Abstract
The linkage between in utero exposure to diethylstilbestrol (DES) and the manifestation of a variety of reproductive disorders and possibly immune alterations in adults (i.e., human and mice) is suggestive of a fetal basis of adult disease. While the long-term adverse consequences of prenatal DES-exposure on reproductive disorders are well known, there is a paucity of data with regard to immune outcome. We hypothesize that prenatal DES-exposure “imprints” the immune system, altering the response to subsequent exposure to DES in adult mice. In this pilot study, C57BL/6 mice were prenatally exposed to DES or vehicle only (oil) and then exposed to DES at 1 year of age. Potential alterations in the spleen were then examined. Female DES-exposed mice (DESprenatal/DESadult) or femaleDES had higher serum levels of interferon-gamma (IFNγ) in response to administration of an IFNγ -inducer (soluble proteins-derived from Toxoplasma gondii), compared to female controls, which received oil during prenatal life (Oilprenatal/DESadult). Splenic lymphocytes from female DESprenatal/DESadult mice, when activated with Concanavalin A (ConA), also secreted higher levels of IFNγ compared to female controls (Oilprenatal/DESadult) when examined at 14-months of age. This increase in IFNγ in prenatal DES-exposed mice is not due to enhanced numbers of splenocytes or increased relative percentages of CD4+ or CD8+ cells. ConA-activated T-cells from female DESprenatal/DESadult had increased expression of the co-stimulatory molecule, CD28. These above immune changes were not evident in the males prenatally exposed to DES. Prenatal DES exposure also did not induce autoimmunity in non-autoimmune C57BL/6 mice. Overall, results from these prefatory studies suggest that prenatal DES exposure may have long-term immune alterations, which become evident following a secondary exposure to DES in adult life.
INTRODUCTION
Fetal development must be carefully controlled to ensure that the complex developmental pathways proceed in an ordered and regulated fashion. These tightly controlled pathways are critical for the birth of a healthy offspring. Thus, alterations in the in utero environment during the critical developmental period can have profound consequences after birth. For instance, alcohol consumption during pregnancy has been linked to abnormal neurological development such as fetal alcohol syndrome (Guerri, Citation2002). Recent studies show that malnourishment early in pregnancy is associated with the development of high blood pressure in adult life (Osmond and Barker, Citation2000). Of a particular concern is the inadvertent exposure of developing fetuses to chemicals, which can cross the placenta and alter the functions of developing cells (Ahmed et al., Citation1999). One such functional alteration may be a deviant response to chemical exposure later in life (Tchernitchin et al., Citation1999; Ahmed, Citation2000; Karpuzoglu-Sahin et al., 2001).
Diethylstilbestrol (DES), a prototype transplacental chemical, is a potent synthetic estrogenic compound. Prenatal exposure to DES has generated significant interest since it is regarded as not only a transplacental carcinogen/teratogen, but also an immune-altering compound. Administration of DES dates back to 1940 when it was primarily given to pregnant women who were at risk of developing serious complications and spontaneous abortions (Marselos and Tomatis, Citation1992). It was originally thought that administration of DES would help stabilize the natural estrogen levels and alleviate the risk of pregnancy complications. DES-related health problems began to be reported in the late 1960s when young women exposed to DES in utero were diagnosed with a rare carcinoma, clear cell vaginal adenocarcinoma. Decades of retrospective studies have shown adverse reproductive effects including infertility and a variety of carcinomas in both men and women were related to exposure to DES during prenatal development (Herbst et al., Citation1971; Hammes and Laitman, Citation2003). A recent questionnaire study of 4821 DES-exposed daughters and 2095 unexposed women reported an increased risk of developing breast cancer by the age of 40 and over (Palmer et al., Citation2002).
Immune-related abnormalities have also been reported in DES-exposed mothers, daughters and sons including the development of autoimmunity (arthritis and lupus), asthma, increased susceptibility to respiratory infections and altered lymphocyte functions (Ways et al., Citation1987; Wingard and Turiel, Citation1988; Burke et al., Citation2001). Laboratory animals exposed to DES during neonate development or later in adult life likewise have shown immune alterations. Neonatal DES treatment in mice has led to reduced proliferative response to T-cell mitogens (Kalland et al., Citation1979), decreased natural killer cell functions (Kalland, Citation1984), and induced autoantibodies to the phospholipid, cardiolipin (Forsberg, Citation2000).
Thus, it is conceivable that exposure to immunomodulatory DES during a critical fetal developmental period may have long lasting immune consequences. Our previous studies suggested that prenatal exposure to DES may imprint the immune system (Karpuzoglu-Sahin et al., Citation2001a). It is likely that DES-induced immune modulation during the critical fetal period may alter the response pattern of lymphoid cells when a subsequent exposure to the hormone occurs later in life. Moreover, it is not known whether these prenatal DES effects can be evident as late as over 1 year of age. The principal objective of the present study was to determine whether the response of splenic lymphocytes from prenatal DES-exposed mice is altered when mice are exposed to DES during late adult life. We found that DESprenatal/DESadult female mice had altered splenic immune changes. These studies may have future implications in areas of research in cancer, infection immunity, and autoimmune diseases.
MATERIALS AND METHODS
Animal Breeding and Experimental Design
All mice used in this study were of the C57BL6 strain obtained from the Charles River Laboratories (Wilmington, MA). A standard timed-pregnancy protocol was followed in which 15 female C57BL/6 mice were bred to male C57BL/6 mice. Of the 15 females that were bred, 7 became pregnant (47% pregnancy rate). The selection for treatment of the pregnant mothers was as follows: 2 females received vehicle (corn oil), 3 received 0.25 μ g of DES and 2 females received 2.5 μ g of DES. On day 14 of gestation, the mice were given a subcutaneous injection of 0.25 μg or 2.5 μg of DES (Sigma, St. Louis, MO) in 0.05 ml autoclaved tocopherol-stripped corn oil (ICN, Auro, OH) or corn oil only (served as a control). The offspring were fed a commercial pelleted diet devoid of synthetic estrogens (Special diet #7013 Harlan Teklad, Madison, WI) and given water ad libitum. All animals were housed in a 14-hour light:10-hour dark cycle.
A total of 18 pups (10 female and 8 male) born to pregnant mothers that received corn oil (vehicle only) survived to 1 year of age for these experiments. A total of 18 pups (7 females and 11 males) born to pregnant mothers that received 0.25 μg of DES survived to 1 year of age. Since there were limited numbers of mice born to mothers given 2.5 μg of DES, they were not included in the main part of the study. These mice were instead used in preliminary studies to determine the kinetics of IFNγ induction in response to the administration of proteins-derived from T. gondii. Current studies in our laboratory have shown that estrogenic compounds modulate IFNγ (Karpuzoglu-Sahin et al., Citation2001a, Citation2001b; Calemine et al., Citation2002; Fenaux et al., Citation2004). Therefore, in this study to examine the in vivo changes in IFNγ levels, we utilized soluble protein antigens from T. gondii, which are excellent inducers of IFNγ. For the in vitro study, splenocytes were cultured with Con A, which has been shown to induce IFNγ (Karpuzoglu-Sahin et al., Citation2001b). All animal procedures were in accordance with the guidelines of the animal care committee at Virginia Polytechnic Institute and State University. This experiment was performed on 2 separate days with an equal number of mice from each treatment group on each day. The error control structure for the experiment was a generalized randomized complete block design (Lenter and Bishop, Citation1993).
Preparation of Soluble T. gondii
Tachyzoites of the RH strain of T. gondii were maintained by serial passage in bovine monocyte cells (a gift from John Dame, University of Florida). Host bovine monocyte cells were grown to monolayers in 75 cm2 plastic cell culture flasks in RPMI medium containing 10% (v/v) fetal bovine serum, 100 U penicillin G per ml, and 100 μg of dihydrostreptomycin (GIBCO, Grand Island, New York) per mL. Cell cultures were incubated at 37°C in a 95% air, 5% CO2 atmosphere. Tachyzoites were collected for antigen preparation by first removing the cell culture medium and replacing it with calcium and magnesium free Hank's balanced salt solution (HBSS). The bovine monocyte cells were then scraped off the plastic growth surface of the 75 cm2 flask into the HBSS, and the suspension forced through a 27-gauge needle to rupture the majority of the bovine monocyte cells. Free tachyzoites were separated from intact cells and most cellular debris by filtration through a 3-mm pore-size polycarbonate filter (Nuclepore Corporation, Pleasanton, California). The numbers of tachyzoites recovered were determined by counting in a Neubauer hemacytometer. The organisms were suspended in injectable saline and frozen at −95°C for 3–5 days to ensure there were no viable organisms. Whole organisms were then sonicated on ice for six 30-second pulses to obtain the soluble T. gondii antigenic preparation. The protein concentration was quantified using a Bradford protein assay and diluted to a stock concentration of 2 mg/mL in sterile saline (0.9% sodium chloride injection USP).
Measurement of IFNγ Following T. gondii Protein Extract Administration
When the mice (DES (0.25 μg) (nfemale = 7, nmale = 11) or oil (nfemale = 10, nmale = 8) reached 1 year of age, they received one subcutaneous injection of 30 μg/kg BW of DES dissolved in 0.05 mL corn oil (vehicle). Six days later, each mouse was given an intraperitoneal (IP) injection of 20 μg of soluble proteins (derived from RH strain T. gondii) in 10 μl sterile saline. Six hours following the T. gondii protein administration, blood was collected through the retrorbital sinus (after halothane anesthesia) using heparin-coated glass microcapillary tubes. This time period was selected based on a previously published study that reported high levels of IFNγ in sera of mice, 6 hours after administration of soluble protein antigens-derived from T. gondii (Lighvani et al., Citation2001). Two months after T. gondii administration, the mice were anesthetized with halothane, and terminated by cervical dislocation.
Reproductive and Lymphoid Organs Weights
Reproductive organs (uterus and ovaries from females, seminal vesicles and testes from males) and lymphoid organs (thymus and spleen) were isolated, trimmed of all excess body fat and blotted dry of any excess blood using sterile gauzes. The tissues were weighed as described previously (Calemine et al., Citation2002). DES treatment did not significantly alter the body weights or reproductive organ weights (). The uterus was processed for histological analysis of uterine epithelial cell height, which was not significantly altered by prenatal DES treatment (data not shown).
TABLE 1 Effects of DES on body weight and organ weight
Analysis of Salivary Glands, Thyroid, and Kidneys for Lymphocyte Infiltration
Since estrogenic compounds can influence autoimmune pathology (Ahmed, Citation1994), potential target tissues (salivary glands, thyroids, kidneys) were collected, fixed in 10% neutral buffered formalin and embedded in paraffin. Five-micron sections were cut, stained with H&E, and submitted for pathologic analysis.
Isolation of Lymphocytes
Lymphocytes from thymus and spleen were aseptically dissociated by gently grinding the organs on a sterile steel sieves screen (Sigma) in phenol red-depleted RPMI-1640 incomplete media (Mediatech, Herndon, VA) by procedures previously reported (Calemine et al., Citation2002). Care was taken to ensure that cells were not inadvertently exposed to estrogenic compounds. This includes culturing cells in the phenol-red depleted media, since phenol red has been shown to be estrogenic and using charcoal- and dextran-pretreated 10% heat-inactivated fetal bovine serum to eliminate any estrogenic compounds in the serum. Tris-ammonium chloride lysis buffer (pH 7.2) was added to the splenocyte suspension to remove erythrocytes before culturing. The cell suspensions were washed twice with RPMI-1640 at 200 × g for 5–8 minutes at 4°C and resuspended in complete media. The media contained 10% heat-inactivated fetal bovine serum (pretreated) 200 mML-glutamine, 5000 IU/mL penicillin, 5000 μg/mL streptomycin, and 100 × non-essential amino acids. Cell numbers were assessed by a Cell Counter and Analyzer System (CASY-1) (Scharfe System GmbH, Reutingen, Germany) and adjusted to 5 × 106 cells/mL as reported in our previous studies (Calemine et al., Citation2002).
Phenotyping of Lymphocytes by Flow Cytometric Analysis
One hundred microliters of freshly isolated splenocytes at a concentration of 5 × 106 cells/ml (5 × 105 cells/well) were plated in 96-well round-bottom tissue-culture plates (Corning, Corning, NY). To determine phenotypic changes in prenatal DES-treated mice, cells were washed in PBS to avoid any inadvertent stimulation from the FBS-supplemented media. Aliquots of splenocytes were dual-color stained (0.5 μg of fluorescent-labeled antibody to 5 × 105 cells) immediately after harvesting and 24 hours after culture with ConA with various combinations of fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated anti-mouse antibodies (Pharmingen, San Diego, CA). Dual color staining combinations included: (1) FITC anti-CD4 with PE anti-CD25 antibodies; (2) FITC anti-CD19 with PE anti-CD40L antibodies; (3) FITC anti-CD90 with PE anti-CD28 antibodies; and, (4) FITC anti-CD4 or FITC anti-CD8 with PE anti-CDVβ8 antibodies. Splenocytes were also stained with the relevant isotype-matched FITC and PE anti-rat IgG2aκ control antibodies and subjected to flow cytometric analysis by procedures reported in our earlier studies (Ahmed and Talal, Citation1989; Ahmed et al., Citation1994; Verthelyi and Ahmed, Citation1998; Donner et al., Citation1999). The data were analyzed with the Immuno-4 software program.
7-AAD Staining of Lymphocytes
Staining of cells with 7-aminoactinomycin D (7-AAD) allows for the identification of cells at 3 stages of cell viability/ apoptosis; these include: 7-AADdull (live), 7-AADintermediate (early apoptosis), and 7-AADbright (late apoptosis/necrotic) (Schmid et al., Citation1994; Donner et al., Citation1999; Gogal et al., Citation2000). In our previous studies, we have shown that 7-AAD analysis closely match with FITC-annexin V and is superior to propidium iodide and forward/side scatter analyses (Donner et al., Citation1999; Gogal et al., Citation2000). Splenocytes were triple stained with FITC- anti-CD8 antibodies, PE-anti-CD4 antibodies and 7-AAD immediately after harvest and 24 hours after culturing in ConA stimulation. Each subset of T-cells (CD4−8−, CD4+8+, CD4+8−, CD4−8+) was gated and the percentages of live and apoptotic cells were determined by 7-AAD analyses.
Cytokine Analysis of Serum and Cell Culture Fluids
Sandwich enzyme-linked immunosorbent assay (ELISA) was used to detect IFNγ protein levels in cell culture fluids as per procedures reported in our previous publication (Karpuzoglu-Sahin et al., Citation2001b). ELISA procedures for serum samples involved coating 96-well Maxisorp high-binding immunoassay plates (Fisher Scientific, Sowanee, GA) with 4.0 μg/mL of purified anti-IFNγ clone R4-6A2 antibody (Pharmingen) diluted in 1 × PBS. The plates were coated at 4°C overnight. The plates were washed twice with PBS + 0.0005% Tween 20 and incubated in blocking buffer (1 × PBS + 1% BSA) at room temperature for 2 hours. Plates were blotted dry and serum samples were added to wells at 1:2 dilution and incubated at 4°C for 18–22 hours. After 5 washes, the plates were incubated with 0.5 μg/mL of biotin conjugated anti-IFNγ antibody (Pharmingen). After 6 washes, the plates were incubated with horseradish peroxidase solution (Vector Labs, Burlingame, CA) for 30 minutes at room temperature. Plates were washed 8 times and allowed to develop with TMB substrate (KPL, Gaithersburg, MD). The plates were read at 450 nm using a spectrophotometer reader (Molecular Devices, Sunnyvale, CA). The IFNγ protein levels were extrapolated using the linear region of the standard curve calculated by the SOFTMAX PRO, Molecular Devices Inc. Software.
Serum Immunoglobin Analysis
A sandwich enzyme linked immunosorbent assay was employed to measure IgG and IgM levels and levels of anti-cardiolipin autoantibodies in the blood per our previously reported procedures (Verthelyi and Ahmed, Citation1997). Briefly, peripheral blood obtained from each C57BL/6 mouse and sera collected from each sample were frozen at −70°C until analyzed. Medium binding plates (Costar, Cambridge, MA) were coated with 50 μl of IgG or IgM (0.25 μg/ml, Southern Biotech, Birmingham, AL) in bicarbonate buffer and incubated overnight at 4°C. The plates were rinsed with PBS containing Tween 20 between each step. The plates were blocked with 1% BSA in PBS and incubated at room temperature for 1 hour in a humidified chamber. After blocking, the sera and standards were diluted in 1% BSA in PBS. A 100 μL aliquot of sera or standard was added to each well and incubated for 3 hours at room temperature in a humidified chamber. Then 100 μL of AP-conjugated goat anti-mouse IgG or IgM (Southern Biotech) in 1% BSA PBS were added per well and incubated at 37°C for 1 hour in a humidified chamber. Plates were washed with PBS with 5% Tween between each step. The color was determined using pNPP substrate (Sigma) after incubating for 45 minutes and the plates were measured at 405 nm with an ELISA reader (Molecular Devices). The IgG and IgM antibody levels were calculated by the formula obtained from standards using SoftMax Pro software from Molecular Devices Inc.
Statistics
An ANOVA for a split-plot experiment in generalized randomized complete blocks was performed to test for main effects of prenatal DES treatment and gender as well as their interaction. Whole plot variability (dam-to-dam variability) was used to test for the main effect of prenatal treatment while subplot variability (pup-to-pup variability nested within a dam) was used to test for the main effect of gender and its interaction with prenatal DES treatment. Post hoc mean separation was conducted using Bonferroni multiple comparisons. The MIXED procedure of the SAS System (ver. 8.1, SAS Institute Inc., Cary, NC 27513) was used to perform the calculations. p-Values less than 0.05 were considered significant. Data are presented as mean ± standard error of the mean (SEM).
RESULTS
Alterations in Serum IFNγ Levels in Prenatal DES-Exposed Mice Given Protein Extract Derived from T. gondii Antigens
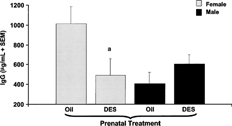
In our preliminary kinetic studies, in response to in vivo administration of T. gondii antigens, we found that serum IFNγ was highest at 6 hours, declined by 24 hours to a lowest level by 48 hours. Therefore, in subsequent studies, serum IFNγ levels were determined 6 hours after To gondii antigen administration. We found that female prenatal DES-exposed mice had higher levels of IFNγ in their serum compared to prenatal oil treated female mice 6 hours after in vivo administration of T. gondii antigens (). In contrast, male prenatal DES-exposed mice did not show a similar increase in serum IFNγ levels compared to their control counterparts (). Female prenatal DES-exposed mice had a decrease in IgG levels compared to their gender-matched controls. IgG levels were unchanged in male mice given either prenatal oil or prenatal DES treatment (). There were no changes in IgM levels in males or females (data not shown). There were no detectable levels of anticardiolpin autoantibodies in either prenatal DES or oil-exposed mice (data not shown).
FIG. 1. IFNγ levels in serum 6 hours after administration of soluble T. gondii protein. C57BL/6 mice were exposed to either DES (0.25 μg) (nfemale = 7, nmale = 11) or oil (nfemale = 10, nmale = 8) during prenatal development. One year later, mice were given 1 subcutaneous injection of DES (30 μg/kg BW). Mice were then challenged with 20 μg of soluble proteins derived from the R.H. strain of T. gondii. Serum samples were collected 6 hours after the T. gondii antigen administration. Mean data are presented in pg/ml ± SEM. Differences between genders and treatment groups were considered significant when p < 0.05. a denotes significant difference between females given prenatal oil and females given prenatal DES treatment. b denotes significant difference between females given prenatal DES and males given prenatal DES treatment.
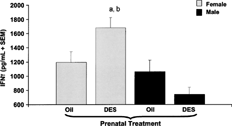
FIG. 2. IgG levels in serum six hours after T. gondii antigen administration. C57BL/6 mice were exposed to either DES (0.25 μg) (nfemale = 7, nmale = 11) or oil (nfemale = 10, nmale = 8) during prenatal development. One year later, mice were given one subcutaneous injection of (30 μg/kg bw). Mice were then challenged with 20 μg of soluble protein antigen derived from the R.H. strain of T. gondii. Serum samples were collected 6 hours after the T. gondii antigen administration. Mean data are presented in pg/ml ± SEM. Differences between genders and treatment groups were considered significant when p < 0.05. a denotes significant difference between females given DES and females given oil prenatal treatment (p = 0.001).
Enhanced Secretion of IFNγ in In Vitro Activated Splenocytes from Prenatal DES-Exposed Mice
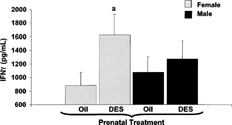
Two months post-injection of protein extracts from T. gondii, at a time when there were no detectable serum levels of IFNγ, mice were terminated to determine ConA-induced IFNγ. ConA activated splenic lymphocytes from female prenatal DES-exposed mice (DESprenatal/DESadult) secreted higher levels of IFNγ when compared to cultures from female prenatal vehicle only treated mice (oilprenatal/DESadult) (). This increase in the levels of IFNγ secreted by ConA-activated splenocytes was not evident in male prenatal DES-treated mice.
FIG. 3. Secreted IFNγ levels after 24 hour stimulation with ConA. C57BL/6 mice were exposed to either DES (0.25 μg) (nfemale = 7, nmale = 11) or oil (nfemale = 10, nmale = 8) during prenatal development. At 1 year of age, all mice were given 30 μg DES/kg BW. Two months later, mice were euthanized to collect splenocytes. Splenocytes were cultured with an optimal concentration of ConA (10 μg/ml). The supernatants were collected after 24 hours of culture and analyzed for IFNγ levels by ELISA. Mean data are represented in pg/ml ± SEM. Differences between gender and prenatal treatments were considered significant when p < 0.05. a denotes significant difference between females given DES and females given oil prenatal treatment.
Effects of Prenatal Exposure to DES on the Splenic Weight and Cellularity
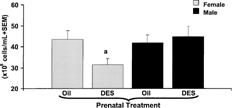
We next investigated whether the enhanced ability of splenocytes from female prenatal DES-exposed mice (DESprenatal/DESadult) to secrete IFNγ was due to an increase in splenic lymphocyte cellularity. Interestingly, female prenatal DES-exposed mice had decreased splenic cellularity compared to female prenatal vehicle only treated controls (). There were no changes in splenic lymphocyte cellularity in the males. The splenic weight was not significantly altered by prenatal DES treatment in either females or males (Femaleoil 0.09 ± 0.004 g, FemaleDES 0.08 ± 0.005 g, Maleoil 0.07 ± 0.004 g, MaleDES 0.08 ± 0.004 g). Similarly, splenic weight to body weight ratios were also not markedly altered by prenatal DES exposure (Femaleoil 0.37 ± 0.01, FemaleDES 0.33 ± 0.02) (Maleoil 0.21 ± 0.01, MaleDES 0.24 ± 0.01). Males, in general, had decreased splenic weight to body-weight ratios compared to females, which was due to the increased body weights in males relative to females. Since female prenatal DES-exposed mice had decreased numbers of splenic lymphocytes, we next determined whether there were any alterations in T-cells.
FIG. 4. Splenic lymphocyte count of adult C57BL/6 mice prenatally exposed to oil or DES treatment. C57BL/6 mice were exposed to either DES (0.25 μg) (nfemale = 7, nmale = 11) or oil (nfemale = 10, nmale = 8) during prenatal development. At 1 year of age, all mice were given 30 μg DES/kg BW. Immediately after harvesting the spleen, splenocyte suspensions were counted on a CASY-1 Cell Counter and Analyzer System (Scharfe System GmbH, Reutingen, Germany). Data presented as mean cellularity ± SEM. Differences between gender and prenatal treatments were considered significant when p < 0.05. a denotes significant difference between females given DES and females given oil prenatal treatment.
Effects of Prenatal Exposure to DES on Splenic T-cell Subpopulations
To investigate whether the differences in secreted IFNγ levels from prenatal DES-exposed mice were due to changes in the numbers of CD4- and CD8-positive T-cells, freshly-isolated splenocytes from mice were stained for flow cytometric analysis of T-cell subsets. There was no significant change in the relative percentages of T-cell subsets (CD4+CD8−, CD4−CD8+, CD4+CD8+, and CD4−CD8−) in either prenatal oil or DES-treated female and male mice (). As expected, the majority of T-cell subsets, which may have migrated to the spleen, were CD4+CD8− and CD4−CD8+ cells. There were virtually no immature CD4+CD8+ T-cells. Freshly isolated splenic T-cells were also analyzed for staging of cell death using 7-AAD, a DNA binding dye. No significant change in the relative percentages of viable, apoptotic and late apoptotic/necrotic T-cells in the spleen of prenatal oil or DES-treated mice were observed (data not shown).
TABLE 2 Percent expression of CD4 and CD8 splenocytes from DES or oil prenatally exposed mice immediately after harvest
CD4+CD8− and CD4−CD8+ cells were analyzed in ConA stimulated splenocyte cultures. Oil or DES prenatal treatment did not have an effect on the relative percentages of CD4+CD8− and CD4−CD8+ in 24-hour ConA-activated cultured cells (CD4+CD8−: Femaleoil 15.9 ± 1.0, FemaleDES 16.6 ± 0.9, Maleoil 14.8 ± 1.0, MaleDES 15.8 ± 0.9) (CD4−CD8+: Femaleoil 5.7 ± 1.4, FemaleDES 6.8 ± 1.3, Maleoil 8.1 ± 1.4, MaleDES 7.8 ± 1.3). However, relative percentages of apoptotic T-cells in ConA-stimulated splenocyte cultures showed a significant increase in late apoptotic or 7-AADbright CD4+CD8− from male mice given prenatal DES-treatment (Maleoil 32.3 ± 3.3, MaleDES 42.0 ± 3.8, p < 0.05). The relative percentage of apoptotic CD4+CD8− and CD4−CD8+ cells were not changed in ConA-stimulated splenocyte cultures from female mice given prenatal DES (data not shown).
Effects of Prenatal Exposure to DES on Splenic T-cell Functionality
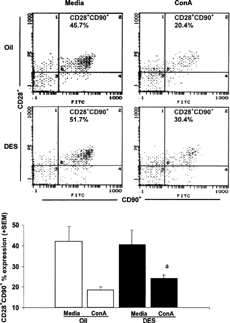
It is likely that the DES-induced production of IFNγ in response to in vivo and in vitro stimulations may be due to altered T-cell functions. Thus, we next examined whether T-cells have increased expression of CD28 co-stimulatory cell surface marker since this molecule is a well-known co-stimulator for T-cells. shows that upon ConA stimulation, there was a statistically significant increase in expression of CD28 on T-cells in prenatal DES-treated female mice. There were no significant differences in CD28 expression on T-cells from male mice given prenatal DES or oil treatments.
FIG. 5. CD28+ CD90+ unstimulated and ConA stimulated splenocytes from female mice given prenatal oil or DES treatment. C57BL/6 mice were exposed to either DES (0.25 μg) (nfemale = 6) or oil (nfemale = 6) during prenatal development. At 1 year of age, all mice were given 30 μg DES/kg BW. Splenocytes were stained for CD28 co-stimulatory marker expressed on T-cells (CD90). The top panel () shows representative data of female splenocytes. The bottom panel () shows data presented as mean ± SEM of CD28 expression on T-cells cultured in the presence or absence of ConA for 24 hours in female mice. Differences between prenatal treatments were considered significant when p < 0.05. The relative percentages of CD28 expression on T-cells in media cultures included (Femaleoil = 49.4 ± 7.7, FemaleDES = 40.7 ± 7.1, Maleoil = 49.4 ± 7.0, MaleDES = 48.5± 7.3). a denotes significant difference between cultured splenocytes stimulated with ConA from females given DES and females given prenatal treatment.
Effects of Prenatal Exposure to DES on Autoimmune Parameters
Salivary glands, kidney, and thyroid were examined for elevated lymphocyte infiltration, which is suggestive of autoimmune disease. As has been reported in our earlier study (Ahmed et al., Citation1989), some aged mice had lymphocytic sialoadenitis (inflammation of salivary glands) and mild pyelitis (inflammation of renal pelvis) which was unrelated to the treatment or gender.
Splenocytes were analyzed for CD4+CD25+ regulatory cells, which are now thought to play a critical role in regulation of autoimmunity (Shevach, Citation2002). These cells consist of only 1–2% of the total splenic cellularity. There was no significant difference in the relative percentage of regulatory cells in prenatal oil or DES-treated mice (Femaleoil 1.5 ± 0.3, FemaleDES 1.8 ± 0.3, Maleoil 1.2 ± 0.3, MaleDES 1.4 ± 0.3). Splenocytes cultured in media or ConA for 24 hours were stained for CD4+CD25+ cells. shows the relative percentages of CD4+CD25+ populations in prenatal oil and DES-treated mice. Interestingly, splenocytes from male mice had more CD4+CD25+ cells in response to ConA stimulation compared to females. Prenatal DES did not alter the relative percentages of CD4+CD25+ cells in ConA activated splenocytes.
TABLE 3 Relative percentages of CD4+CD25+ splenocytes after 24-hour incubation from female and male mice exposed to either oil or DES prenatally
Autoreactive lymphocytes in the spleen were also examined by cytometric analysis. Autoreactive T-cells (CD4+CD8+CDV β8+) consisted of 1–3% of the total splenic cellularity. Prenatal exposure to DES followed by adult DES treatment did not enhance the relative percentage of autoreactive T-cells (Femaleoil 1.8 ± 0.5, FemaleDES 1.8 ± 0.5, Maleoil 2.8 ± 0.4, MaleDES 1.8 ± 0.5). CD19+CD40 ligand+ cells, which presumably include autoreactive B-cells, also made up a small percentage of total splenic lymphocytes (1.3 ± 0.2% in DES-treated mice and 1.2 ± 0.2% in oil-treated mice) and appeared to not be affected by the prenatal DES-treatment. Further, autoantibodies specific for cardiolipin were not significantly different from controls (data not shown).
DISCUSSION
Natural and synthetic estrogenic hormones have played a wide role in the treatment of reproductive disorders such as breast cancer and prostate cancer as well as contraceptives (ethinyl estradiol), menopausal therapies (hormone replacement therapy) and reduction of premature births (progesterone) (Meis et al., Citation2003). Diethylstilbestrol was the first synthetic hormone administered to high risk pregnant women in rigorously untested belief that it had therapeutic value. Neoplastic and non-neoplastic reproductive abnormalities noticed in prenatal DES-exposed humans and experimental animals are now well characterized. However, immunological abnormalities are only beginning to be recognized. There are few studies that explore the long-term immunological effects of prenatal exposure to DES.
In a recent series of studies, we have shown that estrogenic hormones regulate the cytokine, IFNγ (Ahmed et al., Citation1999; Karpuzoglu-Sahin et al., 2001). In this study, we further extend our earlier observations to show that there was an increased level of expression of IFNγ in prenatal DES-exposed female mice in response to both in vivo (T. gondii soluble antigen administration) and in vitro (ConA) stimulation. The levels of the prototype TH1 type cytokine, IFNγ, induced by T. gondii antigens in serum were higher in female DESprenatal/DESadult exposed mice than female controls. The serum level of total IgG in female prenatal DES-exposed mice was significantly lower compared to female controls. Males, on the other hand, that received DES prenatal treatment did not have any significant changes in IFNγ or IgG levels in serum after T. gondii challenge. The mechanisms induced by prenatal exposure to DES in females that drive the enhanced IFNγ response warrants further investigation. Interestingly, an increase in IFNγ levels in female prenatal DES-exposed mice was evident despite the decreased numbers of splenic lymphocytes and unaltered CD4+CD8− or CD4−CD8+ relative percentages. It is likely that this increase in IFNγ may be due to increase in other IFNγ producers such as natural killer cells, macrophages, dendritic cells, γ δTCR+ cells or B-cells, an issue that is not addressed in the present studies. It is also likely that there may be gender differences in either the levels and/or response to IL-12, IL-18, IL-15, or IL-23 cytokines that are known to induce IFNγ. In this regard, others have shown that there are sex differences in the levels of IL-12 and IL-10. Female mice had increased ability to secrete IL-12 (an IFNγ inducer) and less ability to secrete IL-10 (Wilcoxen et al., Citation2000).
Enhanced secretion of IFNγ in the cell culture fluids of ConA-activated splenocytes from female prenatal DES-exposed mice compared to controls was also noted. This increase in IFNγ was noticed despite a decrease in splenic lymphocyte cellularity. We are currently examining differences in T-cell activation signaling in cells obtained from prenatal DES-exposed mice when compared to control mice. In support of qualitative activation differences, in this study we note that ConA-activated splenic T-cells from female prenatal DES-exposed mice had an increased expression of CD28 compared to controls. The interaction of CD28 on T-cells with B7 molecules (B7-1 and B7-2) on antigen presenting cells is necessary for optimal T-cell activation. It is possible that in female prenatal DES-exposed mice there is an enhanced co-stimulatory effect via CD28 and B7 molecular interactions. Additional studies are necessary to address CD28-mediated signaling, including Pi3 kinase activation and NF-κB that may play an important role in IFNγ induction.
Since estrogenic hormones have been associated with the induction of autoimmunity, we also investigated whether in utero exposure to DES has any influences on the development of autoimmunity in adult life. Prenatal DES exposure did not correlate with the induction of lymphocyte infiltration of salivary glands, kidneys, and thyroid. Since these mice were already aged over 1 year, it is not uncommon to see lymphocytic sialoadenitis and pyelitis in C57BL/6 mice (Ahmed, Citation1994). It is conceivable that prenatal DES may induce autoimmunity in other strains, notably in autoimmune prone strains, an aspect that merits investigation. Since CD4+CD25+ regulatory T-cells have now been shown to be involved in the regulation of autoimmunity, we also investigated whether prenatal DES-exposure alters this subset. In our model, there were no measurable differences in the numbers of CD4+CD25+ regulatory T-cells in prenatal DES-exposed mice. However, it is possible that DES may alter the functions of CD4+CD25+ regulatory cells, an aspect currently being examined. Further, prenatal DES-exposure had no noticeable effects on the autoreactive T-cells (CD4+CD8+Vβ8+).
It is important to mention that these long-term prenatal DES studies (over 1 year of age) are difficult to perform for several reasons. First, C57BL/6 mice, in general, tend to be poor breeders (only 7 out of 15 female mice became pregnant—often in this strain their pregnancy rates can be as low as 35%). C57BL/6 mice are also not particularly good mothers (since cannibalism of their offspring is not uncommon irrespective of DES treatment). In addition, a percentage of pups born to prenatal DES-treated mothers die within a few days of birth. Further, it was not uncommon to notice that the mothers injected with DES during pregnancy tended to neglect their offspring. Thus, these offspring were likely deprived of vital nutrition during this critical period of growth. Of note, there were no physical abnormalities noticed in the offspring other than low body weight. While mortality in prenatal oil-treated mice was not a significant concern, there was a high mortality in pups born to pregnant mothers given either 0.25 μg or 2.5 μ g DES, 33 and 46%, respectively. As a result of this high mortality in the DES-treated groups, it is possible there was an unintentional biased selection of the remaining viable mice. However, the mice that survived the critical neonatal period continued to survive as long as 1 year of age. Another caveat of this study is that limited availability of large numbers of aged mice prevented performance of kinetics and dose response studies. Additionally, the possibility that male prenatal DES-exposed mice may have increased IFNγ levels compared to females at certain stages of life or at other doses cannot be excluded. Differences in utero position of male and female mice may have also been a variable in the amount of actual DES exposure.
Overall, our studies show that female mice exposed to DES during fetal development have decreased splenic lymphocyte cellularity, enhanced IFNγ responses to in vitro and in vivo stimulants and altered splenic T-cell expression of CD28. Males appeared to be less sensitive to immunological changes due to prenatal exposure to DES compared to females. Therefore, the results from our studies suggest that fetal exposure to DES may have long-term immune consequences.
Abbreviations: 7-AAD: 7-aminoactinomycin D; ConA: Concanavalin A; DES: Diethylstilbestrol; IFNγ : Interferon-gamma; IL-10: Interleukin 10.
These studies were supported by a grant from NIEHS/NIH 1 RO1-ES08043. J. Fenaux was supported by VMRCVM graduate student stipend scholarship program. The authors would like to thank Joan Kalnitsky for performing flow cytometric studies, Mary Nickel and Crystal Boyle for animal care, and the advice of Dr. S. Holladay, Dr. W. Huckle, and Dr. M. Fenaux.
Robert M. Gogal, Jr. is also affiliated with the Department of Biomedical Sciences, Virginia College of Osteopathic Medicine, Blacksburg, Virginia.
REFERENCES
- Ahmed S. A. Autoimmune lesions: Is the C57BL/6 mouse strain normal or autoimmunity-prone?. Immunol. Today 1994; 15: 389–390, [PUBMED], [INFOTRIEVE], [CSA]
- Ahmed S. A. The immune system as a potential target for environmental estrogens (endocrine disrupters): A new emerging field. Toxicology 2000; 150: 191–206, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ahmed S. A., Aufdemorte T. B., Chen J. R., Montoya A. I., Olive D., Talal N. Estrogen induces the development of autoantibodies and promotes salivary gland lymphoid infiltrates in normal mice. J. Autoimmun. 1989; 2: 543–552, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ahmed S. A., Gogal R. M., Jr., Walsh J. E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H]-thymidine incorporation assay. J. Immunol. Meth. 1994; 170: 211–224, [CSA], [CROSSREF]
- Ahmed S. A., Hissong B. D., Verthelyi D., Donner K., Becker K., Karpuzoglu-Sahin E. Gender and risk of autoimmune diseases: Possible role of estrogenic compounds. Environ. Health Perspect. 1999; 107(Suppl 5)681–686, [PUBMED], [INFOTRIEVE], [CSA]
- Ahmed S. A., Talal N. Immune-endocrine interaction and autoimmune diseases. Autoimmunity and Toxicology, M. E. Kammuller, N. Bloksma, W. Seinen. Elsevier Science Publishers, Holland 1989; 415
- Burke L., Segall-Blank M., Lorenzo C., Dynesius-Trentham R., Trentham D., Mortola J. F. Altered immune response in adult women exposed to diethylstilbestrol in utero. Am J. Obstet. Gynecol. 2001; 185: 78–81, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Calemine J., Gogal R., Lengi A., Sponenberg P., Ahmed S. A. Immunomodulation by diethylstilbestrol is dose and gender related: Effects on thymocyte apoptosis and mitogen-induced proliferation. Toxicology 2002; 178: 101–118, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Donner K. J., Becker K. M., Hissong B. D., Ahmed S. A. Comparison of multiple assays for kinetic detection of apoptosis in thymocytes exposed to dexamethasone or diethylstilbesterol. Cytometry 1999; 35: 80–90, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Fenaux J. B., Gogal R. M., Jr., Ahmed S. A. Diethystilbesterol exposure during fetal development affects thymus: Studies in fourteen-month-old mice. J. Repro. Immunol. 2004; 64: 75–90, [CSA], [CROSSREF]
- Forsberg J. G. Neonatal estrogen treatment and its consequences for thymus development, serum level of autoantibodies to cardiolipin, and the delayed-type hypersensitivity response. J. Toxicol. Environ. Health Part A 2000; 60: 185–213, [CSA], [CROSSREF]
- Gogal R. M., Jr., Smith B. J., Kalnitsky J., Holladay S. D. Analysis of apoptosis of lymphoid cells in fish exposed to immunotoxic compounds. Cytometry 2000; 39: 310–318, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Guerri C. Mechanisms involved in central nervous system dysfunctions induced by prenatal ethanol exposure. Neurotox. Res. 2002; 4: 327–335, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Hammes B., Laitman C. J. Diethylstilbestrol (DES) update: Recommendations for the identification and management of DES-exposed individuals. J. Midwifery Womens Health 2003; 48: 19–29, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Herbst A. L., Ulfelder H., Poskanzer D. C. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N. Engl. J. Med. 1971; 284: 878–881, [PUBMED], [INFOTRIEVE], [CSA]
- Kalland T. Exposure of neonatal female mice to diethylstilbestrol persistently impairs NK activity through reduction of effector cells at the bone marrow level. Immunopharmacology 1984; 7: 127–134, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kalland T., Strand O., Forsberg J. G. Long-term effects of neonatal estrogen treatment on mitogen responsiveness of mouse spleen lymphocytes. J. Natl. Cancer Inst. 1979; 63: 413–421, [PUBMED], [INFOTRIEVE], [CSA]
- Karpuzoglu-Sahin E., Hissong B. D., Ahmed S A. Interferon-γ levels are up-regulated by 17β -estradiol and diethylstilbestrol. J. Repro. Immunol. 2001a; 52: 113–127, [CSA], [CROSSREF]
- Karpuzoglu-Sahin E., Zhi-Jun Y., Lengi A., Sriranganathan N., Ahmed S A. Effects of long-term estrogen treatment on IFNγ, IL-2 and IL-4 gene expression and protein synthesis in spleen and thymus of normal C57BLF/6 mice. Cytokine 2001b; 14: 208–217, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Experimental Design and Analysis, M. Lenter, T. Bishop. Valley Book Company, Blacksburg, VA 1993; 223
- Lighvani A. A., Frucht D. M., Jankovic D., Yamane H., Aliberti J., Hissong B. D., Nguyen B. V., Gadina M., Sher A., Paul W. E., O'Shea J. J. T-bet is rapidly induced by interferon-γ in lymphoid and myeloid cells. Proc. Natl. Acad. Sci. USA 2001; 98: 15137–15142, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Marselos M., Tomatis L. Diethylstilbestrol: I, Pharmacology, toxicology and carcinogenicity in humans. Eur. J. Cancer 1992; 28A: 1182–1189, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Meis P. J., Klebanoff M., Thom E., Dombrowski M. P., Sibai B., Moawad A. H., Spong C. Y., Hauth J. C., Miodovnik M., Varner M. W., Leveno K. J., Caritis S. N., Iams J. D., Wapner R. J., Conway D., O'Sullivan M. J., Carpenter M., Mercer B., Ramin S. M., Thorp J. M., Peaceman A. M., Gabbe S. Prevention of recurrent pre-term delivery by 17α -hydroxyprogesterone caproate. N. Engl. J. Med. 2003; 348: 2379–2385, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Osmond C., Barker D. J. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ. Health Perspect. 2000; 108(Suppl 3)545–553, [PUBMED], [INFOTRIEVE], [CSA]
- Palmer J. R., Hatch E. E., Rosenberg C. L., Hartge P., Kaufman R. H., Titus-Ernstoff L., Noller K. L., Herbst A. L., Rao R. S., Troisi R., Colton T., Hoover R. N. Risk of breast cancer in women exposed to diethylstilbestrol in utero: Prelimiinary results (United States). Cancer Causes Control 2002; 13: 753–758, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Schmid I., Uittenbogaart C. H., Keld B., Giorgi J. V. A rapid method for measuring apoptosis and dual-color immunofluorescence by single laser flow cytometry. J. Immunol. Meth. 1994; 170: 145–157, [CSA], [CROSSREF]
- Shevach E. M. CD4+CD25+ suppressor T-cells: More questions than answers. Nat. Rev. Immunol. 2002; 2: 389–400, [PUBMED], [INFOTRIEVE], [CSA]
- Tchernitchin A. N., Tchernitchin N. N., Mena M. A., Unda C., Soto J. Imprinting: Perinatal exposures cause the development of diseases during the adult age. Acta Biol. Hung. 1999; 50: 425–440, [PUBMED], [INFOTRIEVE], [CSA]
- Verthelyi D., Ahmed S. A. Characterization of estrogen-induced autoantibodies to cardiolipin in non-autoimmune mice. J. Autoimmun. 1997; 10: 115–125, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Verthelyi D. I., Ahmed S. A. Estrogen increases the number of plasma cells and enhances their autoantibody production in non-autoimmune C57BL/6 mice. Cell. Immunol. 1998; 189: 125–134, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ways S. C., Mortola J. F., Zvaifler N. J., Weiss R. J., Yen S. S. Alterations in immune responsiveness in women exposed to diethylstilbestrol in utero. Fertil. Steril. 1987; 48: 193–197, [PUBMED], [INFOTRIEVE], [CSA]
- Wilcoxen S. C., Kirkman E., Dowdell K. C., Stohlman S. A. Gender-dependent IL-12 secretion by APC is regulated by IL-10. J. Immunol. 2000; 164: 6237–6243, [PUBMED], [INFOTRIEVE], [CSA]
- Wingard D. L., Turiel J. Long-term effects of exposure to diethylstilbestrol. West. J. Med. 1988; 149: 551–554, [PUBMED], [INFOTRIEVE], [CSA]