Abstract
Indirubin-3-monoxime is an aryl hydrocarbon receptor (AhR)-binding, idole-derived compound known to be a potent inducer of CYP1A1, at least in hepatic cell lines. To date, very little is known about the effects of indirubin-3-monoxime on cells relevant to the immune system. We treated human U937 histiocytic lymphoma cells with indirubin-3′-monoxime in four different regimens, representing various stages of PMA-induced differentiation and further activation with LPS. Gene expression profiles were monitored using commercial macroarrays for 96 select stress and toxicity genes. The four treatment regimens were (A) the monocyte model: U937 cells were treated with 1 μM indirubin-3′-monoxime or carrier control for 24 hrs; (B) the differentiated model: PMA-differentiated U937 cells were treated with 1 μM indirubin-3′-monoxime or carrier control for 24 hrs; (C) the differentiation process model: U937 cells were treated for 24 hours with 1 μM indirubin-3′-monoxime or carrier control, followed by PMA-induced differentiation over a 24-hr period; and (D) the LPS-activated model: PMA-differentiated and LPS-activated U937 cells were treated with 1 μM indirubin-3′-monoxime or carrier control for 24 hrs. Indirubin-3′-monoxime treatment caused a 12.7-, 2.1-, 3.2-, and 4.5-fold increases in CYP1A1 in regimes A, B, C, and D, respectively. Indirubin-3′-monoxime treatment also enhanced cyclo-oxygenase 2 expression in PMA-differentiated cells, but reduced the expression of macrophage inflammatory protein, catalase, heme oxygenase-1, glutathione peroxidase, and manganese superoxide dismutase; a scenario consistent with oxidative stress. Macroarray validity was confirmed by RT-PCR using CYP1A1, COX-2, MIP-β, and GADPH as target genes, and at the protein level for CYP1A1, COX-2, and β-actin. To our knowledge, this is the first study to examine the effects of indirubin-3-monoxime in a human cell line relevant to the immune system. Overall, our study suggests that indirubin-3′-monoxime is either a classical AhR ligand, or that it modulates endogenous AhR-regulated activity.
INTRODUCTION
The aryl hydrocarbon receptor (AhR) is a cytoplasmic ligand-activated transcription factor belonging to the PER, ARNT and SIM (PAS) superfamily of proteins. Like most members of the PAS superfamily, the AhR contains a basic-helix-loop-helix motif (bHLH) (Gu et al., Citation2000). The basic region of the motif contains a DNA binding capacity and the helix-loop-helix contributes the ability to form the requisite heterodimer with aryl hydrocarbon receptor nuclear translocator (ARNT) in order to fully function as a transcription factor (Hoffman et al., Citation1991). Upon ligand binding, the AhR translocates to the nucleus where it forms a heterodimer with ARNT and alters the transcription of a battery of genes including phase 1 and phase 2 drug-and steroid-metabolizing enzymes (Hahn, Citation1998; Nebert and Dieter, Citation2000). Some of the most potent xenobiotoic AhR ligands are 2, 3, 7, 8-tetrachloroedibenzo-p-dioxin (TCDD) and the structurally related halogenated aromatic hydrocarbons (HAHs), as well as select polycyclic aromatic hydrocarbons (PAHs). Each of these AhR ligands strongly induce the CYP1A family of Phase I enzymes (Hahn, Citation1998).
Although the AhR remains an orphan receptor in that the natural endogenous ligand(s) are not completely identified, metabolites of tryptophan and other indole-containing compounds have been shown to bind and induce translocation of the receptor to the nucleus. Several metabolic derivatives of tryptophan, including tryptamine and indole acetic acid, as well as metabolites of UV-irradiated tryptophan, are AhR ligands (Helferich and Denison, Citation1991; Heath-Pagliuso et al., Citation1998). It is now believed that gut microflora convert tryptophan into compounds that function as AhR ligands (Perdew and Babbs, Citation1991). Recent evidence suggests that the indole-containing compound 2-(1′H-indole-3′carbonyl)-thiazole-4-carboxylic acid methyl ester may be one of endogenous ligands for the AhR (Song et al., Citation2002).
Indirubin is thought to be at least one of the active ingredients in Danggui Longhui Wan, an ancient Chinese herbal remedy for neoplasia derived, in part, from the plant Polygonum tinctorium (Hoessel et al., Citation1999; Leclerc et al., Citation2001). Through fermentation, oxidation, and dimerization processes, indirubin can be produced from P. tinctorium (Leclerc et al., Citation2001), bacteria (Bhushan et al., Citation2000) and mollusks (Cooksey, Citation2001; Meijer et al., Citation2003). Indirubin is an AhR-binding idole-derived indigoid also present in humans through the dimerization and oxidation of indoxyl, and thus may be an endogenous ligand for the AhR (Adachi et al., Citation2001; Spink et al., Citation2003; Sugihara et al., Citation2004; Guengerich et al., Citation2004; Schnitzer et al., Citation2005). Moreover, cyclin-dependent kinase is strongly inhibited by indirubin, suggesting that this compound is the active anti-neoplastic agent in P. tinctorium (Leclerc et al., Citation2001). Polygonum tinctorium is also used for enhancing detoxication processes and reducing inflammation, suggesting that this plant contains compounds that induce cytochrome P450 enzymes. Indirubin is the most likely candidate because AhR ligands are potent inducers of the CYP1A family of cytochrome-P450s.
Exposure to anthropogenic AhR ligands, such as 2,3,7,8-tetracholordibenzo-p-dioxin (TCDD), co-planar polychlorinated biphenyls, and select PAHs, is associated with immune suppression and reproductive and developmental abnormalities (Holsapple et al., Citation1996). To date, nutraceutical-derived AhR ligands have not been examined in detail with regard to their potential to modulate immune function, either directly through the AhR, or by modulating endogenous AhR function(s), thus the impetus for our study described herein. Kunikata et al. (Citation2000) purified indirubin directly from P. tinctorium to subsequently show that IFN-γ and IL-6 production by HBL-38 human myelomonocytes were suppressed following treatment in the nM range. However, preparations of natural compounds like indirubin are notoriously unstable, and they may contain impurities which can influence the model at hand. Indirubin-3-monoxime is a stable form of indirubin, is commercially available, and has been used as a surrogate for indirubin in several studies cited herein. At this point, we do not know if natural indirubin is more biologically active than indirubin-3-monoxime over the short period of stability of the former. For experimental purposes, the stability of indirubin-3′-monoxime offers continuity throughout multiple experiments within a given study.
U937 histiocytic lymphoma cells have been used as a putative monocyte model in their undifferentiated state, or as a differentiated cell upon treatment with phorbol esters (Nilsson et al., Citation1981; Littman et al., Citation1983; Hewison et al., Citation1992). Furthermore, differentiated U937 cells are reported to be activated in the presence of LPS (Caron et al., Citation1994). This cell line has also been shown to express high levels of the AhR (Hayashi et al., Citation1995) and the AhR co-factor protein ARNT (Komura et al., Citation2001), thus U937 cells should be a good model for understanding the effects of AhR ligands on the basic biology of human monocytes and similar cells undergoing differentiation. Due to the high affinity of indirubin-3′-monoxime for the AhR, we examined the differential expression patterns for 96 human stress and toxicity genes in U937 cells under four different stages of differentiation and activation. The study presented herein, therefore, describes the effects of indirubin-3′-monoxime on the putative human monocyte cell line U937 at the molecular level (gene expression), and CYP1A1 and COX-2 protein expression.
MATERIALS AND METHODS
Cells and Cell Culture
U937 human histiocytic lymphoma cells (Sundstrom and Nilsson, Citation1976) were obtained from American Type Cell Culture (ATCC, Manassas, VA). For routine growth and maintenance, U937 cell were grown in T-75 Costar tissue culture flasks (Corning International Corporation, Corning, NY) under a humidified atmosphere of 5% CO2 at 37°C in RPMI 1640 medium (Cellgro) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μ g/ml streptomycin, 10 mM HEPES solution, 1% of a 7.5% sodium bicarbonate solution, and 100 mM L-glutamine, each from Sigma Chemical Company (St Louis, MO). Cell cultures were split by dilution three times per week to maintain logarithmic growth.
Chemicals
Indirubin-3′-monoxime, a stable form of indirubin (Marko et al., Citation2001; Guengerich et al., Citation2004), was obtained from A.G. Scientific, Inc. San Diego, and dissolved in DMSO (Sigma) to a concentration of 10−2 M and stored in a cryovial at −20°C, then diluted to final working concentrations in culture media just prior to each experiment.
Establishing the Effective Dose of Indirubin-3′-Monoxime
To establish an effective dose of indirubin-3-monoxime for all experiments, U937 cells were treated with a range of concentrations and examined for maximum CYP1A1 protein induction. U937 cells were seeded into 24-well plates at a concentration of 106 cells per well in 1 ml of media and incubated in the presence of 0.1 μ M phorbol myristate acetate (PMA) (Sigma) over a 24-hr period. Cultures were then treated with 10, 1, 0.1, or 0.01 μ M indirubin-3′-monoxime, or 0.01% DMSO carrier control for 48 hrs. The overlying media from adherent differentiated U937 cells was removed and replaced with lysis buffer (150 mM NaCl, 50 mM Tris-HCl, 1 mM PMSF, [pH 8.0], with 1% NP-40). After for 20 min at 4°C, the lysates were centrifuged for 20 min at 14,000 × g. The supernatants were then collected and the protein content determined. Ten μg of protein from each lysate were subjected to SDS-PAGE on 10% gels and transferred overnight to 0.45 μ m nitrocellulose membranes. The membranes were blocked for 1 hr with 3% bovine serum albumin (BSA) in 0.01 M phosphate-buffered saline pH 7.2 (PBS), washed extensively with PBS containing 0.5% Tween-20 (PBS-TW20), and probed for the relative expression of CYP1A1 protein using antibody G-18 (Santa Cruz Biotechnology, Santa Cruz, CA). As an internal loading control, the blots were also probed for expression of β -actin protein using antibody AA20-33 (Sigma). The appropriate alkaline phosphatase (AP)-conjugated secondary antibody was used for each primary antibody and AP activity was visualized using NBT/BCIP. Immunoblots were then scanned for documentation and further presentation.
Upon examination of the CYP1A1 immunoblots, it became clear that 1 μ M indirubin-3′-monoxime induced the maximum amount of specific protein in differentiated U937 cells when treated for 48 hrs. One μ M, indirubin-3′-monoxime, and one higher level at 10 μ M were subsequently examined for their potential cytotoxicity in four treatment regimes covering the various stages of differentiation and activation of U937 cells.
Cytotoxicity of Indirubin-3′-Monoxime in U937 Cells
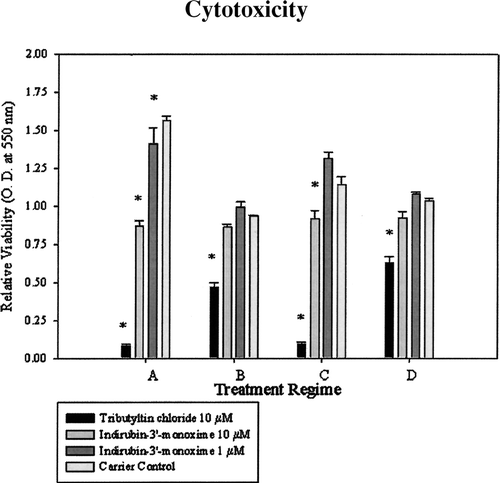
U937 cells were seeded into 96-well Costar plates at 2 × 105 cells per well in 200 μl of media. The four treatment regimens are as follows: (A) undifferentiated cells (monocytes) treated with 10 μM tributyltin-chloride (TBT), a well characterized and highly leukocytotoxic agent (Kergosien and Rice, Citation1998), as a positive control for cytotoxicity, 10 μM indirubin-3′-monoxime, 1 μM indirubin-3′-monoxime or DMSO as carrier control for 24 hrs; (B) U937 cells were differentiated for 24 hrs in the presence of 0.1 μ M PMA, then treated with 10 μ M TBT, 10 μ M indirubin-3′-monoxime, 1 μ M indirubin-3′-monoxime, or DMSO as carrier control for 24 hrs; (C) undifferentiated U937 cells were treated with 10 μM TBT, 10 μ M indirubin-3′-monoxime, 1 μ M indirubin-3′-monoxime or DMSO as carrier control for 24 hrs followed by a 24-hr period of differentiation in the presence of PMA (effects of treatment on the differentiation process); and (D) U937 cells were differentiated in the presence of 0.1 μM PMA for 24 hrs, the culture medium removed and replaced with fresh media containing 0.1 μM PMA and 1 μg/ml LPS (Salmonella typhimurium; Sigma) for 24 hrs to yield differentiated and activated cells, followed by a 24-hr exposure to 10 μM TBT, 10 μ M indirubin-3′-monoxime, 1 μ M indirubin-3′-monoxime or DMSO as carrier control.
All treatments, including the DMSO carrier control, were conducted in six replicated wells of a 96-well plate per treatment. The cytotoxicity of indirubin-3′-monoxime to U937 cells was determined using a MTT (3-[4, 5]-2,5-diphenyltetrazolium bromide) assay for cell death/viability (Barile et al., Citation1993; Husoy et al., Citation1993). MTT was dissolved in non-supplemented media at 5 mg/ml and 20 μ l of the MTT solution were added per well. The plates were incubated for four hours at 37°C, then centrifuged for 10 min and the supernatant removed by aspiration using a 26-gauge needle and 1 ml syringe. The precipitate was then solubilized with 100 μ l of acidified isopropanol. Following a brief period of shaking, the plates were read at 550 λ and the optical density for each well was recorded and averaged over the six wells per treatment.
Differential Gene Expression (Macroarrays)
Eight GEArray Q series arrays for human stress and toxicity pathways (#HS-112-2, Superarray) () were used to analyze gene expression by utilization of RNA from indirubin-3′-monoxime- and carrier control-treated U937 cells under the four aforementioned regimens as summarized in . Five million U937 cells were seeded into T-25 tissue culture flasks in a total volume of 5 ml of culture media. The cells were then treated with 1 μ M indirubin-3′-monoxime or DMSO control according to the aforementioned states of differentiation and activation. The cells were then lysed by addition of Tri-reagent (Sigma) directly over the adherent differentiated macrophages or onto pelleted monocytes and the RNA isolated following the instructions suggested by the manufacturer. The purity of each RNA preparation was assessed by spectrophotometry and a 1.2% formaldehyde-agarose gel prior to initiating the macroarray procedure.
TABLE 1 Superarray Macroarray Table for 96 human stress and toxicity genes tetra-spotted on the arrays
TABLE 2 Indirubin-3′-treatment regimens
AmpoLabeling-LPR kits from Superarray were used to reverse transcribe and label 3 μ g of RNA from each treatment and regimen. Macroarrays consisted of 96 genes tetra-spotted onto the commercial array plus three pUC18 tetraspots and three blanks as negative controls to monitor background levels of hybridization and chemiluminescence. The housekeeping genes glyceraldehyde-3-phosphate dehydrogenase, cyclophilin A, ribosomal protein L13a, and beta actin were also included as positive controls and for normalization procedures. The arrays were developed and chemiluminescent hybridization signals were monitored and recorded using a FujiFilm Intelligent Dark Box II equipped with a Fuji CCD camera (LAS-1000) cooled to −25°C prior to attaining saturated signals. Signal intensities were saved as 16 bit Tiff files and analyzed using the accompanying software packages (ScanAlyze and GEArray analyzer) provided by the manufacturer. Arrays were analyzed in duplicate for each treatment regime. Background chemiluminescence was subtracted and data sets were normalized according to manufacturer's instructions and recommendations.
RT-PCR
Reverse transcription using oligo d(T) primers, followed by polymerase chain reaction analysis, was performed following macroarray analysis to confirm array validity using RNA obtained for the macroarray. Primers for PCR (listed in ), and optimal conditions for use, were selected from the literature or generated by PrimerQuest at 〈http://biotools.idtdna.com/biotools/primer_quest/primer_quest.asp〉 and synthesized by Sigma-Genosys (The Woodlands, TX). PCR products were run through 2% TAE agarose gels to size-fractionate products. Gels were stained with ethidium bromide to visualize PCR product banding patterns and then documented by photography.
TABLE 3 List of PCR primers, cycling conditions and amplicon size for each gene used to validate the macroarray results from treating U937 cells with indirubin-3′-monoxime
Western Blots for Specific Protein
Cells were treated with 1 μ M indirubin-3′-monoxime in each of the four treatment regimens as described above. The media was removed from adherent differentiated cells and replaced with un-supplemented RPMI. The cells were then scraped from the plate bottom and pelleted by centrifugation. Non-adherent monocytes were pelleted and resuspended in un-supplemented RPMI and centrifuged again. The cell pellet from each regimen was frozen overnight at −80°C to assist in the lysis step. The cell pellet was then lysed in 1 ml of lysis buffer for 20 min at 4°C, then centrifuged for 10 min at 14,000 × g. The supernatants were collected and the protein content determined. Procedures for immunoblotting were the same as those described above for CYP1A1 expression profiles in the range-finding experiments. Antibodies G-18 and M-19, both from Santa Cruz Biotechnology were used to detect CYP1A1 and COX-2 proteins, respectively. Antibody AA20-33 from Sigma was used to detect β-actin protein as an internal loading control. The appropriate AP-conjugated secondary antibody was used, followed by NBT/BCIP visualization and documentation of alkaline phosphatase activity.
RESULTS
Effective Dose of Indirubin-3-Monoxime and Cytotoxicity
Following a 48 hr exposure period, it became clear that a concentration of 1 μ M indirubin-3-monoxine induced the maximum amount of CYP1A1 protein expression in PMA-differentiated U937 cells (). Compared to the carrier control, indirubin-3′-monoxime exposure at 1 μ M caused slight cytotoxicity in the monocyte regimen (regimen A). Neither of other treatment regimens exposed to 1 μ M indirubin-3′-monoxime exhibited cytotoxicity (). Furthermore, 10 μ M indirubin-3′-monoxime exposure was cytotoxic to U937 cells in only two of the treatment regimes representing undifferentiated monocytes (Regimen A) and U937 cells undergoing differentiation (Regimen C). Based on the CYP1A1 protein levels and cytotoxicity data, subsequent experiments to monitor gene expression and to determine COX-2 and CYP1A1 protein expression used a working concentration of 1 μ M indirubin-3′-monoxime.
FIG. 1 CYP1A1 protein expression in PMA-differentiated U937 cells following exposure to indirubin-3′-monxime treatment for 48 hrs. Differentiated U937 cells were treated with indirubin-3′-monoxime, lysed, and subjected to SDS-PAGE/immunoblots steps and probed for CYP1A1 protein (a) or β-actin protein (b). Lane 1, carrier control; lane 2, 10 μ M; lane 3, 1 μ M; lane 4, 0.1 μ M; lane 5, 0.01 μ M.
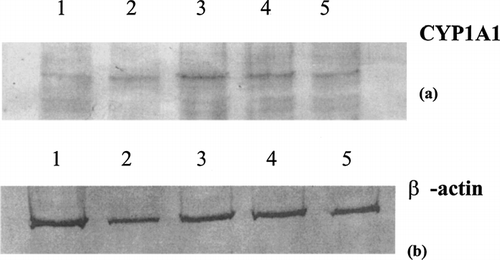
FIG. 2 Cytotoxicity of 10 μ M and 1 μ M indirubin-3′-monoxime to U937 cells under different stages of differentiation and activation. Regimes, A representing monocytes, B representing macrophages, C representing differentiation process, and D representing activated macrophages. Data represent means of three experiments +/− standard error. Viability as determined by MTT assay. Tributyltin chloride was included as a positive control showing cellular toxicity.
Macroarray
Superarray macroarrays consisting of 96 genes associated with stress and toxicity was used to determine the effect of 1 μ M indirubin-3′-monoxime on U937 cells under the conditions of all four of the above treatment regimens as compared to the DMSO carrier control. The most notable effect of indirubin-3′-monoxime on undifferentiated U937 monocytes is a 12-fold increase in expression of the CYP1A1 gene (). Genes for glutathione reductase, the heat-stress protein HSPA9B, and DNA damage and repair protein UNG were also increased under this regime. Two other genes related to oxidative stress, glutathione peroxidase and EPHX2, were reduced as were the RAD23A gene for DNA damage and repair and the apoptosis signaling gene BAX.
TABLE 4 Altered gene expression in U937 cells following exposure to 1 μ M indirubin-3′-monoxime (regimen A) as determined by macroarray analysis
Indirubin-3′-monoxime and DMSO were then examined for their effects of U937 cells in a PMA-differentiated state. The most notable effect in Regimen B was an increase in the mRNA levels for the growth arrest/senescence gene p21, also named CDKN1A:P21Wafl(p21Cip1), the gene for CYP1A1, as well as BCL-X and caspase 8, two genes for apoptosis signaling (). Expression of the proinflammatory gene MIP-1β was reduced by indirubin-3′-monoxime, as were the oxidative and metabolic stress genes catalase, heme oxygenase-1, and metallothionein. Expression of the gene for heat shock protein 70 (Hsp70) was also suppressed.
TABLE 5 Altered gene expression in PMA-differentiated U937 cells (regimen B) following exposure to 1 μ M indirubin-3′monoxime as determined by macroarray analysis
Monocyte differentiation is a critical and necessary step in the overall immune response, especially in proinflammatory responses, thus this process may be sensitive to xenobiotic perturbation. U937 cells were treated with indirubin-3′-monoxime or carrier control for 24 hrs, then differentiated in the presence of PMA for 24 hrs. The genes for the growth arrest and senescence-related gene p21, the gene for the proinflammatory protein PAI-1, and both CYP1A1 and COX-2 genes were increased (). However, catalase and superoxide dismutase gene expression, as well two heat shock genes, were reduced.
TABLE 6 Altered gene expression in U937 cells exposed to 1 μM indirubin-3′-monoxime and then differentiated with PMA (regimen C) as determined by macroarray analysis
Lastly, indirubin-3′-monoxime was examined for its effects on gene expression in PMA-differentiated U937 cells that were activated in the presence of LPS. Of the 96 genes available on the macroarray, only three were significantly altered by exposure to indirubin-3′-monoxime. As expected, the gene for CYP1A1 was induced in this regimen, while the expression of Hsp70 and IκBα genes were inhibited ().
TABLE 7 Altered gene expression in PMA-differentiated and LPS-activated U937 exposed to 1 μ M indirubin-3-monoxime (regimen D) as determined by macroarray analysis
RT-PCR
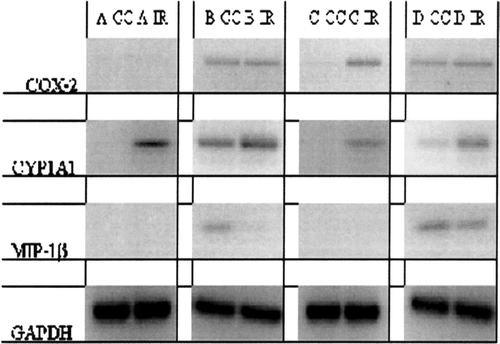
RT-PCR for CYP1A, COX-2, and MIP-1β genes was selected based on intensity of illumination and the alteration of illumination on the four macroarrays. GAPDH was used as a positive control due to its intense and uniform illumination in all four treatment regimes. Duplicate RT-PCR reactions from two independent sets of RNA yielded confirmatory results and validation for the macroarrays ().
FIG. 3 Photograph of RT-PCR for COX-2, CYP1A1, MIP-1β, and GAPDH expression in U937 cells treated with 1 μ M indirubin-3′-monoxime under four different regimens. Regimen A, U937 cells treated with indirubin-3′-monoxime (IR) or carrier control (CC); Regimen B, effects of indirubin-3′-monoxime (IR) and carrier control (CC) on PMA-differentiated U937 cells; Regimen C, effects of indirubin-3′-monoxime (IR) and carrier control (CC) on PMA-induced differentiation of U937 cells; and Regimen D, effects of indirubin-3′-monoxime treatment (IR) and carrier control (CC) on differentiated and LPS-activated cells.
Immunoblots
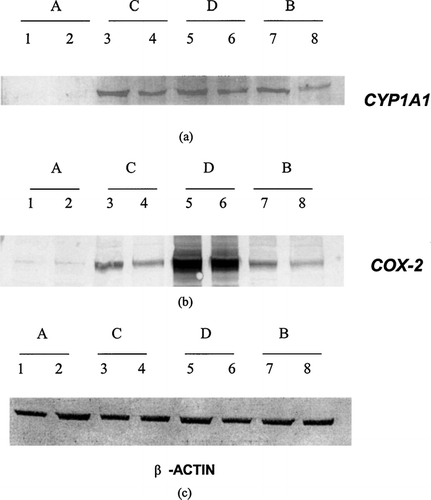
Immunoblotting confirmed that the high expression of the CYP1A1 gene in U937 cells following exposure to indirubin-3′-monoxime extended to the protein level (). Likewise, the increased expression of the COX-2 gene in U937 cells following exposure to indirubin-3′-monoxime seen in the macroarray and by RT-PCR extends to the protein level as shown in the immunoblots of COX-2 protein ().
FIG. 4 Immunoblots for CYP1A1 (a), COX-2 (b), and β -actin (c) in U937 cells treated with 1 μ M indirubin-3′-monoxime under four different regimens. Regimen A, U937 cells treated with indirubin-3′-monoxime (lane 1) and carrier control (lane 2) for 24 hrs; Regimen C, effects of indirubin-3′-monoxime (lane 3) and carrier control (lane 4) on PMA-induced differentiation of U937 cells; Regimen D, effects of indirubin-3′-monoxime (lane 5) and carrier control (lane 6) on differentiated and LPS-activated cells; and Regimen B, effects of indirubin-3′-monoxime (lane 7) and carrier control (lane 8) on PMA-differentiated U937 cells.
DISCUSSION AND CONCLUSIONS
The studies described herein regarding the biological properties of indirubin-3′-monoxime show clearly that this compound is active through the AhR in U937 cells, as evidence by CYP1A1 induction at both the gene and protein level. All AhR ligands examined to date induce the CYP1A-family of cytochrome-P450 enzyme systems, albeit with different levels of potency dictated by the affinity of the compound for the AhR. For example, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) has the highest affinity for the AhR of any known exogenous agent. In comparison, the coplanar PCBs (IUPAC 77, 126, and 169) bind the AhR with roughly 1/10–1/1000th the affinity of TCDD (Kafafi et al., Citation1993). The relative affinity of indirubin-3′-monoxime for the AhR remains in debate. Using yeast reporter systems, Adachi et al. (Citation2001) reported that natural indirubin binds to the AhR with an affinity 50-fold higher than that of TCDD. It has been shown, however, that yeast reporter systems underestimate the potency of TCDD (Miller, Citation1999).
Indirubin-3′-monoxime at 1 μ M was slightly cytotoxic to undifferentiated U937 cells; however, the observed cytotoxicity was minimal compared to that of tributyltin chloride, a well-known cytotoxic organotin (Kergosien and Rice, Citation1998). Furthermore, indirubin-3′-monoxime exhibited no cytotoxicity at either 1 μ M or 10 μ M in regimens B and D (PMA-differentiated, and PMA-differentiated + LPS-activated U937 cells, respectively).
Indirubin-3′-monoxime induced CYP1A1 mRNA levels in U937 cells in all four treatment regimens and increased COX-2 mRNA levels when followed by PMA-induced cellular differentiation. Also, the most significantly induced gene in either of the regimens was that of CYP1A1 by indirubin-3′-monoxime in undifferentiated U937 cells (Regimen A). CYP1A1 protein levels were increased in all regimes treated with 1 μ M indirubin-3′-monoxime except that of the undifferentiated cells (Regimen A). This striking induction of both CYP1A1 gene and protein suggests that indirubin-3′-monoxime functions as an AhR ligand in U937 cells in all four growth regimes representing various stages of differentiation in this particular model.
As an alternative interpretation of the data, it is possible that indirubin-3′-monoxime may modulate endogenous AhR functions and pathways, including the availability of ARNT due to increased expression of hypoxia-inducing factor-alpha (HIF-α), which by nature competes with the AhR for ARNT. Neither HIF-α mRNA nor protein were quantified in this particular study, and it is not clear at this point whether or not the redox state of U937 cells is altered by indirubin-3′-monoxime. However, catalase mRNA levels were reduced by indirubin-3′-monoxime treatment either prior to, or following PMA-induced differentiation of U937 cells. This is of interest due to the role of catalase in the detoxication of H2O2. Levels of heme oxygenase 1 (HO-1) mRNA, another marker of oxidative stress, were reduced by indirubin treatment in differentiated U937 cells. Together, these data suggest that indirubin-3′-monoxime suppresses the ability of differentiated U937 cells to regulate oxidative stress. When treated with indirubin-3′-monoxime prior to differentiation, mRNA levels for both catalase and manganese superoxide dismutase (SOD2) were reduced. Glutathione peroxidase was also reduced in undifferentiated U937 cells treated with indirubin-3′-monoxime. Collectively, these observations do suggest that indirubin-3′-monoxime alters the redox state of U937 cells, as previously demonstrated in PCB-126-treated liver cells (Hennig et al., Citation2002).
Indole-containing compounds, such as indirubin and indigo, and/or their metabolites, may be the particular class of compounds responsible for driving the evolution, selection, and development of the AhR, and its role in normal cellular function (Hahn, Citation1998). Crawford et al. (Citation1997) showed that the AhR is activated and translocated to the nucleus simply by activating leukocytes with phorbol esters and calcium ionophores, leading to CYP1A1 induction even in the absence of exogenous AhR ligands. These findings suggest that merely activating immuno-competent cells involves the release or production of a natural AhR ligand.
Recent studies reveal that tryptophan levels are critical to the functional integrity of T-cells (Munn, Citation2002; Mellor et al., Citation2004), and that the enzyme indoleamine 2,3,-dioxygenase (IDO) is responsible for the metabolism and clearance of this amino acid. IDO expression is increased in activated macrophages and dendritic cells, as well as developing uterine and placental tissues in pregnant mammals (Mellor et al., Citation2001; Schnitzer et al., Citation2005). To date, two hypotheses have emerged to explain the protective effects of increased (induced) IDO levels on reducing T-cell activity, thereby protecting the developing xenographic offspring: the tryptophan reduction hypothesis, and the utilization hypothesis (Grohmann et al., Citation2003). The reduction hypothesis is based on the observation that by reducing tryptophan levels, IDO reduces T-helper cell activity, particularly TH1 activities, and is thus a regulatory mechanism for inflammatory responses. The utilization hypothesis is based on the alternative hypothesis that tryptophan is metabolized by IDO to various kynurenines (metabolites of tryptophan), that are in themselves the active immunomodulating compounds that down regulate TH1 responses and thus inflammation (Grohmann et al., Citation2003; CitationLigam et al., 2005). Regardless of which hypothesis is true, modulated IDO expression seems to be influential, if not critical, in inflammation. With regards to indirubin, this plant-derived compound is an intermediate indole metabolite of tryptophan, and as such may modulate IDO expression. Furthermore, since indirubin-3′-monoxime induces CYP1A1 gene and protein as our study herein shows, it is possible that IDO expression may be linked to the activation of the AhR, as the AhR is known to be activated in proinflammatory responses even in the absence of TCDD (Crawford et al., Citation1997).
Tryptophan is the precursor for a variety of indoles that are now known to bind and activate the AhR. For example, the indole-containing compound 2-(1′ H-indole-3′carbonyl)-thiazole-4-carboxylic acid methyl ester may be one of several endogenous ligands for the Ah receptor (Song et al., Citation2002). Moreover, other classical properties of indirubin-3-monoxime, such as cyclin-dependent kinase inhibition, could also contribute to toxicity.
Macrophage inflammatory protein (MIP)-1α is a chemokine that attracts natural killer cells (Sherry et al., Citation1988; Kitaya et al., Citation2003). MIP-1α mRNA levels were reduced in U937 cells by indirubin-3′-monoxime treatment following differentiation of U937 cells. Reduced MIP-1β levels may explain some of the anti-inflammatory properties of the herbal source of indirubin.
Previously, it had been shown by Hoessel et al. (Citation1999) that indirubin-3-monoxime inhibits cyclin-dependent kinases, thereby effecting cell cycling through an interaction with the ATP binding site on these kinases. A possible alternative mechanism by which indirubin-3′-monoxime inhibits cyclin-dependent kinases is demonstrated in our U937 cells undergoing PMA-induced differentiation. Under these conditions, indirubin-3′-monoxime increases the mRNA levels for p21, a cyclin-dependent kinase inhibitor that acts by binding to the CDK and inhibiting its function (Coqueret, Citation2003). This is a novel finding with regard to the effects of indirubin-3′-monoxime on immune cells.
Interestingly, in differentiated U937 cells indirubin-3-monoxime increased the expression of bcl-xL that encodes for a protein involved in cell survival following growth factor withdrawal, and thus, avoidance of apoptosis (Boise et al., Citation1993). Caspase 8 mRNA levels were also induced by indirubin-3′-monoxime in differentiated U937 cells. Caspase 8, a cysteine protease, is the key-initiator caspase in the apoptotic pathway (Fernandes-Alnemri et al., Citation1994). It is not clear how an increase in expression of one anti-apoptosis gene and the increase of a pro-apoptosis gene is manifested in normal cell physiology, or if this observation is even unique to AhR-ligands.
Based on the macroarray data and RT-PCR results, it is not surprising to see that both CYP1A and COX-2 proteins are increased in U937 cells by indirubin-3-monoxine. The present study clearly shows that indirubin-3′-monoxime, a stable form of indirubin, is an AhR ligand, and based on the work described herein using U937 cells, this compound may have immunotoxic properties. To further characterize the potential immunotoxicity of indirubin-3′-monoxime, more in vitro studies using primary human and mouse leukocytes are needed, as are whole animal studies.
ACKNOWLEDGMENTS
This work was supported by NIH grants R15-ES10556-01 and R15-ES013471-01. We appreciate the advice and technical expertise of the Clemson University Genomics Center (CUGI) faculty and staff.
REFERENCES
- Adachi J., Mori Y., Matsui S., Takigami H., Fujino J., Kitagawa H., Miller C. A., Kato T., Saeki K., Matsuda T. Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine. J. Biol. Chem. 2001; 276: 31475–31478, [PUBMED], [INFOTRIEVE], [CSA]
- Barile F. A., Arjun S., Hopkinson D. In vitro cytotoxicity testing: Biological and statistical significance. Toxicol. In Vitro 1993; 7: 111–116, [CSA], [CROSSREF]
- Bhushan B., Samanta S. K., Jain R. K. Indigo production by naphthalene-degrading bacteria. Lett. Appl. Microbiol. 2000; 31: 5–9, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Boise L. H., Gonzalez-Garcia M., Postema C. E., Ding L., Lindsten T., Turka L. A., Mao X., Nunez G., Thompson C. B. Bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 1993; 74: 597–608, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Caron E., Liautard J. P., Kohler S. Differentiated U937 cells exhibit increased bactericidal activity upon LPS activation and discriminate between virulent and avirulent Listeria and Brucella species. J. Leukocyte Biol. 1994; 56: 174–181, [PUBMED], [INFOTRIEVE], [CSA]
- Cooksey C. J. Tyrian purple: 6,6′-Dibromoindigo and related compounds. Molecules 2001; 6: 736–769, [CSA]
- Coqueret O. New roles for p21 and p27 cell-cycle inhibitors: A function for each cell compartment?. Trends Cell Biol. 2003; 13: 65–70, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Crawford R. B., Holsapple M. P., Kaminski N. E. Leukocyte activation induces aryl hydrocarbon receptor up-regulation, DNA binding, and increased Cyp1a1 expression in the absence of exogenous ligand. Mol. Pharmacol. 1997; 52: 921–927, [PUBMED], [INFOTRIEVE], [CSA]
- Fernandes-Alnemri T., Litwack G., Alnemri E. S. CPP32, a novel human apoptotic protein with homology to Caenorhabditits elegans cell death protein Ced-3 and mammalian interleukin-1 β-converting enzyme. J. Biol. Chem. 1994; 269: 30761–30764, [PUBMED], [INFOTRIEVE], [CSA]
- Grohmann U., Fallarino F., Puccetti P. Tolerance, DCs and tryptophan: Much ado about IDO. Trends Immunol. 2003; 34: 242–248, [CSA], [CROSSREF]
- Gu Y., Hogenesch J. B., Bradfield C. A. The PAS Superfamily: Sensors of environmental and developmental signals. Ann. Rev. Pharmacol. Toxicol. 2000; 40: 519–561, [CSA], [CROSSREF]
- Guengerich F. P., Martin M. V., McCormick W. A., Nguyen L. P., Glover E., Bradfield C. A. Aryl hydrocarbon receptor response to indigoids in vitro and in vivo. Arch. Biochem. Biophys. 2004; 423: 309–316, [CSA], [CROSSREF]
- Hahn M. E. The aryl hydrocarbon receptor: A comparative perspective. Comp. Biochem. Physiol. Part C. 1998; 121: 23–53, [CSA]
- Hayashi S., Okabe-Kado J., Honma Y., Kawajiri K. Expression of Ah receptor (TCDD receptor) during human monocytic differentiation. Carcinogenesis 1995; 16: 1403–1409, [PUBMED], [INFOTRIEVE], [CSA]
- Heath-Pagliuso S., Rogers W. J., Tullis K., Seidel S. D., Cenijn P. H., Brouwer A., Denison M. S. Activation of the Ah receptor by tryptophan and tryptophan metabolites. Biochemistry 1998; 37: 11508–11515, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Helferich W. G., Denison M. S. Ultraviolet photoproducts of tryptophan can act as dioxin agonists. Mol. Pharmacol. 1991; 40: 674–678, [PUBMED], [INFOTRIEVE], [CSA]
- Hennig B. L., Meerarani P., Slim R., Toberk M., Daugherty A., Silverstone A. E., Robertson L. W. Proinflammatory properties of coplanar PCBs: In vitro and in vivo evidence. Toxicol. Appl. Pharmacol. 2002; 181: 174–183, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Hewison M., Brennan A., Singh-Ranger R., Walters J. C., Katz D. R., O'Riordan J. L. The comparative role of 1,25-dihydroxycholecalciferol and phorbol esters in the differentiation of the U937 cell line. Immunology 1992; 77: 304–311, [PUBMED], [INFOTRIEVE], [CSA]
- Hoessel R., Leclerc S., Endicott J. A., Nobel M. E. M., Lawrie A., Tunnah P., Leost M., Damiens E., Marie D., Marko D., Niederberger E., Tang W., Eisenbrand G., Meijer L. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat. Cell Biol. 1999; 1: 60–67, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Hoffman E. C., Reyes H., Chu F. F., Sander F., Conley L. H., Brooks B A., Hankinson O. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science 1991; 252: 954–958, [PUBMED], [INFOTRIEVE], [CSA]
- Holsapple M. P., Karras J. G., Ledbetter J. A., Schieven G. L., Burchiel S. W., Davila D. R., Schatz A. R., Kaminski N. E. Molecular mechanisms of toxicant-induced immunosuppression: Role of second messengers. Ann. Rev. Pharmacol. Toxicol. 1996; 36: 131–159, [CSA], [CROSSREF]
- Husoy T., Syversen T., Jenssen J. Comparisions of four in vitro cytotoxicity tests: MTT assay, NR assay, uridine incorporation and protein measurements. Toxicol. In Vitro 1993; 7: 149–154, [CSA], [CROSSREF]
- Kafafi S. A., Afeefy H. Y., Ali A. H., Said H. K., Kafafi G. Binding of polychlorinated biphenyls to the aryl hydrocarbon receptor. Environ. Health Perspect. 1993; 101: 422–428, [PUBMED], [INFOTRIEVE], [CSA]
- Kergosien D H., Rice C. D. Macrophage secretory function is enhanced by low levels of tributyltin-oxide (TBTO), but not tributyltin-chloride (TBTCl). Arch. Environ. Contam. Toxicol. 1998; 34: 223–228, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kitaya K., Nakayama T., Okubo T., Kuroboshi H., Fushiki S., Honjo H. Expression of macrophage inflammatory protein-1β in human endometrium: Its role in endometrial recruitment of natural killer cells. J. Clin. Endocrinol. Metab. 2003; 88: 1809–1814, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Komura K., Hayashi S., Makino I., Poellinger L., Tanaka H. Aryl hydrocarbon receptor/dioxin receptor in human monocytes and macrophages. Mol. Cell Biochem. 2001; 226: 107–118, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kunikata T., Tatefuji T., Aga H., Iwaki K., Ikeda M., Kurimoto M. Indirubin inhibits inflammatory reactions in delayed-type hypersensitivity. Eur. J. Pharmacol. 2000; 410: 93–100, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Leclerc S., Garnier M., Hoessel R., Marko D., Bibb J. A., Snyder G. L., Greengard P., Biernat J., Wu Y., Mandelkow E., Eisenbrand G., Meijer L. Indirubins inhibit glycogen synthase kinase-3β and CDK5/P25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer's disease. J. Biol. Chem. 2001; 276: 251–260, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ligam P., Manuelpillai U., Wallace E. M., Walker D. Localisation of indoleamine 2,3-dioxygenase and kynurenine hydroxylase in human placenta and decidua: Implications for role of the kynurenine pathway in pregnancy 2005. Placenta. 2004; 26: 498–504, [CSA], [CROSSREF]
- Littman B. H., Hall R. E., Muchmore A. V. Lymphokine and phorbol (PMA) regulation of complemnet (C2) synthesis using U937. Cell Immunol. 1983; 76: 189–195, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Marko D., Schatzle S., Friedel A., Genzlinger A., Zankl H., Meijer L., Eisenbrand G. Inhibition of cyclin-dependent kinase 1 (CDK1) by indirubin derivatives in human tumour cells. Br. J. Cancer. 2001; 84: 283–289, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Meijer L., Skaltsounis A., Magiatis P., Polychronopoulos P., Knockaert M., Leost M., Ryan X. P., Vonica C. A., Brivanlou A., Dajani R., Crovace C., Tarricone C., Musacchio A., Roe S. M., Pearl L., Greengard P. GSK-3-selective inhibitors derived from tyrian purple indirubins. Chem. Biol. 2003; 10: 1255–1266, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Mellor A. L., Sivakumar J., Chandler P., Smith K., Molina H., Mao D., Munn D. H. Prevention of T-cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat. Immunol. 2001; 2: 64–68, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Mellor A. L., Chandler P., Baban B., Hansen A. M., Marshall B., Pihkala J., Waldmann H., Cobbold S., Adams E., Munn D. H. Specific subsets of murine dendritic cells acquire potent T-cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int. Immunol. 2004; 16: 1391–1401, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Miller C. A. A human aryl hydrocarbon receptor signalling pathway constructed in yeast displays additive responses to ligand mixtures. Toxicol. Appl. Pharmacol. 1999; 160: 297–303, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Munn D. H., Sharma M. D., Lee J. R., Jhaver K. G., Johnson T. S., Keskin D. B., Marshall B., Chandler P., Antonia S. J., Burgess R., Slingluff C. L., Mellor A. L. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science 2002; 297: 1867–1870, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Nebert D. W., Dieter M. Z. The evolution of drug metabolism. Pharmacology 2000; 3: 124–135, [CSA], [CROSSREF]
- Nilsson K., Forsbeck K., Gidlund M., Sundstrom C., Totterman T., Sallstrom J., Venge P. Surface characteristics of the U-937 human histiocytic lymphoma cell line: Specific changes during inducible morphologic and functional differentiation in vitro. Haematol. Blood Transfus. 1981; 26: 215–221, [PUBMED], [INFOTRIEVE], [CSA]
- Perdew G. H., Babbs C. F. Production of Ah receptor ligands in rat fecal suspensions containing tryptophan or indole-3-carbinol. Nutr. Cancer 1991; 16: 209–218, [PUBMED], [INFOTRIEVE], [CSA]
- Schnitzer S. E., Schmid T., Zhou J., Eisenrand G., Brune B. Inhibition of GSK3 by indirubin restores HIF-α accumulation under prolonged periods of hypoxia/anoxia. FEBS Lett. 2005; 579: 529–533, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sherry B., Tekamp-Olson P., Gallegos C., Bauer D., Davatelis G., Wolpe S. I., Masiarz F., Coit D., Cerami A. Resolution of the two components of macrophage inflammatory protein 1, and cloning and characterization of one of those components, macrophage inflammatory protein 1 β. J. Exp. Med. 1988; 168: 2251–2259, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Song J., Clagett-Dame M., Peterson R. E., Hahn M. E., Westler W. M., Sicinski R. R., DeLuca H. F. A ligand for the aryl hydrocarbon receptor isolated from lung. Proc. Natl. Acad. Sci., USA 2002; 99: 14694–14699, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Spink B. C., Hussain M. M., Katz B. H., Eisele L., Spink D. C. Transient induction of cytochromes P450 1A1 and 1B1 in MCF-7 human breast cancer cells by indirubin. Biochem. Pharmacol. 2003; 66: 2313–2321, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sugihara K., Kitamura S., Yamada T., Okayama T., Ohta S., Yamashita K., Yasuda M., Fuji-Kuriyama Y., Saeki K., Matsui S., Matsuda T. Aryl hydrocarbon receptor-mediated induction of microsomal drug-metabolizing enzyme activity by indirubin and indigo. Biochem. Biophys. Res. Commun. 2004; 318: 571–578, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sundstrom C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int. J. Cancer. 1976; 17: 565–577, [PUBMED], [INFOTRIEVE], [CSA]