Abstract
Sodium methyldithiocarbamate (SMD) is an agricultural fumigant-type pesticide commonly used as a pre-plant biocide. SMD is currently listed by the US Environmental Protection Agency (EPA) as the third most commonly used conventional pesticide. Previously, SMD has been shown to be immunotoxic in mice. Initial immunotoxicological studies indicated that thymocytes are major targets of SMD or its breakdown products in mice. The purpose of the present study was to determine if this effect was mediated by an SMD-induced stress response. The decrease in thymus weight correlated to a decrease in all thymocyte subpopulations. However, as seen in earlier studies, the double positive (CD4+CD8+) thymocyte subpopulation was more selectively decreased than the other subpopulations. The double negative (CD4−CD8−) and single positive (CD4+CD8− or CD4−CD8+) thymocyte subpopulations decreased in absolute numbers while increasing in percentage of the remaining cells in the thymus after SMD intoxication. In the current study, SMD caused an increase in serum corticosterone, a stress-related hormone. Blocking corticosterone either by 1) adrenalectomy or by 2) chemically blocking synthesis of nascent corticosterone in adrenal-competent animals abrogated the thymocyte atrophy caused by SMD. However, an additional stressor (restraint) did not act additively or synergistically to increase atrophy. Therefore, it is likely that SMD causes thymic atrophy by increasing serum corticosterone.
INTRODUCTION
Sodium methyldithiocarbamate (SMD) is a soil fumigant that is commercially used for the eradication of nematodes, bacteria, fungi, and other life-forms. SMD was listed by the U.S. Environmental Protection Agency (EPA) as the third most commonly used conventional pesticide in 2001 (by weight) with approximately 60 million pounds used (Anonymous, Citation2004). The second most commonly used pesticide, methyl bromide, has been phased out by the EPA due to reports of ozone depletion. Public concerns over methyl bromide use prompted the EPA to encourage increased use of SMD as an alternative to methyl bromide (Sauerhoff, Citation1998; CitationAnonymous, 2002). Increased SMD usage could allow SMD to increase to the first or second most commonly used conventional pesticide in the United States. With increased use of SMD, the risk of exposure to the general public increases proportionally.
There have been several reported human exposures to SMD both in peer-reviewed literature and in the popular press. The most notable reported exposure was in Dunsmuir, California. On July 14, 1991, a train carrying ∼ 19,000 gallons of a 32% solution of SMD derailed near Dunsmuir and spilled SMD into the Sacramento River (1991; Koehler and Van Ness, 1993; Bowler et al., 1994; Cone et al., 1994; Koo et al., 1995; Kreutzer et al., 1996; Hanley, 1999). Many residents in Dunsmuir and other nearby towns were exposed to high levels of SMD and its major volatile breakdown product, methylisothiocyanate (MITC). For up to one week following the accident, residents reported a rotten egg/sulfur smell, indicative of sulfur compounds from SMD breakdown. After the incident, 705 people sought medical care for health problems with symptoms including: headache (64%), nausea (46%), eye irritation/blurring of vision (40%), dizziness (30%), shortness of breath (27%), and diarrhea (25%) (Bowler et al., Citation1994). Furthermore, there have been several other reports of SMD exposures. Improper application of SMD in agricultural settings caused the evacuation of several small towns and also elementary schools that were downwind from sites of SMD application (Hanley, Citation1999; Maxwell, Citation1999a, Citation1999b).
Although no lymphocyte counts have been reported from SMD-exposed people, lymphocyte populations were modulated in rodent models of SMD exposure. Initial immunotoxicological studies in mice indicated that major targets of SMD or its breakdown products were thymocytes (Pruett et al., Citation1992a). The decrease in thymus weight correlated directly to a decrease in all thymocyte subpopulations. Most notably, the double positive (DP; CD4+CD8+) thymocytes decreased in both percentage and total number in the thymus after in vivo exposure to SMD. SMD also depleted the double negative (DN; CD4−CD8−) and single positive (SP; CD4+CD8− or CD4−CD8+) thymocytes, but to a lesser extent than the DP thymocytes (Pruett et al., Citation1992a).
Thymic atrophy had been commonly linked to increases in serum corticosterone. Several models of thymic atrophy including chemical exposures (ethanol, propanil, and morphine) and neurogenic/psychogenic stress (restraint stress) indicated that stress-induced increases in serum corticosterone caused thymic atrophy (Saad and Jerrells, Citation1991; Freier and Fuchs, Citation1993; Zhao et al., Citation1995; Tarcic et al., Citation1998). Further, this increase in corticosterone could be linked to the depletion in DP thymocytes. DP thymocytes, which were in the process of thymic selection, were very sensitive to increases in either serum corticosterone or synthetic glucocorticoids (Screpanti et al., Citation1989; Sei et al., Citation1991; Fuchs and Pruett, Citation1992; Jondal et al., Citation1993; Chow et al., Citation1997; Groves et al., Citation1997). It seemed likely that SMD would cause stress-induced increases in serum corticosterone and that this might be responsible for general thymic atrophy with strong selective depletion of DP thymocytes.
This study was performed to elucidate the mechanism by which SMD caused thymic atrophy. SMD caused an increase in serum corticosterone, a stress-related hormone. Since increases in serum corticosterone had been associated with thymic atrophy, increases in serum corticosterone were blocked by 1) adrenalectomy and 2) inhibition of synthesis of nascent corticosterone in adrenal-competent animals. Both methods completely abrogated SMD-mediated thymic atrophy. Therefore, it was likely that SMD caused thymic atrophy by increasing serum corticosterone.
MATERIALS AND METHODS
Animals and Treatment
All experiments involving mice were conducted in accordance with the National Institutes of Health standards for care and use of experimental animals and the Louisiana State University Health Sciences Center Institutional Animal Care and Use Committee (IACUC). All mice were obtained Charles River Labs (Wilmington, MA) via the National Cancer Institute's animal program. Female B6C3F1 (C57BL/6 × C3H, F1 generation) mice were purchased at 6–8 weeks of age and quarantined for 2 weeks to allow them to recover from shipping stress before being used in experiments at approximately 8–12 weeks of age. Animals were group-housed, maintained on a 12-hour light/dark cycle, and had access to standard rodent chow and water ad libitum. Adrenalectomized (ADX) and sham-ADX mice were obtained after surgery (performed by Charles River Labs). ADX mice were housed identically to other mice except that they were given 0.9% saline drinking water to maintain physiological salt balance.
SMD-Preparation and Administration
SMD was purchased in crystalline form at a purity > 98% (Catalog #PS-221, ChemService, Westchester, PA). The SMD dosage varied between studies from 300, 200, and 100 mg/kg as indicated in the Results section. The SMD was diluted in distilled, endotoxin-free water (Sigma, St. Louis, MO) and administered in 0.2 ml volumes by oral gavage using an 18-gauge stainless steel gavage needle with a rounded tip. All SMD solutions were made immediately before administration.
Restraint Stress
Mice were restrained for 2 hours (9:00 PM–11:00 PM) in a conical 50 mL centrifuge tube containing a 2-centimeter lengthwise slit for ventilation. After 2 hours the mice were released into their cages.
Administration of Aminoglutethimide
Aminoglutethimide (Sigma, St. Louis, MO) was administered subcutaneously with a 22-gauge needle in a volume of 0.2 ml/mouse. The dosage was 30 mg/kg, and the aminoglutethimide was dissolved in trace amounts of dimethyl sulfoxide (60 μl per 7.5 mg of aminoglutethimide in a total volume of 1.2 ml) in food grade corn oil as a vehicle. The oil was sterile filtered (0.22 μm Steriflip, Millipore Corp., MI) before use. Mice treated with SMD received the vehicle for aminoglutethimide to control for possible vehicle effects.
Organ Cell Counts and Flow Cytometry
Mice were asphyxiated in 100% CO2, and spleens and thymi were removed. Single cell suspensions were washed once by centrifugation before resuspension and enumeration using a Coulter Z1 electronic particle counter (Coulter Corp., Miami, Florida). Cells were adjusted to 106/ml and resuspended in 100 μl of FACS buffer (phosphate-buffered saline without calcium or magnesium, 0.1% fatty acid-free bovine serum albumin, 0.1% sodium azide). Lymphocytes were stained with fluorescent-labeled antibodies for the following cell surface markers: CD3ε-PE, CD4-FITC, CD8α-cychrome, B220-PE, and DX5-PE (BD Pharmingen, San Diego, CA). Splenocytes were blocked for nonspecific binding with antibodies against CD16/CD32. Cellular subpopulations were analyzed by flow cytometry (FacScan, Becton Dickinson). Nonspecific labeling (based on the isotype control cells) was less than 1% for all samples.
Corticosterone Assay
Serum corticosterone was measured using a commercial corticosterone radioimmunoassay (Coat-A-Count Rat Corticosterone kit, Diagnostic Products Corporation, Los Angeles, CA) as previously described (Myers et al., Citation2001). Briefly, a standard curve was prepared in duplicate using corticosterone at concentrations of 1000, 500, 200, 50, and 0 ng/ml. Twenty-five μ l of serum or standard was pipetted into precoated tubes. One thousand μ l of 125I-corticosterone was added to each tube and incubated at room temperature for 2 hours. All liquid was decanted and tubes were blotted dry before reading on a gamma counter (Wallac 1470, Perkin-Elmer, Gaithersburg, MD). Sample concentrations were calculated from the standard curve and the r2 values for the standard curve were > 0.98 for all experiments.
Statistics
Data were analyzed by ANOVA with Bonferroni's posttest using Prism 3.0 software (GraphPad Software, Inc., San Diego, CA). Data is presented as the mean ± the standard deviation.
RESULTS
SMD-Depleted Thymocytes in Mice
SMD (300 mg/kg) was administered in vivo either as a single dosage (1×) on day 1 or 1 dose each day for 3 consecutive days (3×). Thymocytes and splenocytes were then analyzed for alterations in thymic cellularity and subpopulations on day 4. As reported previously (Pruett et al., Citation1992a), SMD caused a depletion of thymocytes, including a depletion of DP thymocytes. SMD caused depletion of thymocytes whether administered 1× or 3×. Only DP thymocytes were depleted in mice treated once (1×) or three times (3×) with SMD at 300 mg/kg (). The sensitivity of DP thymocytes (CD4+CD8+) was notable because a single dose of SMD suppressed the total DP thymocyte cell count by 50%. Further, the other populations of thymocytes were not significantly suppressed. This was important because it identified the DP thymocytes as being more sensitive to SMD. Unlike thymic subpopulations, splenic subpopulations were not significantly altered by either a single or multiple administrations of SMD ().
TABLE 1 Thymocyte and splenocyte subpopulation percentages and cell counts after SMD exposure
SMD-Depleted Thymocytes in a Dose-Dependent Manner
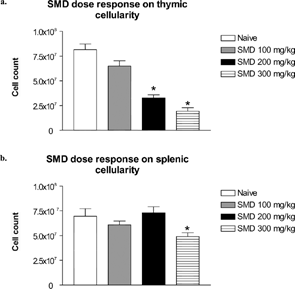
Mice were treated once each day with SMD at a concentration of 100, 200, or 300 mg/kg for 3 days. On day 4, samples were collected for analysis. Thymic cellularity decreased in a dose-dependent manner with maximum atrophy at the SMD concentration of 300 mg/kg (). Splenocytes were significantly decreased in mice treated with SMD at 300 mg/kg. SMD at a dosage of 200 or 100 mg/kg, however, did not alter splenic cellularity ().
FIG. 1 Dose-response effect of SMD on thymic and splenic cellularity. Mice were administered SMD orally at 9:00 PM daily for 3 days. On day 4 thymi (a) and spleens (b) were collected and cellularity was determined. N = 5 mice per group. Asterisks (*) indicate significant difference from naïve control (p < 0.05).
SMD-Induced Increases in Serum Corticosterone
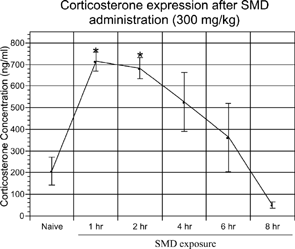
It was speculated that SMD depleted thymocytes by an indirect mechanism induced by an increase in serum corticosterone, which has been reported with other chemicals (Pruett et al., Citation1992b, Citation2000; Han et al., Citation1993). In order to examine the indirect effects of SMD on the immune system, a time course experiment was performed. The mice were dosed with SMD (300 mg/kg) and blood serum samples were collected at various times after SMD administration. Mice were administered SMD in order to sacrifice all groups at the same time (2:00 PM). Serum corticosterone from naïve mice was significantly lower when compared to serum corticosterone at 1 hour or 2 hours post-SMD administration (). However, the value for naïve mice was not significantly different from the values for mice treated with SMD 4 hours, 6 hours, or 8 hours earlier. Serum corticosterone began decreasing at 4 and 6 hours and approached a baseline level. At 8 hours post-SMD intoxication, serum corticosterone levels had dropped slightly below naïve corticosterone levels and significantly different from the values at 1 hour, 2 hours, or 4 hours after SMD treatment. Although the values at 4 and 6 hours were not significantly different from naïve animals, the levels of serum corticosterone were above baseline levels. Therefore, it appeared that a single administration of SMD at 300 mg/kg caused an increase in serum corticosterone, indicative of a stress response, which returned to baseline levels by 6–8 hours post-SMD administration.
FIG. 2 SMD-increased serum corticosterone in mice. Mice were administered SMD (300 mg/kg) orally at 9:00 PM. After 1, 2, 4, 6, or 8 hours, trunk blood was collected and serum was isolated for corticosterone analysis by RIA. N = 5 mice per group. Asterisks (*) indicate significant difference from naïve control (p < 0.05).
Thymic Atrophy Was Prevented by Adrenalectomy
Since SMD caused an increase in serum corticosterone, it was likely that SMD induced thymic atrophy by increasing serum corticosterone. To test this hypothesis, the effects of increased serum corticosterone on thymocytes were eliminated by using ADX mice. ADX mice were incapable of producing high levels of serum corticosterone in response to stressors. Furthermore, ADX mice were also known to be more sensitive to toxic compounds than normal mice due to the abrogated corticosterone production. Therefore, the dosage of SMD for the ADX mice was reduced from 300 mg/kg to 200 mg/kg.
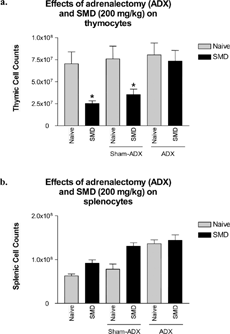
As in the previous experiment, SMD administration to wild-type animals (both normal animals or animals with sham adrenalectomies) decreased thymocyte cell counts, which was significantly decreased compared to naïve, naïve ADX, or naïve sham-ADX groups ().
FIG. 3 SMD did not cause thymic atrophy in adrenalectomized mice. Adrenalectomized mice were administered SMD (200 mg/kg) orally at 9:00 PM on day 1 only. On day 3, thymi and spleens were removed and analyzed for alterations in cellularity. Corticosterone levels were analyzed for all samples. Two samples (each from a different group) were omitted due to the presence of serum corticosterone, indicative of adrenal regeneration. N = 5 mice per group. Asterisks (*) indicate significant difference from naïve control (p < 0.05).
Serum corticosterone levels were analyzed for each sample. Serum corticosterone levels were undetectable in all ADX animals used for analysis (data not shown). Two samples (one from adrenalectomy/control and one from adrenalectomy/SMD) had detectable serum corticosterone levels. These two samples were omitted from flow cytometric analysis due to the presence of serum corticosterone, which suggested that adrenalectomy was incomplete. The sample size for these two groups was 4, whereas the other group sizes were 5.
The most significant finding of these experiments was that SMD treatment in ADX mice did not cause atrophy. Thymic cell counts in ADX mice treated with SMD were not significantly different from naïve mice. This experiment clearly demonstrated that removing the adrenals, which produce most of the corticosterone detected in serum, completely abrogated the effects of SMD on thymocytes.
Thymic Atrophy Was Prevented by Aminoglutethimide
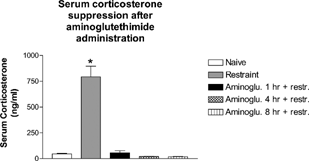
In order to further clarify the mechanism of action of SMD-induced thymic atrophy, a chemical inhibitor of corticosterone synthesis, aminoglutethimide, was used. To verify that the dosage of aminoglutethimide was sufficient to block corticosterone upregulation after the chemical stressor, a time-course study was completed over an 8-hour period. Mice were pretreated with aminoglutethimide up to 8 hours before restraint for 1 hour. Mice were sampled immediately after restraint to determine if aminoglutethimide suppressed stress-induced corticosterone production (). Restraint-stressed mice had significantly more serum corticosterone compared to naïve mice and to mice treated with aminoglutethimide 1, 4, or 8 hours before restraint. This indicated that during the time that SMD caused an induction of serum corticosterone (4–6 hours post-SMD administration), aminoglutethimide could completely block the up-regulation of corticosterone. Therefore, it was likely that aminoglutethimide would block increases in serum corticosterone up-regulation following SMD treatment.
FIG. 4 Aminoglutethimide blocked serum corticosterone for up to 8 hours in vivo. Mice were administered aminoglutethimide for 8, 4, and 1 hour. One hour before collection of serum, all mice (except naïve) were restrained to induce a stress response. Trunk blood was collected at 9:00 AM and serum was isolated for analysis of corticosterone by RIA. N = 5 mice per group. Asterisks (*) indicate significant difference from naïve control (p < 0.05).
To study the role of corticosterone on SMD-induced thymic atrophy, mice were pretreated with aminoglutethimide for 1 hour before SMD treatment to allow for distribution of the inhibitor in vivo (). As expected, aminoglutethimide completely blocked the thymic atrophy caused by SMD. Thymus cellularity in mice dosed with aminoglutethimide and SMD was significantly different than in mice treated with SMD alone. Thymic cellularity in naïve and aminoglutethimide control mice was also significantly different from the SMD-treated group. Further, percentages of T-cells (CD4+ and CD8+), B-cells (B220+), and NK cells (DX5+) in the spleen of aminoglutethimide-treated mice were not significantly altered ( and data not shown). Because SMD did not alter the percentages of splenic cells to a great extent at this time point (1 day after the last SMD treatment), it was not surprising that mice treated with SMD and aminoglutethimide had normal populations of lymphocytes in the spleen.
FIG. 5 SMD did not cause thymic atrophy in combination with aminoglutethimide. Mice were pretreated with aminoglutethimide 1 hour before SMD administration. SMD was administered (200 mg/kg) daily for 3 days. On day 4, thymi (a) and spleens (b) were collected and analyzed for alterations in cellularity. N= 5 mice per group. Asterisks (*) indicate significant difference from naïve control (p < 0.05).
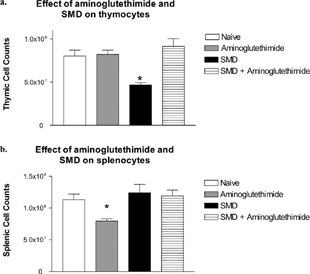
TABLE 2 Aminoglutethimide blocks alterations in thymic subpopulations caused by SMD
Aminoglutethimide Blocked the Up-Regulation of Corticosterone by SMD
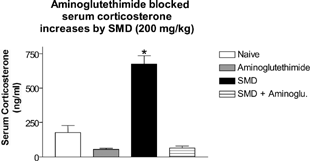
Previous experiments analyzing the role of corticosterone in SMD-induced thymic atrophy included restraint as the positive control (). To demonstrate that aminoglutethimide also blocked SMD-induced up-regulation of corticosterone, serum corticosterone was measured in the mice treated with aminoglutethimide and/or SMD. Mice in the aminoglutethimide experiment were dosed with 200 mg/kg to prevent enhanced toxicity to SMD due to lower serum corticosterone levels.
Naïve, aminoglutethimide, and aminoglutethimide + SMD groups had significantly lower serum corticosterone at 1 hour post-SMD administration compared to mice treated with SMD (). Therefore, as in the restraint-stress experiment, aminoglutethimide also blocked SMD-induced up-regulation of corticosterone.
FIG. 6 Aminoglutethimide blocked serum corticosterone up-regulation by SMD. Mice were pretreated with aminoglutethimide for 1 hour before SMD administration. SMD was administered (200 mg/kg) by oral gavage. After 1 hour, trunk blood was collected and serum was analyzed by RIA for serum corticosterone. N = 5 per group. Asterisks (*) indicate significant difference from naïve control (p < 0.05).
Thymic Atrophy Was Not Enhanced in Mice Treated with SMD and Restraint Stress
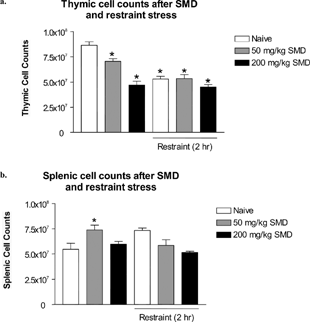
When confronted with a chemical spill or other disaster, people commonly display many stress-related symptoms due to the emotional reaction to the event (Havenaar and van den Brink, Citation1997). It was possible that emotional stress (psychogenic stress) and chemical stress from the xenobiotic could combine synergistically or additively to cause immunosuppression, which was not possible by each stressor alone. To test this possibility, mice were dosed with either a high dosage or a lower dosage of SMD (200 or 50 mg/kg). The low dosage (50 mg/kg) was the lowest dosage reported to cause immunosuppressive effects (Pruett et al., Citation1992a). To mimic the emotional stress exhibited by people during a chemical spill, SMD administration was combined with a short duration of a psychogenic/neurogenic stressor, restraint stress.
Thymic cell counts of naïve mice were significantly different from all of the treated groups (). Mice treated with restraint stress and both dosages of SMD had significant thymic atrophy. The low dosage of SMD caused intermediate levels of thymic atrophy compared to significant thymic atrophy in the restraint, restraint/low SMD, high SMD, or restraint/high SMD groups. Restraint stress combined with any level of SMD did not appear to act synergistically or additively. The thymic atrophy in the higher SMD dosage groups and all restraint groups appeared to plateau around 40–50% cell loss, compared to naïve control.
FIG. 7 SMD intoxication at a high and low dosage in combination with a psychogenic/neurogenic stressor was not synergistic. Mice were dosed with SMD (200 or 50 mg/kg) at 9:00 PM for 3 consecutive days. Immediately after SMD dosage, mice were restrained for 2 hours in their home cages. On day 4, thymi and spleens were removed and analyzed for alterations in cellularity. N = 5 per group. Asterisks (*) indicate significant difference from naïve control (p < 0.05).
DISCUSSION
The results reported here indicated that SMD caused thymic atrophy by an adrenal mediated mechanism. Surgical removal of the corticosterone producing cells (adrenalectomy), or the corticosterone synthesis inhibitor, aminoglutethimide, completely abrogated SMD-induced thymic atrophy.
SMD caused significant thymic atrophy even though it increased serum corticosterone to roughly 700 ng/ml. This is important because corticosterone levels in B6C3F1 mice can peak at 1200–1400 ng/ml when mice are treated with chemical stressors (e.g., morphine) (LeVier et al., Citation1994). Although the peak value of corticosterone did not increase above ∼ 700 ng/ml, the duration of the corticosterone exposure must be taken into account. As shown in a modeling system, both the peak of serum corticosterone and the duration of the exposure are important in the induction of thymic atrophy by a stressor (Pruett et al., Citation1999). Therefore, although the peak was not as high as physiologically possible, its ∼ 4-hour duration at roughly half of the maximal physiological level was sufficient to cause thymic atrophy.
Since thymi naturally atrophy after adolescence in humans (Paul, Citation1998), this increases the potential importance of SMD-induced depletion of thymocytes in both post-adolescents and pre-adolescents. It was previously assumed that the thymus does not play a major role in the production of functional thymocytes in adults as compared to pre-adolescents. This hypothesis developed after studies indicated that both mice that had their thymi removed after 5 days of age and humans thymectomized after 6 months of age “survive normally and display only mild declines in T-cell number” (Mackall and Gress, Citation1997). Further, extrathymic tissues appear to substitute some of the abilities of the thymus in athymic individuals (Mackall and Gress, Citation1997). Therefore, it was possible that the thymus is important in producing thymocytes in pre-adolescents, but not so critical for developing functional thymocytes in adults. Recent data have challenged this assumption and supported the theory that although somewhat atrophied in adults, the mature thymus produces functional thymocytes throughout life, albeit at lower levels in adults (Jamieson et al., Citation1999). Therefore, if the thymus played a role in producing functional T-cells in adults, thymic atrophy caused by SMD could be immunosuppressive in both pre-adolescents and adults.
Exposure data suggest that children could theoretically be exposed to the major breakdown product of SMD, MITC, at dosages approaching 2.5 mg/kg/day in areas near agricultural application (Thongsinthusak, Citation2000). On the basis of body surface area, this is approximately equivalent to a dosage of 17 mg/kg in mice (calculated as described in our previous study) (Padgett et al., Citation1992). This is not much less than dosages that cause thymic atrophy in mice (Keil et al., Citation1996). In addition, we have previously estimated that exposure of a human being to 2.9 ml of a commercial preparation of SMD is roughly equivalent to the 300 mg/kg dosage used in mice in the present study (Padgett et al., Citation1992). These considerations suggest that environmentally relevant dosages of SMD might contribute to thymic atrophy in human beings near sites of usage, and the possibility is even stronger in the case of occupationally relevant dosages.
It was notable that SMD depletes DP thymocytes when exposed after a single administration but repeated administration did not indicate the degree of selective depletion. The single administration of SMD (300 mg/kg × 1) selectively targeted DP thymocytes without altering the cell counts of the other subpopulations (). The higher dosage of SMD (300 mg/kg × 3), on the other hand, decreased the number of all populations, but depleted DP thymocytes more than others. Thymocytes, especially DP thymocytes, are sensitive to corticosterone (Chow et al., Citation1997; Berki et al., Citation2002). Depletion of DP thymocytes has been reported with administration of exogenous corticosterone and dexamethasone, a glucocorticoid receptor (GR) agonist (Screpanti et al., Citation1989). It was not surprising, then, that increases in serum corticosterone by SMD caused initial depletion of DP thymocytes.
There were statistical changes in splenic cellularity associated with SMD administration under some, but not all circumstance ( and ). However, other studies have not demonstrated that decreases in splenic cellularity of this magnitude, in the absence of other effects, are associated with decreased immune function (Padgett et al., Citation1992). Therefore, although there are statistical changes in some splenic cell counts after SMD administration under some circumstances, SMD has not been shown to have a biological significance on splenic immune responses at this time.
There were statistical changes in splenic cellularity associated with SMD administration under some, but not all circumstance ( and ). However, other studies have not demonstrated decreases in splenic cellularity of this magnitude in the absence of other effects that are associated with decreased immune function (Padgett et al., Citation1992). Furthermore, splenic changes were noted in some studies but absent in other studies ( and ). Therefore, although there are statistical changes in some splenic cell counts after SMD administration under some circumstances, SMD has not been shown to have a biological significance on splenic immune responses at this time.
SMD-induced thymic atrophy was completely abrogated in mice using two models of corticosterone inhibition, aminoglutethimide, and adrenalectomy ( and ). First, adrenalectomy completely removes all adrenal products including corticosterone by surgically removing the production of corticosterone. This poses a problem since other adrenal products besides corticosterone (e.g., catecholamines) have been shown to modulate thymic cellularity (Singh, Citation1979; Durant, Citation1986). In order to verify that corticosterone was the likely mediator of the effect, a corticosterone synthesis inhibitor, aminoglutethimide was utilized. Aminoglutethimide modulates corticosterone synthesis by blocking P450scc (cytochrome P450 side chain cleavage), involved in corticosterone biosynthesis from cholesterol (Bastida et al., Citation2001). As was seen in the ADX studies, the aminoglutethimide also blocked thymic atrophy by SMD. Although neither aminoglutethimide nor adrenalectomy abrogated only serum corticosterone production, both methods have mainly the thymic atrophy in common. Therefore, by utilizing both models, it strengthened the case for corticosterone as the major mediator for thymic atrophy after SMD exposure.
In conclusion, these data supported the hypothesis that SMD depleted thymocytes by an adrenal-mediated mechanism. It was highly likely that corticosterone was the indirect mediator of the SMD-induced thymic atrophy in rodents. First, SMD caused a surge in serum corticosterone, indicative of a stress response. Secondly, DP thymocytes, which were sensitive to corticosterone, were selectively depleted with SMD. This pattern of enhanced depletion of DP thymocytes was commonly associated with corticosterone-mediated thymic atrophy. Last, two different mechanisms of corticosterone inhibition, both removal of corticosterone-producing cells and also chemical inhibition of the synthesis of corticosterone, blocked the thymic atrophy by SMD.
This work was supported by NIH grant #ES09158.
REFERENCES
- Anonymous. Dermatitis among workers cleaning the Sacramento River after a chemical spill—California, 1991. MMWR Morb Mortal Wkly Rep 1991; 40: 825–827, 833
- Anonymous. Harmonizing the Clean Air Act & Montreal Protocol Methyl Bromide Phaseouts. US Environmental Protection Agency. 2002
- Anonymous. 2000–2001 Pesticide Market Estimates: Most Commonly Used Conventional Pesticide Active Ingredients: Agricultural Market Sector, 2001, 1999, 1997, and 1987 Estimates: (Table 3.6). United States Environmental Protection Agency, Washington, D.C. 2004
- Bastida C. M., Tejada F., Cremades A., Penafiel R. Aminoglutethimide, a steroidogenesis inhibitor, abolishes hormonal induction of ornithine decarboxylase in steroidogenic tissues: Evidence for its role as cAMP-dependent protein kinase inhibitor. Biochem. Biophys. Res. Commun. 2001; 281: 244–248, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Berki T., Palinkas L., Boldizsar F., Nemeth P. Glucocorticoid (GC) sensitivity and GC receptor expression differ in thymocyte subpopulations. Int. Immunol. 2002; 14: 463–469, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Bowler R. M., Mergler D., Huel G., Cone J. E. Psychological, psychosocial, and psychophysiological sequelae in a community affected by a railroad chemical disaster. J. Trauma Stress 1994; 7: 601–624, [PUBMED], [INFOTRIEVE], [CSA]
- Chow S. C., Snowden R., Orrenius S., Cohen G. M. Susceptibility of different subsets of immature thymocytes to apoptosis. FEBS Lett. 1997; 408: 141–146, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Cone J. E., Wugofski L., Balmes J. R., Das R., Bowler R., Alexeeff G., Shusterman D. Persistent respiratory health effects after a metam sodium pesticide spill. Chest 1994; 106: 500–508, [PUBMED], [INFOTRIEVE]
- Durant S. In vivo effects of catecholamines and glucocorticoids on mouse thymic cAMP content and thymolysis. Cell. Immunol. 1986; 102: 136–143, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Freier D. O., Fuchs B. A. Morphine-induced alterations in thymocyte subpopulations of B6C3F1 mice. J. Pharmacol. Exp. Ther. 1993; 265: 81–88, [PUBMED], [INFOTRIEVE]
- Fuchs B., Pruett S. B. Morphine causes the selective loss of CD4+/CD8+ lymphocytes in the murine thymus through the induction of apoptosis. NIDA Research Monograph Series 1992; 119: 332
- Groves T., Parsons M., Miyamoto N. G., Guidos C. J. TCR engagement of CD4+CD8+ thymocytes in vitro induces early aspects of positive selection, but not apoptosis. J. Immunol. 1997; 158: 65–75, [PUBMED], [INFOTRIEVE]
- Han Y. C., Lin T. L., Pruett S. B. Thymic atrophy caused by ethanol in a mouse model for binge drinking: Involvement of endogenous glucocorticoids. Toxicol. Appl. Pharmacol. 1993; 123: 16–25, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Hanley C. California community humiliated in pesticide scare. The Associated Press, Earlimart, CA 1999
- Havenaar J. M., van den Brink W. Psychological factors affecting health after toxicological disasters. Clin. Psychol. Rev. 1997; 17: 359–374, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Jamieson B. D., Douek D. C., Killian S., Hultin L. E., Scripture-Adams D. D., Giorgi J. V., Marelli D., Koup R. A., Zack J. A. Generation of functional thymocytes in the human adult. Immunity 1999; 10: 569–575, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Jondal M., Okret S., McConkey D. Killing of immature CD4+CD8+thymocytes in vivo by anti CD3 or 5′-(N-ethyl)-carboxamido-adenosine is blocked by glucocorticoid receptor antagonist RU-486. Eur. J. Immunol. 1993; 23: 1246–1250, [PUBMED], [INFOTRIEVE], [CSA]
- Keil D. E., Padgett E. L., Barnes D. B., Pruett S. B. Role of decomposition products in sodium methyldithiocarbamate-induced immunotoxicity. J. Toxicol. Environ. Health 1996; 47: 479–492, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Koehler G. A., Van Ness C. The emergency medical response to the Cantara hazardous materials incident. Prehospital Disaster Med. 1993; 8: 359–365, [PUBMED], [INFOTRIEVE]
- Koo D., Goldman L., Baron R. Irritant dermatitis among workers cleaning up a pesticide spill: California 1991. Am. J. Ind. Med. 1995; 27: 545–553, [PUBMED], [INFOTRIEVE], [CSA]
- Kreutzer R. A., Hewitt D. J., Sun R., Draper W., Mangiamele D., Goldman L., Jackson R., Smith D., Shusterman D. A community-based epidemiologic study of acute health effects from a metam-sodium spill on California's Sacramento River. Toxicol. Ind. Health 1996; 12: 267–275, [PUBMED], [INFOTRIEVE], [CSA]
- LeVier D. G., McCay J. A., Stern M. L., Harris L. S., Page D., Brown R. D., Musgrove D. L., Butterworth L. F., White K. L., Munson A. E. Immunotoxicological profile of morphine sulfate in B6C3F1 female mice. Fundam. Appl. Toxicol. 1994; 22: 525–542, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Mackall C. L., Gress R. E. Thymic aging and T-cell regeneration. Immunol. Rev. 1997; 160: 91–102, [PUBMED], [INFOTRIEVE], [CSA]
- Maxwell L. A. Airborne pesticide sends 21 Earlimart residents to hospitals. The Fresno Bee. Fresno, CA 1999a; A1
- Maxwell L. A. Earlimart pesticide case baffles officials: Application that made dozens ill appears legal, inspectors say. The Fresno Bee. Fresno, CA 1999b; A1
- Myers L. P., Krieg A. M., Pruett S. B. Bacterial DNA does not increase serum corticosterone concentration or prevent increases induced by other stimuli. Int. Immunopharmacol. 2001; 1: 1605–1614, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Padgett E. L., Barnes D. B., Pruett S. B. Disparate effects of representative dithiocarbamates on selected immunological parameters in vivo and cell survival in vitro in female B6C3F1 mice. J. Toxicol. Environ. Health 1992; 37: 559–571, [PUBMED], [INFOTRIEVE]
- Fundamental Immunology, W. E. Paul. Raven Press, New York 1998
- Pruett S. B., Barnes D. B., Han Y. C., Munson A. E. Immunotoxicological characteristics of sodium methyldithiocarbamate. Fundam. Appl. Toxicol. 1992a; 18: 40–47, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Pruett S. B., Collier S., Wu W. J., Fan R. Quantitative relationships between the supression of selected immunological parameters and the area under the corticosterone concentration vs. time curve in B6C3F1 mice subjected to exogenous corticosterone or to restraint stress. Toxicol. Sci. 1999; 49: 272–280, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Pruett S. B., Fan R., Zheng Q., Myers L. P., Hebért P. Modeling and predicting selected immunological effects of a chemical stressor (3,4-dichloropropionanalide) using the area under the corticosterone concentration vs. time curve. Toxicol. Sci. 2000; 58: 77–87, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Pruett S. B., Han Y. C., Fuchs B. A. Morphine suppresses primary humoral immune responses by a predominantly indirect mechanism. J. Pharmacol. Exp. Ther. 1992b; 262: 923–928, [PUBMED], [INFOTRIEVE]
- Saad A. J., Jerrells T. R. Flow cytometric and immunohistochemical evaluation of ethanol-induced changes in splenic and thymic lymphoid cell populations. Alcohol Clin. Exp. Res. 1991; 15: 796–803, [PUBMED], [INFOTRIEVE]
- Sauerhoff M. W. Metam Sodium as an Alternative to Methyl Bromide for Fruit and Vegetable Production and Orchard Replanting. Environmental Protection Agency, Washington, DC 1998; 6
- Screpanti I., Morrone S., Meco D., Santoni A., Gulino A., Paolini R., Crisanti A., Mathieson B. J., Frati L. Steroid sensitivity of thymocyte subpopulations during intrathymic differentiation. Effects of 17 beta-estradiol and dexamethasone on subsets expressing T-cell antigen receptor or IL-2 receptor. J. Immunol. 1989; 142: 3378–3383, [PUBMED], [INFOTRIEVE]
- Sei Y., Yoshimoto K., McIntyre T., Skolnick P., Arora P. K. Morphine-induced thymic hypoplasia is glucocorticoid-dependent. J. Immunol. 1991; 146: 194–198, [PUBMED], [INFOTRIEVE]
- Singh U. Effect of catecholamines on lymphopoiesis in fetal mouse thymic explants. J. Anat. 1979; 129: 279–292, [PUBMED], [INFOTRIEVE]
- Tarcic N., Ovadia H., Weiss D. W., Weidenfeld J. Restraint stress-induced thymic involution and cell apoptosis are dependent on endogenous glucocorticoids. J. Neuroimmunol. 1998; 82: 40–46, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Thongsinthusak T. Evaluation of Methylisothiocyanate as an Air Contaminant. Part B. Exposure Assessment. Department of Pesticide Regulation, Sacramento, CA 2000; 1–52
- Zhao W., Schafer R., Cuff C. F., Gandy J., Barnett J. B. Changes in primary and secondary lymphoid organ T-cell subpopulations resulting from acute in vivo exposure to propanil. J. Toxicol. Environ. Health 1995; 46: 171–181, [PUBMED], [INFOTRIEVE]