Abstract
An epidemic of a multi-systemic disease, known as the toxic oil syndrome (TOS), was caused by consumption of edible oil denatured with 2% aniline. Oleic acid anilide (OAA) has been suggested as one of the most likely etiologic agents responsible for TOS based upon its presence in high quantities in TOS-related oil samples. The aim of this study was to evaluate the immune response of OAA and contribution of its hydrolysis products (aniline and oleic acid) in the immunotoxic response. Female MRL+/+ mice were treated with equimolar doses of OAA, aniline or oleic acid (0.8 mmol/kg), i.p., twice a week for 6 weeks. The levels of immunoglobulins IgE, IgG and its isotypes (IgG1, IgG2a, IgG2 b, and IgG3), and the appearance of antinuclear antibodies (ANA) were determined in the serum. Exposure to OAA and oleic acid caused significant increases in IgG, IgG1, IgG2a, and IgG2b levels as compared to aniline and control groups, whereas IgG3 value increased only in OAA-treated mice. The IgE levels in OAA-, aniline-, and oleic acid-treated groups were higher than the controls. Among the various treatment groups, sera from 50% of the OAA-treated mice gave rise to intense homogenous fluorescence patterns on Hep-2 cells, suggesting the presence of significant levels of antinuclear antibodies (ANA). Furthermore, analysis of serum cytokines showed significant increases in G-CSF levels in OAA- and aniline-treated mice. Among the tissues examined, morphological changes were confined to the spleen, which showed increased lymphocyte population in OAA- and aniline-treated mice. These studies indicate that OAA and its hydrolysis products cause perturbations in the immune response, and could contribute to TOS-related immune derangements.
INTRODUCTION
An epidemic of a multi-systemic disease, toxic oil syndrome (TOS), occurred in Spain in 1981 and caused more than 20,000 illnesses and about 800 deaths (Grandjean, Citation1992). TOS has been epidemiologically related to the ingestion of cooking oil adulterated with aniline (Tabuenca, Citation1981; Pestana and Munoz, Citation1982). The oil denatured with 2% aniline for commercial use was illegally refined to remove aniline for human consumption, which resulted in the formation of several aniline-related compounds. Even after years of extensive research, the etiology and pathogenesis of TOS remain enigmatic, although several mechanisms leading to tissue damage and/or clinical symptoms have been proposed including putative role of leukotrienes, oxidative stress, and involvement of immune system (Balnca et al., Citation1984; Bell et al., Citation1992; Gallardo et al., Citation1994; Heiskanen et al., Citation1995, Citation1997).
The adulterated cooking oil samples responsible for TOS consisted largely of rapeseed oil, animal fat, olive oil, and fatty acid anilides (500–2000 ppm) (Vazquez Roncero et al., Citation1983). Approximately 69% of aniline in the oil samples was found as fatty acid anilides (oleic acid anilide, ∼64%), ∼4% as free aniline, and ∼ 27% as other aniline derivatives (Vazquez Roncero et al., Citation1983). Fatty acid anilides have been suggested as the most likely agents responsible for TOS, especially due to their presence in significant amounts in the case-related cooking oil samples and also on the basis of epidemiological studies (Kilbourne et al., Citation1988).
In earlier studies, we have shown that oleic acid anilide (OAA) exposure in rats results in immunotoxic responses, evident from increased levels of serum IgG and IgA and altered T-helper to T-suppressor cell ratios (Khan et al., Citation1991). However, contribution of expected hydrolysis products of OAA, i.e., aniline and oleic acid (formed due to ubiquitous presence of amidases) in the immunotoxic responses of OAA is not known. Therefore, the focus of the present study was to compare the immune response of OAA and its potential hydrolysis products (aniline and oleic acid) in female MRL+/+ mice, an animal model prone to develop autoimmune disease in the late phase of their life span.
METHODS
Chemicals
Aniline (purity 99.0%), oleic acid (purity 99.0%), and oleoyl chloride (85%) were purchased from Sigma Chemical Co. (St. Louis, MO). Oleic acid anilide was synthesized and purified as described previously (Kaphalia and Ansari, Citation1991).
Animal and Treatments
Female MRL+/+ mice (5 weeks old), purchased from the Jackson Laboratory (Bar Harbor, ME), were acclimatized and maintained in humidity and temperature controlled animal care facility for 1 week and provided lab chow and drinking water ad libitum. The animals were randomly divided into 4 groups and administered intraperitoneal injections. [i.p. to avoid hydrolysis of OAA)] of 0.8 mmol/kg OAA, aniline and oleic acid in corn oil (100 μl per 25 g mouse weight) twice a week for 6 weeks. The fourth group of mice received equal volume of corn oil and served as controls. The animals were euthanized 24 h following the last dose. The blood was withdrawn from the inferior vena cava, and the major organs (heart, lung, thymus, liver, spleen, kidney, pancreas, intestine, and muscle) were removed immediately. Serum was isolated and stored in small aliquots at −80°C till further analysis.
Determination of Serum Immunoglobulins
Immunogloblin IgG and its subclasses IgG1, IgG2a, IgG2b and IgG3 were quantified by using mouse radial immunodiffusion kits (The Binding Site, Birmingham, England). The IgE was quantitated by using an ELISA kit from Bethyl (Montgomery, TX) in 1:25 diluted serum samples at a single dilution due to limited amount of serum. The absorbance was read at 450 nm using a microplate reader (Bio-Rad, Hercules, CA).
Antinuclear Antibodies
The presence of antinuclear antibodies (ANA) in the available sera from 4–5 mice in each group was assessed semi-qualitatively using a kit (FK001.1) from the Binding Site. Diluted serum (1:40) was first incubated with Hep-2 cell slides followed by incubation with goat anti-mouse IgG-FITC conjugated antibodies (Fc-specific; Sigma). The presence of ANA (as well as the location of any binding in the Hep-2 cells) in each serum sample was then assessed using an Olympus BX 51 microscope (Olympus America Inc.) following manufacturer's instructions. Scoring of the slides was subjective; slides were scored based on brightness of staining over multiple fields on each slide using a scale of none (−) to slight (+) to moderate (++) to intense (+++) staining in each field examined.
Measurement of Cytokines
Cytokines G-CSF, MIP-1α, IL-4, IL-6, IL-12 (P40/P70), and IL-13 were analyzed by using protein multiplex immunoassay kits (LMC2031, LMC1021, LMC0041, LMC0061, LMC0121, and LMC0131) as per manufacturer's instructions (Bisource, Camarillo, CA). The fluorescence was measured by Luminex 100 (Bio-Rad).
Histopathology
Heart, lung, thymus, spleen, liver, kidney, pancreas, intestine, and muscle were fixed in 10% neutral buffered formalin. The tissue sections were stained with hematoxylin and eosin (H&E) for morphological evaluations.
Statistical Analysis
The data are presented as mean ± standard error of the mean (SEM). For the determination of statistical significance, the data were subjected to the analysis of variance (ANOVA) followed by Student–Newman–Keuls post hoc test. p values < 0.05 were considered statistically significant.
RESULTS
Changes in the Serum Immunoglobulins
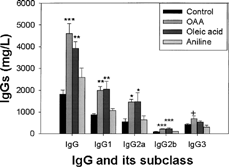
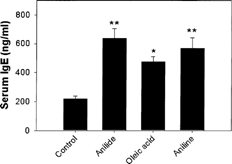
MRL+/+ mice exposed to OAA, aniline or oleic acid did not show any significant differences in their body weights throughout the study. Exposure of the mice to the above-mentioned agents for 6 weeks showed significant changes in serum IgG and its subsets in the OAA and oleic acid groups, but not for aniline. The increases in IgG, IgG1, IgG2a, IgG2b and IgG3 in the OAA and oleic acid groups were 153 and 115, 128 and 135, 169 and 172, 142 and 154, and 64 and 24%, respectively as compared to corresponding control (). The values for IgG and its subsets showed only marginal increases in the aniline-treated mice and could not attain statistical significance. Serum IgE values showed significant increases in all 3 groups, and the increases were 191, 116, and 159% for the OAA-, oleic acid- and aniline-treated mice in comparison to controls ().
Serum Cytokines
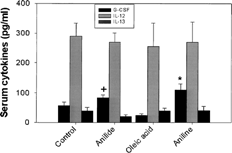
Cytokine G-CSF in serum of mice exposed to OAA and aniline showed significant increases of 47 and 92%, respectively (). Oleic acid group did not show any change in G-CSF. The IL-12 (p40/p70) and IL-13 levels in various treatment groups remained unchanged compared to the controls. The levels of cytokine IL-4, IL-6, and MIP-1α were undetectable with protein multiplex immunoassay kit.
Antinuclear Antibodies
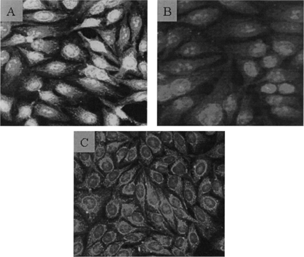
Antinuclear antibodies (ANA) in the serum of the various treatment group mice were detected by following the ultimate development of fluorescence on slides containing Hep-2 cells. Among the available serum samples from the groups, 50% (2/4) of OAA-treated mice had serum that gave rise to an intense nuclear staining pattern for ANA (). This result suggested the possible involvement of dsDNA, ssDNA, and/or histone antigens. In the oleic acid-treated group, only 25% (1/4) of the available serum samples yielded a slight homogenous staining pattern. The sera from control and aniline-treated mice failed to give rise to similar fluorescence patterns. With the control mice sera, the very slight staining near the Hep-2 cell nuclei most likely reflects the fact that these mice are autoimmune-prone.
Histopathology
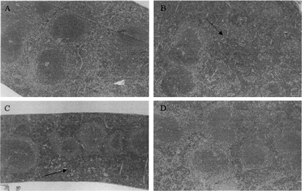
The morphological evaluations of various tissues showed changes only in the spleen, and were confined to OAA- and aniline-treated groups. Spleens in these mice showed increased lymphocyte populations in the red pulp areas ().
DISCUSSION AND CONCLUSIONS
Ever since the occurrence of TOS over two decades ago, the immune system has been presumed to be involved in the syndrome's pathogenesis. This presumption is based on the clinical data obtained from different phases of TOS. The acute phase of TOS was characterized by fever, rash, eosinophilia, pulmonary edema, myalgia, lymphadenopathy, and increased serum IgE levels (Gomez-Reino, Citation1987; Abaitua Borda and Posada de la Paz, Citation1992; Bell et al., Citation1992), whereas the chronic phase was characterized by scleroderma-like lesions, hepatitis, peripheral neuropathy, and pulmonary hypertension (Lahoz et al., Citation1983, Citation1992; Gomez-Reino, Citation1987). In addition, the chronic phase was characterized by the production of antinuclear and antinucleolar antibodies (Arnaiz-Villena et al., Citation1982; Pereira et al., Citation1985). Because of the similarities of TOS to other prominent autoimmune diseases (ADs), including systemic lupus erythematosus (SLE) and systemic scleroderma, there has been considerable interest in developing an animal model to identify the etiologic agent (Bell et al., Citation1998; Weatherill et al., Citation2003).
Immunogenetic factors, age, and sex of the animals are important determinants in the development of ADs (Ahmed et al., Citation1985). Therefore, use of appropriate species and strain of animals are critical in studying autoimmunity and/or ADs. In this study, we used MRL+/+ mice, which develop spontaneous SLE in their second year of life span, possess low levels of anti-ssDNA and anti-dsDNA antibodies at the age of 6 months and have antinuclear antibodies at the age of 10 months (Andrews et al., Citation1978; Murphy and Roths, Citation1978; Izui et al., Citation1984). Thus, these MRL+/+ mice could serve as an excellent model for studying the effect of OAA and its hydrolysis products because these mice are susceptible to develop ADs, many of the autoantibodies appear late, while the disease develops only in the second year. This slow development of disease could allow the evaluation of specific effects of chemicals on immune parameters in relatively young animals. Our decision to use female mice was due to higher susceptibility and prevalence of ADs among females in general (Ahmed et al., Citation1985) and TOS patients in particular (World Health Organization Report, Citation1984).
Due to the ubiquitous presence of amidases/esterases in tissues, it seems likely that OAA can be hydrolyzed, and the hydrolysis products could also contribute to the immunological responses of OAA. Therefore, mice were exposed to equimolar doses of OAA, oleic acid or aniline in this study. Both OAA and oleic acid induced similar increases in total serum IgG and its isotypes (IgG1, IgG2a, IgG2b, and IgG3), whereas increases in aniline-treated mice were minimal. Only noticeable increase due to aniline treatment was in serum IgE that also showed significant increases similar to OAA- and oleic acid-treated groups. Interestingly, increases in IgE levels have also been reported in TOS patients (Gallardo et al., Citation1994). From the data it seems OAA and oleic acid are capable of stimulating both TH1 and TH2 responses as evident from the increases in IgG2a (TH1) and IgG1 and IgE (Th2) (Koller et al., Citation2002). Our data on serum immunoglobulins in response to OAA treatment in mice are also in agreement with the findings in rats (Khan et al., Citation1991) and Swiss mice (Blasco and Ruiz-Gutierrez, Citation1992). Granulocyte colony-stimulating factor (G-CSF), which showed increases in OAA- and aniline-treated mice, appears to be due to activation of macrophages, endothelial cells and fibroblasts. It regulates the production of neutrophilic ganulocytes and also activation of mature neutrophils. G-CSF levels were generally higher in TOS patients (Gallardo et al., Citation1994). However, the exact role of G-CSF in OAA-induced immunotoxicity remains to be established.
The presence of ANA in half of the available OAA-mice serum samples (which gave rise to an intense homogenous ANA fluorescence pattern [solid nuclear staining] on the Hep-2 cell slides) is reflective of a likely involvement of dsDNA, ssDNA or histone antigens that are associated with SLE and other connective tissue disorders (Notmant et al., Citation1975). This is consistent with the reports on TOS patients (Arnaiz-Villena et al., Citation1982; Noriega, 1982; Pereira et al., Citation1985; Lahoz et al., Citation1992). Our data suggest that OAA exposure can accelerate autoimmune responses in these genetically-susceptible mice. It is well documented that there is a genetic link to TOS susceptibility, and that women under the age of 40 are at high risk for developing the disease (Abaitua Borda et al., Citation1998). In addition, there was an increased prevalence of the MHC Class II genes HLA-DR3 and HLA-DR4 in female TOS patients (Vicario et al., Citation1982; Bell et al., Citation1992). The morphological assessment of increased splenic lymphocyte population in the OAA-and aniline-treated mice in this study further suggests the involvement of immune system. Earlier studies have also reported increases in splenocyte proliferation following OAA treatment (Bell et al., Citation2002).
Autoimmunity arises when immune tolerance to self-antigens breaks down, either triggering disease and/or provoking pathogenesis of ADs. Thus, ADs can develop due to pathogenic effects of either autoantibodies or autoreactive T-cells and pathogenic disease can occur from an imbalance of cytokine activity (Koller et al., Citation2002). OAA, being small molecules, will not be antigenic by themselves. However, after metabolism in the body and binding to a larger molecule, they can stimulate an immune response (Kubicka-Muranyi et al., Citation1993; Goebel et al., Citation1995; Von Schmiedeberg et al., Citation1996).
In conclusion, our studies suggest that OAA and oleic acid elicit an immune response in female MRL+/+ mice. These responses, especially the IgG and IgE were stronger in OAA-treated mice than in oleic acid- and aniline-treated mice. Although we observed increases in ANA in OAA-treated mice, this observation needs a detailed investigation. It is also evident from our results that these mice could serve as an excellent model to study the early induction of immunotoxic responses. Dose and time-response studies with OAA, its hydrolysis products as well as their secondary metabolites with larger number of animals will shed more light on their potential in inducing an autoimmune response and lead to a better understanding of their role in the pathogenesis of TOS.
This article is not subject to U.S. Copyright laws.
This publication was made possible by grant ES04815 and ES11584 from the National Institute of Environment Health Sciences (NIEHS) and its contents are solely the responsibility of the authors and do not necessarily represent the views of the NIH or NIEHS.
REFERENCES
- Abaitua Borda I., Posasa de la Paz M. Clinical Findings. WHO Regional Publications European Series, No. 42, 1992; 27–38
- Abaitua Borda I., Philen R M., Posada de la Paz M., Gomez de la Camara A., Diez Ruiz-Navarro M., Gimenez Ribota O., Alvargonzalez Soldevilla J., Terracini B., Severiano Pena S., Fuentes Leal C., Kilbourne E. M. Toxic oil syndrome mortality, the first 13 years. Int. J. Epidemiol. 1998; 27: 1057–1063, [PUBMED], [INFOTRIEVE], [CSA]
- Ahmed S. A., Penhale W. H., Talal N. Sex hormones, immune responses, and autoimmune diseases: Mechanisms of sex hormone action. Am. J. Pathol. 1985; 121: 531–551, [CSA]
- Andrews B. S., Eisengerg R. A., Theofilopolos A. N., Izui J. B., Dixon F. J. Spontaneous murine lupus-like syndromes: Clinical and immunopathological manifestations in several strains. J. Exp. Med. 1978; 148: 198–215, [CSA], [CROSSREF]
- Arnaiz-Villena L., Vicario J. L., Serrano-Rios M., Bellas C., Mampaso F. Glomerular basement-membrane anti-bodies and HLA-DR2 in Spanish rapeseed oil desease. New Engl. J. Med. 1982; 307: 1404–1405, [PUBMED], [INFOTRIEVE], [CSA]
- Balnca M., Boulton P., Brostoff J. Toxic oil syndrome, clinical and immunological characteristics: A review. Clin. Allergy 1984; 14: 165–168, [CSA]
- Bell S. A., Faust H., Schmid A, Merurer M. Autoantibodies to C-reactive protein (CRP) and other acute-phase proteins in systemic autoimmune diseases. Clin. Expt. Immunol. 1998; 113: 327–332, [CSA], [CROSSREF]
- Bell S. A., Hobbs M. V., Rubin R. L. Isotype-restricted hyperimmunity in a murine model of the toxic oil syndrome. J Immunol. 1992; 148: 3369–3376, [PUBMED], [INFOTRIEVE], [CSA]
- Bell S. A., Kuntze I., Caputo A., Chatelain R. Strain-dependent in vitro and in vivo effects of oleic acid anilides on splenocytes and T-cells in a murine model of the toxic oil syndrome. Food Chem. Toxicol. 2002; 40: 19–24, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Blasco R., Ruiz-Gutierrez V. Effect of fatty acid anilides on immune responses of Swiss mice. Clin. Exp. Immunol. 1992; 89: 131–135, [PUBMED], [INFOTRIEVE], [CSA]
- Gallardo S., Pozo V. D., Cárdaba B., Andrés B D., Martin-Orozco E., Fermandez J. C., Tramon P., Psada M., Abaitua I., Palomin P., Lahoz C. Immunological basis of toxic oil syndrome (TOS). Toxicology 1994; 93: 289–299, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Goebel C., Kubicka-Muranyi M., Tonn T., Gonazlez J., Gleichmann E. Phagocytes render chemicals immunogenic: Oxidation of gold (1) to the T-cell sensitizing gold (III) metabolite generated by mononuclear phagocytes. Arch. Toxicol. 1995; 69: 450–459, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Gomez-Reino J. J. Immune system disorders associate with adulterated cooking oil. UNEP, ILO, WHO International Program on Chemical Safety IPCS Commission of the European Communities, A. J. Berlin, M. H. Dean, E. M. Draper, F. Smith, Spreafico. Martin Nijhoff Publishers, DordechtThe Netherlands 1987; 376–389
- Grandjean E. C. Introduction. Toxic Oil Syndrome Current Knowledge and Future Perspectives. European Series, No. 42, WHO Regional Publications. 1992; 1–2
- Heiskanen K. M., Naarala J., Savolainen K. M. The effects of N-(5-vinyl-1,3-thiazolidin-2-ylidene) phenylamine (5-VTPA) on the changes in free intracellular calcium and the production of reactive oxygen metabolites in human leukocytes. Toxicology 1995; 100: 195–202, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Heiskanen K. M., Savolainen K. M. Effects of 3-phenylamino-1,2-propanediol and its mono- and dioleylesters on the production of reactive oxygen metabolites in human polymoronuclear leukocytea. Toxicol. Lett. 1997; 91: 39–45, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Izui S., Kelley V. E., Masuda K., Youshida H., Roths J. B., Murphy E. D. Induction of various autoantibodies by mutant gene lpr in several strains of mice. J. Immunol. 1984; 133: 227–233, [PUBMED], [INFOTRIEVE], [CSA]
- Kaphalia B. S., Ansari G. A. S. Rapid chromatographic analysis of fatty acid anilides suspected of causing toxic oil syndrome. J. Anal. Toxicol. 1991; 14: 1–5, [CSA]
- Khan M. F., Kaphalia B. S., Palafox A., Jerrells T. R., Ansari G. A. S. Toxicity of oleic acid anilide in rats. Arch. Environ. Contam. Toxicol. 1991; 21: 571–577, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kilbourne E. M., Bernert Posada de la Paz J. T., Jr., Hill M., Jr R. H., Abaitua Borda I., Kilbourne B. W., Zack M M. Chemical correlates of pathogenicity of oil related to the toxic oil syndrome epidemic in Spain. Am. J. Epidemiol. 1988; 127: 1210–1227, [PUBMED], [INFOTRIEVE], [CSA]
- Koller L. D., Stang B. V., Hall J. A., Posasa de la P. M., Ruiz Mendez M. V. Immunoglobulin and autoantibody responses in MRL/lpr mice treated with ‘toxic oils’. Toxicology 2002; 178: 119–133, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kubicka-Muranyi M., Goebels R., Goebel C., Uetrecht J., Gleichmann E. T-Lymphocytes ignore procaimanide, but respond to its reactive metabolites in peritoneal cells: demonstration by the adoptive transfer popliteal lymph node assay. Toxicol. Appl. Pharmcol 1993; 122: 88–94, [CSA], [CROSSREF]
- Lahoz C., Rose N. R., Robinson C. J. Immunology. WHO Reg Publ Eur Ser. 1992; 42: 143–152, [PUBMED], [INFOTRIEVE], [CSA]
- Lahoz C., Tricas L., Lauzurica P., Gurbindo C., Garcia R. Hyper IgE, eosinophilia and immunological hyperreactivity due to ingestion of adulterated rapeseed oil (toxic oil syndrome). Eur. J. Respir. Dis 1983; 64(Suppl. 126)415–418, [CSA]
- Murphy E. D., Roths J. B. Autoimmunity and lymphoproliferation: Induction by mutant gene lpr, and acceleration by a male-associated factor in strain BXSB mice. Genetic Control of Autoimmune Disease, N. R. Rose, P. E. Bigazzi, N. L. Warner. Elsevier/North-Holland, New York 1978; 207
- Noreiga A. R. Group TESS: Toxic epidemic syndrome, Spain 1981. Lancet 1982; 2: 697–702, [CSA]
- Notmant D. D., Kurat N., Tan E. M. Profiles of antinuclear antibodies in progressive systemic sclerosis. Arthritis Rheum. 1975; 23: 617–625, [CSA]
- Pereira R. S., Black C. M., Arnaiz-Villena A., Vicario J. L., Gomez-Reino J. J. Collagen antibodies in toxic oil disease. Lancet 1985; 2: 273, [CSA], [CROSSREF]
- Pestana A., Munoz E. Anilides and the Spanish toxic oil syndrome (news). Nature 1982; 298: 608, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Tabuenca J. M. Toxic-allergic shock syndrome caused by ingestion of rapeseed oil denatured with aniline. Lancet 1981; 2: 567–568, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Vazquez Roncero A., Janer Del Valle C., Maestro Duran R., Graciani Constante E. New aniline derivatives in cooking oils associated with the toxic oil syndrome. Lancet 1983; 2: 1024–1025, [PUBMED], [INFOTRIEVE], [CSA]
- Vicario J. L., Serrano-Rios M., San Andres F., Arnaiz-Villena A. HLA-DR3, DR4 increase in chronic stage of Spainsh oil disease. Lancet 1982; 1: 276, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Von Schmiedeberg S., Hanten U., Goebel C., Schuppe H. C., Uetrcht J., Gleichmann E. T-cells ignore the parent drug propylthiouracil but are sensitized to a reactive metabolite generated in vivo. Clin. Immunol. Immunopathol. 1996; 80: 162–170, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Weatherill A. R., Stand B. S., O'Hara K., Koller L. D., Hall J. A. Investigating the onset of autoimmunity in A.SW mice following treatment with ‘toxic oils‘. Toxicol. Lett 2003; 236: 205–216, [CSA], [CROSSREF]
- World Health Organization Report. World Health Organization Regional Office for Europe. WHO, Copenhagen 1984; 8