Abstract
Particulate matter (PM) components of air pollution have been associated with mortality and health risks in susceptible populations including asthmatics. More than a decade of PM research has demonstrated that these effects do not occur indiscriminately and are related to particle size, surface area, and chemical composition. Experimental evidence in rodents indicates that inhaled or instilled diesel exhaust particles (DEPs) increase lung injury, inflammation, and allergic airway responses, and that ultra-fine carbon black (UFCB) particles cause more pulmonary inflammation than fine carbon black (FCB) particles in a dose-dependent manner. Our preliminary work determined that a dose of 100 μg of FCB, UFCB, or DEPs (NIST SRM 2975) was sufficient to enhance pulmonary inflammation in Brown Norway (BN) rats 24 hours after intratracheal (IT) instillation of the particles. In the current investigation, we sought to compare, on a mass basis, the effects of a 100 μg dose of these particles on allergic sensitization to house dust mite (HDM) antigen. Immediate airway responses (IAR) to HDM challenge and a battery of proinflammatory, allergic, and acute injury responses in the lung were then measured two and seven days post-challenge. DEPs exposure increased 8 of 10 responses including IAR and levels of IL-4, IL-13, TNFα, total protein, cysteinyl leukotrienes, and eosinophils in bronchoalveolar lavage fluid (BALF). UFCB and FCB significantly enhanced 4 of 10 and 2 of 10 of these responses compared to saline, respectively. Among other responses that were not statistically elevated with particle treatment, mean values for FCB were higher than for UFCB. Particles administered prior to challenge rather than prior to sensitization did not significantly enhance any of these responses above levels of saline controls. We conclude that on a mass basis, DEPs had the greatest potential to enhance allergic induction, indicating that chemical composition is more important than particle size in determining potency for this health effect.
INTRODUCTION
Many studies over the last decade have shown that diesel exhaust particles (DEPs) from a variety of sources can act as adjuvants to enhance immune responses to common allergens. While these reports have arisen mainly from animal experiments (Fujimaki et al., Citation1997; Miyabara et al., Citation1998; Hashimoto et al., Citation2001; Ichinose et al., Citation2003), a human clinical study has confirmed this effect (Diaz-Sanchez, Citation1997), and epidemiological surveys are beginning to report that the incidence of asthma may be associated with proximity to highways with high diesel traffic (Proietti et al., Citation2003; Heinrich and Wichmann, Citation2004). Considerable evidence now exists that supports the role of DEPs in the rising incidence of asthma and allergic lung disease (Casillas et al., Citation1999; Nel et al., Citation2001; Xia et al., Citation2004). DEPs are complex mixtures containing varying amounts of elemental carbon and many hundreds of bioactive organic compounds. One way to more fully understand the adjuvant properties of DEPs has been to study one or more of their components.
Several reports have shown that organic extracts of DEPs possess adjuvant activity (Diaz-Sanchez, Citation1997; Tsien et al., Citation1997; Devouassoux et al., Citation2002). These findings have been extended to show biochemical effects of DEPs at the level of cellular mitochondria. According to the mechanisms proposed by Nel and colleagues (2001), metabolically-activated organic compounds forming quinones and semiquinones cause oxidative damage in airway epithelial cells, which in turn increases chemokine production and polarizes the immune response to a TH2 phenotype (Nel et al., Citation2001; Xia et al., Citation2004). Other studies have reported that the elemental carbon core of these particles alone boosts allergic immune responses to the same degree as whole DEPs (Granum and Lovik, Citation2002). Thus, carbon black (CB) particles, which lack the chemical complexity of DEPs, also behave as adjuvants albeit at high concentrations (Al-Humadi et al., Citation2002). Although the mechanisms by which CB particles enhance allergic responses in the lung are not well understood, it appears that some level of injury and inflammation initiated by these particles leads to the observed adjuvancy. It has been suggested that ultrafine carbon black (UFCB) induces more pulmonary injury and inflammation than fine carbon black (FCB) at low doses, due to the enhanced free radical activity and greater surface area of UFCB (Li et al., Citation1999). Furthermore, ultrafine PM may be of greater health concern due to having a larger surface area and heightened oxidant capacity, compared to larger PM, with subsequent increased potential to induce pulmonary inflammation (Pietropaoli et al., Citation2004).
Sensitized Brown Norway (BN) rats display an atopic TH2-prone phenotype including high inducible levels of airway eosinophils, proinflammatory and TH2 cytokines, elevated levels of allergen-specific serum IgE, and immediate airway responses (IAR) to house dust mite (HDM) challenge (Singh et al., Citation2003). Our laboratory has previously demonstrated that BN rats exposed to residual oil fly ash prior to sensitization have stronger allergic immune responses to HDM than saline-treated, sensitized controls (Lambert et al., Citation1999). Preliminary experiments showed that a low dose of 100 μg of FCB, UFCB, or DEPs was sufficient to induce pulmonary inflammation in BN rats 24 hours after intratracheal (IT) instillation. We hypothesized that stimulation of inflammation and injury in the lung are prerequisites to adjuvancy. To test this, we measured lung injury, IAR, and proinflammatory and allergic responses after allergen challenge in HDM-sensitized BN rats exposed to equal mass doses (100 μg) of FCB, UFCB, and DEPs 24 hours prior to sensitization. Furthermore, we sought to determine the relative adjuvancy produced by both sizes of CB particles and DEPs compared to saline alone, when particles were administered prior to allergen challenge.
MATERIALS AND METHODS
Animals
Inbred, female Brown Norway (BN) (strain BN/Ss Nhsd; 160–180 g) rats were purchased from Charles River Laboratories, Inc. (Wilmington, MA) and allowed to acclimate for a minimum of 1 week prior to exposures. Animals were housed in AAALAC-approved facilities fitted with high-efficiency particulate air filters to minimize external contamination. The use of all rats in these studies was reviewed by the U.S. Environmental Protection Agency's Animal Care and Use Committee. Rats (used at 8–10 weeks old) had ad libitum access to rat chow and water. Rats were free of Sendai virus, pneumonia virus, and a variety of other rodent viruses and Mycoplasma sp. as determined by serological testing of randomly selected rats and sentinels monitored throughout the study.
Antigen
House dust mite (HDM) antigen derived from Dermatophagoides farinae was purified from ground, whole-bodied mites after defatting, extraction in 0.125 M ammonium bicarbonate, and dialysis with distilled water (Greer Laboratories, Lenoir, NC). Extract purification was achieved by a combination of DEAE ion exchange chromatography and gel filtration, with the final preparation containing > 75% of group I allergen (Derf1), as determined by the vendor.
Particle Samples
Bulk diesel exhaust particles (DEPs) (Standard Reference Material (SRM) 2975), were purchased from the National Institute of Standards Technology (NIST) (Gaithersburg, MD) and have been described elsewhere (Singh et al. Citation2004). The certified analysis of these particles is available online 〈http://patapsco.nist.gov/srmcatalog/certificates/2975.pdf〉. The reported mean diameter of these particles was 11.2 ± 0.1 μ m by area distribution, and the surface area, as determined by nitrogen adsorption, was 91 m2/g (see NIST Certificate of Analysis SRM 2975). It is possible that smaller-sized particles present in the sample could not be detected by the methods used. The certified analysis contains 11 certified concentrations and 28 reference concentrations for selected PAHs found in the DEPs. There is no information provided on metal content for the DEPs sample. The fine carbon black (FCB) (Huber, 990) and ultrafine carbon black (UFCB) (Printex 90) samples were a gift from Dr. Vicki Stone, Napier University (Edinburgh, UK) and have been characterized elsewhere (Li et al., Citation1999). The reported size (by mean diameter in nm) and surface area (by nitrogen adsorption in m2/g) determined by the original supplier of the FCB was 320 (nm) and 9 (m2/g), respectively. The same reported values for the UFCB were 14 (nm) and 300 (m2/g). There were considerable differences reported in metal content for these 2 samples. Briefly, the UFCB sample had higher amounts of beryllium, chromium, copper, iron, lead, thallium, and zinc than the FCB (see Li et al. (Citation1999) for exact quantities). No additional analysis of metal content was performed in the present study. The 3 particle samples ordered by size are: DEPs ≫ FCB > UFCB. When ordered by surface area, the samples are arranged as follows: UFCB > DEPs > FCB. All particle suspensions were disaggregated by sonication prior to intratracheal instillation.
Experimental Design
Rats were instilled intratracheally (IT) with 100 μ g of either FCB, UFCB, or DEPs in 250 μl of instillation grade saline or saline only (as negative control) either on day 0, 24 hours prior to sensitization (IT, 5 μg HDM in saline on days 1 and 3), or on day 14, 24 hours prior to challenge. On day 15, rats were challenged IT with 10 μg HDM in saline. Following allergen challenge, immediate airway responses (IAR) were assessed using a whole body plethysmograph. Rats were euthanized (IP, 200 mg/kg sodium pentobarbital) 2 and 7 days post-challenge and bled by cardiac puncture. Serum and bronchoalveolar lavage fluid (BALF) were collected and stored at −80°C for subsequent assay. HDM-specific IgE was measured in the serum and additional responses were measured in the BALF including total eosinophils, total protein, cysteinyl leukotrienes, ascorbic acid (AA), TNFα, IL-13, and IL-4. Mediastinal lymph nodes were collected and processed for lymphoproliferative assays.
Immediate Airway Responses to House Dust Mite
Two weeks after sensitization, animals were placed in a whole-body plethysmograph (Buxco Electronics, Troy, NY) equipped with a pneumotachograph and pressure transducer to monitor pulmonary ventilation responses as previously described (Lambert et al., Citation1998, Citation1999, Citation2000). Baseline ventilatory readings were measured for 10 minutes prior to challenge. Animals were then removed from the plethysmograph, anesthetized with halothane, IT instilled with 10μ g HDM in 250 μl saline and then placed in the plethysmograph for an additional 20 minutes to evaluate the IAR following challenge. These responses are expressed as the enhanced pause (Penh), which is a derived value that provides an index of airflow obstruction, which has been correlated with changes in airway resistance (Hamelmann et al., Citation1997). Penh values were averaged during the baseline (control) periods and the post-challenge periods to obtain mean values for each event and are represented as change from the mean during the baseline period to the mean during the post-challenge period.
Antigen-Specific Serum IgE
Antigen-specific serum IgE production was measured by enzyme-linked immunosorbent assay (ELISA) as previously described (Gilmour et al., Citation1996). Briefly, 96-well flat-bottom ELISA plates were coated with 100 μl/well of mouse anti-rat IgE heavy chain antibody (Serotec, Ltd., Oxford, UK) at a concentration of 2.5 μ g/ml in coating buffer (Pierce, Rockford, IL) and incubated overnight at 4°C. The following day, after a nonspecific protein blocking step using bovine serum albumin (BSA) and washing, 100 μl of each serum sample (diluted 1:5 in blocking buffer) were added in duplicate wells to the plates. Following an overnight incubation at 4°C and washing, the plates were treated successively with 100 μl/well of biotinylated HDM (2 μ g/ml, prepared using Sulfo-NHS-LC-Biotinylation Kit, Pierce, Rockford, IL) and horseradish peroxidase-streptavidin (diluted 1:1500), with washes and incubation for 1 hour at room temperature between each of these steps. Finally, 100 μl/well TM Blue (Dako Corporation, Carpinteria, CA) was added as a substrate for horseradish peroxidase and reactions were allowed to develop at room temperature for at least 10 minutes. Plates were read at 650 nm by a Spectromax ELISA plate reader (Molecular Devices, Menlo Park, CA).
Bronchoalveolar Lavage and Lavage Cell Differentials
The trachea of each rat was surgically exposed, cannulated, and tied off with a silk thread suture. The right lobe was lavaged 3 times with a single volume of warmed saline (0.035 ml/kg × body wt. × 0.55). The left lung was inflated with 4% paraformaldehyde for histopathological analysis. Total white blood cell counts were obtained using a hemocytometer, and cell viability was assessed by trypan blue exclusion. Approximately 50,000 cells from each sample were centrifuged onto duplicate glass slides using a Cytospin (Shandon, Pittsburgh, PA) and stained with Diff Quik (American Scientific Inc., Sewickley, PA) for identification of eosinophils, macrophages, neutrophils and lymphocytes. At least 200 cells were counted on each slide to obtain percent values for each leukocyte subpopulation.
BALF Biochemical Analysis
BALF was centrifuged (1500 RPM, 10 minutes, 4°C) and the supernatant was analyzed. Total protein levels were determined using Coomassie Plus reagent (Pierce, Rockford, IL). The assay was adapted for automated analysis using a Cobas Fara II centrifugal spectrophotometer (Hoffman-La Roche, Branchburg, NJ). Perchloric acid (PCA) was added to a separate aliquot of BALF for antioxidant analysis at a final concentration of 3% and samples were centrifuged at 20,000 × g for 30 minutes at 4°C then stored at −80°C. Ascorbic acid (AA) analysis was performed by liquid chromatography with electrochemical detection (LCEC) as described elsewhere (Kutnink et al., Citation1987). The detection limit for AA was 0.2 nmol/ml. Cysteinyl leukotriene concentrations were measured as described in (Matthay et al., Citation1984) using an ELISA kit from Cayman Chemical (Ann Arbor, MI). BALF supernatant was analyzed for concentrations of the TH2 cytokines, IL-4 and IL-13, and the proinflammatory cytokine TNFα by ELISA using rat Cytoscreen kits purchased from Biosource International (Camarillo, CA).
Lymphocyte Proliferation Assay
Lung-associated mediastinal lymph nodes were removed from the right mainstem bronchus on days two and seven, following HDM challenge. Single-cell suspensions were prepared using ground-glass homogenizers, and HDM-specific lymphocyte proliferation was assessed in 96-well cell culture plates by measuring cellular incorporation of 3H-thymidine as described previously (Gilmour et al., Citation1996).
Statistical Analysis
The data were analyzed using a one way analysis of variance (ANOVA) model (SAS version 6.02, SAS Institute Inc., Cary, NC). Following an overall statistically significant finding, pairwise comparisons between particle treatment groups and control were performed. Significance levels were adjusted for multiple comparisons using a Bonferroni technique. In cases where the assumptions of the usual ANOVA were not met, a nonparametric procedure (Kruskal-Wallis procedure) was substituted. The level of significance was set at 0.05.
RESULTS
Pre-Sensitization Particle Exposures
Immediate Airway Response to HDM Challenge
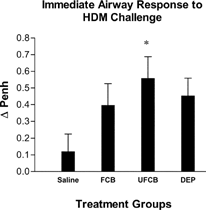
Intratracheal instillation of either UFCB particles or DEPs prior to HDM sensitization significantly enhanced the IAR to allergen challenge (). FCB particles did not produce a statistically significant rise in the allergen-mediated IAR, yet the mean value for this group was about three times higher than that for the control group.
FIG. 1Immediate airway responses to HDM (10 μ g in 250 μ l saline) challenge (by IT instillation) in BN rats exposed to saline, fine carbon black (FCB), ultrafine carbon black (UFCB), or diesel exhaust particles (DEPs) 24 hours prior to allergic sensitization. All particles were administered in a 100 μ g dose (IT) suspended in sterile saline. Baseline ventilatory readings were measured for 10 minutes prior to challenge in each rat and airway responses following challenge were averaged over a period of 20 minutes. Responses are expressed as change in arbitrary units of enhanced pause (PenH) from baseline to post-challenge. Data are presented as mean ± SEM for each treatment group (n = 5) and were analyzed for statistical differences by 1-way ANOVA. Significance was determined at p = 0.05. *Significantly different from rats exposed to saline prior to sensitization.
HDM-Specific Lymphocyte Proliferation in Pulmonary Lymph Nodes and Serum Antibody Production
Allergen-specific serum IgE and lymphoproliferative responses were measured in all rats 2 and 7 days post-challenge. Particle treatment did not significantly enhance these responses compared to control values on days 2 or 7 (). However, on day seven post-challenge, the mean lymphoproliferative response values of particle-treated groups exceeded the mean value for saline control. FCB- and DEPs-treated rats had higher average lymphoproliferative responses than UFCB-treated rats. At the same time point, the mean value for HDM-specific serum IgE was nearly 5 times higher in the FCB group than in the saline control group. This was due to 2 high responders in the FCB group, and was reflected in the variance (high standard error). UFCB-treated rats also had elevated HDM-specific serum IgE, but again, the increase was not statistically significant.
HDM-specific lymphocyte proliferation and HDM-specific serum IgE production 2 and 7 days after HDM challenge
Pulmonary Inflammation, BALF Biochemistry, and Lung Injury
Pulmonary inflammatory responses were measured 2 and 7 days after allergen challenge to understand the kinetics of lung injury. Instillation of particle suspensions prior to sensitization increased leukocyte influx into the BALF on day 2 compared to saline controls (). Of these increases, only the DEPs-exposed rats demonstrated a significant change in total numbers of eosinophils, neutrophils, and lymphocytes. By far, the cellular inflammatory response was dominated by influx of eosinophils on day 2. On day 7, the only indication of continued cellular inflammation was a significantly greater number of macrophages in the BALF of DEPs-exposed rats compared to saline-exposed rats. Histopathological analyisis of the left lung lobes showed many hallmarks of allergic lung disease including peribronchiolar and perivascular eosinophil and lymphocyte accumulation, and edema. Semiquantitative scoring suggested these effects were stronger in the DEPs-exposed rats although no statistical analyses were performed on the graded scores (data not shown).
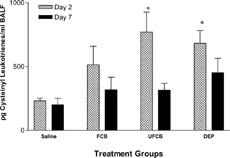
While all particle treatments appeared to increase total protein in the BALF, the only significant increases were in DEPs-exposed rats on day 2, and by day 7, total protein levels in all groups were equivalent to sensitized saline controls (). Cysteinyl leukotrienes, measured as an indicator of bronchial smooth muscle stimulatory activity, were significantly elevated on day 2 in the BALF of UFCB- and DEPs- but not FCB-treated rats. By day 7, these levels were not significantly different from sensitized saline control levels ().
FIG. 2Total protein concentrations in bronchoalveolar lavage fluid (BALF) of BN rats exposed to saline, fine carbon black (FCB), ultrafine carbon black (UFCB), or diesel exhaust particles (DEPs) 24 hours prior to allergic sensitization. Total protein levels were measured on post-challenge days 2 and 7. All particles were administered in a 100 μg dose (IT) suspended in sterile saline. Data are presented as mean ± SEM for each treatment group (n = 5) and were analyzed for statistical differences by 1-way ANOVA. Significance was determined at p = 0.05. *Significantly different from rats within the same timepoint exposed to saline prior to sensitization.
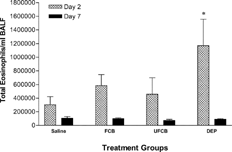
FIG. 3Cysteinyl leukotriene concentration in bronchoalveolar lavage fluid (BALF) of BN rats exposed to saline, fine carbon black (FCB), ultrafine carbon black (UFCB), or diesel exhaust particles (DEPs) 24 hours prior to allergic sensitization. Cysteinyl leukotrienes levels were measured on post-challenge days 2 and 7. All particles were administered in a 100 μg dose (IT) suspended in sterile saline. Data are presented as mean ± SEM for each treatment group (n = 5) and were analyzed for statistical differences by 1-way ANOVA. Significance was determined at p = 0.05. *Significantly different from rats within the same timepoint exposed to saline prior to sensitization.
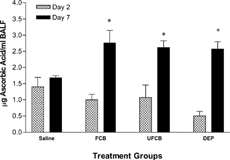
As a measure of antioxidant capacity either in response to injury or as a marker of protection, ascorbic acid (AA) was measured in the BALF. No differences among the groups were observed on post-challenge day 2 (); however, on day 7, AA levels in DEPs-, FCB-, and UFCB-exposed rats were significantly increased compared to saline control.
FIG. 4Ascorbic acid concentrations in bronchoalveolar lavage fluid (BALF) of BN rats exposed to saline, fine carbon black (FCB), ultrafine carbon black (UFCB), or diesel exhaust particles (DEPs) 24 hours prior to allergic sensitization. Ascorbic acid levels were measured on post-challenge days 2 and 7. All particles were administered in a 100 μg dose (IT) suspended in sterile saline. Data are presented as mean ± SEM for each treatment group (n = 5) and were analyzed for statistical differences by 1-way ANOVA. Significance was determined at p = 0.05. *Significantly different from rats within the same timepoint exposed to saline prior to sensitization.
Cellular inflammation in bronchoalveolar lavage fluid 2 and 7 days after HDM challenge
Cytokine Concentrations in BALF
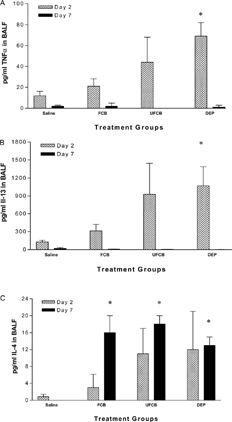
Only DEPs significantly increased TNFα concentrations in the BALF compared to saline on day 2. Concentrations of TNFα on day 7 were very low or undetectable in all particle-exposed groups (). Similarly, IL-13 levels on post-challenge day 2 were significantly elevated above saline control levels only in DEPs-exposed rats, while on day 7, IL-13 concentrations were nearly undetectable across all treatment groups and controls (). BALF concentrations of IL-4, another TH2 cytokine, did not demonstrate this pattern. All 3 particle-exposed groups had significantly greater amounts of IL-4 in the BALF compared to saline-treated sensitized controls on day 7. There were no significant differences among the groups on day 2, despite some high responders in the UFCB- and DEPs-exposed groups ().
FIG. 5Pro-inflammatory and TH2 cytokines in bronchoalveolar lavage fluid (BALF) of BN rats exposed to saline, fine carbon black (FCB), ultrafine carbon black (UFCB), or diesel exhaust particles (DEPs) 24 hours prior to allergic sensitization. (A) TNFα, (B) IL-13, and (C) IL-4 levels were measured on post-challenge days 2 and 7. All particles were administered in a 100 μg dose (IT) suspended in sterile saline. Data are presented as mean ± SEM for each treatment group (n = 5) and were analyzed for statistical differences by 1-way ANOVA. Significance was determined at p = 0.05. * Significantly different from rats within the same timepoint exposed to saline prior to sensitization.
Pre-Challenge Particle Exposures
IAR, Pulmonary Eosinophils, BALF Leukotriene Levels, and Lung Injury
HDM-sensitized BN rats exposed to FCB, UFCB, or DEPs prior to allergen challenge did not show significant changes in HDM-induced IAR compared to saline controls (), unlike when particles were administered prior to sensitization (). Also, there were no significant differences in total numbers of eosinophils, total protein, or cysteinyl leukotriene levels in the BALF of particle-exposed rats compared to saline-exposed rats ().
Lung responses to allergen in sensitized rats exposed to particles 24 hours prior to HDM challenge
DISCUSSION
Several reports have demonstrated that, in addition to diesel exhaust particles, simple elemental carbon particles display adjuvant activities important in the promotion of allergies and asthma. The purpose of this study was to compare the adjuvant effects of different-sized carbon particles to a well-characterized DEPs sample in a pulmonary allergic sensitization model. The results demonstrate many significant effects in BN rats exposed to a relatively low dose of DEPs (100 μg), and fewer increases in responses with the two different sized CB particles as summarized below: 1) DEPs exposure 24 hours prior to HDM sensitization produced the greatest number of significantly increased responses across all endpoints measured; 2) adjuvant effects were seen with both fine and ultrafine CB particles, but fewer increased responses reached statistical significance; 3) enhanced responses were confined to the lung with no significant alterations of allergen-specific serum IgE for any of the treatment groups; and, 4) exposure to DEPs or CB particles 24 hours prior to allergen challenge did not significantly alter pulmonary allergic responses in sensitized rats. These results suggest that chemical composition is a stronger determinant of adjuvant activity than particle size, and that the timing of particle exposure in relation to allergen sensitization and challenge is important.
Although the exact mechanisms by which elemental carbon produces adjuvant effects are not clear, it has been proposed that mucosal inflammation is a prerequisite (Granum and Lovik, Citation2002). Since UFCB has been shown to cause increased inflammation compared to FCB (Li et al., Citation1999), we reasoned that the smaller particles would be a more potent adjuvant. Preliminary instillation experiments, however, showed that 100 μg of each of the three samples caused mild, statistically nonsignificant differences in pulmonary edema, LDH release, and cell numbers 24 hours after exposure. Despite these modest and equivocal changes, the samples induced, on a mass basis, quite different levels of adjuvant activity across the measured responses with DEPs > > UFCB > FCB. This would suggest that the PAHs or other chemicals in the DEPs augmented the adjuvant effects over and above the carbon core, and that the enhanced activity was not directly related to particle size or the initial pro-inflammatory activity after particle exposure. Although no studies until now have reported the effects of ultrafine particles on allergic sensitization in vivo, fine and coarse ambient PM samples from several different regions in Europe had equivalent adjuvant activity when administered intranasally during OVA sensitization in mice (Steerenberg et al., Citation2004). Using the same PM samples in a murine footpad immunization model, it was further shown that coarse or fine fractions of ambient PM with OVA did not consistently change serum IgE levels or TH2 cytokine production in draining lymph nodes (Nygaard et al., Citation2005). In our study, the CB-treated animals displayed much larger variability in responses compared to saline instilled controls or, indeed, the DEPs-treated rats, indicating that this apparently milder treatment may have enhanced sensitization in some, but not all, of the animals in the CB treatment groups.
It is quite well-established that components of DEPs, such as polycyclic aromatic hydrocarbons (PAHs) and their quinone derivatives generate reactive oxygen species (ROS) that, in turn, lead to the activation of cytokines and chemokines. These signaling molecules are then available to trigger and regulate TH2 cytokine production and allergic inflammation in the lung (Nel et al., Citation2001). In addition, organic rich DEPs can induce production of mediators of allergic lung disease, such as IL-5; this was observed in the lung lavage fluid of DEPs-instilled mice in the absence of allergen (Singh et al., Citation2004). Although ROS were not measured in this study, they may have played a more significant role in the DEPs' adjuvant effects due to the presence of PAHs. To support this notion, ultrafine ambient particles that contain higher levels of organics have been shown to produce greater amounts of ROS than fine or coarse ambient particles (Li et al., Citation2003). Future efforts to investigate the activity of ambient ultrafine particles that are rich in organic compounds are warranted.
The BN rat model of sensitization to HDM has been a useful surrogate for testing the adjuvant effects of a variety of different air pollutants on the development of allergic asthma (Gilmour et al., Citation1996; Lambert et al., Citation1999, Citation2000) and provides information on both immune and airway responses. Examination of these responses at 2 and 7 days post-challenge allows assessment of primary immune-mediated responses and the development of secondary, specific immune responses, respectively. The results of this study clearly show that DEPs produced many more significant effects at the day 2 time point, while effects with the CB samples were limited to changes in BALF IL-4 and anti-oxidant activity at the day 7 timepoint and additionally enhanced IAR and cysteinyl leukotrienes with UFCB. Measurement of the IAR, pulmonary inflammation and cysteinyl leukotrienes, which have a role in bronchial hyperresponsiveness and allergic airways disease (Wardlaw et al., Citation1989; Mitsunobu et al., Citation2000, Citation2001), provide endpoints which are characteristic of asthma in humans. The model provides a link between these endpoints and the immunological mediators that are suggestive of mechanisms for increased disease.
In addition to investigating the effects of particles on allergic sensitization, this study was also designed to investigate whether particle exposure would affect responses to a subsequent allergen challenge 24 hours later. Although a trend of enhanced responses in particle-exposed rats occurred, saline-administered allergic rats had unusually elevated responses (compared to other studies performed in our laboratory), which may have masked particle-induced effects and prevents us from drawing definitive conclusions with respect to this question.
In summary, our comparison of the adjuvant activity of three carbon-rich PM samples on an equal mass basis indicates that, at a low dose of 100 μg, all 3 particle samples could enhance allergic sensitization as measured by subsequent airway responses to antigen challenge, however, the effects were more pronounced with DEPs than with UFCB and FCB. These differences did not appear to be related to differences in particle size or pro-inflammatory activity. The presence of PAHs in the DEPs may have been responsible for their increased adjuvant activity, possibly via the production of ROS. The effects of DEPs on allergic sensitization were more evident in the primary immune responses, whereas the effects of FCB were more evident at the 7-day (secondary response) timepoint. Achieving more accurate hazard identification based on relationships between particle composition and adjuvant effects will require further study of the threshold doses and underlying cell signaling events that enhance immune mediated diseases such as allergic asthma.
This manuscript does not reflect EPA policy.
REFERENCES
- Al-Humadi N. H., Siegel P. D., Lewis D. M., Barger M. W., Ma J. Y., Weissman D. N., Ma J. K. The effect of diesel exhaust particles (DEP) and carbon black (CB) on thiol changes in pulmonary ovalbumin allergic sensitized Brown Norway rats. Exp. Lung Res. 2002; 28: 333–349, [PUBMED], [INFOTRIEVE], [CSA]
- Casillas A. M., Hiura T., Li N., Nel A. E. Enhancement of allergic inflammation by diesel exhaust particles: Permissive role of reactive oxygen species. Ann. Allergy Asthma Immunol. 1999; 83: 624–629, [PUBMED], [INFOTRIEVE], [CSA]
- Devouassoux G., Saxon A., Metcalfe D. D., Prussin C., Colomb M. G., Brambilla C., Diaz-Sanchez D. Chemical constituents of diesel exhaust particles induce IL-4 production and histamine release by human basophils. J. Allergy Clin. Immunol. 2002; 109: 847–853, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Diaz-Sanchez D. The role of diesel exhaust particles and their associated polyaromatic hydrocarbons in the induction of allergic airway disease. Allergy 1997; 52: 52–56, discussion 57–58[PUBMED], [INFOTRIEVE], [CSA]
- Fujimaki H., Saneyoshi K., Shiraishi F., Imai T., Endo T. Inhalation of diesel exhaust enhances antigen-specific IgE antibody production in mice. Toxicology 1997; 116: 227–233, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Gilmour M. I., Park P., Selgrade M. K. Increased immune and inflammatory responses to dust mite antigen in rats exposed to 5 ppm NO2. Fundam. Appl. Toxicol. 1996; 31: 65–70, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Granum B., Lovik M. The effect of particles on allergic immune responses. Toxicol. Sci. 2002; 65: 7–17, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Hamelmann E., Schwarze J., Takeda K., Oshiba A., Larsen G. L., Irvin C. G., Gelfand E. W. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 1997; 156: 766–775, [PUBMED], [INFOTRIEVE], [CSA]
- Hashimoto K., Ishii Y., Uchida Y., Kimura T., Masuyama K., Morishima Y., Hirano K., Nomura A., Sakamoto T., Takano H., Sagai M., Sekizawa K. Exposure to diesel exhaust exacerbates allergen-induced airway responses in guinea pigs. Am. J. Respir. Crit. Care Med. 2001; 164: 1957–1963, [PUBMED], [INFOTRIEVE], [CSA]
- Heinrich J., Wichmann H. E. Traffic-related pollutants in Europe and their effect on allergic disease. Curr. Opin. Allergy Clin. Immunol. 2004; 4: 341–348, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ichinose T., Takano H., Sadakane K., Yanagisawa R., Kawazato H., Sagai M., Shibamoto T. Differences in airway-inflammation development by house dust mite and diesel exhaust inhalation among mouse strains. Toxicol. Appl. Pharmacol. 2003; 187: 29–37, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kutnink M. A., Hawkes W. C., Schaus E. E., Omaye S. T. An internal standard method for the unattended high-performance liquid chromatographic analysis of ascorbic acid in blood components. Anal. Biochem. 1987; 166: 424–430, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Lambert A. L., Dong W., Selgrade M. K., Gilmour M. I. Enhanced allergic sensitization by residual oil fly ash particles is mediated by soluble metal constituents. Toxicol. Appl. Pharmacol. 2000; 165: 84–93, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Lambert A. L., Dong W., Winsett D. W., Selgrade M. K., Gilmour M. I. Residual oil fly ash exposure enhances allergic sensitization to house dust mite. Toxicol. Appl. Pharmacol. 1999; 158: 269–277, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Lambert A. L., Winsett D. W., Costa D. L., Selgrade M. K., Gilmour M. I. Transfer of allergic airway responses with serum and lymphocytes from rats sensitized to dust mite. Am. J. Respir. Crit. Care Med. 1998; 157: 1991–1999, [PUBMED], [INFOTRIEVE], [CSA]
- Li N., Sioutas C., Cho A., Schmitz D., Misra C., Sempf J., Wang M., Oberley T., Froines J., Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003; 111: 455–460, [PUBMED], [INFOTRIEVE], [CSA]
- Li X. Y., Brown D., Smith S., MacNee W., Donaldson K. Short-term inflammatory responses following intratracheal instillation of fine and ultrafine carbon black in rats. Inhal. Toxicol. 1999; 11: 709–731, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Martin J. G., Xu L. J., Toh M. Y., Olivenstein R., Powell W. S. Leukotrienes in bile during the early and the late airway responses after allergen challenge of sensitized rats. Am. Rev. Respir. Dis. 1993; 147: 104–110, [PUBMED], [INFOTRIEVE]
- Matthay M. A., Eschenbacher W. L., Goetzl E. J. Elevated concentrations of leukotriene D4 in pulmonary edema fluid of patients with the adult respiratory distress syndrome. J. Clin. Immunol. 1984; 4: 479–483, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Mitsunobu F., Mifune T., Hosaki Y., Ashida K., Tsugeno H., Okamoto M., Harada S., Takata S., Tanizaki Y., Harada M. Enhanced peripheral leukocyte leukotriene production and bronchial hyperresponsiveness in asthmatics. Eur. Respir. J. 2000; 16: 504–508, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Mitsunobu F., Mifune T., Hosaki Y., Ashida K., Tsugeno H., Okamoto M., Takata S., Tanizaki Y. Enhanced production of leukotrienes by peripheral leukocytes and specific IgE antibodies in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2001; 107: 492–498, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Miyabara Y., Takano H., Ichinose T., Lim H. B., Sagai M. Diesel exhaust enhances allergic airway inflammation and hyperresponsiveness in mice. Am. J. Respir. Crit. Care Med. 1998; 157: 1138–1144, [PUBMED], [INFOTRIEVE], [CSA]
- Nel A. E., Diaz-Sanchez D., Li N. The role of particulate pollutants in pulmonary inflammation and asthma: Evidence for the involvement of organic chemicals and oxidative stress. Curr. Opin. Pulmon. Med. 2001; 7: 20–26, [CROSSREF]
- Nygaard U. C., Alberg T., Bleumink R., Aase A., Dybing E., Pieters R., Lovik M. Ambient air particles from four European cities increase the primary cellular response to allergen in the draining lymph node. Toxicology 2005; 207: 241–254, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Pietropaoli A. P., Frampton M. W., Hyde R. W., Morrow P. E., Oberdorster G., Cox C., Speers D. M., Frasier L. M., Chalupa D. C., Huang L. S., Utell M. J. Pulmonary function, diffusing capacity, and inflammation in healthy and asthmatic subjects exposed to ultrafine particles. Inhal. Toxicol. 2004; 16(Suppl 1)59–72, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Proietti L., Spicuzza L., Polosa R. Urban air pollution at the crossroads of the allergic pandemic. Ann. Ital. Med. Int. 2003; 18: 64–72, [PUBMED], [INFOTRIEVE], [CSA]
- Singh P., Daniels M., Winsett D. W., Richards J., Doerfler D., Hatch G., Adler K. B., Gilmour M. I. Phenotypic comparison of allergic airway responses to house dust mite in three rat strains. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003; 284: L588–598, [PUBMED], [INFOTRIEVE], [CSA]
- Singh P., DeMarini D. M., Dick C. A., Tabor D. G., Ryan J. V., Linak W. P., Kobayashi T., Gilmour M. I. Sample characterization of automobile and forklift diesel exhaust particles and comparative pulmonary toxicity in mice. Environ. Health Perspect. 2004; 112: 820–825, [PUBMED], [INFOTRIEVE], [CSA]
- Steerenberg P. A., Withagen C. E., van Dalen W. J., Dormans J. A., Cassee F. R., Heisterkamp S. H., van Loveren H. Adjuvant activity of ambient particulate matter of different sites, sizes, and seasons in a respiratory allergy mouse model. Toxicol. Appl. Pharmacol. 2004; 200: 186–200, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Tsien A., Diaz-Sanchez D., Ma J., Saxon A. The organic component of diesel exhaust particles and phenanthrene, a major polyaromatic hydrocarbon constituent, enhances IgE production by IgE-secreting EBV-transformed human B-cells in vitro. Toxicol. Appl. Pharmacol. 1997; 142: 256–263, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Wardlaw A. J., Hay H., Cromwell O., Collins J. V., Kay A. B. Leukotrienes, LTC4 and LTB4, in bronchoalveolar lavage in bronchial asthma and other respiratory diseases. J. Allergy Clin. Immunol. 1989; 84: 19–26, [PUBMED], [INFOTRIEVE], [CSA]
- Xia T., Korge P., Weiss J. N., Li N., Venkatesen M. I., Sioutas C., Nel A. Quinones and aromatic chemical compounds in particulate matter induce mitochondrial dysfunction: Implications for ultrafine particle toxicity. Environ. Health Perspect. 2004; 112: 1347–1358, [PUBMED], [INFOTRIEVE], [CSA]