Abstract
Increasing the understanding of how metal ions/complexes react in situ will allow for the improved specificity and controlled toxicity of novel synthetic metallocompounds that will be used as inhaled diagnostics or therapeutics. Our previous work showed that inhalation of select metals (e.g., chromium, vanadium, nickel, iron) caused alterations in lung immune cell function and in local bacterial resistance. The data also suggested that variations in the degree of immuno-modulation induced were not solely dependent on the amount of metal deposited in the lung, but also on the specific compound. If specificity governs immunomodulatory potential, it follows that physicochemical properties inherent to the metal may have a role in the elicited effects. We hypothesize that major determinants of any metal compound's immunomodulatory potential in situ are its redox behavior, valency, and/or solubility. Using changes in local bacterial resistance as an endpoint, differences in immunotoxic potential in the lungs were quantified for a range of chromium agents (insoluble calcium chromate(VI), and soluble sodium chromate(VI), potassium bis(dipicolinato)chromate(III) and sodium bis(dipicolinato)chromate(II)). Results indicated that among the latter three forms of Cr, strongly oxidizing hexavalent Cr (Cr[VI]) had the greatest impact on resistance, while reducing divalent and fairly unreactive trivalent forms of Cr had no effect at an equal exposure level (i.e., 100 μg Cr/m3, 5 hr/d, for 5 d). Insoluble Cr(VI) had a greater effect than its soluble form. When data was analyzed in the context of pre-infection lung Cr burdens, it was seen that immunomodulatory potentials for both Cr(VI) agents did not differ significantly; however, complexes with different oxidation states did induce varying responses, suggesting that differences in potential might be attributed to redox behavior. From this it was concluded that for Cr, certain physicochemical properties are likely more important to any in situ pulmonary immunotoxicity than others (i.e., redox behavior is more critical than solubility). Our findings, in part, will help provide a basis for understanding why certain metals could be a greater health risk than others, even when encountered in equal amounts. This, in turn, will help researchers in the design of inhalable diagnostic/therapeutic metallopharmaceuticals by pre-empting the selection of certain metal ions/complexes for potential use in these products.
Keywords :
INTRODUCTION
The presence of select metals (as both free ions and as complexes) in polluted air may account for a large number of exposure-associated respiratory diseases (Lippmann, Citation1999; Lippmann et al., Citation2003; Cohen et al., Citation2000; Cohen, Citation2004; EPA, Citation2004). As the lung is a target organ, with each breath, local cell populations are directly exposed to the various metals present. Individual metals can then, in turn, trigger a wide variety of biological effects that may be involved in the pathogeneses of these disease states. For example, impaired immunocompetence can evolve as a result of modifications in the structural, functional, and/or biochemical properties of local immune cells, primarily alveolar macrophages (AM) and neutrophils (Graham and Gardner, Citation1985; Cohen et al., Citation2000; Cohen, Citation2004). Any induced change in activity as secretory cells during initiation/propagation of immune responses, alone or in conjunction with effects on phagocytic, intracellular killing, or reactive intermediate formation capacities, may ultimately lead to increases in infectious lung diseases.
Many in vivo and in vitro studies have addressed whether, the extent, and the means by which individual metals induce these effects (reviewed in Cohen et al., Citation2000; Cohen, Citation2004; Cohen and Costa, Citation2006). It is clear from these studies that dose (i.e., amount delivered to lung) was a determinant in the degree of immunomodulation observed. However, potential for a metal to be an immuno-toxicant also appeared to depend on the particular agent used. This suggests that certain inherent properties of metal compounds or metals themselves were determinative in their toxicity. Among the numerous possible physicochemical properties are solubility, valency, and redox activity.
A key physicochemical property that would likely have a great impact upon the immunotoxicity of any airborne metal compound is its solubility. In general, solubility of a metal alone or a metal compound is dependent on molecule size, ligand type, charge, and nuclearity (Lay and Lavina, Citation2004). Given the inherent difficulties in testing the effects from exposure to insoluble compounds, most metal compounds that have been investigated in inhalation studies have been in the soluble form (reviewed in Cohen et al., Citation2000; Cohen, Citation2004; Cohen and Costa, Citation2006). Rarely have the effects of soluble and insoluble compounds been compared directly. In the case of airborne materials, it seems paramount that compound solubility may be critical to the affects induced in the lungs. This is because insoluble materials will continue to persist for some time following entrainment, and therefore may give rise to more prolonged states of toxicity than corresponding particles of soluble compounds that are more readily cleared.
Two other physiochemical properties likely to impact on immunotoxicity are valency and redox behavior. Many metals exist in several oxidation states; for example, iron has several oxidation states, but predominantly exists in nature in the +2 and +3 forms. Chromium can exist in states ranging from −2 to +6, but only +3, and +6 are commonly encountered in nature. While the fundamental difference between a +2 and a +3 state is only one electron, the difference is profound and affects how the metal forms complexes, as well as structures and properties of these complexes (Purcell and Kotz, Citation1977; Cotton and Wilkinson, Citation1980; Lay and Lavina, Citation2004). Importantly, the ligands coordinated to the metal can affect the valence state that predominates under these conditions. The inherent ability of a metal to change valence (also referred to as redox behavior) generally can occur in shuttling-type processes or via unidirectional ones. Examples of unidirectional mechanisms are more abundant in biology, with most metal ions having one preferred valence. Fundamental chemistry dictates when a metal accepts an electron, it is reduced and if it donates one, it is oxidized. A metal's valency is also critical as it is a factor in determining the: types of coordinating ligands; stereochemistry of complexes formed; solubility; reactivity (intra- and extracellular); and, ultimately, mechanisms by which the metal may gain entry to cells. Together, valency and redox properties undoubtedly also play important roles in the toxicity of a metal in situ.
In the studies reported here, immunotoxic effects were examined in vivo for each of four chemically distinct Cr compounds. Chromium was selected for analyses since, among the many metals routinely present in urban atmospheres, it is one of the best studied for (immuno)toxic effects. Chemically, the highest oxidation state of Cr (i.e., hexavalent Cr(VI)) is generally considered to undergo unidirectional redox processes (although exceptions to this view do exist [Lay and Lavina, Citation2004]). In this process, once an electron is accepted, the instability of the pentavalent Cr(V) intermediate leads to rapid acceptance of electrons until the ion that will not readily accept (or donate) further is formed (i.e., trivalent Cr(III)). Valency dictates that at neutral pH, Cr(VI) compounds are mostly tetrahedral; this is critical in that it allows their anions to enter cells via the same transport systems used by other tetrahedral (e.g., SO42− and PO43−) ions (Arslan et al., Citation1987). Once in a cell, the Cr(VI) is highly oxidizing and readily reduces under the physiological conditions around it (Standeven and Wetterhan, Citation1989). Though oxidation states of +5 and +4 have been reported as forming and/or existing in biological systems, they are generally considered highly reactive, unstable, and short-lived with half-lives of only minutes (Nag and Bose, Citation1985; CitationFederal Register, 2004; Lay and Lavina, Citation2004).
In the cellular environment, the most compatible oxidation state of chromium is +3. Unlike Cr(VI), most Cr(III) complexes are octahedral cations (Lay and Lavina, Citation2004) that only cross cell membranes very slowly by diffusion; with less soluble forms, the metal enters via endocytosis or pinocytosis. Lastly, divalent Cr(II) compounds are reducing, and so the free cation readily oxidizes under physiological conditions. Like Cr(III) agents, Cr(II) compounds exist primarily as octahedral complexes (Cotton and Wilkinson, Citation1980; Lay and Lavina, Citation2004) and their abilities to enter cells are also likely to be constrained. Thus, as with solubility, both redox behavior and valency are likely to be important factors in the immunotoxic effect of any Cr agent taken into the lungs.
Many metals, including Cr, have been shown in various studies to be immunotoxicants whose effects in the lungs appear to vary with the compound tested (reviewed in Cohen et al., Citation2000; Cohen, Citation2004; Cohen and Costa, Citation2006). Several mechanisms have been postulated in those studies to explain how metals act directly (i.e., damage to membranes/organelles, inhibition of critical enzymes, interference with surface receptor binding/post-binding processing and signal transduction) or indirectly (i.e., displacement of endogenous metals from biomolecules resulting in toxicity from the liberated metals, neurotoxicity leading to stress responses) to bring about effects on immune cells. Novel pathways for each metal, as well as potential common mechanisms, are likely to exist but as yet have not been fully defined. By examining the extent to which inherent physico-chemical characteristics influence the toxicity of metal ions/complexes on lung immune cells, the studies here document whether these properties may play a role in mechanisms of respiratory disease pathogeneses. These studies should help provide clearer rationales for the differing immunotoxicities of commonly encountered ambient metals, yield information that can lead to a better appreciation for potential reactions of metals in living systems, and enable better design of new inhalable metallopharmaceuticals.
MATERIALS AND METHODS
Experimental Animals
Ten-week-old inbred pathogen-free male F344 rats (≈225 g, Charles River Wilmington, MA) were used in exposures to each compound. Upon arrival as 8-wk-olds, the rats were quarantined for 2 wk. Rats were housed individually in Cr-free cages in temperature (20°C)- and humidity (50% RH)-controlled rooms, and provided Purina Rodent Chow and water ad libitum. Rats underwent routine clinical screening under veterinary supervision prior to initiation of exposures to each Cr agent (each at 100 μg Cr/m3) for 5 hr/d for five consecutive days. All facilities and experimental procedures were approved by the NYU Medical Center Committee on Animal Care and Use.
Chemical Agents
To analyze the effects from soluble Cr agents with varying valences, sodium chromate(VI) (Na2CrO4), potassium bis(dipicol-inato) chromate(III) (K[Cr(III)(dipic)2]), also referred to as potassium bis (pyridine-2,6-dicarboxylato)chromate(III) (hereafter as Cr(III)dipic) and sodium bis(dipicolinato)chromate(II) (Na2[Cr(II)(dipic)2] · 1.5H2O), also referred to as sodium bis(pyri-dine-2,6-dicarboxylato) chromate(II) (hereafter as Cr(II)dipic), were employed in the exposures. These soluble forms of Cr(III) and Cr(II) were used since halogen salts of each Cr ion could not be prepared at neutral pHs required for exposure (i.e., CrCl3 and CrCl2 form precipitates at pH > 4.0). To compare effects of a soluble vs. an insoluble form of Cr(VI), insoluble calcium chromate (CaCrO4) was used as a counterpart to Na2CrO4. Control rats were exposed to filtered air only.
Both Cr(VI) agents were purchased from Sigma (St. Louis, MO). The Cr(II) and Cr(III) dipic complexes ( and ) were synthesized de novo. Potassium bis(dipicolinato)-chromate(III) was prepared utilizing the method of CitationHoggard and Schmidtke (1986). The purity of the K[Cr(III)(dipic)2] used for the exposures was 100%; purity was determined by ESI MS (mass spectroscopy) and the material had a base peak at the predicted m/z of 382.07. Sodium bis(dipicolinato)chromate(II) was synthesized as follows:, dipicolinic acid (1.00 g, 6.0 mmole) and Na2CO3 (0.64 g, 6.0 mmole) were added to a 25 ml flask; deionized water (15 ml) was added and the mixture heated until no more CO2 evolved. This sodium 2,6-dipicolinate solution was then degassed with argon for 2 hr. Chromium(II) acetate dihydrate (0.56 g, 1.5 mmole) was added to another 25 ml flask with a stirring bar and degassed 2 hr. The sodium dipicolinate was syringed under argon onto the chromium(II) acetate dihydrate; this mixture was stirred under argon for 2 hr during which a violet precipitate formed. The latter was filtered off, washed with acetone, and air-dried. In this reaction, product yield was 0.36 g (27%). The purity of the Na2[Cr(II)(dipic)2] · 1.5H2O used for the exposures was 100% (Methods of purity determination include analyses of isolated product: Extinction coefficient (ε552 [H2O], UV/VIS) = 242 M−1 cm−1. m/z, 428.00 (100%, base peak, M + H+). Elemental composition calculated for C14CrH9Na2N2O9.5 (as %) C = 36.94; Cr = 11.42, H = 1.99, and N = 6.16; found C = 37.14; Cr = 11.19; H = 2.29, and N = 6.34).
Generation and Characterization of Exposure Atmospheres and Exposure System
Using previously described procedures (Cohen et al., Citation1996, Citation1997a, Citation2003), atmospheres of each soluble agent were generated by nebulizing a dilute solution (pH 7.2–7.4) via a Collison nebulizer (BGI, Waltham, MA). Atmospheres of insoluble particles were similarly generated, except that insoluble CaCrO4 was purchased in the appropriate size. Each aerosol was mixed with filtered air and directly introduced into the exposure system. Target concentration was always 100 μg Cr/m3; if there was a significant effect, subsequent exposures would then utilize atmospheres ranging down to 0.001 μg/m3. This range was selected as it encompassed Cr levels used in previous/ongoing studies, approached levels routinely encountered in select occupational settings (i.e., welding, mining, and steel refining), and was representative of Cr levels routinely encountered in urban atmospheres. Aerodynamic size distribution of each aerosol generated was confirmed via an 8-stage multiple orifice impactor (MSP, St. Paul, MN) after exposure (due to flow requirement). Mass concentration was assessed during exposure by particle weight (using a Cahn Electrobalance with 1 μg sensitivity) collected on 47 mm filters (Type FG, 0.2 μm pore, Millipore, Bedford, MA). Representative filters were later re-assessed for Cr content by graphite atomic absorption spectroscopy (AAS).
All exposures were nose-only, using a radial, flow past design 50-port exposure system. Each atmosphere entered through a top port and was radially distributed to each station. Internal surfaces were constructed of Teflon to prevent agent-wall interaction/reaction. Exhaust air from each station returned to a central point for removal. Rats were housed in plastic restraint tubes during each 5 hr exposure; earlier work has shown that rats do not undergo undue stress under these conditions. All dilution and nebulizer air was passed through a cleansing system to remove ambient pollutants. Initial air was pre-filtered and temperature-controlled; relative humidity of dilution air was maintained at 50% (±5%). Delivery of aerosol to each port was highly reproducible within and between exposure groups (a 2% CV).
Studies of Host Resistance/In Situ Bacterial (Listerial) Clearance After Cr Agent Exposure
To determine effects on in situ antibacterial responses, resistance to pneumonia-inducing Gram-positive Listeria monocytogenes (LM, strain L242/73 type 4b), was assessed. Listeria (from stock held on trypticase soy agar [TSA]/0.6% yeast extract held at 4°C) was grown for 16 hr in trypticase soy broth at 37°C. Bacterial concentration was then spectrophotometrically determined using a calibration curve prepared at 540 nm. Based on the value obtained, an aliquot was removed and diluted with phosphate-buffered saline to the needed concentration for intratracheal instillation (110 μl/rat) under light halothane anesthesia. Earlier studies indicated that use of this extrapolation to predict Listeria concentration was within 90% of predicted values (Cohen et al., Citation1989, Citation2001, Citation2002b).
One day after their final agent exposure, cohorts of 24 rats per Cr group and 15 air controls were infected with 4 × 106 bacteria/rat (< LD10 in this strain at this age [Cohen et al., Citation2001, Citation2002b]). A set of six naive rats was infected in parallel; three were analyzed immediately to establish baseline bacterial burdens, and the others sacrificed 72 hr later to monitor virulence consistency. A separate set of 10 Cr-exposed rats and 5 controls remained uninfected and were sacrificed to obtain baseline lung Cr burdens at the time of infection (i.e., Day 0 rats). Within each infected set, cohorts of 6, 8, and 10 Cr-exposed rats (along with 5 air controls) were then euthanized by Nembutol (100 mg/kg, IP) overdose at 24, 48, and 72 hr post-infection, respectively—a period encompassing the innate response to Listeria in the lung. The lungs of each rat were then isolated en bloc; after the trachea and extrapulmonary bronchi were removed via tungsten blade, the tissue was weighed and processed for estimation of listerial burden by homogenization and subsequent plating of serial dilutions (triplicate) on TSA plates for 24 hr at 37°C. The remaining homogenate volume was then measured and the material placed at 4°C for later use in determining lung Cr burden at each sacrifice timepoint.
Both total number of Listeria and number of Listeria/g lung were calculated for each rat. The percentage change in total Listeria and in total Listeria/g lung from those of each parameter obtained with control rats at each timepoint were used as indices of the modulation of listerial clearance induced by each test agent. Because of potential differences in deposition and/or clearance of each agent, these percentages were further analyzed in the context of total amount of Cr present in the lungs at sacrifice and pre-infection timepoints.
Assessment of Lung Metal Burden
Our NIEHS Center Analytical Core procedures used in earlier experiments (Cohen et al., Citation1997a, Citation2003) were applied to determine the amount of Cr (for each agent) in homogenized lung samples isolated at each timepoint (as well as on Day 0 in uninfected lungs). Each final isolate (in 0.5 ml 2% nitric acid) was analyzed for Cr using a Solaar Model M6 AA spectrophotometer (with Zeeman background correction) with GF95 graphite furnace. The minimal detectable Cr value was 1 ppb; extraction efficiency, regardless of matrix, was > 99%. All materials used were reagent grade and standards NISTS (National Institute of Standards and Technology) traceable. Reagents and standards dilutions were made up in ultrapure H2O. Standard curves consisted of 5-point calibration with a standard blank to assure accurate baselines.
Data Analysis
Effects from each Cr agent upon each test endpoint were analyzed by two-way ANOVA (analysis of variance) with appropriate data transformations; individual factors were exposure group (air alone or specific Cr agent) and assessment time. All data were tested to assure assumptions of normality and homogeneity of variance were met, and transformations applied as needed. All data were also screened for outliers using Dixon and Grubb's analyses (Taylor, Citation1990). Significant time or group effects, or effects associated with interaction between the two were sub-tested using t-tests corrected for multiple comparisons. Outcomes were considered significant at p < 0.05.
RESULTS
Lung Cr Burdens as Function of Test Agent
Rats in each Cr treatment group were thought to be exposed to 100 μg Cr/m3 during each 5-hr regimen. Analyses of filter samples collected during the exposures indicated that rats in the soluble Cr(II) (Na2[Cr(II)(dipic)2] · 1.5H2O) and Cr(III) (K[Cr(III)(dipic)2]) groups experienced average levels of 104.56 (±2.64 [SE]) and 99.22 (±2.07) μg Cr/m3, respectively. Animals in the soluble (Na2CrO4) and insoluble (CaCrO4) Cr(VI) groups received 110.08 (±3.52 [SE]) and 118.57 (±2.88) μg Cr/m3, respectively. Thus, rats exposed to CaCrO4 received slightly (but, still statistically significantly) more Cr over the five exposure days than any Cr-exposed counterpart. Average particle sizes delivered to each rat were consistent; mass median aerodynamic diameters were 0.21 μm (σg = 1.9) for the Cr(II)dipic, 0.22 μm (σg = 1.8) for the Cr(III)dipic, 0.34 μm (σg = 1.7) for the Na2CrO4, and 0.27 μm (σg = 2.7) for the CaCrO4 atmospheres.
Twenty-four hr after the final exposure (i.e., Day 0), rats in each group were either infected or sacrificed for analyses of lung Cr burdens. The data shown in indicates that rats in the CaCrO4-exposed group had the highest total lung Cr content among all treatment groups on Day 0. The Cr levels in these rats were 96, 67, and 13% greater than those in Na2CrO4-, Cr(II)dipic-, and Cr(III)dipic-exposed rats, respectively. Initial pulmonary levels of Cr(III) were 47 and 72% greater than those seen in the rats exposed to the Cr(II) and soluble Cr(VI) agents, respectively. When the lungs of infected rats in each treatment group were analyzed after 3 d of infection, there were again significant differences in total Cr content. Levels of Cr in the lungs of rats from the insoluble CaCrO4 group were the greatest (e.g., 108, 39, and 40% greater than in lungs of Na2CrO4-, Cr(II)dipic-, and Cr(III)dipic-exposed rats, respectively). Levels of Cr in rats that received soluble Na2CrO4 group were even significantly lower (≈ 50%) than that of rats that were exposed to either dipic complex.
FIG. 2 Lung Cr burdens at Day 0 (i.e., pre-infection) and Day 3 of infection with Listeria. Each bar represents the average burden ([A] ng Cr; [B] ng Cr/g lung) (± SE) in the lungs of 5 (Day 0; solid bar) or 10 (Day 3; hatched bar) rats/treatment regimen. In Day 0 sets; ‡value significantly (p < 0.05) different from that in Cr(II) and soluble Cr(VI) rats. In Day 3 sets, *value significantly (p < 0.05) different from that in rats in all other groups; #value significantly (p < 0.05) different from that in Cr(II) and Cr(III) rats.
![FIG. 2 Lung Cr burdens at Day 0 (i.e., pre-infection) and Day 3 of infection with Listeria. Each bar represents the average burden ([A] ng Cr; [B] ng Cr/g lung) (± SE) in the lungs of 5 (Day 0; solid bar) or 10 (Day 3; hatched bar) rats/treatment regimen. In Day 0 sets; ‡value significantly (p < 0.05) different from that in Cr(II) and soluble Cr(VI) rats. In Day 3 sets, *value significantly (p < 0.05) different from that in rats in all other groups; #value significantly (p < 0.05) different from that in Cr(II) and Cr(III) rats.](/cms/asset/e8eded58-70be-4ffd-bfea-3fc9552e3926/iimt_a_171811_uf0002_b.gif)
Analyses of the Day 0 Cr burdens in the context of lung weight revealed the same trends and relative differences noted above among all of the treatment groups (). Levels of Cr/g lung in rats that received CaCrO4 were 99, 71, and 12% greater than those in rats exposed to Na2CrO4, Cr(II)dipic, and Cr(III)dipic, respectively. However, when Cr levels at Day 3 of infection were similarly analyzed, the relative differences among the groups were very different from those obtained using the absolute burdens. For example, while rats in the CaCrO4 regimen still had the greatest Cr/g lung levels, these levels were now 101, 16, and 11% greater than those of the Na2CrO4-, Cr(II)dipic-, and Cr(III)dipic-exposed rats, respectively.
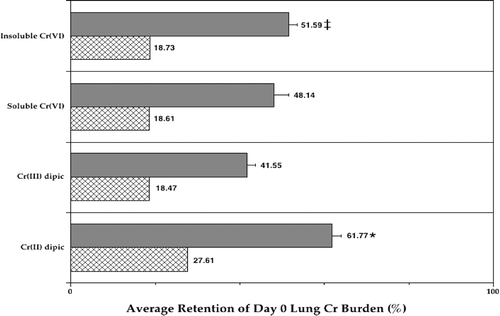
It is yet uncertain to what extent the presence of the Listeria impacted on clearance of each agent (i.e., as opposed to what would occur had uninfected rats been monitored on Day 3). When the data was evaluated in the context of absolute Cr content, they indicated that retention of Cr when delivered as soluble Cr(II)dipic was significantly greater (≈62% of Day 0 level) than with any of the other compounds (). Exposure to soluble Cr(III)dipic yielded the least retention (≈42%) of the metal after the 3 d period, whereas both Cr(VI) agents gave rise to ≈50% values. In contrast, if analyses were performed in the context of Cr/g lung, the retention values for Cr from the CaCrO4−, Na2CrO4−, and Cr[III]-exposed rats were all now 18–19%, while that of from the Cr(II)-exposed hosts dropped to 28%. Thus, it appears that analyses of Cr burdens in the context of lung mass is inappropriate during the infection-to-resolution process.
FIG. 3 Average retention of Cr in the lungs of Listeria-infected rats in each Cr treatment group. Each bar represents the average retention (%; ± SE) of Day 0 burden in the lungs of 10 Day 3 rats per treatment regimen. Data analyzed in terms of ng Cr (solid bar) or of ng Cr/g lung (hatched bar). *Value significantly (p < 0.05) different from that in rats in all other groups; ‡value significantly different from that in rats in the Cr(III) group.
Lung Listeria Burdens as Function of Test Agent
Following the 5 d exposures and infection with viable Listeria on Day 0, rats in each exposure group were assessed for listeric burdens at 24, 48, and 72 hr post-infection. All data were then compared to the burdens measured in the lungs of air-exposed infected rats to determine whether a particular Cr compound could induce significant immunomodulation at any of the observed timepoints. In no case did exposure to any of the four Cr agents cause significant effects on listeric burdens 24 or 48 hr post-infection (, , , ). Only on Day 3 did it become apparent that of the four agents analyzed, both compounds containing the strongly oxidizing chromium(VI), e.g., soluble Na2CrO4 and insoluble CaCrO4, brought about a significant reduction in pathogen clearance, and that CaCrO4 had the greatest effect. When evaluated using the lower Cr exposure dose of 10 μg/m3, neither the CaCrO4 nor the Na2CrO4 retained the effect on resistance seen at the higher dose. Corresponding patterns were apparent when the data were analyzed in the context of lung weight at the time of sacrifice. Analyses of effects produced by exposure to the dipic alone (as sodium dipic) indicated to be no effect on host resistance to Listeria.
FIG. 4 Listeric (bacterial) burdens in the lung of rats at each day post-infection. (A) CaCrO4; (B) Na2CrO4; (C) Cr(III)dipic; (D) Cr(II)dipic regimen. Values shown with ♦ represent the mean (±SE) of 6, 8 and 10 rats at 24, 48, and 72 hours post-infection/Cr regimen, respectively; values indicated with ○ represent mean (±SE) of 5–6 air control rats at same timepoints. Statistical significance (p value) of result (Cr vs. air)—when present—is indicated.
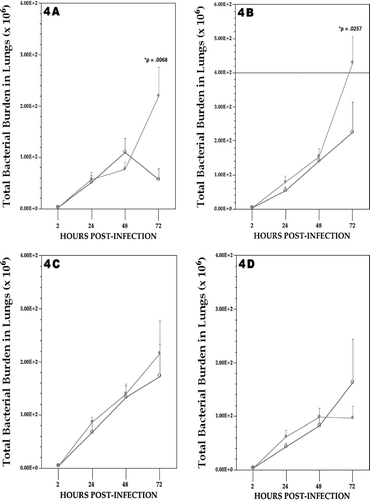
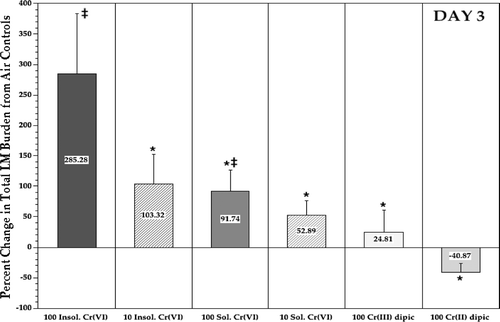
Rats that had received insoluble CaCrO4 displayed a 286% greater total lung burden of Listeria compared to their time-matched air control counterparts (); these levels were also significantly greater than those in all other Cr treatment groups as well. Inhalation of soluble Na2CrO4 led to a significant ≈92% increase in listeric burden compared to the air controls; rats exposed to the soluble reducing Cr(II)dipic or the fairly unreactive Cr(III)dipic had burdens similar to those of the controls. When all burden data were re-analyzed to take into account changes in lung size that could reflect increases in mass due to bacteria, edema, and/or an increased presence of immune cells that had migrated to the infection site, the same pattern of results were obtained.
FIG. 5 Relative difference in listeric (LM) burden in the lungs of rats at Day 3 post-infection. Each bar represents the mean (± SE) average percentage differences in Listeria levels (compared to those in air controls) in the lungs of 10 Day 3 rats/indicated treatment regimen. *Value significantly (p < 0.05) different from that in rats in 100 μg Cr/m3 insoluble CaCrO4 group. ‡Value significantly (p < 0.05) different from that in air control rats.
Estimation of Relative Immunomodulatory Potential of Each Test Agent
Analyses of the percentage change in listeric burden (as either total burden or burden/g lung compared to that in infected control counterparts) in the context of the total Cr present in the lungs yielded a weak negative correlation between lung Cr burden on Day 3 and relative change in Listeria burden. That is, rats with the highest Cr burdens appeared to have the lowest percentage changes in Listeria levels compared to controls. Similar patterns were also observed when correlating total listeric burden, listeric burden/g lung, or lung weight vs. Day 3 lung Cr burden. Analyses of the relationships between extent of infection and increase in lung weight, as well as between the apparent loss of Cr burden and increase of the mass of and/or damage to the lung during the infection process both yielded positive correlations. Because of this latter set of correlations, it became apparent that the use of the Day 3 Cr burden as a factor for assessing the relative immunomodulatory potentials of each Cr agent was not reliable due to its likely status as an “after-the-fact” endpoint.
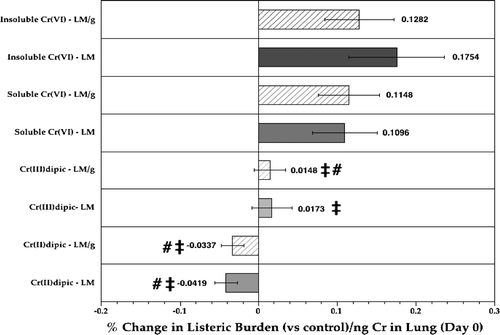
In contrast, if the initial burden (i.e., Day 0) was used as a predictor for the ultimate change in host resistance to Listeria challenge, clear (potential) immunomodulation patterns became apparent (). When the percentage change in listeric burdens from air control levels (as absolute numbers or in terms of per gram lung tissue) were estimated, the data demonstrate that in both cases the insoluble CaCrO4 had the greatest effect on resistance as measured at a per ng Cr burden pre-infection. For both estimate types, relative changes in listeric burdens were significantly greater than that following exposures with the soluble reducing Cr(II)dipic or the fairly unreactive Cr(III)dipic. Values for the soluble strongly oxidizing Na2CrO4-exposed rats were always, albeit not significantly, less (i.e., 60%) than for the CaCrO4-exposed hosts also, but still significantly greater than those observed with either Cr-dipic agent. Under the experimental conditions in this study, these results suggest that insoluble hexavalent CaCrO4 was the most potent immunomodulant of the four agents analyzed. However, the results shown in also indicate that the immunomodulatory potentials for effects from the two Cr(VI) compounds did not significantly differ. In contrast, this data show that complexes in different oxidation states induced varying responses. Specifically, the results indicate clear differences in potentials among the soluble forms that could be attributed to their respective oxidation states and thus, their redox behavior.
FIG. 6 Relative difference in listeric burden in the lungs of rats at Day 3 post-infection as a function of Day 0 lung Cr burdens. Each bar represents the mean (n = 10, Day 3 rats/indicated treatment regimen; ±SE) average percentage differences in Listeria levels (LM; solid bar) or of total Listeria/g lung (LM/g; hatched bar) compared to respective values in air controls, in the context of ng Cr in lungs at Day 0. ‡Value significantly (p < 0.05) different from that in rats in insoluble CaCrO4 group; #value significantly (p < 0.05) different from that in Na2CrO4 rats.
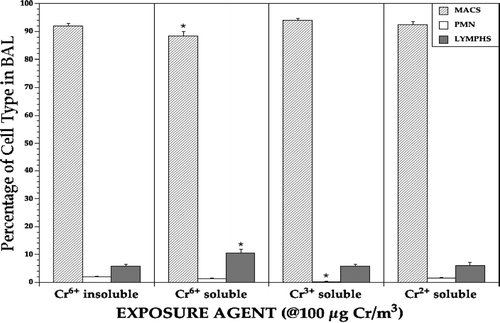
Lung immune cell population profiles on Day 0 were also examined to determine whether these might be a useful indicator of any pre-infection predisposition for an alteration in resistance to Listeria among the rats in each exposure regimen (). The results indicated that inhalation of soluble hexavalent Na2CrO4 slightly (albeit significant) reduced (i.e., 3.8–5.7%) the percentage of pulmonary macrophages while concurrently increasing (i.e., 75–84%) the percentage of lymphocytes as compared to rats in any other exposure group.
FIG. 7 Immune cell population distribution in the lavages of lungs of rats at Day 0 pre-infection. Each bar represents the mean (±SE) average percentage of the indicated cell type among all cells recovered from the lungs of 5 Day, 0 rats/indicated treatment regimen. *Value significantly (p < 0.05) different from that in rats in the other treatment regimens.
DISCUSSION
We have hypothesized that physicochemical properties are major determinants of any metal's pulmonary immunomodulating potential in situ. The results of this study on Cr agents—which had proposed analyzing a range of atmospheric levels of Cr that spanned levels routinely encountered in certain occupational settings (i.e, welding, mining, steel refining), through and down to those that have been measured in several urban centers in the United States (CitationDoherty et al., 2005; Prophete et al., Citation2006)—demonstrated that at least two properties, redox behavior and solubility, likely govern these potentials. This conclusion is based upon studies using an infectivity model that employed the facultative intracellular bacteria Listeria monocytogenes. This host resistance model is well suited for studies of lung cell-mediated components (neither B-lymphocytes nor antibodies are essential for resistance) and their dysregulation by inhaled chemicals (van Loveren et al., Citation1988, Citation1996; see North and Condlan, Citation1998; Antonini et al., Citation2001a, Citation2001b).
A complex array of cytokines, leukocytes, and lymphocytes is involved in cell-mediated anti-listerial responses (Czuprynski and Haak-Frendscho, Citation1997; Unanue, Citation1997, Citation1998; Cohen et al., Citation2001, Citation2002b). Initially, Listeria are phagocytized by local alveolar macrophages (AM) that, in turn, release autostimulatory interleukin-1 α (IL-1 α), tumor necrosis factor-α (TNFα), and interferon-α (IFNα), as well as IL-6 and -12. The monokines also interact with T-lymphocytes to cause them to: up-regulate localization of receptors for interactions with AM; develop increased IL-1 and -2 receptor expression; and, undergo proliferation and differentiation. In conjunction with stimulated local natural killer cells, the lymphocytes release IFNγ that feeds back to primed AM to enhance IL-1 α, TNFα, and reactive oxygen/nitrogen intermediate (ROI/RNI) production. If unperturbed, within 48–72 hr of infection, listeric burdens peak and resolution of infection follows thereafter. Clearly, there are multiple targets in these processes for metal agents, including Cr, to affect. The results of the studies reported herein only reflect the cumulative effects on one or more critical immune parameters as a result of inhalation of each Cr agent. Ongoing studies to examine AM and neutrophil functions, as well as expression of several critical cytokines during the 72-hr post-infection period, will help define further if there are agent-specific effects, and if these outcomes are governed by physicochemical properties.
In the analyses of the role of solubility in immunomodulation, the agents selected for study reflect a wide spectrum of solubilities. At one extreme, the poor solubility of non-hydrated-CaCrO4 is 0.11 g/L (0.0007 mol/kg H2O, at 25°C; Katz and Salem, Citation1993), while very soluble Na2CrO4 has a value of 842.40 g/L (5.2000 mol/kg; CitationDean, 1999). The solubility values for the Cr(III)dipic and Cr(II)dipic agents fall between these two values (i.e., measured to be 10.60 g/L [0.0252 mol/kg] and 29.70 g/L [0.0652 mol/kg], respectively). Differences in how these soluble and insoluble Cr particles are handled in the lung are reflected by their retention patterns. After 5 d of exposure to ≈100 μg Cr/m3, rats that received insoluble CaCrO4 had the greatest lung Cr burdens (both in absolute amount and as per gram lung tissue), while those exposed to soluble Na2CrO4, had the least. The soluble Cr(III)dipic exposure led to better retention in the lung as compared to its Cr(II)dipic analog. Macrophages provide the major means for clearance of insoluble/poorly soluble particles from the lung; insoluble Cr enters cells by endocytosis after adherence to cation binding sites or directly by phagocytosis (see Cohen and Costa, Citation2006). Upon entry, Cr then becomes localized in phagolysosomes where it is dissolved and converted to soluble ions. Because of these temporal processes, rapid diffusion of CaCrO4 through the lung epithelia does not occur and clearance of the agent out of the lungs ultimately relies on mucociliary transport of any uningested particles and the CaCrO4-bearing AM. In contrast, Cr derived from soluble agents is readily absorbed through the lung epithelia, with the limiting step being uptake via non-selective channels. Unless bound to biomolecules within cells (including AM, thereby inducing subsequent intracellular toxicities), the Cr would then rapidly enter the blood to be bound by serum agents or carried to extra-pulmonary sites by erythrocytes.
Solubility also influences inflammation and several AM functions (i.e., cytokine, ROI, and RNI formation) (Cohen et al., Citation1998) that are critical for host resistance against Listeria. Production of IL-1 β by AM from rats exposed for 2 wk to 350 μg Cr/m3 (as insoluble BaCrO4) was unaffected compared to that by cells from air-exposed rats; in contrast, production of TNFα by AM from Cr-exposed hosts was reduced. After host exposure to soluble K2CrO4, IL-1β and TNFα release by AM was reduced, but only after exposures for longer than 2 wk. Regarding ROI, only those AM from BaCrO4-treated rats demonstrated a reduced ability to form these products (spontaneously or after IFNγ priming). Both hexavalent Cr agents caused increases in total lung immune cell levels, but only K2CrO4 led to elevated percentages of neutrophils in the lungs. Though the current study did not indicate any major effects from inhaled Cr on immune cell profiles, the short exposure duration and low Cr concentration used (compared to those in the 1998 studies by Cohen et al.) might be a major reason why this solubility-related, potentially predisposing factor for altered resistance, did not occur. Nevertheless, even in the absence of any significant solubility-related alteration in lung immune cell profiles, the previously-reported effects of insoluble Cr on AM provide a basis to explain why the rats that inhaled CaCrO4 for 1 wk in the current study had a much greater relative change in Listeria levels (compared to air controls at 72 hr) than their Na2CrO4-exposed counterparts.
The observations in these current studies about the extent and direction of immuno-modulation induced by the three soluble Cr agents confirmed expectations about the likely critical role of redox potentials. For example, the strongly oxidizing Na2CrO4 had a much greater impact upon host resistance as compared to the fairly unreactive Cr(III)dipic as well as against the reducing Cr(II)dipic. Yet, there were also unexpected observations. For example, rats that inhaled the soluble Cr(II)dipic had listeric burdens less than that of the air controls. Few studies have specifically examined immunotoxicologic effects of Cr in the lung as they pertain to redox behavior (reviewed in Cohen, Citation2004). Glaser et al. (Citation1985) noted that rats had enhanced numbers of AM with increased phagocytic activity after a 4-wk exposure to 50 μg Cr(VI)/m3 (as soluble dichromate), but activity was suppressed at a level of 200 μg Cr/m3. In rabbits exposed to 900 μg Cr/m3 (as Na2CrO4; Johansson et al., Citation1987), there was also AM influx, but no altered function. In contrast, exposure of rabbits to 600 μg Cr(III)/m3 (as chromic nitrate) had no effect on AM numbers, but did cause decreases in their function. Based on these aforementioned findings, it could have been expected that inhalation of Na2CrO4 in the current studies might have been without consequence, while exposure to soluble Cr(III) dipic would be deleterious to the host.
Since Listeria relies on macrophage parasitization for proliferation, any increase in AM numbers (not percentages; such as that seen with Na2CrO4) with normal phagocytic activity could potentially worsen the prospects for resistance. Once infected, normal “healthy” AM (like other macrophages; Cohen et al., Citation1989) would rapidly kill the bacteria to prevent/minimize intracellular replication; any dysfunctional AM would be less able to resist the bacteria and would soon attain high burdens. Upon AM death, progeny bacteria would then be released to (re)infect neighboring cells. In the current studies, AM from Na2CrO4-exposed rats were likely to have several functions critical for killing Listeria compromised due to increasing Cr burdens; as a result, the AM from these rats could ingest the bacteria but then do little to prevent replication. In contrast, because Cr(III) ions only cross cell membranes very slowly, the AM of rats that received Cr(III)dipic are less likely to have substantive Cr levels. Even if phagocytic activity among AM bearing Cr(III) was depressed, this would mean that most Listeria would remain extracellular (thereby, reducing replicative capacity) or be ingested and killed by normal non-Cr-bearing AM.
The predictions as to how AM from rats exposed to soluble Cr agents function are tied to both the redox properties of each Cr agent and the role of reducing equivalents in normal AM antibacterial function. Electrochemical potentials clearly indicate that Cr(VI) is a strong oxidant and unidirectionally reduces under physiological conditions to Cr(III)(E° (CrVI/CrIII) = +1.41 V; E°(CrV/CrIV) = +1.34 V; E°(CrIV/CrIII) = +2.10 V; Katz and Salem, Citation1993). Upon entry into an AM, Cr(VI) is reduced by (or has ligand displacement reactions with) sulfhydryl-bearing glutathione (GSH), NADH, and NADPH (Arslan et al., Citation1987; Standeven and Wetterhan, Citation1989). Loss of nicotinamide equivalents could impair ROI formation via the NAD(P)H oxidase system. Similarly, loss of GSH (due to oxidation to GSSG or complexation) would impair the GSH redox cycle used for protection against peroxidative damage that arises during intracellular killing, as well as from an increased presence of reactive species derived from Cr(VI) reactions with cellular oxygen/thiol moieties. Increased GSH oxidation could force the AM to consume most of the available NAD(P)H equivalents for GSSG reduction rather than for use in ROI formation. Based on these scenarios, it could be assumed that the presence of oxidizing Cr(VI) in the AM of Na2CrO4-exposed rats may have decreased the killing of ingested Listeria.
Unlike Cr(VI), Cr(III) is not an oxidant (E°(CrIII/CrII) = −0.41 V). However, the reverse of this reaction suggests that Cr(II) is an effective reductant. The data herein suggest that as a reductant, Cr(II) might actually help maintain or promote formation of the reduced species cited above. No one has yet described the specific reactions between Cr(II) and GSSG or NAD(P)+ in detail. However, Cr(II)-induced reductions (Chau et al., Citation1971; Chau and Wilkinson, Citation1972), as well as simple electron transfer reactions with proteins (i.e., cytochromes; Grimes et al., Citation1974; Greenwood et al., Citation1977; Jones and Wilson, Citation1984), have been shown to occur under cell-like conditions. If Cr(II) deposited in AM is processed in this manner, it is feasible that reducing equivalent availability for NAD(P)H oxidase could be enhanced. It would follow then that there would also potentially be a greater overall formation of the ROI required for killing Listeria within phagolysosomes in the AM from these Cr(II)dipic-exposed hosts, even as compared to within the AM from air-exposed controls.
In summary, these studies of lung listeric burdens in rats exposed to Cr agents differing in solubilility (for a fixed valence) or redox behavior (for soluble forms only) have shown that both physicochemical properties can govern eventual immunomodulatory potentials in situ. It was apparent that at equivalent levels of exposure, inhalation of insoluble hexavalent Cr (i.e., Cr(VI)) was more detrimental than soluble hexavalent Cr. Overall, insoluble hexavalent CaCrO4 was seen to be the most potent immunomodulant of the four agents analyzed. Still, as analyses of the immunomodulatory potentials for effects from the two Cr(VI) compounds did not significantly differ while those among the complexes in different oxidation states did, it is concluded here that redox behavior might be a more critical determinant than solubility with respect to pulmonary immunomodulatory potentials of Cr agents in situ.
Ongoing studies with other metal (e.g., vanadium, zinc, lead) agents of varied solubility and redox behaviors should provide further information about the significance of physico-chemical properties to observed toxic outcomes after inhalation. Specifically, these studies are addressing the possibility whether oxidizing metals that undergo redox shuttling and cycling are more toxic in situ than corresponding oxidizing metals that undergo unidirectional processes. In fact, the studies with soluble pentavalent oxidizing vanadium (V(V)), which—when present as a free ion in biologic media or within cells—can shuttle back-and-forth between V(V) and the tetravalent (V(IV)) state (Cohen, Citation1996) have already shown that V(V) has an even greater impact on host resistance than either soluble or insoluble unidirectional Cr(VI) agents examined in the this report.
Once completed, these lines of investigaton should provide researchers a better basis for understanding why certain metals could be a greater health risk than others, even when encountered in equal amounts. This improved understanding, in turn, will help researchers in the design of inhalable diagnostic/therapeutic metallopharmaceuticals by preempting the selection of certain metal ions/complexes for potential use in these products.
ACKNOWLEDGMENT
This study was supported primarily by funds from the NIGMS/NIH Grant GM065458 and Grant GM40525. The Authors are also grateful to services/assistance provided, in part, by the Center Programs in the NYU Department of Environmental Medicine that is supported by NIEHS (Grant ES0260) and by the USEPA/PM Center Grant R82735101.
REFERENCES
- Antonini J. M., Roberts J. R., Clarke R. W. Strain-related differences of nonspecific respiratory defense mechanisms in rats using a pulmonary infectivity model. Inhal. Toxicol. 2001a; 13: 85–102, [CSA]
- Antonini J. M., Roberts J. R., Clarke R. W., Yang H. M., Barger M. W., Ma J. Y., Weissman D. N. Effect of age on respiratory defense mechanisms: Pulmonary bacterial clearance in Fischer 344 rats after intratracheal instillation of Listeria monocytogenes. Chest 2001b; 120: 240–249, [CSA]
- Arslan P., Beltrame M., Tomasi A. Intracellular chromium reduction. Biochim. Biophys. Acta 1987; 931: 10–15, [CSA]
- Chau A. S., Wilkinson R. J. Chromous chloride reduction. VII. Stereochemistry and structure of the major product employed in the confirmation of endrin residues. Bull. Environ. Contam. Toxicol. 1972; 8: 105–108, [CSA]
- Chau A. S., Won H., Cochrane W. P. Chromous chloride reductions. V. Reaction of endrin ketone (II) with chromous chloride solution. Bull. Environ. Contam. Toxicol. 1971; 6: 481–484, [CSA]
- Cohen M. D. Vanadium and its immunotoxicology. Toxicol. Ecotoxicol. News 1996; 3: 132–135, [CSA]
- Cohen M. D. Pulmonary immunotoxicology of select metals: Aluminum, arsenic, cadmium, chromium, manganese, nickel, vanadium, and zinc. J. Immunotoxicol. 2004; 1: 39–70, [CSA]
- Cohen M. D., Becker S., Devlin R., Schlesinger R. B., Zelikoff J. T. Effects of vanadium upon polyI:C-induced responses in rat lung and alveolar macrophages. J. Toxicol. Environ. Health 1997b; 51: 591–608, [CSA]
- Cohen M. D., Costa M. Chromium Compounds. Environmental and Occupational Medicine, 4th Edition, W. Rom. Lippincott-Raven, Philadelphia, PA 2006, in press
- Cohen M. D., Chen C. M., Wei C. I. Decreased resistance to Listeria monocytogenes in mice following vanadate exposure: Effects upon the function of macrophages. Int. J. Immunopharmacol. 1989; 11: 285–292, [CSA]
- Cohen M. D., Chen L., Zelikoff J. T., Schlesinger R. B. Pulmonary retention and distribution of inhaled chromium: Effects of particle solubility and ozone co-exposure. Inhal. Toxicol. 1997a; 9: 843–865, [CSA]
- Cohen M. D., Sisco M., Baker K., Bowser D., Chen L. C., Schlesinger R. B. Impact of co-exposure to ozone on the carcinogenic potential of inhaled chromium. I. Effects on retention and on extra- and intracellular distribution. J. Toxicol. Environ. Health 2003; 66: 39–56, [CSA]
- Cohen M. D., Sisco M., Baker K., Chen L. C., Schlesinger R. B. Effect of inhaled chromium on pulmonary A1AT. Inhal. Toxicol. 2002a; 14: 765–771, [CSA]
- Cohen M. D., Sisco M., Baker K., Li Y., Lawrence D., van Loveren H., Zelikoff J. T., Schlesinger R. B. Effect of inhaled ozone on pulmonary immune cells critical to antibacterial responses in situ. Inhal. Toxicol. 2002b; 14: 599–619, [CSA]
- Cohen M. D., Sisco M., Li Y., Zelikoff J. T., Schlesinger R. B. Ozone-induced modulation of cell-mediated immune responses in the lungs. Toxicol. Appl. Pharmacol. 2001; 171: 71–84, [CSA]
- Cohen M. D., Yang Z., Zelikoff J. T., Schlesinger R. B. Pulmonary immunotoxicity of inhaled ammonium metavanadate in Fisher 344 rats. Fundam. Appl. Toxicol. 1996; 33: 254–263, [CSA]
- Cohen M. D., Zelikoff J. T., Chen L. C., Schlesinger R. B. Immunotoxicologic effects of inhaled chromium: Role of particle solubility and co-exposure to ozone. Toxicol. Appl. Pharmacol. 1998; 152: 30–40, [CSA]
- Pulmonary Immunotoxicology, M. D. Cohen, J. T. Zelikoff, R. B. Schlesinger. Kluwer Academic Publishers, Norwell, MA 2000
- Advanced Inorganic Chemistry, Fourth Edition, F. Cotton, G. Wilkinson. John Wiley and Sons, New York 1980
- Czuprynski C. J., Haak-Frendscho M. Non-specific resistance mechanisms to listeriosis: Implications for experimental and naturally occurring infection. Immunol. Rev. 1997; 158: 47–56, [CSA]
- Lange's Handbook of Chemistry, 15th Edition, J. A. Dean. McGraw Hill, New York, 3.22
- Doherty S. P., Prophete C., Zelikoff J., Maciejczyk P., Salnikow K., Gould T., Larson T., Jaques P., Koenig J., Sioutas C., Lippmann M., Cohen M. Effects of select particulate matter (PM)-associated metals on macrophage iron homeostasis. The Toxicologist 2004; 78(S1)181, [CSA]
- EPA (United States Environmental Protection Agency). Air Quality Criteria for PM, 2004. Washington, DC 2004, United States Environmental Protection Agency, EPA/600/P-99/002aF, bF
- Occupational Exposure to Hexavalent Chromium. Proposed Rule. October 4, 2004; 69: 59305–59474, Federal Register: 29 CFR Parts 1910, 1915, 1917, 1918, and 1926, #191
- Glaser U., Hochrainer D., Kloppel H., Kuhnen H. Low level chromium(VI) inhalation effects on alveolar macrophages and immune functions in Wistar rats. Arch. Toxicol. 1985; 57: 250–256, [CSA]
- Graham J. A., Gardner D. E. Immunotoxicity of air pollutants. Immunotoxicology and Immunopharmacology, J. Dean, M. I. Luster, A. E. Munson, H. Amos. Raven Press, New York 1985; 367–380
- Greenwood C., Brittain T., Brunori M., Wilson M. T. Chromous ion reduction of mammalian cytochrome oxidase and some of its derivatives. Biochem. J. 1977; 165: 413–416, [CSA]
- Grimes C. J., Piszkiewicz D., Fleischer E. B. Electron transfer reactions in biological systems: Reduction of ferricytochrome c by chromous ions. Proc. Natl. Acad. Sci. USA 1974; 71: 1408–1412, [CSA]
- Hoggard P. E., Schmidtke H. H. Luminescence spectrum of bis(2,6-pyridinedicar-boxylato)chromate(III). Inorg. Chem. 1973; 12: 1986–1990, [CSA]
- Johansson A., Robertson B., Curstedt T., Camner P. Alveolar macrophage abnormalities in rabbits exposed to low concentrations of trivalent chromium. Environ. Res. 1987; 44: 279–293, [CSA]
- Jones G. D., Wilson M. T. A comparison of the reactions of cytochrome c oxidase, cytochrome c and azurin with Cr2+ ions. J. Inorg. Biochem. 1984; 21: 147–158, [CSA]
- Katz S. A., Salem H. The toxicology of chromium with respect to its chemical speciation: A review. J. Appl. Toxicol. 1993; 13: 217–224, [CSA]
- Lay P. A., Levina A. Chromium. Comprehensive Coordination Chemistry II – From Biology and Nanotechnology, Volume 4 Transition Metal Groups 3-6, J. A. McCleverty, T. J. Meyer. Elsevier Pergamon, San Diego, CA 2004; 313–413
- Environmental Toxicants: Human Exposures and Their Health Effects, 2nd Edition, M. Lippmann. Van Nostrand Reinhold, New York 1999
- Lippmann M., Frampton M., Schwartz J., Dockery D., Schlesinger R., Koutrakis P., Froines J., Nel A., Finkelstein J., Godleski J., Kaufman J., Koenig J., Larson T., Luchtel D., Liu S., Oberdorster G., Peters A., Sarnat J., Sioutas C., Suh H., Sullivan J., Utell M., Wichmann E., Zelikoff J. USEPA Particulate Matter Health Effect Research Centers Program: A midcourse report of status, progress, and plans. Environ. Health Perspect. 2003; 111: 1074–1092, [CSA]
- Nag K., Bose S. N. Chemistry of tetra- and pentavalent chromium. Struct. Bond. 1985; 63: 153–197, [CSA]
- North R. J., Conlan J. W. Immunity to Listeria monocytogenes. Immunology of Intracellular Parasitism. Chemical Immunology, F. Y. Liew, F. E. Cox. Karger, Basel 1998; Volume 70: 1–20
- Prophete C., Maciejczyk P., Salnikow K., Gould T., Larson T., Koenig J., Jaques P., Sioutas C., Lippmann M., Cohen M. Effects of PM-associated metals on alveolar macrophage phosphorylated ERK-1/-2 and iNOS expression during ongoing alteration in iron homeostasis. J. Toxicol. Environ. Health 2006, In Press[CSA]
- Purcell K., Kotz J. Inorganic Chemistry. W.B. Saunders Comp., Philadelphia 1977
- Standeven A. M., Wetterhahn K. E. Chromium(VI) toxicity: Uptake, reduction, and DNA damage. J. Am. Coll. Toxicol. 1989; 8: 1275–1283, [CSA]
- Statistical Techniques for Data Analysis, J. K. Taylor. Lewis Publishers, Chelsea, MI 1990
- Unanue E. R. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T-cell response. Immunol. Rev. 1997; 158: 11–25, [CSA]
- Unanue E. R. Interrelationship among macrophages, natural killer cells, and neutrophils in early stages of Listeria resistance. Curr. Opin. Immunol. 1998; 9: 35–43, [CSA]
- van Loveren H., Rombout P. J., Wagenaar S. S., Walvoort H. C., Vos J. G. Effects of ozone on the defense to a respiratory Listeria monocytogenes infection in the rat. Suppression of macrophage function and cellular immunity and aggravation of histopathology in lung and liver during infection. Toxicol. Appl. Pharmacol. 1988; 94: 374–393, [CSA]
- van Loveren H., Steerenberg P. A., Garssen J., van Bree L. Interaction of environmental chemicals with respiratory sensitization. Toxicol. Lett. 1996; 86: 163–167, [CSA]