863 test subjects (17 individuals) may have also developed IgE antibody. Therefore, the minimum incidence rate of sensitization to the enzyme in the body lotion was 0.46% (4/863) with a potential high rate of 2.4% (21/863). The measured rate of sensitization was compared to estimate rates of sensitization to enzymes among screened populations using Poisson regression analysis (Pepys et al., Citation1973; Sarlo et al., Citation2003). The rate of sensitization was at least 31 times greater than estimated rates of sensitization among the screened populations (p < 0.0001) adding further support to the conclusion that the allergic antibody was due to the exposure to enzyme in the body lotion.
Exposure assessments for enzyme-containing body lotions showed that exposure could occur from a variety of sources, including the shower. Even though the enzyme level in product was low, there was sufficient aerosolization to lead to levels of enzyme in air of 0.25 ng/m3 to 0.50 ng/m3. These enzyme levels are very low in comparison to exposures in occupational settings (> 5 ng/m3) and in the bar soap study (average = 10 ng/m3). However, the shower may create aerosol particles that are efficiently delivered to the airway, thereby providing sufficient delivered dose of enzyme to lead to sensitization. So while the concentration in air is low compared to known effect concentrations, the delivered dose may be comparable to what is needed to sensitize due to the mode of delivery. The work done by other investigators that showed that immunologically active enzyme was associated with shed skin squames in bedding leads one to assume that there is some exposure from the bed (sleeping, changing linen, etc). Finally, we cannot rule out the possibility that enzyme lotion on the hands led to direct inoculation of the eyes or nasal mucosa through hand-to-face contact. Therefore, it is not possible to determine if one or all of these modes of exposure were responsible for causing the sensitization.
The results of this study show that it is crucial to do a thorough risk assessment for enzyme-containing consumer products. Personal care products that come into intimate contact with the individual present unique exposure scenarios that must be carefully assessed. While we believed that we had reduced the risk of inducing enzyme-specific IgE antibody by using low level of enzyme in an intermittent-use lotion, this was not sufficient enough to prevent sensitization. Fortunately, none of the sensitized test subjects developed skin or respiratory symptoms consistent with allergy to the enzyme-lotion. However, they were removed from exposure as soon as the IgE was detected so we cannot predict what would have occurred if they had continued to use the product.
This work was funded by The Procter & Gamble Company.
REFERENCES
- American Thoracic Society, 1995. Standardization of spirometry: 1994 update. Am. J. Resp. Crit. Care Med.. 52: 1107–1136
- Belin L., Hoborn J., Falsen E., Andre J.. 1970. Enzyme sensitization in consumers of enzyme-containing washing powder. Lancet. 2: 1153–1157. [PUBMED], [INFOTRIEVE]
- Bernstein D. I., Gallagher J. S., Grad M., Bernstein I. L.. 1984. Local ocular anaphylaxis to papain enzyme contained in a contact lens cleansing solution. J. Allergy Clin. Immunol.. 74: 258–260. [PUBMED], [INFOTRIEVE]
- Blaikie L., Richold M., Whittle E., Lawrence R. S., Keech S., Basketter D. A.. 1999. Airborne exposure from topically applied protein (proteolytic enzyme). Human Exp. Toxicol. 18: 528, (abstract).
- Cormier E., Sarlo K., Scott L.. 2004. Lack of type 1 sensitization to laundry enzymes among consumers in the Philippines: Results of a two-year clinical study in atopic subjects. Annals Allergy Asthma Immunol.. 92: 549–557
- Fisher A. A.. 1985. Allergic reactions to contact lens solutions. Cutis. 36: 209–211. [PUBMED], [INFOTRIEVE]
- Hildebrandt D., Ziegler K., Wollina U.. 1998. Electrical impedance and transepidermal water loss of healthy human skin under different conditions. Skin Res. Tech.. 4: 130–134
- Johnston G., Innis J. D., Mills K. J., Bielen F., Date R. F., Weisgerber D., Sarlo K.. 1999. Safety assessment for a leave-on personal care product containing a protease enzyme. Human Exp. Tox.. 18: 527, (abstract).
- Kelling C. K., Bartolo R. G., Ertel K. D., Smith L. A., Watson D. D., Sarlo K.. 1998. Safety assessment of enzyme-containing personal cleansing products: Exposure characterization and development of IgE antibody to enzymes after a 6-month use test. J. Allergy Clin. Immunol.. 101: 179–187. [PUBMED], [INFOTRIEVE]
- Martinsen O. G., Grimnes S., Karlsen J.. 1995. Electrical methods for skin moisture assessment. Skin Pharmacol.. 8: 237–245. [PUBMED], [INFOTRIEVE]
- Pepys J., Wells I. D., D'Souza M. F., Greenberg M.. 1973. Clinical and immunological responses to enzymes of Bacillus subtilis in factory workers and consumers. Clin. Allergy. 3: 143–160. [PUBMED], [INFOTRIEVE]
- Pepys J.. 1975. Skin testing. Br. J. Hosp. Med.. 14: 412–415
- Pocalyko D. J., Chander P, Harding C. R., Blaikie L., Watkinson A., Rawlings A. V.. 2002. The efficacy, stability and safety of topically applied protease in treating xerotic skin, In. Skin Moisturization, J. J., Leyden, A. V., Rawlings, pp. 365–384, Marcel Dekker, New York
- Rothgeb T. M., Goodlander B. D., Garrison P. H., Smith L. A.. 1988. The raw material, finished products and dust pad analysis of detergent proteases using a small synthetic substrate. J. Am. Oil Chem. Soc.. 65: 806–810
- Sarlo K., Kirchner D. B., Parker R., Troyano E., Rodriguez C., Stachlewitz R.. 2003. Exposure to enzymes in detergents does not lead to the development of IgE antibody among consumers. Toxicol. Lett.. 144(Suppl. 1)S32, (abstract).
- Schweigert M. K., MacKenzie D. P., Sarlo K.. 2000. Occupational asthma and allergy associated with the use of enzymes in the detergent industry—A review of the epidemiology, toxicology and methods of prevention. Clin. Exp. Allergy. 30: 1511–1518. [CROSSREF], [PUBMED], [INFOTRIEVE]
- Zetterstrom O.. 1977. Challenge and exposure test reactions to enzyme detergent in subjects sensitized to subtilisin. Clin. Allergy. 7: 355–363. [PUBMED], [INFOTRIEVE]
- Zetterstrom O., Wide L.. 1974. IgE antibodies and skin test reactions to a detergent enzyme in Swedish consumers. Clin. Allergy. 4: 273–280. [PUBMED], [INFOTRIEVE]