Abstract
The current study was designed to develop and test a T-cell dependent antibody response to keyhole limpet hemocyanin (KLH) in cynomolgus monkeys. In an optimization experiment, monkeys (3/sex) were given a single intramuscular injection of KLH at 10 mg/animal to evaluate the kinetics of the antibody response. Serum samples were collected pretest, and on Days 4, 6, 8, 11, 15 and 22 for measurement of anti-KLH IgM and IgG endpoint titers. In a subsequent experiment, female monkeys (3/group) were treated once daily by gavage with the immunosuppressive agent cyclosporine (Neoral) at 0, 10 and 50 mg/kg for 21 days, and the effects of drug treatment on anti-KLH IgM and IgG responses were determined. The effects of cyclosporine on hematology, biochemistry, bone marrow, organ weights, gross and histopathology, and peripheral lymphocyte subsets also were evaluated. Robust anti-KLH IgM and IgG responses were seen in monkeys given a single intramuscular injection of KLH at 10 mg/animal, with peak antibody responses at approximately 10–14 days post-immunization for anti-KLH IgM, and 14–21 days for anti-KLH IgG. Decreases in anti-KLH IgG endpoint titers were seen in 1 monkey given cyclosporine at 10 mg/kg, and 1 monkey dosed at 50 mg/kg. Relative to vehicle control animals, mild lymphoid depletion was evident in lymph nodes and tonsil of monkeys with suppressed anti-KLH IgG titers. Collectively, these findings in individual animals provided evidence of cyclosporine-induced immunosuppression. Cyclosporine at 10 and 50 mg/kg did not alter anti-KLH IgM production, hematology, biochemistry, bone marrow, organ weights, or peripheral lymphocyte subsets. Lastly, the results of this study demonstrated that KLH immunization at 10 mg/animal did not alter the standard toxicity endpoints evaluated in control animals.
BACKGROUND
The Committee for Medicinal Products for Human Use (CHMP), formally the Committee for Proprietary Medicinal Products (CPMP), and the Food and Drug Administration (FDA) have provided guidance for immunotoxicity testing of new drug candidates (CPMP, Citation2000; FDA, Citation2002). Both agencies recommend evaluating a primary T-cell-dependent antibody response when assessing potential drug effects on immune function. Evaluation of a T-cell-dependent antibody response provides an overall measure of host immune function, and requires the active participation of T-lymphocytes, B-lymphocytes, and macrophages (Luster et al., Citation1992). When assessing the immunotoxicity potential of test compounds, this assay may be used to supplement standard immunotoxicity endpoints including hematology, lymphoid organ weights, histological evaluation of lymphoid tissues, and bone marrow cellularity. If evidence of immunosuppression is observed in initial studies, additional mechanistic studies may be required to further characterize the altered immune response.
Cynomolgus monkeys are used routinely in preclinical toxicity testing as a non-rodent species. Evaluation of immunotoxicity in cynomolgus monkeys may be needed preclinically when a test compound has pharmacological activity in non-human primates but not in rodents (e.g., biologicals), or when evidence of immunotoxicity is seen in repeated-dose toxicity studies. Although the antibody responses to sheep red blood cells in monkeys have been reported previously (Tryphonas et al., Citation1989; Arnold et al., Citation1999), responses to other T-cell-dependent antigens such as keyhole limpet hemocyanin (KLH) have not been fully characterized. The current study developed and tested a T-cell-dependent antibody response to KLH in adult cynomolgus monkeys. This study evaluated the timing of the anti-KLH IgM and IgG responses, and the effects of cyclosporine treatment on antibody production. The study also investigated the effects of cyclosporine on hematology, biochemistry, bone marrow, organ weights, gross and histopathology, and peripheral lymphocyte subsets. In addition, the effects of KLH immunization alone on these parameters are discussed.
METHODS
Treatment Protocol
Male and female cynomolgus monkeys from Mauritius were obtained from Charles River Laboratories. Animals were approximately 3–7 years of age, and weighed 3–10 kg at study initiation. In a pilot experiment, male monkeys were given a single 2 mL intramuscular (IM) injection of KLH (Pierce, Rockford, IL) at 3, 10 and 30 mg/animal, with 1 animal per dose. Blood was collected by femoral venipuncture twice pretest (day −6 and day 1 [i.e., immediately before KLH injection]), and on days 4, 6, 8, 11, 15, and 22. In a subsequent experiment, male and female monkeys (3/sex/group) were given a single 1 mL IM injection of KLH at 10 mg/animal. As in the pilot experiment, blood also was collected twice pretest (day −7 and day 1 [i.e., immediately before KLH injection]), and on days 4, 6, 8, 11, 15, and 22. In both experiments, serum was isolated and stored at −20°C prior to analysis.
In the validation study investigating immunosuppression, female monkeys were treated by gavage once daily with cyclosporine (Neoral) at 0, 10, and 50 mg/kg for 21 days. Neoral was purchased from AmerisourceBergen (Toledo, OH), and was diluted in distilled water. Control monkeys received vehicle (distilled water) alone. Doses were selected based on reports investigating the effects of cyclosporine on renal allograft survival in cynomolgus monkeys. A daily dose of 10 mg/kg is considered suboptimal in preventing renal allograft rejection in monkeys; however, short-term allograft survival can be obtained in some animals at this dose (Schuurman et al., Citation1996, Citation2001). Doses of cyclosporine above 30 mg/kg are sufficient to prolong renal allograft survival, although doses as high as 100–150 mg/kg are typically needed for long-term graft survival greater than 100 days (Schuurman et al., Citation1996, Citation2001). In the current study, the 10 mg/kg dose of cyclosporine was selected to test the sensitivity of the experimental model. The 50 mg/kg dose was expected to induce immunosuppression without causing overt systemic toxicity, which may occur at doses of ≥ 100 mg/kg. All monkeys were given a single IM dose of KLH (10 mg/animal) on day 8, and the effects of cyclosporine on the production of IgM and IgG against KLH were evaluated 10–14 days post-immunization. Additional toxicity endpoints evaluated are summarized in . For histopathologic evaluation, representative tissue samples were collected after a post-mortem examination. Tissues were fixed in 10% buffered formalin, embedded in paraffin, section, and stained with hematoxylin and eosin. Slides were examined by two Board Certified Veterinary Pathologists (REG and JFR).
TABLE 1 The effects of cyclosporine treatment of cynomolgus monkeys—Endpoints evaluated
Anti-KLH Antibody Determination
Serum anti-KLH IgM and anti-KLH IgG were measured by enzyme-linked immunosorbent assay (ELISA). Validation of these methods included evaluation of specificity, sensitivity, plate positioning, inter- and intra-assay precision, and sample stability (Piccotti; serum samples from Pfizer Protocol #3169 and #DI 0103). For determination of anti-KLH IgM endpoint titers, serum samples were serially diluted from 1/400–1/102,400. For determination of anti-KLH IgG endpoint titers, sera were serially diluted from 1/100–1/25,600. For samples with high IgG concentrations, sera were diluted up to 1/102,400. Endpoint titers were defined as the reciprocal of the greatest dilution of serum at which antibody reactivity was detected following background correction (i.e., minus the absorbance of the negative control sera + 3 standard deviations). Endpoint titers were reported as the reciprocal of the dilution and the log base 2 (log2) of the dilution.
RESULTS
Evaluation of Anti-KLH Antibody Responses
This phase of the study evaluated the kinetics of the anti-KLH IgM and IgG responses following immunization with KLH. In a pilot study that evaluated KLH at 3, 10, and 30 mg/animal, a single IM dose of KLH elicited robust anti-KLH IgM and IgG responses (). In this experiment, anti-KLH IgM endpoint titers were similar at 10 and 30 mg/animal (). The highest anti-KLH IgM endpoint titers were seen in the monkey given 3 mg of KLH, although this animal had the highest pretest IgM levels. The presence of anti-KLH IgM in unimmunized monkeys is discussed next. Anti-KLH IgG endpoint titers were comparable at 3 and 10 mg/animal, but decreased at 30 mg (). Based on the results of this pilot experiment, a dose of KLH of 10 mg/animal was selected for subsequent experiments.
FIG. 1. Evaluation of KLH dose and timing of immunization. Male cynomolgus monkeys (1 animal/dose) were given a single IM injection of KLH at 3, 10, or 30 mg per animal. Blood was collected by femoral venipuncture twice pretest (days −6 and 1), and on days 4, 6, 8, 11, 15, and 22 (3, 5, 7, 10, 14, and 21 days post-immunization). Serum anti-KLH IgM and IgG endpoint titers (EPT) were measured by ELISA. Results are report as log2 values for (A) anti-KLH IgM and (B) anti-KLH IgG.
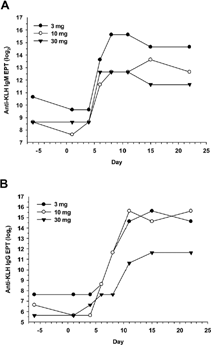
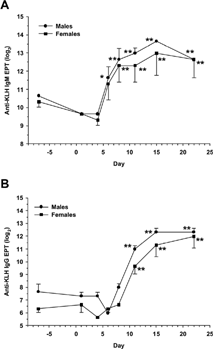
In the optimization experiment, statistically significant increases in anti-KLH IgM endpoint titers were seen in male monkeys from days 6 through 22 compared to pretest, and in females from days 8 through 22 (). The IgM responses peaked on approximately days 11–15 (10–14 days post-immunization) in both males and females. Statistically significant increases in anti-KLH IgG endpoint titers were seen in male and female monkeys from days 11 through 22 (). The IgG responses reached a maximum on approximately days 15–22 (14–21 days post-immunization) in both sexes. The timing of the anti-KLH IgM and IgG responses in this experiment was similar in male and female monkeys, although responses appeared slightly greater in males. The results of the optimization experiment were consistent with the data obtained from the male monkey given 10 mg of KLH in the pilot experiment ().
FIG. 2. Kinetics of the anti-KLH IgM and IgG responses in monkeys. Male and female cynomolgus monkeys (3/sex) were given a single IM injection of KLH at 10 mg/animal on day 1. Blood was collected by femoral venipuncture twice pretest (days −7 and 1), and on days 4, 6, 8, 11, 15, and 22. Serum anti-KLH IgM and IgG endpoint titers were measured by ELISA, and are reported as log2 values. Results are expressed as mean ± standard error for (A) anti-KLH IgM and (B) anti-KLH IgG. For statistical analysis, antibody concentrations following KLH immunization were compared to levels in unimmunized animals (day 1 predose) using pairwise comparisons within one-factor analysis of variance (ANOVA). *Statistically significant difference compared to pretest, p ≤ 0.05. **Statistically significant difference compared to pretest, p ≤ 0.01.
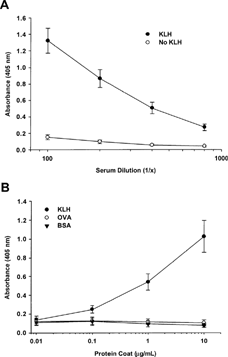
In experiments evaluating the dose of KLH and the kinetics of the IgM and IgG responses, anti-KLH IgM was detected in pretest samples, suggesting preexisting antibodies in cynomolgus monkeys. To further investigate this possibility, anti-KLH IgM and IgG endpoint titers were measured in unimmunized male and female monkeys (9/sex). The mean anti-KLH IgM levels (log2 values) were 10.09 and 10.20 in male and female monkeys, respectively, corresponding to a serum dilution of approximately 1:1,200. In contrast, the levels of IgG specific for KLH in unimmunized males and females were near or below the assay's limit of detection (dilution of 1:100). Additional experiments demonstrated that the anti-KLH IgM detected in sera of unimmunized monkeys was specific for KLH, since antibody was undetectable in uncoated ELISA wells (i.e., no KLH), and sera from unimmunized monkeys did not cross-react with irrelevant proteins such as ovalbumin (OVA) or bovine serum albumin (BSA) ().
FIG. 3. Specificity of anti-KLH IgM detected in unimmunized monkeys. Serum samples were obtained from unimmunized male and female cynomolgus monkeys (6–9/sex). (A) ELISA plates were coated with KLH, or wells were left uncoated. Sera were tested at dilutions ranging from 1:100 to 1:800. (B) Plates were coated with KLH, OVA, or BSA at 0.01, 0.1, 1, and 10 μ g/mL. Serum samples were tested at a dilution of 1:400. Data are expressed as mean absorbance ± standard error.
Evaluation of the Immunosuppressive Effects of Cyclosporine
No clinical signs of toxicity were seen in monkeys given cyclosporine at 10 or 50 mg/kg/day for 21 days. No drug-related changes in body weight, food consumption, hematology, biochemistry, bone marrow, peripheral lymphocyte subpopulations, organ weight, gross pathology, or histology of non-lymphoid tissues were seen. In addition, KLH immunization alone did not alter these parameters in control monkeys (data not shown).
Consistent with previous findings, anti-KLH IgM was present in all animals pretest. Endpoint titers ranged from 8.64–11.64 in these samples. In vehicle control animals, anti-KLH IgM endpoint titers increased from a mean of 9.97 at pretest to 13.31 on days 18 and 22 (). Anti-KLH IgM in animals given cyclosporine at 10 and 50 mg/kg appeared similar to vehicle control animals. In contrast to IgM, anti-KLH IgG was undetectable in pretest samples. The mean anti-KLH IgG endpoint titer in vehicle control monkeys was 11.31 on day 22. Anti-KLH IgG levels were decreased to 8.97 in monkeys given 50 mg/kg, although this change did not reach statistical significance (p = 0.08). In a high dose animal (No. 3070), the anti-KLH IgG titer was only 7.64 (). This titer corresponded to a serum dilution of 1:200, compared to an average dilution of 1:3,200 in vehicle control monkeys. In addition, a single animal (No. 3066) given cyclosporine at 10 mg/kg had an anti-KLH IgG titer of 8.64 (dilution of 1:400). Although the effects of drug treatment on antibody production did not appear to be dose-dependent, these observations suggested that cyclosporine suppressed anti-KLH IgG responses in individual monkeys, but not IgM responses.
TABLE 2 Effects of cyclosporine on T-cell-dependent antibody response to KLH in monkeys
TABLE 3 Inhibition of anti-KLH IgG by cyclosporine—Individual animal data
Mild lymphoid depletion was evident in animals No. 3066 (10 mg/kg) and No. 3070 (50 mg/kg) relative to vehicle control monkeys. Lymphoid depletion was noted in the axillary lymph node, inguinofemoral lymph node, bronchial lymph node and/or tonsil (), and was characterized by decreased cellularity in the cortical and paracortical regions with a decrease in the number and size of follicles with germinal centers. These findings were consistent with suppressed anti-KLH IgG titers in these animals. No histopathologic findings were seen in thymus or spleen of these monkeys. Lymphoid tissues from the remaining animals dosed at 10 and 50 mg/kg had no collective pattern of lymphoid changes or were considered within limits of controls (data not shown).
TABLE 4 Histopathologic evaluation of lymphoid tissue
SUMMARY
Regulatory agencies recommend evaluating a primary T-cell-dependent antibody response when assessing the immunotoxicity potential of new drug candidates (CPMP, Citation2000; FDA, Citation2002). The current study developed a T-cell-dependent antibody response to KLH in cynomolgus monkeys, and investigated the effects of the immunosuppressive agent cyclosporine on IgM and IgG responses against KLH, along with additional immune-related and non-immune endpoints. Robust anti-KLH IgM and IgG responses were seen in animals following immunization with KLH. An important consideration to take into account when evaluating antibody responses to KLH is the possible presence of preexisting IgM antibodies, which appear specific to KLH. Although significant increases in anti-KLH IgM endpoint titers were seen following KLH immunization in the current study, the magnitude of the increase over pretest was not as pronounced as anti-KLH IgG, which was near or below limits of detection in unimmunized monkeys. The explanation for the presence of anti-KLH IgM in sera of unimmunized monkeys is unclear, although it may represent the presence of natural antibodies (Guilbert et al., Citation1982). Since cynomolgus monkeys eat crab and other shellfish, a specific antibody response to KLH obtained from diet is another possible explanation.
Cyclosporine administration suppressed anti-KLH IgG production in 1 monkey (No. 3066) given 10 mg/kg and 1 monkey (No. 3070) dosed at 50 mg/kg. The effects of cyclosporine on anti-KLH IgG production did not appear to be dependent on trough cyclosporine concentrations. Although monkey No. 3070 had the highest cyclosporine concentration recorded in the study, levels in animal No. 3066 were below the limit of detection. The anti-KLH IgM endpoint titers in animals with suppressed IgG were similar to control, although monkey No. 3070 had a high pretest titer that made it difficult to interpret the results. Although the suppressed anti-KLH IgG responses were seen in a limited number of animals, these results were consistent with the findings reported in dog (Finco-Kent and Kawabata, Citation2005). In contrast to the findings in dog and monkey, cyclosporine suppresses anti-KLH IgM and IgG responses in rat (Gore et al., Citation2004; Piccotti, unpublished data). Importantly, a histopathology correlate to the reduced antibody response was seen in lymphoid tissues in the current study. Relative to control animals, mild lymphoid depletion of lymph nodes and tonsil was seen by light microscopy in monkeys with suppressed anti-KLH IgG titers. These findings were supported by immunohistochemical analyses of lymph node tissues, which showed a reduction in the staining of CD20+ B-cells compared to control animals (data not shown). Immunohistochemical analysis of T-cells in lymphoid tissues is ongoing.
In our experience, there is substantial inherent variability in histologic appearance of lymphoid tissues in “normal” or control cynomolgus monkeys. This variability can hamper the detection of patterns of drug-related immunologic effects, particularly when responses in a group are not homogenous or substantial. Because of the low incidence and lack of a dose-response, the relationship of the histopathologic findings to drug treatment in the current study was uncertain when evaluated independent of other study results. However, these findings provided good evidence of drug-related immunosuppression in individual animals when interpreted in combination with reduced anti-KLH IgG production. The results of this study also suggested that higher doses of cyclosporine and/or longer exposures may be needed to evaluate immunotoxicity in cynomolgus monkeys, although doses resulting in overt toxicity should be avoided to prevent secondary effects (e.g., stress) on immune function.
Last, the current study evaluated the effects of KLH immunization alone on hematology, biochemistry, bone marrow, organ weights, gross and histopathology, and peripheral lymphocyte subsets. Parameters in control monkeys given KLH at 10 mg/animal were comparable to pretest and/or remained within historical ranges, indicating that KLH did not alter the immune or non-immune endpoints evaluated. Although these findings were limited to observations in 3 animals, the results suggested that standard toxicity endpoints may be evaluated in animals immunized with KLH. Additional studies are needed to substantiate this possibility; however, these data appear consistent with observations from other meeting participants.
The authors would like to thank Dr. Michael Bleavins for review of the manuscript, and Kiara Donahoo and Carrie-Anne Malinczak for technical support.
REFERENCES
- Arnold D. L., Bryce F., Mes J., Tryphonas H., Hayward S., Malcolm S. Toxicological consequences of feeding PCB congeners to infant rhesus (Macaca mulatta) and cynomolgus (Macaca fascicularis) monkeys. Food Chem. Toxicol. 1999; 37: 153–167, [PUBMED], [INFOTRIEVE], [CSA]
- CPMP (Committee for Proprietary Medicinal Products). Note for Guidance on Repeated Dose Toxicity. 2000, CPMP/SWP/1042/99
- FDA (Food and Drug Administration, Center for Drug Evaluation and Research). Guidance for Industry. Immunotoxicology Evaluation of Investigational New Drugs. 2002
- Finco-Kent D., Kawabata T. T. Development and validation of a model to measure the T-dependent antibody response (TDAR) in canines. J. Immunotoxicol. 2005; 2: 197–201, [CSA]
- Gore E. R., Gower J., Kurali E., Sui J. L., Bynum J., Ennulat D., Herzyk D. J. Primary antibody response to keyhole limpet hemocyanin in rat as a model for immunotoxicity evaluation. Toxicology 2004; 197: 23–35, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Guilbert B., Dighiero G., Avrameas S. Naturally-occurring antibodies against nine common antigens in human sera. I. Detection, isolation, and characterization. J. Immunol. 1982; 128: 2779–2787, [PUBMED], [INFOTRIEVE], [CSA]
- Luster M. I., Portier C., Pait D. G., White K. L., Gennings C., Munson A. E., Rosenthal G. J. Risk assessment in immunotoxicolgy. I. Sensitivity and predictability of immune tests. Fundam. Appl. Toxicol. 1992; 18: 200–210, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Schuurman H. J., Hengy J. C., Ringers J., Vonderscher J., Schuler W., Jonker M. Neoral pharmacokinetics in cynomolgus monkeys: Relation to efficacy in renal allografting. Transpl. Proc. 1996; 28: 3142–3144, [CSA]
- Schuurman H. J., Slingerland W., Menninger K., Ossevoort M., Hengy J. C., Dorobek B., Vonderscher J., Ringers J., Odeh M., Jonker M. Pharmacokinetics of cyclosporine in monkeys after oral and intramuscular administration: Relation to efficacy in kidney allografting. Transpl. Int. 2001; 14: 320–328, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Tryphonas H., Hayward S., O'Grady L., Loo J. C. K., Arnold D. L., Bryce F., Zawidzka Z. Z. Immunotoxicity studies of PCB (aroclor 1254) in the adult rhesus (Macaca mulatta) monkey—Preliminary report. Int. J. Immunopharmacol. 1989; 11: 199–206, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]