Abstract
Hexachlorobenzene (HCB) is an environmental pollutant that induces adverse immune effects in humans and rats. Brown Norway rats (BN) appear to be very susceptible to HCB-induced immune effects. Oral exposure causes inflammatory skin and lung lesions, enlarged spleen and lymph nodes (LN) and elevated humoral responses. This review describes recent experiments that were performed to elucidate the mechanisms underlying these adverse immune effects. The metabolites tetrachlorohydroquinone and tetrachlorobenzoquinone are capable of generating neoantigen-specific T-cells in the mouse reporter antigen popliteal lymph node assay with 2,4,6-trinitrophenyl-Ficoll. Additional experiments in BN rats have shown that T-cells are involved in the development of HCB-induced skin lesions, the increase in auricular LN weight, lung eosinophilia, and humoral responses. However, splenomegaly and macrophage infiltration in the spleen and lung occur independent of T-cells. Remarkably, HCB does not induce T-cell sensitization in BN rats, suggesting that T-cells are activated nonspecifically, for example by macrophages. Transcriptome profiles obtained after subchronic HCB exposure to BN rats reveal that HCB induces a systemic inflammatory response in which several innate immune cells, such as macrophages, and pro-inflammatory cytokines are involved. Additional experiments have shown that macrophages are implicated in HCB-induced skin lesions, in particular in the aggravation of skin effects. Also, HCB-induced lung eosinophilia and elevation of anti-ssDNA IgM antibodies are less pronounced after macrophage elimination. Presumably, HCB activates macrophages, thereby generating pro-inflammatory adjuvant signals that induce a systemic inflammatory response. Subsequently, this may lead to polyclonal activation of T- and B-cells, eosinophilia, and eventually visible clinical effects.
INTRODUCTION
Hexachlorobenzene (HCB; C6Cl6) is a low molecular weight compound (LMWC; molecular weight: 285) that has been used in the past as a fungicide for seed grains. In the 1970s such a use was prohibited in most countries, but HCB is still generated as a by-product of several industrial processes. Furthermore, trace amounts of HCB are present as contaminants in some chlorine-containing pesticides. Although emission of HCB has decreased substantially compared to the 1970s, residues can still be found throughout the environment due to its chemical stability and high persistence.
The toxic effects of HCB on humans were first noted in the eastern part of Turkey in the 1950s. Seed grain treated with HCB was unfortunately used as food, resulting in a poisoning of approximately 3,000–5,000 people. Victims developed a syndrome that has been called Porphyria Turcica, characterized by hepatic porphyria (Cam, Citation1958). Other clinical features were skin lesions in sun-exposed areas, caused by photochemical activation of accumulated porphyrins (Bickers, Citation1987), hyperpigmentation, hirsutism, painless arthritis, small hands, enlarged liver, spleen, lymph nodes (LN), and thyroid and neurological symptoms (Gocmen et al., Citation1986; Peters et al., Citation1987). Breast-fed infants born from mothers exposed to HCB developed a syndrome that was different from Porphyria Turcica. Since these children developed skin lesions on arms and legs that were rose-red, this disease was called Pembe Yara (pink sore). Histology of skin biopsies showed hyperkeratosis and infiltrations of lymphocytes and macrophages. Other clinical symptoms were fever, diarrhea, and hepatomegaly. Thorax radiography revealed the presence of pulmonary infiltrates of unknown etiology. These infants did not develop porphyria and most victims (> 95%) died due to heart–lung failure (Cam, Citation1960). Follow-up studies among 204 victims, 20–30 years after the poisoning have shown that arthritis, enlarged thyroid, neurological, and dermatological symptoms still persisted (Cripps et al., Citation1984).
Experiments in laboratory animals confirmed several of the toxic effects observed in humans, such as hepatic porphyria (Elder, Citation1978; Daniell et al., Citation1997; Franklin et al., Citation1997), immunotoxicity (Vos, 1986; Michielsen et al., Citation1999b), neurotoxic effects (Courtney, Citation1979) and effects on the reproductive system (Foster et al., Citation1995; Alvarez et al., Citation2000; Ralph et al., Citation2003).
Following a short overview of the immune effects observed in rats after oral exposure to HCB, this review focuses on the mechanisms behind these immune effects, especially on the role of both T-cells and macrophages.
ADVERSE IMMUNE EFFECTS
HCB has immunostimulatory effects in both humans and rats. In mice, however, immunosuppression is observed (Loose et al., Citation1978; Barnett et al., Citation1987). In humans, immunotoxic effects were seen among the victims of the poisoning in Turkey and also in workers exposed occupationally to HCB in a chemical plant in Brazil. In these workers impaired functions of neutrophilic granulocytes and increased serum IgM and IgG levels were observed (Queiroz et al., Citation1998a, 1998b). When comparing the mouse and rat model with effects observed in humans, the rat seems a more relevant model. Furthermore, most research on the immunotoxicity of HCB has been performed in rats. Therefore, this review only focuses on the immune effects observed in rats.
Oral exposure of rats to HCB also induced a stimulation of the immune system (Vos et al., Citation1979a, 1979b, 1983; Schielen et al., Citation1993; Michielsen et al., Citation1997). In Wistar rats exposed to HCB, a dose-dependent elevation of the number of peripheral neutrophilic and basophilic granulocytes and monocytes and an increase in spleen and LN weights was observed. Histopathology showed increased marginal zones and follicles and extramedullary hemopoiesis in the spleen and increased numbers of high endothelial venules (HEVs) in mesenteric LN (MLN) and popliteal LN (PLN). The primary and secondary IgM and IgG responses to tetanus toxoid, a thymus-dependent antigen, were elevated in HCB-exposed rats (Vos et al., Citation1979a). Schielen et al. (Citation1993) have shown that HCB increased the number of ED3+ macrophages in the spleens of Wistar rats. These macrophages are associated with experimentally induced autoimmune diseases such as rheumatoid arthritis (Dijkstra et al., Citation1987, 1992) and are thought to be capable of activating B-1 cells (Damoiseaux et al., Citation1991), which are known to produce natural antibodies, such as anti-DNA antibodies. In Wistar rats, oral exposure to HCB not only increased ED3+ macrophages but also the number of splenic B-1 cells and serum IgM levels against autoantigens. (Schielen et al., Citation1993, 1995b). These results might be indicatory of an autoimmune component in HCB-induced immunostimulation.
In a study comparing 3 different rat strains (Wistar, Brown Norway (BN), and Lewis) the BN rat appeared to be the most susceptible strain for HCB-induced immunotoxicity (Michielsen et al., Citation1997). BN rats are known to be more prone to develop type-2 dependent autoimmunity whereas Lewis rats are more susceptible to develop Type-1-mediated autoimmune diseases (Donker et al., Citation1984). Drugs known to induce adverse immune effects such as D-penicillamine (Donker et al., Citation1984) and nevirapine (Shenton et al., Citation2003) induced autoimmunity in BN rats and tolerance in Lewis rats. Effects induced by HCB are less polarized, since adverse immune effects were induced in both strains (Michielsen et al., Citation1997). summarizes the immunotoxic effects of HCB in the BN rat and shows the characteristic picture of HCB-induced immunopathology.
Figure 1 Representative HE stained lung sections of female BN rats exposed to the control diet (A, ×200) or 450 mg HCB/kg diet (B, C) for 21 days. HCB exposure caused marked perivascular infiltrates of predominantly eosinophinilic granulocytes (B, ×200). Granulomas consisting of monocytes and macrophages and Langerhans-type giant cells were also observed (C, ×40).
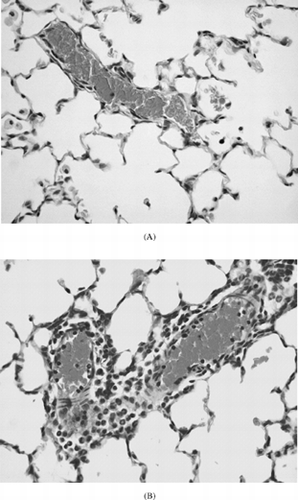
TABLE 1 Summary of immunotoxic effects of HCB in the Brown Norway rata
MECHANISMS OF HCB-INDUCED ADVERSE IMMUNE EFFECTS
Many LMWCs, such as drugs, metals, and environmental pollutants, are able to induce systemic allergy or autoimmune-like phenomena. Much research has been done to elucidate the mechanisms of LMWC-induced adverse immune effects (for reviews on this topic see Gleichmann et al., 1989; Griem et al., Citation1998; Pichler, Citation2001; Pieters et al., Citation2003; Seguin and Uetrecht, 2003).
Immunomodulatory Effects of the Oxidative Metabolites of HCB
LMWC themselves are too small to be recognized by T-cells, but can activate the immune system by binding to proteins, thereby forming neoantigens. HCB itself is not able to bind to proteins, but its oxidative metabolite tetrachlorobenzoquinone (TCBQ) is protein-reactive (Van Ommen et al., Citation1985, 1986). Recently, we have used the mouse reporter antigen popliteal lymph node assay with 2,4,6-trinitrophenyl (TNP)-Ficoll as a reporter antigen to study the immunostimulatory properties of HCB, TCBQ, and tetrachlorohydroquinone (TCHQ). The inert chemical silica was also included to serve as a model compound with adjuvant properties. In this assay TNP-Ficoll is co-injected with the chemical to obtain more mechanistic information. TNP-Ficoll is a sugar moiety that cannot be recognized by T-cells. So, an isotype switch to IgG1 indicates that the injected chemical induced neoantigen-specific T-cell stimulation (Albers et al., Citation1997). This experiment has shown that TCHQ and TCBQ as well as silica caused significant increases in PLN cell number whereas HCB caused only a slight but not significant increase. Both metabolites induced the formation of germinal centers and also an isotype switch to IgG1 in response to TNP-Ficoll. Accordingly both TCHQ and TCBQ were able to form neoantigens and stimulate T-cells. Silica and HCB were unable to do so. To confirm that the results of the primary response were compound-specific, we primed mice with TCHQ and challenged them 4 weeks later with a suboptimal dose of TCHQ together with TNP-Ficoll. A significant increase in TNP-specific IgG1 antibody secreting cells was only found in mice that were primed and challenged with TCHQ, showing that the response was specific for TCHQ (Ezendam et al., Citation2003).
Involvement of T-Cells in Adverse Immune Effects
To further investigate the role of T-cells in HCB-induced immune effects experiments were performed in BN rats. The immunosuppressive drug Cyclosporin A (CsA) was used to decrease peripheral T-cell number and inhibit T-cell activation. This study has shown that T-cells were involved in the onset of HCB-induced skin lesions, since CsA treatment delayed the development and decreased the severity of these skin effects, but did not affect the incidence. Furthermore, T-cells were necessary for the increase in auricular LN (ALN) weight, lung eosinophilia and the increases in serum IgE and anti-ssDNA IgM. Remarkably, splenomegaly was not affected and macrophage infiltrations into lung and spleen were independent of T-cells. Together, these data show that the immunopathological effects of HCB were to a large extent mediated by T-cells, but that macrophages might account for at least a part of the observed immune effects (Ezendam et al., Citation2004a).
In additional studies the role of T-cells has been investigated further. Since we have demonstrated that the metabolite TCBQ can induce noncognate T-cell help in the RA-PLNA and thus form hapten-carrier complexes or neo-epitopes (Ezendam et al., Citation2003), we wanted to determine if HCB could induce immunological memory after oral exposure of BN rats. However, by using a challenge with TCBQ on the ears of HCB-exposed BN rats, no hapten-specific T-cells could be detected in the draining lN. Furthermore, HCB-induced immune effects could not be transferred to naive recipients (Ezendam, Citation2004). Hence, although HCB-induced pathology could be partly prevented by inhibition of T-cells with CsA, HCB exposure does not lead to (specific) T-cell sensitization and immunological memory. Thus it seems that haptenization of TCBQ to self-proteins is not involved in the activation of T-cells (Ezendam, Citation2004). This is in agreement with previous work, that has shown that reactive metabolites formed by cytochrome P450-catalyzed conversion of HCB (Schielen et al., Citation1995a) or generated in the mercapturic acid pathway (Michielsen et al., Citation2000) were not involved in the immune effects. Furthermore, additional experiments were performed to investigate if myeloperoxidase (MPO), an enzyme present in granulocytes, could oxidize HCB. MPO is able to convert other chemicals implicated in adverse immune reactions into reactive and thus possible immunogenic intermediates (for reviews see Uetrecht Citation1990, 1997). However, no evidence was found for the capability of MPO to convert HCB into reactive intermediates (Ezendam, Citation2004). Together, these data argue against an involvement of reactive metabolites as initiators of HCB-induced immune effects.
Adjuvant Effect of HCB
Some LMWC (e.g., the inert chemical silica) can induce adverse immune effects, because they have adjuvant activity. Silica specifically activates macrophages, leading to the release of pro-inflammatory cytokines, such as TNF-α and IL-1 and reactive oxygen species (ROS) (Driscoll et al., Citation1990; Gossart et al., Citation1996). Release of these pro-inflammatory mediators can elicit a cascade of events, such as expression of cell adhesion molecules and release of chemokines leading to the recruitment of inflammatory cells. Thus, silica can be considered as an adjuvant and exposure can eventually lead to inflammation and secondary cell damage. In vitro data have shown that HCB (added to the cells as a suspension) activated rat alveolar macrophages (NR8383 cell line) similar to silica, inducing TNF-α and IL-6 production, suggesting that HCB might also have adjuvant activity (Ezendam, Citation2004). As a consequence, these adjuvant or pro-inflammatory mediators might activate autoreactive T-cells or elicit immune response against (auto)antigens that under normal circumstances are ignored (Stoy, Citation2002).
Toxicogenomics
DNA micoarray experiments have provided additional evidence for the significance of macrophages and granulocytes in HCB-induced immune effects. BN rats were exposed to 150 and 450 mg/kg diet HCB for 28 days. Thereafter, transcriptome profiling with DNA microarrays from Affymetrix was performed in spleen, mesenteric lymph node (MLN), thymus, blood, liver, and kidney. Data confirmed known effects of HCB such as stimulatory effects on the immune system and induction of enzymes involved in drug metabolism, porphyria, and the reproductive system. New findings include the upregulation of genes encoding pro-inflammatory cytokines, antioxidants, acute phase proteins, mast cell markers, complement, chemokines, and cell adhesion molecules. Furthermore, this inflammatory response is not only confined to the immune organs, but also occurs in liver and kidney. Thus, HCB induced a systemic inflammation. (Ezendam et al., Citation2004b).
HCB-Induced Immune Effects Are Probably Initiated by Macrophages
More evidence for the importance of macrophages has been obtained in a kinetic study. Macrophages were observed in the lung early after HCB exposure and both skin and lung pathology were early inflammatory events. Furthermore, macrophage infiltrations in the spleen seemed to precede the increase in spleen weight (Ezendam, Citation2004). To study the importance of macrophages in the observed immunopathology, macrophages were eliminated with clodronate-liposomes. The principle of this so-called macrophage “suicide”technique is based on the capacity of macrophages to eliminate foreign bodies. After phagocytosis of the liposomes, the toxic compound clodronate is released inside in the macrophage, leading to cell death (Van Rooijen, Citation1989). These experiments have shown that macrophages were involved in HCB-induced skin lesions, in particular in the aggravation of skin lesions. Also, HCB-induced lung eosinophilia and elevation of anti-ssDNA IgM antibodies were less pronounced after depletion of macrophages (Ezendam, Citation2004). summarizes the results of the experiments with CsA and clodronate-liposomes.
TABLE 2 Summary of results of T-cell- and macrophage-dependency of HCB-induced adverse immune effects in the BN rat
These findings provide additional information on the mechanism of HCB-induced immune derangements. We postulate that initially HCB activates macrophages in several organs, in particular the lung and skin. Notably, a toxicokinetic study has shown that as soon as 3 hours after a single oral dose of HCB peak, concentrations of HCB were detected in the lungs (personal communication, D. Overstreet). This means that HCB is rapidly absorbed into the systemic circulation and distributed to the lungs. Thus, early deposition of HCB may indeed be associated with the early presence of macrophages in the lungs. This accumulation of macrophages and also the formation of granulomas in the lung occurred independently of T-cells (Ezendam et al., Citation2004a).
Also in the spleen, macrophage infiltration and activation occurred early after exposure and appeared to precede the increase in spleen weight (Ezendam, Citation2004). Moreover, both HCB-induced splenomegaly and splenic macrophage influxes were T-cell-independent (Ezendam et al., Citation2004a). Based on these findings we suggest that after HCB exposure T- and B-cells are polyclonally activated by macrophages. The polyclonal nature of the B-cell response was illustrated by previous studies that have shown that HCB increased serum levels of IgM against ssDNA and total IgM, IgG, and IgE (Schielen et al., Citation1995b; Michielsen et al., Citation1997). Notably, antibodies against ssDNA are produced by B-1 cells that are presumably activated by MZ macrophages (Damoiseaux et al., Citation1991). In addition, transcriptome profiles have shown that HCB increased expression of antibodies against the acetylcholine receptor in spleen, thymus, liver, and kidney and of nerve growth factor in spleen and liver (Ezendam et al., Citation2004b). The absence of IgG against ssDNA in HCB-treated rats (Michielsen et al., Citation1997) indicates that specific T-cell help is not sufficiently available and hence supports the idea of polyclonal T-cell activation.
CONCLUSIONS
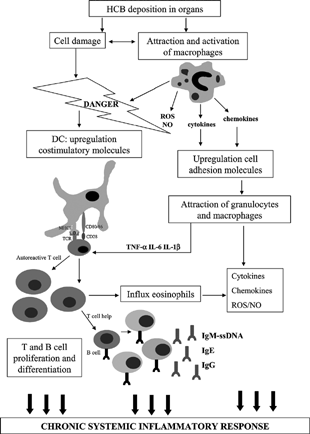
In conclusion, we propose that after HCB exposure pro-inflammatory adjuvant signals are generated that induce a systemic inflammatory response with influxes of neutrophils and macrophages into various nonimmune and immune organs. Subsequently, this leads to polyclonal activation of T- and B-cells, eosinophilia, and eventually to visible clinical effects.
What exactly elicits the attraction of macrophages is quite speculative, but HCB deposition can directly induce cell damage, or elicit damage by interfering with the integrity of cell membranes due to its lipophilic nature. This could mean that HCB-induced immune effects may be explained by the danger hypothesis proposed by Matzinger (Citation1994). This hypothesis is based on the assumption that the immune system does not discriminate between self and nonself, but rather between harmless and dangerous. According to this hypothesis, danger signals can activate antigen-presenting cells that subsequently can deliver costimulatory signals to T-cells, and danger signals can be derived from damaged cells, but also heat shock proteins or cytokines are considered as inducible danger signals (Anderson and Matzinger, 2000). It has been shown that necrotic cells can provide maturation signals to dendritic cells (DCs) (Gallucci et al., Citation1999; Sauter et al., Citation2000) and macrophages (Barker et al., Citation1999), resulting in upregulation of costimulatory molecules and enhancement of T-cell proliferation. Furthermore, necrotic cells induced NF-κ B and chemokine gene expression in DCs, macrophages, and fibroblasts (Li et al., Citation2001). HCB exposure may lead to cell death and generation of danger signals that can activate macrophages. Furthermore, these danger signals could be involved in the activation of T-cells by providing costimulatory signals and could also induce several pro-inflammatory mediators leading to the observed inflammation.
All these events are summarized in to show the possible mode of action of HCB-induced immune effects. Activated macrophages release pro-inflammatory mediators, such as TNF-α, IL-1β, IL-6, ROS, and chemokines. These proinflammatory mediators are known to be capable of inducing numerous events, including upregulation of cell adhesion molecules, thereby facilitating recruitment of other inflammatory cells. Furthermore, pro-inflammatory cytokines may also stimulate T-cell responses (Curtsinger et al., Citation1999). Immunostimulation of autoreactive T-cells by adjuvant or danger signals is explained by the so-called bystander hypothesis. This hypothesis postulates that dysregulated macrophage activation can lead to expansion of preexisting autoreactive T-cells and B-cells (reviewed in Stoy, Citation2002). Evidently in such a situation, regulatory mechanisms (i.e., regulatory T-cells) are not sufficient to control these preexisting autoreactive T-cells and B-cells (Fournie et al., Citation2001).
In conclusion, the HCB-induced immunopathology supports the notion that the etiology of immune derangements induced by xenobiotics is very complex. In general, the BN rat model for HCB exposure is an interesting addition to other existing BN rat models, e.g., models to study the drugs D-penicillamine (Donker et al., Citation1984) and nevirapine (Shenton et al., Citation2003). Furthermore, this model illustrates that this strain can be very useful as a candidate for predictive testing of possible immunostimulatory xenobiotics.
REFERENCES
- Albers R., Broeders A., van der Pijl A., Seinen W., Pieters R. The use of reporter antigens in the popliteal lymph node assay to assess immunomodulation by chemicals. Toxicol. Appl. Pharmacol. 1997; 143(1)102–109
- Alvarez L., Randi A., Alvarez P., Piroli G., Chamson-Reig A., Lux-Lantos V., Kleiman de Pisarev D. Reproductive effects of hexachlorobenzene in female rats. J. Appl. Toxicol. 2000; 20(1)81–87
- Anderson C. C., Matzinger P. Danger: The view from the bottom of the cliff. Semin. Immunol. 2000; 12(3)231–238
- Barker R. N., Erwig L., Pearce W. P., Devine A., Rees A. J. Differential effects of necrotic or apoptotic cell uptake on antigen presentation by macrophages. Pathobiology 1999; 67(5–6)302–305
- Barnett J. B., Barfield L., Walls R., Joyner R., Owens R., Soderberg L. S. The effect of in utero exposure to hexachlorobenzene on the developing immune response of BALB/c mice. Toxicol. Lett. 1987; 39: 263
- Bickers D. R. The dermatologic manifestations of human porphyria. Ann. N.Y. Acad. Sci. 1987; 514: 261–267
- Cam C. Cases of skin porphyria related to hexachlorobenzene intoxication. Saglik Dergisi 1958; 32: 215–216
- Cam C. Une nouvelle dermatose épidémique des enfants. Anales de Dermatologie 1960; 87: 393–397
- Courtney K. D. Hexachlorobenzene (HCB): A review. Environ. Res. 1979; 20(2)225–266
- Cripps D. J., Peters H. A., Gocmen A., Dogramici I. Porphyria turcica due to hexachlorobenzene: A 20 to 30 year follow-up study on 204 patients. Br. J. Dermatol 1984; 111(4)413–422
- Curtsinger J. M., Schmidt C. S., Mondino A., Lins D. C., Kedl R. M., Jenkins M. K., Mescher M. F. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T-cells. J. Immunol. 1999; 162(6)3256–3262
- Damoiseaux J. G., Dopp E. A., Dijkstra C. D. Cellular binding mechanism on rat macrophages for sialylated glycoconjugates, inhibited by the monoclonal antibody ED3. J. Leukoc. Biol. 1991; 49(5)434–441
- Daniell W. E., Stockbridge H. L., Labbe R. F., Woods J. S., Anderson K. E., Bissell D. M., Bloomer J. R., Ellefson R. D., Moore M. R., Pierach C. A., Schreiber W. E., Tefferi A., Franklin G. M. Environmental chemical exposures and disturbances of heme synthesis. Environ. Health Perspect. 1997; 105(1)37–53, Suppl.
- Dijkstra C. D., Dopp E. A., Huitinga I., Damoiseaux J. G. Macrophages in experimental autoimmune diseases in the rat: A review. Curr. Eye. Res. 1992; 11: 75–79, Suppl.
- Dijkstra C. D., Dopp E. A., Vogels I. M., Van Noorden C. J. Macrophages and dendritic cells in antigen-induced arthritis. An immunohistochemical study using cryostat sections of the whole knee joint of rat. Scand. J. Immunol. 1987; 26(5)513–523
- Donker A. J., Venuto R. C., Vladutiu A. O., Brentjens J. R., Andres G. A. Effects of prolonged administration of D-penicillamine or captopril in various strains of rats. Brown Norway rats treated with D-penicillamine develop autoantibodies, circulating immune complexes, and disseminated intravascular coagulation. Clin. Immunol. Immunopathol. 1984; 30(1)142–155
- Driscoll K. E., Lindenschmidt R. C., Maurer J. K., Higgins J. M., Ridder G. Pulmonary response to silica or titanium dioxide: inflammatory cells, alveolar macrophage-derived cytokines, and histopathology. Am. J. Respir. Cell. Mol. Biol. 1990; 2(4)381–390
- Porphyria caused by hexachlorobenzene and other polyhalogenated aromatic hydrocarbons, G. H. Elder. Springer-Verlag, New York, Heidelberg 1978; 157–200
- Ezendam J. Mechanisms of Hexachlorobenzene-Induced Adverse Immune Effects. Utrecht University, Utrecht 2004
- Ezendam J., Hassing I., Bleumink R., Vos J. G., Pieters R. Hexachlorobenzene-induced immunopathology in Brown Norway rats is partly mediated by T-cells. Toxicol. Sci. 2004a; 78: 88–95
- Ezendam J., Staedtler F., Pennings J., Vandebriel R. J., Pieters R., Boffetta P., Harleman J. H., Vos J. G. Toxicogenomics of subchronic hexachlorobenzene exposure in Brown Norway rats. Environ. Health Perspect. 2004b; 112(7)782–791
- Ezendam J., Vissers I., Bleumink R., Vos J. G., Pieters R. Immunomodulatory effects of tetrachlorobenzoquinone, a reactive retabolite of hexachlorobenzene. Chem. Res. Toxicol. 2003; 16(6)688–694
- Foster W. G., McMahon A., Younglai E. V., Jarrell J. F., Lecavalier P. Alterations in circulating ovarian steroids in hexachlorobenzene-exposed monkeys. Reprod. Toxicol. 1995; 9(6)541–548
- Fournie G. J., Mas M, Cautain B., Savignac M., Subra J. F., Pelletier L., Saoudi A., Lagrange D., Calise M., Druet P. Induction of autoimmunity through bystander effects. Lessons from immunological disorders induced by heavy metals. J. Autoimmun. 2001; 16(3)319–326
- Franklin M. R., Phillips J. D., Kushner J. P. Cytochrome P450 induction, uroporphyrinogen decarboxylase depression, porphyrin accumulation and excretion, and gender influence in a 3-week rat model of porphyria cutanea tarda. Toxicol. Appl. Pharmacol. 1997; 147(2)289–299
- Gallucci S., Lolkema M., Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 1999; 5(11)1249–1255
- Gleichmann E., Kimber I., Purchase I. F. Immunotoxicology: Suppressive and stimulatory effects of drugs and environmental chemicals on the immune system. A discussion. Arch. Toxicol. 1989; 63(4)257–273
- Gocmen A., Peters H. A., Cripps D. J., Morris C. R., Dogramaci I. Porphyria turcica: hexachlorobenzene-induced porphyria. Hexachlorobenzene: Proceedings of an International Symposium, C. Morris, J. R. Cabral. IARC Scientific Publications No. 77, Lyon. 1986; 567–573
- Gossart S., Cambon C., Orfila C., Seguelas M. H., Lepert J. C., Rami J., Carre P., Pipy B. Reactive oxygen intermediates as regulators of TNF-alpha production in rat lung inflammation induced by silica. J. Immunol. 1996; 156(4)1540–1548
- Griem P., Wulferink M., Sachs B., Gonzalez J. B., Gleichmann E. Allergic and autoimmune reactions to xenobiotics: How do they arise? Immunol. Today 1998; 19(3)133–141
- Li M., Carpio D. F., Zheng Y., Bruzzo P., Singh V., Ouaaz F., Medzhitov R. M., Beg A. A. An essential role of the NF-kappa B/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J. Immunol. 2001; 166(12)7128–7135
- Loose L. D., Silkworth J. B., Pittman K. A., Benitz K. F., Mueller W. Impaired host resistance to endotoxin and malaria in polychlorinated biphenyl- and hexachlorobenzene-treated mice. Infect. Immun. 1978; 20(1)30–35
- Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994; 12: 991–1045
- Michielsen C. C., Bloksma N., Klatter F. A., Rozing J., Vos J. G., van Dijk J. E. The role of thymus-dependent T-cells in hexachlorobenzene-induced inflammatory skin and lung lesions. Toxicol. Appl. Pharmacol. 1999a; 161(2)180–191
- Michielsen C. P., Bloksma N., Ultee A., van Mil F., Vos J. G. Hexachlorobenzene-induced immunomodulation and skin and lung lesions: A comparison between Brown Norway, Lewis, and Wistar rats. Toxicol. Appl. Pharmacol. 1997; 144(1)12–26
- Michielsen C., Boeren S., Rietjens I., van Mil F., Vos J., Bloksma N. The mercapturic acid biotransformation pathway of hexachlorobenzene is not involved in the induction of splenomegaly, or skin and lung lesions in the Brown Norway rat. Arch. Toxicol. 2000; 74(10)609–617
- Michielsen C. P., Leusink-Muis A., Vos J. G., Bloksma N. Hexachlorobenzene-induced eosinophilic and granulomatous lung inflammation is associated with in vivo airways hyperresponsiveness in the Brown Norway rat. Toxicol. Appl. Pharmacol. 2001; 172(1)11–20
- Michielsen C. C., van Loveren H., Vos J. G. The role of the immune system in hexachlorobenzene-induced toxicity. Environ. Health Perspect 1999b; 107: 783–792, Suppl 5
- Michielsen C., Zeamari S., Leusink-Muis A., Vos J., Bloksma N. The environmental pollutant hexachlorobenzene causes eosinophilic and granulomatous inflammation and in vitro airways hyperreactivity in the Brown Norway rat. Arch. Toxicol. 2002; 76(4)236–247
- Peters H., Cripps D., Gocmen A., Bryan G., Erturk E., Morris C. Turkish epidemic hexachlorobenzene porphyria. A 30-year study. Ann. N.Y. Acad. Sci. 1987; 514: 183–190
- Pichler W. J. Drug allergy. Curr. Opin. Allergy Clin. Immunol. 2001; 1(4)285–286
- Pieters R., Ezendam J., Nierkens S. Chemical-specific properties co-determine the type of adverse immune response. Autoimmun. Rev. 2003; 2(1)25–29
- Queiroz M. L., Bincoletto C., Perlingeiro R. C., Quadros M. R., Souza C. A. Immunoglobulin levels in workers exposed to hexachlorobenzene. Hum. Exp. Toxicol. 1998a; 17(3)172–175
- Queiroz M. L., Quadros M. R., Valadares M. C., Silveira J. P. Polymorphonuclear phagocytosis and killing in workers occupationally exposed to hexachlorobenzene. Immunopharmacol. Immunotoxicol. 1998b; 20(3)447–454
- Ralph J. L., Orgebin-Crist M. C., Lareyre J. J., Nelson C. C. Disruption of androgen regulation in the prostate by the environmental contaminant hexachlorobenzene. Environ. Health Perspect. 2003; 111(4)461–466
- Sauter B., Albert M. L., Francisco L., Larsson M., Somersan S., Bhardwaj N. Consequences of cell death: Exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 2000; 191(3)423–434
- Schielen P., Den Besten C., Vos J. G., Van Bladeren P. J., Seinen W., Bloksma N. Immune effects of hexachlorobenzene in the rat: Role of metabolism in a 13-week feeding study. Toxicol. Appl. Pharmacol. 1995a; 131(1)37–43
- Schielen P., Schoo W., Tekstra J., Oostermeijer H. H., Seinen W., Bloksma N. Autoimmune effects of hexachlorobenzene in the rat. Toxicol. Appl. Pharmacol. 1993; 122(2)233–243
- Schielen P., Van Rodijnen W., Pieters R. H., Seinen W. Hexachlorobenzene treatment increases the number of splenic B-1-like cells and serum autoantibody levels in the rat. Immunology 1995b; 86(4)568–574
- Seguin B., Uetrecht J. The danger hypothesis applied to idiosyncratic drug reactions. Curr. Opin. Allergy. Clin. Immunol. 2003; 3(4)235–242
- Shenton J. M., Teranishi M., Abu-Asab M. S., Yager J. A., Uetrecht J. P. Characterization of a potential animal model of an idiosyncratic drug reaction: Nevirapine-induced skin rash in the rat. Chem. Res. Toxicol. 2003; 16(9)1078–1089
- Stoy N. S. Monocyte/macrophage initiation of organ-specific autoimmunity: The ultimate ‘bystander’ hypothesis?. Med. Hypotheses 2002; 58(4)312–326
- Uetrecht J. Drug metabolism by leukocytes and its role in drug-induced lupus and other idiosyncratic drug reactions. Crit. Rev. Toxicol. 1990; 20(4)213–235
- Uetrecht J. P. Current trends in drug-induced autoimmunity. Toxicology 1997; 119(1)37–43
- Van Ommen B., Adang A. E., Brader L., Posthumus M. A., Muller F., van Bladeren P. J. The microsomal metabolism of hexachlorobenzene. Origin of the covalent binding to protein. Biochem. Pharmacol. 1986; 35(19)3233–3238
- Van Ommen B., van Bladeren P. J., Temmink J. H., Muller F. Formation of pentachlorophenol as the major product of microsomal oxidation of hexachlorobenzene. Biochem. Biophys. Res. Commun. 1985; 126(1)25–32
- Van Rooijen N. The liposome-mediated macrophage ‘suicide’ technique. J. Immunol. Meth. 1989; 124(1)1–6
- Vandebriel R. J., Meredith C., Scott M. P., van Dijk M., van Loveren H. Interleukin-10 is an unequivocal Th2 parameter in the rat, whereas interleukin-4 is not. Scand. J. Immunol. 2000; 52(5)519–524
- Vos J. G. Immunotoxicity of hexachlorobenzene. Hexachlorobenzene: Proceedings of an International Symposium, C. R. Morris, J. R. Cabral. IARC Scientific Publications, LyonFrance 1986; 347–356
- Vos J. G., Brouwer G. M. J., Van Leeuwen F. X. R., Wagenaar S. Toxicity of hexachlorobenzene in the rat following combined pre- and post-natal exposure: Comparison of effects on immune system, liver and lung. Immunotoxicology, D. V. Parke, G. G. Gibson, R. Hubard. Academic Press, London 1983; 219–235
- Vos J. G., Van Logten M. J., Kreeftenberg J. G., Kruizinga W. Hexachlorobenzene-induced stimulation of the humoral response in rats. Ann. N.Y. Acad. Sci. 1979a; 320: 535–550
- Vos J. G., van Logten M. J., Kreeftenberg J. G., Steerenberg P. A., Kruizinga W. Effect of hexachlorobenzene on the immune system of rats following combined pre- and postnatal exposure. Drug. Chem. Toxicol. 1979b; 2(1–2)61–76