Abstract
We hypothesized that acute exercise stress would exacerbate immunosuppressive effects of sub-acute exposure to dietary deoxynivalenol (DON). Male BALB/c mice were fed 0 or 2 mg DON/kg diet for 14 days, 12 animals per dose, and then exercised to fatigue on a treadmill. Mice were euthanized by decapitation, trunk blood and spleens were collected. Single-cell suspensions of splenocytes were used to quantify immune function by plaque hemolysis and conconavalin-A (ConA) stimulated lymphocyte proliferation assays. Serum corticosterone level was determined by enzyme immunoassay. Only the nonexercised DON-fed mice showed significant splenocyte proliferation suppression, 32.9 ± 17.9% of nonexercised controls (p = 0.021). Exercised controls and DON-fed exercised animals showed splenocyte proliferation of 68–75% of nonexercised controls. Antibody response to a T-dependent antigen, sheep red blood cells, was significantly less for exercised DON-fed mice than in controls (p = 0.031). Serum corticosterone levels were significantly higher for both exercised groups compared to the unexercised groups (p < 0.001). IL-4 secretion from mitogen-stimulated splenocytes was elevated by DON alone (p < 0.05) while IL-2 was elevated by DON with exercise stress (p < 0.05). Our hypothesis was confirmed with respect to T-lymphocyte-dependent antibody production, but not for splenocyte proliferation. Exercise stress abrogated DON-mediated suppression of splenocyte proliferation, perhaps mediated by induction of elevated stress hormones counteracting cytokine expression alterations of DON.
INTRODUCTION
Deoxynivalenol (DON) was the most common mycotoxin occurring in the food supply worldwide with most regional diets having less than 1 mg/kg (Pestka and Smolinski, Citation2005). High contamination can occur depending on environmental conditions permitting synthesis of the mycotoxin from Fusarium spp. commonly infecting wheat, corn, barley, oats, rye and triticale (Rotter et al., Citation1996). Human intoxications were correlated with the high incidence of moldy crops (Bhat et al., Citation1989; Li et al., Citation2002) and DON was found in high concentrations in grains collected from these regions. Human illness (nausea, emesis, abdominal pain, bloody stools and increased incidence of secondary infections in children) has been suspected to be caused by DON due to its high incidence in foods in the affected area, low DON concentrations although high prevalence in areas where people were not affected, and low incidence and concentrations of other mycotoxins such as nivalenol, 3-acetylnivalenol, and T-2 toxin. Epidemiologic studies have not been conducted but detection of DON in human urine as a biomarker of exposure (Meky et al., Citation2003) may prove useful to determine if human DON exposure is a disease risk factor.
Toxic effects of dietary DON have been studied in many species with swine being the most sensitive, emesis LOAEL 0.1 mg/kg body weight (BW), and others in the following order: mice = cats = dogs > rats > poultry > ruminants (Pestka and Smolinski, Citation2005). Toxic effects include emesis, reduced feed intake, reduced weight gain and immunotoxicity (Rotter et al., Citation1996). The immune system was sensitive to DON exposure since the replication required upon immune cell activation may be limited by the primary mechanism of action of DON, protein translational inhibition (Rotter et al., Citation1996). Mice are used extensively for evaluating xenobiotic effects on the immune system due to comparability with the human immune system and availability of reagents. Low-level dietary DON has had mixed effects on murine immune function depending on strain, dose and length of exposure. Tryphonas et al. (Citation1986) found no significant effect on plaque-forming cell (PFC) numbers or Con-A, lipopolysaccharide (LPS), or phytohemagglutinin P (PHA-P)-stimulated lymphocyte proliferation at 1, 2 or 4 mg DON/kg diet in Swiss-Webster mice fed for 5 wk. Robbana-Barnat et al. (Citation1988) reported significant reduction in serum levels of antibody to sheep red blood cells (SRBC) when weanling male BALB/c mice were fed 10 mg DON/ kg diet for one or two weeks without significantly inhibited proliferation of splenocytes stimulated with PHA or LPS. Forsell et al. (Citation1986) found significant reduction of body weight after 24 d feeding of 2 mg DON/kg diet to B6C3F1 weanling female mice while the white blood cell count was significantly reduced in mice fed 10 mg DON/kg diet or greater. Greene et al. (Citation1994) found reduced final body weight, rough hair coats and poor grooming in female and male B6C3F1 mice fed 2 mg DON/kg diet for 12 wk. Transient significant reduction in erythropoetic activity in bone marrow and spleens occurred in Swiss-Webster mice fed 6.25 mg DON/kg naturally-contaminated wheat-based diet at 4-wk exposure, but parameters were not different from controls after 18-wk exposure (Arnold et al., Citation1986). Mice have been sensitive for various DON affected parameters but 2 mg/kg in the diet appears to be the overall LOAEL for subchronic studies.
Limited work has investigated interactions of DON with other xenobiotics or stresses on the immune system. Islam et al. (Citation2002) found significant potentiation of glucocorticoid release and trichothecene-induced lymphocyte apoptosis in mice due to a single LPS (0.1 mg/kg BW, intraperitoneally [IP]) and simultaneous DON exposure (12.5 mg/kg BW, orally).
Running to exhaustion on a treadmill by mice was an established model to study acute exercise stress. Hoffman-Goetz et al. (Citation1988) determined that this model of stress produced significant elevation of serum corticosterone in C57BL/6J mice without significant alteration of lymphocyte proliferation with LPS or pokeweed mitogen stimulation compared to sedentary controls. Acute exercise stress in C3He mice caused a significant reduction in the percent of splenic Ig+ B-lymphocytes but not in T-helper (TH), T-suppressor, or total T-lymphocytes (Hoffman-Goetz et al., Citation1989) however when splenocytes were incubated with Con-A, T-suppressor lymphocytes increased significantly and the B-lymphocyte effect was not observed (Randall Simpson et al., Citation1989). Pedersen et al. (Citation1997) proposed more significant immunosuppressive contribution from elevated catecholamine and growth hormone in immediate response to exhaustive exercise and a lag of immunomodulatory effects of glucocorticoids. To our knowledge there are no reported investigations of acute exercise fatigue interaction with any xenobiotic immunotoxicant.
The purpose of this study was to investigate interactive effects of two commonly encountered modulators of the immune system using a murine model. We investigated a dietary concentration of DON which could occur in the human food supply and measured endpoints of immunotoxicity and an indicator of acute exercise fatigue.
MATERIALS
DON was obtained from Sigma (St. Louis, MO). Diet components were obtained from Harlan Teklad (Madison, WI) and ICN Biomedical Inc. HBSS (Hank's balanced salt solution) and AIM-V (Adoptive Immunotherapy Media) media was obtained from GIBCO (Invitrogen Corp., Carlsbad, CA). Guinea Pig Complement Low-Tox was from Accurate Chemical Scientific Corp. (Westbury, NY). OCTEIA Corticosterone (enzymeimmunoassay) was from APLCO Diagnostics (Windham, NH). Sheep red blood cells (SRBC) were obtained from the National Veterinary Service Laboratory (Ames, IA).
METHODS
Animals
The animal protocol was reviewed and approved by Iowa State University Committee on Animal Care. Twenty-four male 8-week-old BALB/c mice were purchased from Harlan (Indianapolis IN). They were housed individually in shoebox cages with hardwood shavings and acclimated for 7 d on AIN-93G diet (Reeves et al., Citation1993). Diet was fed ad libitum, water changed three times a week and animals were exposed to 12-hr reversed light/dark cycle at 72°F and 20% humidity. All animals were trained twice on the treadmill during the acclimation period for 15 min at 10 m/min. Following acclimation all animals were weighed and blocked into three groups by weight then randomly assigned within each block to the treatment groups, two mice per treatment within each block. The treatment groups were: No DON/ No Stress, DON/ No Stress, No DON/ Stress, and DON/ Stress. DON was added to the diet at 2 mg/kg from standard solution, 500 μ g/ml in water. DON concentration in the diet was confirmed by HPLC analysis in our laboratory. The diet was formed into biscuits by mixing the dry diet with adequate water to form dough, which was rolled out, scored and allowed to dry on screen racks at room temperature until firm, about two days. Food was stored in sealed plastic bags at 4°C until use. Food consumption was measured daily for all animals. DON standards and contaminated equipment was handled with use of personal protective equipment. Decontamination of glassware and diet mixing equipment was by soaking in 10% hypochlorite prior to cleaning.
Exercise Stress
The animals were exercised by block, one block per day on three consecutive days, with order determined randomly. The nonexercised mice in the block were exposed to the noise of the treadmill by placing them in close proximity. Exercise was started in the morning toward the end of the set dark cycle to occur during their active nocturnal period. Animals were placed in individual lanes and the treadmill speed was started at 10 m/min with speed increased by 2–3 m increments every 20 min, maximum speed attained was 20 m/min. Mice were removed from the treadmill when they could no longer maintain pace with physical prodding and returned to their cages for 30 min with access to food and water. Time to fatigue was 2.50 to 4.25 hr.
Tissue Collection
Individual cages were transported to the necropsy room where animals were decapitated within 2 min of picking up the cage. Trunk blood was collected, spleens removed aseptically and immediately placed in stomacher bags containing 10 ml HBSS and processed until tissue dissolution with stomacher laboratory blender (Tekmar, Cincinnati, OH). Single cell suspension was achieved by filtering through sterilized nylon mesh and washing cells once in HBSS before suspending in AIM-V media. Cell viability was determined by Trypan Blue dye exclusion (> 95% for all animals) and concurrent cell counting by hemocytometer. Blood was allowed to clot at room temperature, centrifuged at 2500 rpm for 10 min, and serum separated and frozen at −20°C as soon as possible.
Lymphocyte Proliferation
Spleen cell concentration was adjusted to 5 × 106 cells/ml with AIM-V media and 100 μ l was pipetted into 6 replicate wells per animal in a 96-well round bottom plate, Con-A added to three wells (1 μ g/well, 10 μ l), and three wells remained as controls. The plate was incubated at 37°C, 5% CO2 for 72 hr. CellTiter 96 (Promega, Madison, WI; 10 μ l) was added to all wells and incubated for 60 min at 37°C, when well absorbance was read at 450 nm in microplate reader (Benchmark, Biorad Corp., Hercules, CA). The mean absorbance of Con-A-treated wells and control wells for each subject was calculated, difference determined and compared to the control treatment group to assess percent inhibition of lymphocyte proliferation.
Hemolytic Plaque Assay
This assay quantifies the number of B-lymphocytes activated to produce IgM in response to a T-lymphocyte-dependent antigen, SRBC. All mice were primed 4 d prior to sacrifice with an intraperitoneal injection of 0.20 ml of 20% SRBC (vol/vol) in PBS, that had been washed three times with PBS. Spleen cells were diluted with AIM-V media to 3 × 106 cells/ml. An SRBC (4%) suspension in AIM-V media was prepared the day of assay by washing SRBC three times with PBS prior to dilution to final concentration. Guinea pig complement was absorbed to SRBC by rehydrating the lyophilized pellet with 1 ml sterile water then mixing with 250 μ l washed packed SRBC in a microcentrifuge tube, incubating on ice for 10 min, centrifuging at 1500 rpm for 10 min and decanting the complement, repeating three times. Aliquots of the absorbed complement were stored at −20°C until day of use when it was thawed and diluted 3:1 with AIM-V media within 20 min of use. The plaque assay was conducted as per Cunningham and Szenberg (Citation1968). Double-sided microscope slide chambers were constructed the day before the assay. The following components were mixed, 50 μ l each, in 1.5 ml microcentrifuge tubes: spleen cell suspension, 4% SRBC, and absorbed diluted guinea pig complement. Duplicates of each subject were performed and one sample using AIM-V media instead of complement was the negative control. From each replicate 50 μ l was pipetted into each side of one slide assembly, three slide assemblies per subject, four test chambers and two control. Slide edges were sealed with melted paraffin and incubated in humidified boxes for 60 min at 37°C. Lysing of SRBC in response to interaction of complement and IgM produced plaques, with a single plaque forming cell (PFC) in the center, that were counted in each chamber under 4X magnification and means calculated from four chambers per subject. Mean plaques X 20 equaled PFC per 106 spleen cells.
Serum Corticosterone
This assay was conducted according to manufacturer's instructions for OCTEIA kit. Briefly, 30 μ l of thawed mouse serum was diluted with PBS containing horse serum 1:10. Included calibrators, controls and diluted mouse samples were pipetted (100 μ l) in duplicate into provided antibody coated microplate. Enzyme conjugate, corticosterone labeled with horseradish peroxidase, was added to each well (100 μ l) and the plate was incubated for 18 hr at 4°C. The plate was washed three times manually with PBS and 0.05% Tween and tapped dry. Tetramethylbenzidine (TMB) was added to each well (200 μ l) by multichannel pipettor. The plate was incubated for 30 min at room temperature then 100 μ l of hydrochloric acid stop solution was added to all wells by multichannel pipette. Well absorbance was measured at 450 nm by a Biorad microplate reader. A standard curve was constructed with calibrators and serum concentration of corticosterone was read from the curve and corrected for 10X dilution factor.
Cytokine Analysis
Spleen cells, 5 × 106 cells/ml in AIM-V media, were pipetted into 24-well flat bottom plates, 1 ml per well. Three wells per subject were stimulated with Con-A (1 μ g/ml) and two unstimulated control wells. Supernatants were collected from wells at 24 and 48 hr for interleukin (IL)-2, IL-4, and interferon (IFN)-γ determination. Supernatant aliquots were frozen at −20°C until batch ELISA was conducted. Cytokine concentration was determined using mouse monoclonal antibodies in kits (PharMingen, San Diego, CA) and following manufacturer's instructions. Briefly, 96-well ELISA plates (Costar, Corning, NY) were coated with capture antibody for IL-2, IL-4 or IFN-γ overnight at 4°C. Plates were blocked with PBS-10% heat-inactivated fetal bovine serum for one hour at room temperature before the addition of standards and thawed supernatants (100 μ l) and incubation at room temperature for 2 hr. Plates were washed in plate washer between each step with PBS-0.05% Tween. Biotinylated anti-mouse cytokine monoclonal antibody with avidin-horseradish peroxidase conjugate was added to each well (100 μ l) and plates were incubated for 1 hr at room temperature. Plates were washed prior to addition of TMB and hydrogen peroxide substrate, incubated in the dark for 30 min and read at 655 nm on a Biorad microplate reader. Cytokine concentrations were determined from best fit linear regression of blanked mean absorbance of standards against standard dilutions.
Statistics
One-way analysis of variance conducted with SAS with Student's t-test for pair-wise contrasts. Cytokine comparisons were evaluated by repeated measures analysis with Tukey-Kramer adjustment for post hoc pairwise comparison in SAS (SAS 9.1, Cary NC). A p value less than 0.05 was considered significant.
RESULTS
There were no significant differences between treatment groups for initial (22.9 ± 1.1 g) or final body weight (24.5 ± 1.0 g), average daily feed intake (3.25 ± 0.22 g), body weight gain (1.6 ± 0.8 g), or total spleen cells (2.21 ± 0.70 × 108).
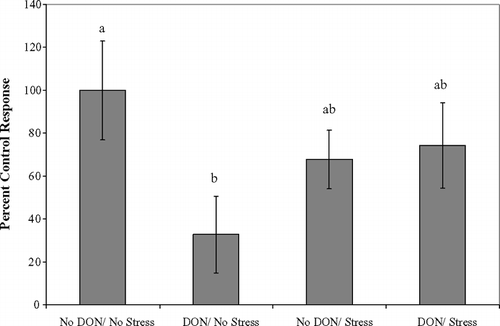
A significant difference was found between the treatment groups for Con-A-stimulated lymphocyte proliferation (). An error occurred in Con-A dilution for one block of animals resulting in complete cell death and plate exclusion, therefore n = 4 for each treatment group. Mice fed 2 mg DON/kg diet and not exercised had significant inhibition of proliferation compared to the control group.
FIG. 1 Inhibition of Con-A stimulated splenocyte proliferation in response to sub-acute dietary DON. Spleen cells (5 × 106), from BALB/c mice after completion of acute exercise fatigue ± 2 mg/kg dietary DON for 14 d, were incubated with Con-A for 72 hr. Proliferation assessed by MTS dye reduction assay. Values are mean ± SEM. Different letters indicate results significantly different from each other, p = 0.021, n = 4 per treatment.
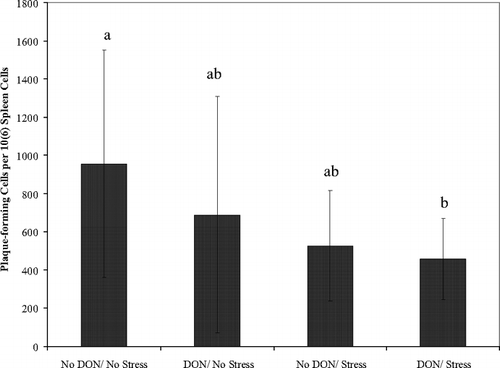
There was significant reduction of B-lymphocytes expressing antibodies specific for SRBC in mice fed 2 mg DON/kg diet and stressed compared to the control group with the other two groups falling in between (). Neither DON nor exercise stress alone significantly depressed PFCs but together appeared to produce and additive effect.
FIG. 2 Dietary DON (2 mg/kg) with acute exercise stress inhibits plaque-forming cells in spleen to SRBC. Spleen cells (3 × 106 cells/ml) from BALB/c mice after completion of acute exercise fatigue ± 2 mg/kg dietary DON for 14 d, were assessed for number of PFCs. Mean PFCs ± SEM based on four chambers per mouse. Different letters indicate results significantly different from each other, p = 0.05, n = 6 per treatment.
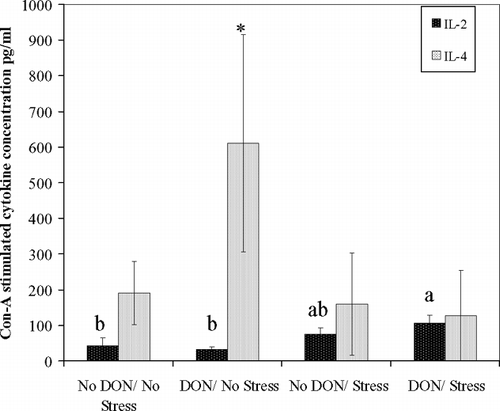
There was a significant main effect of stress on serum corticosterone levels (). ELISA analysis of supernatant from Con-A-stimulated splenocytes revealed a significant increase in IL-4 secretion after 48 hr of culture in cells harvested from DON fed non-exercised mice compared to all other treatments (). IL-2 was significantly elevated from DON-fed stressed mice compared to either nonstressed groups after 48 hr of culture (). No treatment differences in IL-2 or IL-4 expression were observed after 24 hr of culture or for IFN-γ at either time point.
FIG. 3 Serum corticosterone significantly elevated by acute exercise stress in BALB/c mice. Serum corticosterone determined by enzyme immunoassay from blood collected 30 min after completion of acute exercise fatigue of BALB/c mice ± 2 mg/kg dietary DON for 14 d. Values are means ± SEM, n = 6. *Significant main effect of acute exercise stress (p < 0.001).
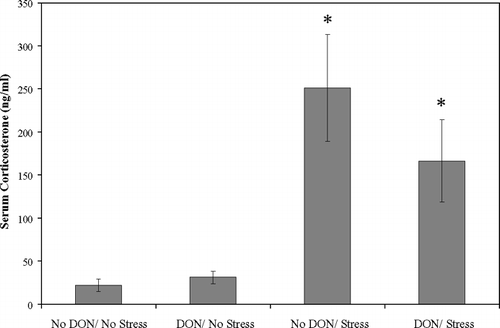
FIG. 4 Dietary DON alone stimulates IL-4 while DON with acute exercise stress stimulates IL-2, in Con-A stimulated spleen cells. Con-A-stimulated spleen cell supernatants, from BALB/c mice after completion of acute exercise fatigue ± 2 mg/kg dietary DON for 14 d. IL-4 and IL-2 determined by murine monoclonal antibody ELISA. Values are means ± SEM for two replicates per subject, n = 4 mice per treatment. For IL-2, different letters indicate results significantly different from each other at 48 hr of incubation (p < 0.05). For IL-4, *denotes treatment significantly different from other treatments (p < 0.05). No significant difference occurred between treatment groups at 24 hr of incubation for either cytokine (data not shown).
DISCUSSION
BALB/c mice were chosen for our study because they had shown sensitivity to DON at low dietary doses and are used commonly in immunotoxicity studies. BALB/c male mice have been used in two DON feeding studies (Robbana-Barnat et al., Citation1987, Citation1988) where the earlier trial found significantly reduced food intake with 2.5 mg/kg dietary DON (p < 0.05, n = 12) for one week while the second study found a trend toward reduced weight gain and reduced anti-SRBC serum antibodies but no effect on feed intake in the 5 mg/kg diet group (n = 8) for one or two week feedings. There were no apparent differences between these two studies by the same laboratory indicating sensitivity of this mouse strain but potential variability within the strain. The dietary dose chosen for our study seemed likely to produce an observable effect without overt clinical illness.
Significant inhibition of proliferation of spleen cells stimulated by Con-A, a polyclonal T-lymphocyte mitogen, in mice fed DON and not exercised might be due to the effect of elevated IL-4 since this cytokine promotes differentiation of B-cells and inhibits TH1 cells (Elenkov and Chrousos, Citation2002). IL-4 has been shown to be expressed in vitro from Con-A stimulated CD4+ T-lymphocytes from B6C3F1 mice after 7 d of incubation with 100 ng DON/ml media (Ouyang et al., Citation1995). Rat peripheral blood lymphocyte proliferation following PHA mitogen stimulation was inhibited by 50 and 100 ng DON/ml media (Miller and Atkinson, Citation1986). Protein synthesis (measured by a [14C]-leucine incorporation assay) and proliferation (measured by [3H]-thymidine incorporation) of LPS-stimulated splenocytes from female B6C3F1 mice were inhibited by 100 ng DON/ml media (Warner et al., Citation1994), with elevated IL-4 in macrophage-depleted lymphocytes with 25 to 100 ng DON/ml. This study indicated that protein synthesis inhibition occurred concurrent with increased secretion of IL-4 in response to DON exposure in vitro, although the in vivo situation is much more complicated this finding may help to explain the results observed in our study.
Acute exercise ameliorated the effect of DON on splenocyte proliferation, perhaps because exercise is known to affect T-lymphocyte subpopulations. Mice exhaustively exercised had significantly increased percentage of T-suppressor splenocytes when stimulated in culture with Con-A (Randall Simpson et al., Citation1989). IL-2 was significantly elevated in our study by dietary DON + exercise stress compared to non-exercised mice, although elevation could have been a response to the stress. Lymphocyte proliferation in this study for the stressed groups was not different from control yet IL-2 is a potent T-lymphocyte and NK cell growth factor (Handa et al., Citation1983). An increased percentage of T-suppressor lymphocytes in response to catecholamine-induced lymphocyte trafficking (Pedersen and Hoffman-Goetz, Citation2000) could be responsible for this IL-2 release. DON has stimulated IL-2 release and increased expression of IL-2 mRNA after 10 mg/kg dietary DON for 4 wk in male B6C3F1 mice (Zhou et al., Citation1998), however lower doses were not tested. Increased IL-2 may be an additive effect of DON and acute exercise stress.
Our results indicated significant inhibition of PFCs by 2 mg/kg DON with acute exercise stress, which has not been reported at this dose in mice or any other species. Plaque-forming cells are differentiated B-lymphocytes producing mostly IgM since primary immunization of antigen was 4 d prior. SRBC are T-lymphocyte-dependent antigens thus for antibody expression the antigen must be processed by antigen-presenting cells, MHC II recognition and activation of T-helper lymphocytes, and antigen presentation for B-lymphocyte activation (Abbas et al., Citation1996). Numerous cytokines must also be produced by these cells to promote activation, differentiation, and clonal expansion of the different cell populations. IL-2 has an inhibitory feedback on TH2 cells that may have been additively stimulated by DON and acute exercise stress. DON and other trichothecene mycotoxins inhibit protein synthesis (Rotter et al., Citation1996), which may further inhibit the ability of cells to produce antibody although cytokine production was stimulated.
The same acute stress model in male C3He mice resulted in a trend toward decreased Ig+ cells in splenic cultures with Con-A (Randall Simpson et al., Citation1989). Conversely, stress hormones (such as glucocorticoids and catacholamines) can inhibit release of cytokines (IL-12, IL-1) from antigen-presenting cells, inhibiting cellular and promoting humoral immune functions (Elenkov and Chrousos, Citation2002) as well as inhibiting end-stage differentiation of B-lymphocytes (Madden et al., Citation1995). Elevated plasma corticosterone confirmed significant stress in the acutely exercised mice in our study. New cytokines have been discovered recently (IL-23, IL-27) that have overlapping functions with IL-12 (Agnello et al., Citation2003), but their response to stress hormones has not yet been revealed.
The effects observed in our study could be a combined effect of acute exercise-induced reduction of B-lymphocyte numbers, differentiation of B-lymphocytes to antibody producers in the spleen, and/or altered expression of inhibitory or stimulatory cytokines. Phenotypic evaluation of spleen cell populations with a cytokine assessment battery would define this interaction more clearly. To date, inhibition of synthesis of specific proteins by DON has not been determined. Genomic or proteomic global investigation to identify possible low-dose sensitive protein targets is warranted.
CONCLUSIONS
To our knowledge, this is the first report of an immunotoxicity interaction between a low-level dietary exposure to DON, or any other xenobiotic, and acute exercise stress. Acute exercise stress may protect the mouse from DON immunotoxicity due to differential effects on lymphocyte subsets and cytokine cell signaling proteins. Acute exercise stress combined with dietary DON to cause a significant reduction in PFCs, which may be a result of stress hormone activity and DON effects on one or more cells involved in the T-dependent response to SRBC. Effects seen in this study were observed at a lower level of DON than has been previously reported and may reflect special sensitivity in BALB/c male mice. Further investigation on immune function is warranted with this model of two commonly occurring human stressors, acute exercise and low level dietary DON.
Partly funded by Iowa State University Special Research Initiation Grant.
REFERENCES
- Abbas A., Murphy K. M., Sher A. Functional diversity of helper T-lymphocytes. Nature 1996; 383: 787–793, [INFOTRIEVE], [CSA]
- Agnello D., Lankford C. S., Bream J., Morinobu A., Gadina M., O'Shea J. J., Frucht D. M. Cytokines and transcription factors that regulate T-helper cell differentiation: New players and new insights. J. Clin. Immunol. 2003; 23: 147–161, [INFOTRIEVE], [CSA], [CROSSREF]
- Arnold D. L., McGuire P. F., Nera E. A., Karpinski K. F., Bickis M. G., Zawidzka Z. Z., Fernie S., Vesonder R. F. The toxicity of orally-administered deoxynivalenol (vomitoxin) in rats and mice. Food Chem. Toxicol. 1986; 24: 935–941, [INFOTRIEVE], [CSA], [CROSSREF]
- Bhat R. V., Beedu S. R., Ramakrishna Y., Munshi K. L. Outbreak of trichothecene mycotoxicosis associated with consumption of mould-damaged wheat products in Kashmir Valley, India. Lancet 1989, 8628: 35–37, [CSA], [CROSSREF]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology 1968; 14: 599–600, [INFOTRIEVE], [CSA]
- Elenkov I. J., Chrousos G. P. Stress hormones, pro-inflammatory and anti-inflammatory cytokines, and autoimmunity. Ann. N.Y. Acad. Sci. 2002; 966: 290–303, [INFOTRIEVE], [CSA]
- Forsell J. H., Witt M. F., Tai J. H., Pestka J. J. Effects of 8-week exposure of the B6C3F1 mouse to dietary deoxynivalenol (vomitoxin) and zearalenone. Food Chem. Toxicol. 1986; 24: 213–219, [INFOTRIEVE], [CSA], [CROSSREF]
- Greene D. M., Azcona-Olivera J. I., Pestka J. J. Vomitoxin (deoxynivalenol)-induced IgA nephropathy in the B6C3F1 mouse: Dose response and male predilection. Toxicology 1994; 92: 245–260, [INFOTRIEVE], [CSA], [CROSSREF]
- Handa K., Sukuki R., Matsui H., Shimizu Y., Kumagai K. Natural killer (NK) cells as responders to interleukin-2 (IL-2) II. IL 2-induced interferon-γ Production. J. Immunol. 1983; 130: 988–992, [INFOTRIEVE], [CSA]
- Hoffman-Goetz L., Thorne R. J., Houston M. E. Splenic immune responses following treadmill exercise in mice. Can. J. Physiol. Pharmacol. 1988; 66: 1415–1419, [INFOTRIEVE], [CSA]
- Hoffman-Goetz L., Thorne R., Simpson J. A., Arumugam Y. Exercise stress alters murine lymphocyte subset distribution in spleen, lymph nodes, and thymus. Clin. Exp. Immunol. 1989; 76: 307–310, [INFOTRIEVE], [CSA]
- Islam Z., Moon Y. S., Zhou H. R., King L. E., Fraker P. J., Pestka J. J. Endotoxin potentiation of trichothecene-induced lymphocyte apoptosis is mediated by up-regulation of glucocorticoids. Toxicol. Appl. Pharm. 2002; 180: 43–55, [CSA], [CROSSREF]
- Li F. Q., Li Y. W., Luo X. Y., Yoshizawa T. Fusarium toxins in wheat from an area in Henan province, PR China, with a previous human Red Mould intoxication episode. Food Addit. Contam. 2002; 19: 163–167, [INFOTRIEVE], [CSA], [CROSSREF]
- Madden K. S., Sanders V. M., Felten D. L. Catecholamine influences and sympathetic neural modulation of immune responsiveness. Ann. Rev. Pharmacol. Toxicol. 1995; 35: 417–448, [CSA], [CROSSREF]
- Meky F. A., Turner P. C., Ashcroft A. E., Miller J. D., Qiao Y. L., Roth M. J., Wild C. P. Development of a urinary biomarker of human exposure to deoxynivalenol. Food Chem. Toxicol. 2003; 41: 265–273, [INFOTRIEVE], [CSA], [CROSSREF]
- Miller K., Atkinson H. A. The in vitro effects of trichothecenes on the immune system. Food Chem. Toxicol. 1986; 24: 545–549, [INFOTRIEVE], [CSA], [CROSSREF]
- Ouyang Y. L., Azcona-Olivera J. I., Pestka J. J. Effects of trichothecene structure on cytokine secretion and gene expression in murine CD4+ T-cells. Toxicology 1995; 104: 187–202, [INFOTRIEVE], [CSA], [CROSSREF]
- Pedersen B. K., Bruunsgaard H., Klokker M., Kappel M., MacLean D. A., Nielsen H. B., Rohde T., Ullum H., Zacho M. Exercise-induced immunomodulation—Possible roles of neuroendocrine and metabolic factors. Int. J. Sports Med. 1997; 18: S2–S7, [INFOTRIEVE], [CSA]
- Pedersen B. K., Hoffman-Goetz L. Exercise and the immune system: Regulation, integration, and adaptation. Physiol. Rev. 2000; 80: 1055–1081, [INFOTRIEVE], [CSA]
- Pestka J. J., Smolinski A. T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health, Part B 2005; 8: 39–69, [CSA]
- Randall Simpson J. A., Hoffman-Goetz L., Thorne R., Arumugam Y. Y. Exercise stress alters the percentage of splenic lymphocyte subsets in response to mitogen but not in response to interleukin-1. Brain Behav. Immun. 1989; 3: 119–128, [INFOTRIEVE], [CSA], [CROSSREF]
- Reeves P. G., Nielsen F. H., Fahey G. C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993; 123: 1939–1951, [INFOTRIEVE], [CSA]
- Robbana-Barnat S., Loridon-Rosa B., Cohen H., Lafarge-Frayssinet C., Neish G. A., Frayssinet C. Protein synthesis inhibition and cardiac lesions associated with deoxynivalenol ingestion in mice. Food Addit. Contam. 1987; 4: 49–55, [INFOTRIEVE], [CSA]
- Robbana-Barnat S., Lafarge-Frayssinet C., Cohen H., Neish G. A., Frayssinet C. Immunosuppressive properties of deoxynivalenol. Toxicology 1988; 48: 155–166, [INFOTRIEVE], [CSA], [CROSSREF]
- Rotter B. A., Prelusky D. B., Pestka J. J. Toxicology of deoxynivalenol (vomitoxin). J. Toxicol. Environ. Health 1996; 48: 1–34, [INFOTRIEVE], [CSA], [CROSSREF]
- Tryphonas H., Iverson F., So Y., Nera E. A., McGuire P. F., O'Grady L., Clayson D. B., Scott P. M. Effects of deoxynivalenol (vomitoxin) on the humoral and cellular immunity of mice. Toxicol. Lett. 1986; 30: 137–150, [INFOTRIEVE], [CSA], [CROSSREF]
- Warner R. L., Brooks K., Pestka J. J. In vitro effects of vomitoxin (deoxynivalenol) on T-cell interleukin production and IgA secretion. Food Chem. Toxicol. 1994; 32: 617–625, [INFOTRIEVE], [CSA], [CROSSREF]
- Zhou H. R., Yan D., Pestka J. J. Induction of cytokine gene expression in mice after repeated and subchronic oral exposure to vomitoxin (deoxynivalenol): Differential toxin-induced hyporesponsiveness and recovery. Toxicol. Appl. Pharmacol. 1998; 151: 347–358, [INFOTRIEVE], [CSA], [CROSSREF]