Abstract
Previous studies in a guinea pig model of asthma have suggested that age and sex contribute both to the profile of asthma symptoms, i.e., asthma heterogeneity, as well as to the success of primary prevention strategies. The present study investigated the contributions of age and sex to the severity of central vs. peripheral airway hyperresponsiveness as well as to the effectiveness of secondary preventions strategies for asthma as modeled in the guinea pig. Experimental groups: Young/Young, sensitized and challenged before sexual maturity; Young/Adult, sensitized young and challenged after sexual maturity; Adult/Adult, sensitized and challenged after sexual maturity. Males and females were sensitized IP with 0.5 mg/kg ovalbumin (OVA) and challenged intratracheally with varying doses of OVA. Cellular infiltration into lung and lavage fluid, OVA specific IgG1 as well as airway hyperresponsiveness to intravenous methacholine were determined 24 hr later. Airway hyperresponsiveness in central airways and peripheral lung was assessed by measurement of airway resistance, tissue damping and tissue elastance. Airway hyperresponsiveness with allergen sensitization and challenge was evident in male and female Adult/Adult animals and male Young/Young animals. Airway hyperresponsiveness in female Young/Young animals was not significant, despite marked airway eosinophilia. Changes in tissue elastance were more evident in OVA treated Adult/Adult compared to Young/Young animals. As allergen exposure decreased, a reduction in inflammation was seen in young females before other age sex groups. OVA induced increases in eosinophils were more pronounced in Young/Adult compared to Adult/Adult animals. Our results suggest that in asthmatic children, females may clinically benefit most from secondary prevention strategies to limit allergen exposure. In adult asthmatics, changes in tissue elastance may be significant, and secondary prevention strategies may be more effective in those sensitized as children compared to those sensitized as adults.
INTRODUCTION
Allergen exposure whether environmental or occupational can result in the immunotoxic response of asthma. Regardless of the cause of asthma, treatment strategies generally include pharmacological intervention to alleviate symptoms and, if feasible, efforts to reduce allergen exposure. Current treatment strategies are similar for children and adults, male and female. In current clinical practice, lung function generally defines the severity of the disease, and improvement in lung function is an important goal in controlling asthma. However, studies have shown that patients with a normal forced expiratory volume in one second (FEV1) may still be limited in daily activities (Hanania, Citation2006), suggesting that a single endpoint may not be the best indicator of disease severity for all individuals. A recent study of severe asthma reported that adults had more severe airflow obstruction than children (Jenkins et al., Citation2003). A number of children who were experiencing life threatening asthma episodes had FEV1 values of > 80%. Thus, other factors such as inflammation were likely contributing to disease severity. Our previous studies in a guinea pig model of asthma had indicated that the severity of inflammation did not correspond to the severity of airway hyperresponsiveness as measured by total lung resistance and compliance in different age sex groups, i.e., different age sex groups had different profiles of asthma symptoms (Regal et al., Citation2006). These data suggested that pharmacological intervention should be tailored to target inflammation vs. pulmonary function in different age and sex groups. Given the age and sex differences seen, an initial goal of the present study was to determin e the relative contribution of central vs. peripheral airway events to the severity of airway hyperresponsiveness in different age sex groups, since our previous studies had only considered total lung resistance and compliance.
Besides contributing to asthma heterogeneity, age and sex may also be important determinants of the effectiveness of strategies to limit allergen exposure. In allergic asthma, primary prevention is aimed at reducing sensitization, and secondary prevention is directed at preventing symptoms once sensitization has occurred. Primary prevention strategies are commonly employed in limiting sensitization to workplace exposures (Heederik et al., Citation1999; Baur, Citation2003). Recent recommendations regarding primary prevention of asthma have primarily been concerned with environmental allergens and have concluded that multifaceted interventions to reduce allergen exposure may be of benefit in children, but additional research is needed before specific public health recommendations can be made for either children or adults (King et al., Citation2004; Simpson and Custovic, Citation2004; Arshad, Citation2005; Chan-Yeung et al., Citation2005). As shown by the Childhood Asthma Prevention Study, single interventions to reduce house dust mite allergens in children during the first five years of life are of no clear benefit in preventing the onset of asthma in high-risk children by age 5 (Marks et al., Citation2006). In primary prevention studies as modeled in the guinea pig, our previous study demonstrated that greater reductions in allergen levels are required to limit the inflammatory component of asthma in young females compared to male or female adult animals (Regal et al., Citation2006).
Strategies of secondary prevention are sometimes recommended once sensitization has occurred and asthma is diagnosed, i.e., limiting allergen exposure in individuals already sensitized. In children with asthma, a number of studies have shown success in a multifaceted approach to reduce exposure to multiple allergens/triggers of asthma. For example, the Seattle King County study (Krieger et al., Citation2005) demonstrated a reduction in asthma symptom days and urgent health services use with high intensity interventions aimed at reducing exposure to indoor asthma triggers in children with asthma. In adults with asthma, a secondary prevention study that limited dust mite allergen exposure by encasement of mattresses met with limited success (Woodcock et al., Citation2003). With some allergens such as cat and dog, widespread dissemination of allergens, even in households without pets (Custovic et al., Citation1997), suggests that limiting allergen exposure to a degree that would realistically limit asthma symptoms in those already diagnosed with the disease may not be practical. Occupational exposures are limited to the workplace, and secondary prevention strategies involve moving sensitized workers to jobs with lower exposure or removing them from the workplace exposure all together (Mapp et al., Citation2005).
Given the limitations of conducting controlled prevention studies in human populations, the present study was designed to model secondary prevention strategies in the guinea pig and systematically determine if secondary prevention of asthma (limiting allergen exposure in animals already sensitized by exposure to the same dose of allergen) would be the more effective strategy of asthma control in certain age or sex groups. Since secondary prevention studies in children have met with some success compared to adults (Woodcock et al., Citation2003; Krieger et al., Citation2005), we hypothesized that once sensitized, adult animals would display asthma symptoms in response to lower allergen doses than young animals, indicating that secondary prevention would be more effective in limiting asthma symptoms in young animals compared to adults, whether male or female. Thus, young and adult, male and female guinea pigs with identical exposure to allergen in the sensitization phase were challenged with varying allergen doses to elicit asthma symptoms using cellular infiltration into the lung as the asthma endpoint.
No previous studies have examined the complete dose response relationship to allergen challenge in the guinea pig to assess the effectiveness of secondary prevention strategies in different age and sex groups. Knowledge of the dose-response relationships to allergen in different sex groups has potential applications in risk assessment for occupational allergen exposure. Differences in the dose-response relationships depending on the age or sex would provide evidence that secondary prevention strategies are more effective for particular age or sex groups.
METHODS
Experimental Design
All animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the United States National Institutes of Health. Hartley guinea pigs were purchased from Charles River Laboratories, Kingston, New York facility. All animals were weaned by Charles River and shipped to Duluth on Day 7 postnatal. According to data from Charles River Laboratories (Citation1999), sexual maturity in outbred Hartley male guinea pigs occurs in the range of 56–70 d after birth, after which we refer to an animal as an adult. In female guinea pigs, sexual maturity is achieved from 35 to 42 d after birth. To insure that sexual maturity was reached, adult animals were used 2 wk after the age range at which sexual maturity reportedly occurs.
Three major treatment groups were considered as outlined in : Young/Young (Y/Y), animals sensitized and challenged before sexual maturity; Young/Adult (Y/A), animals sensitized when young and challenged after sexual maturity; Adult/Adult (A/A), animals sensitized and challenged after sexual maturity. All animals were sensitized intraperitoneally (IP) with 0.5 mg/kg ovalbumin (OVA) in saline. OVA challenge occurred 20–21 d (Young/Young), 21–28 d (Adult/Adult), 67–69 d (female Young/Adult) or 96–99 d (male Young/Adult) after sensitization. Of the 283 animals outlined in , a subset of 62 animals (14 Female Y/Y, 14 Male Y/Y, 16 Female A/A, 18 Male A/A) was used for evaluation of asthma heterogeneity with age and sex. For these 62 animals, the contribution of central and peripheral events to airway hyperresponsiveness to methacholine was determined by measuring airway resistance (Rn), tissue damping (G), and tissue elastance (H) in anesthetized animals 24 hr after intratracheal challenge with 400 μ g/kg OVA. The other 221 of the 283 animals were used for assessment of the contributions of age and sex to the effectiveness of secondary prevention measures in different age sex groups. These 221 guinea pigs were evaluated for lung inflammation 24 hr after intratracheal challenge using varying doses of OVA in water (0, 0.4, 4, 40, 400 μ g/kg). Littermates were tracked, and challenge doses were varied across litters and over the time frame of the study. Because antigen challenge can cause severe bronchoconstriction and high mortality in guinea pigs, 30 min prior to intratracheal challenge with OVA, animals were injected IP with 6.1 mg/kg of the H1 antagonist pyrilamine. Guinea pigs were then anesthetized intramuscularly (IM) with a combination of ketamine (35 mg/kg) and xylazine (1 mg/kg) immediately prior to intratracheal challenge with OVA in a volume of 80 μ l/kg.
TABLE 1 Experimental groups of guinea pigs for cellular infiltration and airway hyperresponsiveness
Measurement of Central and Peripheral Components of Airway Hyperresponsiveness
Airway hyperresponsiveness to intravenous (IV) methacholine was measured 23–25 hr after intratracheal challenge with 400 μ g/kg OVA in male and female, Y/Y and A/A guinea pigs sensitized 20-28 d earlier with saline or 0.5 mg/kg OVA IP. Guinea pigs were anesthetized with ketamine/xylazine. The trachea was cannulated, and the animal ventilated at 70 breaths per min, 10 ml/kg room air using a computer controlled piston ventilator (FlexiVent, SciReq, Montreal, Canada) and a water trap to maintain a positive end expiratory pressure of 2.0–2.5 cm water. Pulmonary function measurements were taken immediately before methacholine injection and every 20 sec for 2 min following each dose of methacholine. Methacholine doses were diluted in phosphate buffered saline (PBS) containing heparin and delivered by bolus injection into a dorsal branch of the saphenous vein of the hindfoot. Methacholine doses of 1, 3.3, 10, 33, and 100 μ g/kg were given at ≈ 5-min intervals.
To standardize volume history, each baseline measurement was preceded by two consecutive inflations of the lung to total lung capacity, defined as a tracheal pressure of 30 cm water. Our previous studies (Regal et al., Citation2006) had evaluated total pulmonary mechanics in guinea pigs using a Snapshot 60 maneuver (SciReq FlexiVent 5.0 software) to obtain measurements of total lung resistance and compliance. In the current study, to further assess the heterogeneity of the asthmatic response in the guinea pig, we used the Flexivent 6 sec broadband signal to measure input impedance from 0.5 to 19.75 Hz (Quick Prime 6). The constant phase model then uses the calculated impedance spectrum to calculate Rn (airway resistance), G (tissue damping) and H (tissue elastance) as parameters to distinguish between central and peripheral events in different age sex treatment groups. A coefficient of determination for the constant phase model of 0.8 was used as the cutoff for a reasonable model fit.
Measurement of Cellular Infiltration into the Lung
In studies examining the effectiveness of secondary prevention strategies in different age sex groups, cellular infiltration in response to OVA challenge was evaluated 23–25 hr after antigen challenge as previously described (Fraser et al., Citation1995; Larsen and Regal, Citation2002; Regal et al., Citation2006). Both the airspace [cells in the bronchoalveolar lavage fluid (BAL)] and the tissue compartment were evaluated for cellular infiltration. Four equal volumes of room temperature PBS were used, for a total lavage volume of 60 ml/kg body weight. The BAL was centrifuged to sediment the cells, and the white blood cells in BAL were counted by standard methods in a hemacytometer. Differential counts were obtained from cytospin preparations of BAL cells (3 × 104 cells) stained with a modified Wrights' stain (DiffQuik, American Scientific Products, McGraw Park, IL).
Four-hundred cells were counted and identified as eosinophils, neutrophils, lymphocytes or macrophages. As estimates of the numbers of eosinophils and neutrophils in the lung tissue, the left caudal lung lobe was processed for measurement of eosinophil peroxidase (EPO; total OD/min) and the right caudal lung lobe for myeloperoxidase (MPO; total units of enzyme activity), respectively (Fraser et al., Citation1995). The remainder of the lung was dried at 80°C for 3–5 d for determination of gram dry weight. The BAL cell differential and white blood cell count were used to calculate the total number of each cell type recovered per animal. Since the sizes of animals in this study ranged from ≈ 100–1000 g, all values were normalized using the g dry weight of lung obtained from that animal to approximate the density of a particular cell type in the lung of each animal. Protein in the BAL supernatant was determined by the method of Lowry et al. (Citation1951) and the number of RBC in the BAL was quantified by determining the OD412 of the solution after lysis of the RBC as previously described (Fraser et al., Citation1995). No EPO enzyme activity was detectable in BAL supernatant.
Statistical Analysis
All data were log transformed to equalize variances and allow normal parametric modeling. Figures show the geometric mean +1 SE. Statistical significance was defined as p < 0.05. Statistical analyses used ANOVA with JMP and SAS software (SAS Institute Inc., Cary NC). As tested by ANOVA, litter effects were not significant.
Airway Hyperresponsiveness
ANOVA models were used to examine the effects of sex (male or female), age (Y/Y or A/A) and OVA challenge on baseline measurements. To determine if OVA sensitization and challenge resulted in airway hyperresponsiveness in each of the four treatment groups (* p < 0.05, in , , ), a repeated measures ANOVA used the following two repeated measures: the ratio of the maximum change in the variable after 1 μ g/kg methacholine to the vehicle value and the ratio of the maximum change in the variable after 3.3 μ g/kg methacholine to the vehicle value. In addition, the significance levels of the changes from vehicle control were assessed at each methacholine dose individually (#p < 0.05, in , , ).
FIG. 1 Methacholine-induced changes in airway resistance (Rn) in male (M) and female (F) guinea pigs sensitized with 0.5 mg/kg OVA IP and challenged intratracheally with either saline (NSS) or 400 μ g/kg OVA. Responsiveness to vehicle (Veh; PBS/heparin) and two successive doses of IV methacholine was determined 24 hr after challenge. Details of treatment groups are shown in . Values represent the geometric mean ± SE. * p < 0.05, significant overall OVA effect with repeated measures ANOVA. #p < 0.05, the change in resistance to either 1 or 3.3 μ g/kg methacholine compared to its respective control in animals challenged with OVA is greater than animals challenged with NSS.
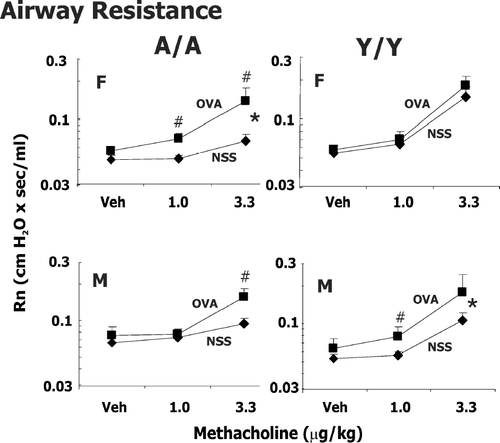
FIG. 2 Methacholine-induced changes in tissue damping (G) in male (M) and female (F) guinea pigs sensitized with 0.5 mg/kg OVA IP and challenged intratracheally with either saline (NSS) or 400 μ g/kg OVA. Responsiveness to vehicle (Veh; PBS/heparin) and two successive doses of IV methacholine was determined 24 hr after challenge. Details of treatment groups are shown in . Values represent the geometric mean ± SE. * p < 0.05, significant overall OVA effect with repeated measures ANOVA. #p < 0.05, the change in tissue damping to either 1 or 3.3 μ g/kg methacholine compared to its respective control in animals challenged with OVA is greater than animals challenged with NSS.
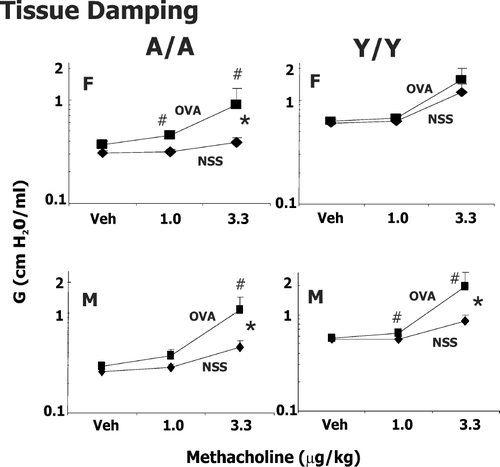
FIG. 3 Methacholine-induced changes in tissue elastance (H) in male (M) and female (F) guinea pigs sensitized with 0.5 mg/kg OVA IP and challenged intratracheally with either saline (NSS) or 400 μ g/kg OVA. Responsiveness to vehicle (Veh; PBS/heparin) and two successive doses of IV methacholine was determined 24 hr after challenge. Details of treatment groups are shown in . Values represent the geometric mean ± SE. * p < 0.05, significant overall OVA effect with repeated measures ANOVA. #p < 0.05, the change in tissue elastance to either 1 or 3.3 μ g/kg methacholine compared to its respective control in animals challenged with OVA is greater than animals challenged with NSS.
Cellular Infiltration
For differential cell counts, if no cells of the cell type of concern were seen in the 400 cells counted, the value used for statistical analysis was 1/2 of 1 cell or 0.125%. This was necessary because of log transformation of the data. Because of the shape and character of the dose-response and variability of the data, statistical analyses examined three different aspects of the dose-response relationships for the variables depicted in , , . Each analysis considered the six different treatment groups (male, female and Y/Y, Y/A, A/A). The first analysis compared the starting values, or the magnitude of the variables in control animals (animals sensitized and challenged with vehicle). Our previous study in primary prevention (Regal et al., Citation2006) had demonstrated differences in resident cell populations with sex and challenge age in unsensitized guinea pigs. Analysis of the current data confirmed our previous results in unsensitized animals. For our second analysis, ANOVA was conducted examining OVA challenge dose, sex, treatment (Y/Y, Y/A, A/A) and interactions (). Finally, given the differences in starting cell populations in the different age sex groups, changes in variables from the appropriate control to the lowest challenge dose (0.4 μ g/kg OVA) and to the challenge dose eliciting apparent maximum eosinophilia (4 μ g/kg) were evaluated ().
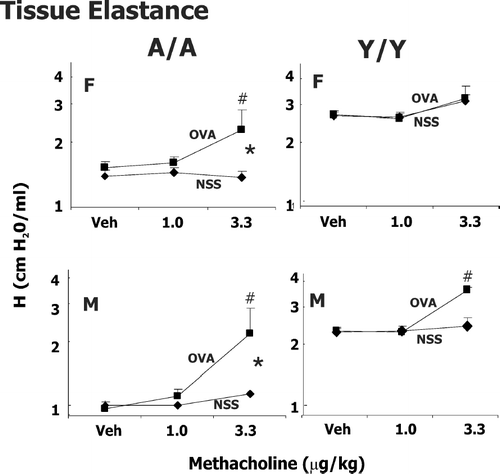
FIG. 4 Eosinophils in lung and BAL of male (M) and female (F) guinea pigs sensitized with 0.5 mg/kg OVA IP and challenged with either water vehicle (Veh) or varying doses of OVA intratracheally. Eosinophils were determined 24 hr after challenge. Details of treatment groups are shown in . Values represent the geometric mean ± SE. * p < 0.05 for the indicated comparisons.
FIG. 5 Neutrophils in lung and BAL of male (M) and female (F) guinea pigs sensitized with 0.5 mg/kg OVA IP and challenged with either water vehicle (Veh) or varying doses of OVA intratracheally. Neutrophils were determined 24 hr after challenge. Details of treatment groups are shown in . Values represent the geometric mean ± SE. * p < 0.05 for the indicated comparisons.
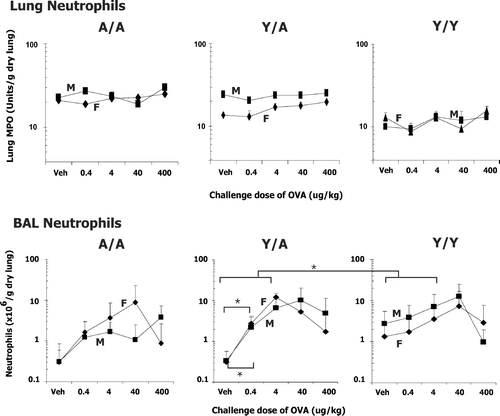
FIG. 6 RBC in BAL and protein in BAL supernatant of male (M) and female (F) guinea pigs sensitized with 0.5 mg/kg OVA IP and challenged with either water vehicle (Veh) or varying doses of OVA intratracheally. Values were determined 24 hr after challenge. Details of treatment groups are shown in . Values represent the geometric mean ± SE. * p < 0.05 for the indicated comparisons.
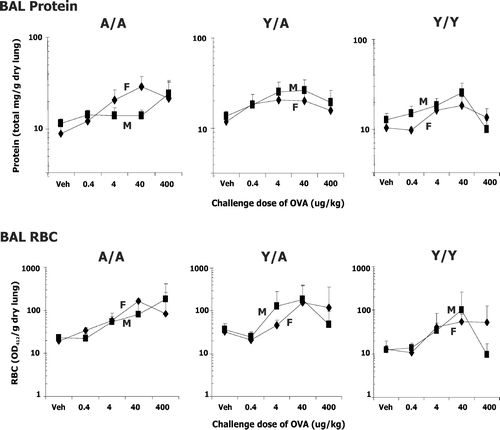
TABLE 2 ANOVA of data in , , and
TABLE 3 Statistical analysis of data in ,
Measurement of OVA-Specific IgG1
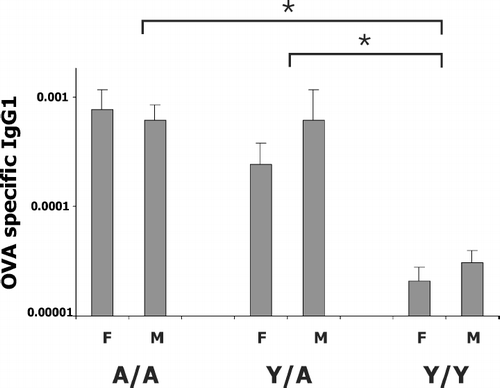
OVA-specific guinea pig IgG1 in serum was measured by ELISA as previously described for the antigen trimellitic anhydride, except ELISA plates in the current study were coated with 2 μ g/ml OVA (Fraser et al., Citation1998; Regal et al., Citation2000). Concentrations of OVA-specific IgG1 in the unknown serum samples were expressed in terms of relative concentrations compared to that of a reference antibody defined as 1. OVA-specific IgG1 data () were analyzed by ANOVA to examine the effects of OVA dose, sex, and treatment group (Y/Y, Y/A, A/A).
FIG. 7 OVA-specific IgG1 antibody in the serum. All animals were sensitized with 0.5 mg/kg OVA and challenged with 0.4–400 μ g/kg OVA intratracheally. Serum was obtained 24 hr after challenge. Details of treatment groups are shown in . Values represent the geometric mean ± SE. *p < 0.05 for ANOVA analysis comparing A/A to Y/Y and Y/A to Y/Y. A/A was not different from Y/A.
RESULTS
Age and Sex Contribute to Heterogeneity of Airway Hyperresponsiveness in Central vs Peripheral Airways
Our previous experiments (Regal et al., Citation2006) in these age sex groups indicated that sensitization with 0.5 mg/kg OVA and challenge with 400 μ g/kg OVA led to significant increases in eosinophils in the lung with minimal lung injury or changes in neutrophils. The profile of eosinophil infiltration and airway hyperresponsiveness differed amongst the treatment groups, suggesting that age and sex contributed to asthma heterogeneity. In particular, at a challenge dose of 400 μ g/kg OVA, Y/Y animals had the greatest increase in eosinophils in the lung but demonstrated less marked changes in total lung resistance and compliance than A/A animals (Regal et al., Citation2006). Thus, experiments were extended to determine if the reactivity of central vs. peripheral airways differed in male and female Y/Y or A/A animals at a challenge dose of 400 μ g/kg. Airway resistance (Rn), tissue damping (G), and tissue elastance (H) were measured in male and female, Y/Y and A/A animals (,, ). Due to extreme reactivity of the guinea pig airways at higher doses of methacholine, the model fit was unacceptable in a significant proportion of OVA sensitized animals at methacholine doses greater than 3.3 μ g/kg, so only data for 1 and 3.3 μ g/kg are shown.
Initial values of Rn, G and H with vehicle administration were compared by ANOVA considering age, sex and OVA treatment. Administration of vehicle alone caused minimal changes in Rn, G and H (data not shown), so vehicle values are used as an indication of baseline pulmonary function in the different age sex groups. A significant age*sex interaction was detected for the Veh value of Rn indicating that the sex effect differed with the age group. That is, males had greater Rn with Veh administration than females in A/A treatment groups, but not in Y/Y treatment groups. Veh values of G and H differed with age and sex, with females having greater tissue damping and tissue elastance than males. In addition, the Veh value for tissue damping and tissue elastance was significantly higher in Y/Y animals compared to A/A animals, reflecting general differences in the mechanical properties of the lung at the different ages. Because of clear differences in the Veh control with age and sex, further statistical analyses considered changes in the value over its respective Veh control at the two different methacholine doses.
With increasing doses of methacholine (1.0 and 3.3 μ g/kg) in OVA and NSS challenged animals, changes in Rn were apparent () and were similar to our previously reported changes in total lung resistance after IV methacholine. Female A/A animals showed the largest OVA effect and female Y/Y no significant OVA effect. Male A/A animals did not have a significant OVA effect by repeated measures, but the change at 3.3 μ g/kg methacholine was significantly greater in OVA challenged animals compared to NSS challenged animals. The significant changes in Rn with OVA challenge were not confined to one sex or one age group.
Tissue damping (G) is a frequency independent reflection of tissue resistance and H an indicator of tissue elastance. As such, G and H reflect events in the lung periphery. Considering tissue damping (), OVA challenge significantly increased responsiveness to methacholine at both doses in A/A animals and male Y/Y animals. No statistically significant effect on tissue damping and elastance was seen with OVA challenge in female Y/Y animals. For tissue elastance () OVA challenge resulted in more marked effects in A/A animals compared to Y/Y animals in methacholine-induced increases. Thus, OVA sensitization and challenge has a more marked effect on elastance in adult animals compared to young animals, regardless of sex. Over the dose range examined, methacholine caused minimal changes in tissue elastance in control animals challenged with NSS.
Changes in Cellular Infiltration Differ with Allergen Avoidance in Age Sex Treatment Groups
In the current study, control animals were sensitized with OVA and challenged with vehicle. Results indicated that: (1) resident populations of eosinophils were greater in females compared to males, and (2) resident populations of neutrophils, along with RBC in the BAL, are significantly greater in adult animals compared to young animals. This analysis of the current data confirmed our previous results in unsensitized animals (Regal et al., Citation2006).
Infiltration of eosinophils and neutrophils into the lung tissue and BAL are shown in and . Since the goal of this study was to determine if reducing allergen exposure would alleviate asthma symptoms preferentially in different age sex groups, our statistical analysis shown in and was designed to determine: (1) does the response change with the challenge dose of allergen? (, significant dose effect); (2) does the dose-response relationship differ with treatment or sex? (, significant OVA dose*Treatment or OVA dose*sex interaction); (3) does the lowest allergen dose cause a significant increase in each of the treatment groups? (, significant change from vehicle to 0.4 μ g/kg OVA); and, (4) does the maximum change in the variable differ between the treatment groups? (, significant change from vehicle to 4 μ g/kg between the age groups).
Eosinophils
Eosinophils in the lung tissue are shown in the upper panel of . ANOVA () revealed a significant dose effect indicating a significant change in lung EPO as the challenge dose of OVA was increased. In addition, the significant sex and treatment effect indicates that females have more tissue eosinophils than males and lung EPO varies with treatment group. As seen in , this sex effect was more evident in the A/A and Y/A groups than the Y/Y animals, though the sex*treatment interaction was not statistically significant. The significant OVA dose by treatment (Y/Y, Y/A, A/A) interaction for lung EPO provides evidence that the dose-response relationship differs with the treatment group. This significant interaction is probably in part due to the marked bell-shaped nature of the dose-response relationship in Y/A animals as compared to A/A and Y/Y treatment groups. Continued analysis of lung EPO determined if the change in lung EPO was significant at the lowest OVA challenge dose (0.4 μ g/kg OVA). This change was significant in all groups except females sensitized and challenged when young (, short brackets in ). These data suggest that a reduction in allergen exposure in Y/Y females is more effective at limiting the allergic response than in other age sex treatment groups. When the changes from vehicle to the maximum response at 4 μ g/kg were compared, a significant difference was revealed when comparing Y/A and A/A animals (, long bracket in ). Thus, animals sensitized when young and challenged as adults (Y/A) have greater increases in lung eosinophils than animals sensitized and challenged as adults.
Because of the large range of animal weights used in this study, eosinophils in the lavage were normalized as eosinophils/g dry lung, and these values are shown in the lower panel of . Presented as total eosinophils per animal lung in vehicle treated animals this represents 2.6 ± 0.4, 1.26 ± 0.2, 2.0 ± 0.4, 1.4 ± 0.2, 1.1 ± 0.2, 1.4 ± 0.4 × 106 eosinophils for female A/A, male A/A, female Y/A, male Y/A, female Y/Y and male Y/Y animals, respectively. When considering eosinophils in the lavage (, lower panel), ANOVA revealed significant sex and dose effects, again indicating that eosinophil density in lavage changed significantly with allergen dose, and females tended to have more eosinophils overall than males. In part this reflects the tendency for females to have more resident eosinophils in vehicle treated animals compared to males (just as with lung EPO). The significant sex*treatment interaction for BAL eosinophils reflects the similarities in the dose-response relationship for Y/Y animals regardless of sex in contrast to the separation of male and female dose-response relationships in the Y/A and A/A animals. The change in BAL eosinophils at the lowest OVA challenge dose (0.4 μ g/kg) was statistically significant only in the male A/A and female Y/A groups. Otherwise, the variability of the response relative to the magnitude of the differences did not allow detection of differences amongst treatment groups for eosinophils in the lavage.
A closer look at the eosinophil data at the 400 μ g/kg OVA challenge dose is warranted because these same sensitization challenge doses were used in the airway hyperresponsiveness experiments shown in through . In , when looking at the change in lung EPO from vehicle to 400 μ g/kg OVA in both male and female Y/Y and A/A animals, a large change was seen in Y/Y animals (female Y/Y, 299 to 596 OD/min/g dry wt or 99% increase; male Y/Y, 178 to 471 OD/min/g dry wt or 165% increase) and male A/A animals (217 to 516 OD/min/g dry wt or 138%) with a less marked change in female A/A animals (531–785 OD/min/g dry wt or 48%). The female A/A animals had a small change in EPO but a marked airway hyperresponsiveness ( through ).
Neutrophils
ANOVA analysis of lung and BAL neutrophils revealed a significant dose effect indicating that neutrophils changed with the OVA challenge dose. As seen in , this change in neutrophils was not as marked as the change in eosinophils depicted in . In addition, note that the number of BAL neutrophils/g dry lung in the vehicle challenged guinea pig lung was ≈ 10-fold fewer than the eosinophils. In vehicle treated animals this represents total neutrophils per lung of 1.2 ± 1.0, 0.4 ± 0.2, 0.3 ± 0.2, 0.2 ± 0.1, 1.6 ± 1.0, 2.5 ± 1.3 × 106 neutrophils for female A/A, male A/A, female Y/A, male Y/A, female Y/Y and male Y/Y animals, respectively. Thus, the neutrophil accumulation was mild, consistent with our previous studies at 400 μ g/kg OVA challenge (Regal et al., Citation2006).
In addition, when looking at the lung MPO as an indicator of tissue neutrophils, it is apparent that the sex and treatment effects detected by ANOVA () are in part due to the differences in vehicle values in the different age sex groups. Again this difference in resident neutrophils is consistent with our previous studies (Regal et al., Citation2006). Comparison of the change in BAL neutrophils from the vehicle control to 4 μ g/kg OVA challenge dose indicated that Y/A animals had a significantly greater change in BAL neutrophils than the Y/Y animals (; , long brackets) and that the female and male Y/A animals had a significant change in BAL neutrophils at the lowest OVA challenge dose of 0.4 μ g/kg (; , short brackets).
Lung Damage
Increased protein and RBCs in the BAL indicate damage due to the allergic response (). ANOVA revealed significant OVA dose and treatment effects for each of these variables (). However, unlike eosinophil and neutrophil infiltration, no clear sex effect or interaction was detected. Also, no statistical differences were detected for the change from vehicle to 0.4 or 4 μ g/kg OVA challenge dose (statistical analysis not shown in ).
OVA-Specific Serum IgG1
All animals in the cellular infiltration study were sensitized with the same dose of OVA (0.5 mg/kg OVA). Our previous studies indicated that the OVA specific serum IgG1 differed with treatment (Y/Y, Y/A, and A/A) but not sex over a range of sensitizing doses. The current study confirmed this observation at the 0.5 mg/kg sensitizing dose. Since ANOVA analysis indicated no significant challenge dose effect, results were combined for all challenge doses within each of the six treatment groups (). Y/Y animals had significantly less OVA specific antibody than the adult animals (Y/A or A/A). No difference was detected between males and females within each treatment group.
DISCUSSION
Once a patient presents with asthma symptoms, treatment involves two major strategies: pharmacological intervention to alleviate symptoms and/or reduction of allergen exposure, i.e., secondary prevention. To direct asthma treatment, the severity of the disease is first assessed by measurement of pulmonary function, and then the severity of the disease dictates the treatment course. Our studies suggest that treatment of disease may be improved by tailoring treatment to the age and sex of the individual rather than generalized for disease severity. Asthma is a heterogeneous disorder, with profiles of inflammation and airway hyperresponsiveness differing in different groups (Bel, Citation2004). In children, for example, severe asthma can occur even in the absence of marked reductions in pulmonary function (Jenkins et al., Citation2003). Our initial studies in a guinea pig model of asthma indicated that the profile of asthma symptoms differed with age and sex (Regal et al., Citation2006). Young female guinea pigs had significant accumulation of eosinophils in the lung with no evident airway hyperresponsiveness. In contrast, adult animals had marked airway hyperresponsiveness as measured by total lung resistance with minimal inflammation in the lung.
The present study extends these observations to show that asthma heterogeneity also includes differences in the reactivity of central vs peripheral airway events. These studies are the first to examine parameters of airway hyperresponsiveness in central vs peripheral lung in the guinea pig model of asthma. Adult guinea pigs tended to have more marked changes in tissue elastance with OVA sensitization and challenge compared to young animals. These data suggest that peripheral airway reactivity may be a greater component of asthma in adult animals compared to young animals, even though young animals, particularly females, have a more marked inflammatory component. Targeting airway hyperresponsiveness in the peripheral lung may be more effective asthma treatment for adults than for children.
Limiting allergen exposure to alleviate asthma symptoms is another strategy used, particularly in occupational asthma where the exposure is limited to the workplace (Mapp et al., Citation2005). Whether these secondary prevention strategies for asthma are more effective in particular age gender groups is unknown. Our results indicate that as allergen exposure is decreased, a noticeable improvement in inflammation will be seen in young females before other age sex groups. In addition, our studies reveal a bell shaped dose response curve, particularly in the Y/A treatment group. A window of allergen exposure causing eosinophilia was evident particularly for animals sensitized when young and challenged as adults. Such attenuation of the allergic response at higher allergen exposures has been noted in clinical studies of cat, dust mite, and lab animal allergic populations (Jeal et al, Citation2006) and has been described as a modified TH2 response. It is associated with lower rates of specific IgE and an increased frequency of specific IgG production.
In the guinea pig model, no clear differences in OVA-specific IgG1 were detected over the dose range to indicate changes in specific antibody accounted for the difference. Studies in mice have indicated that neonatal exposure with allergen prevents experimental allergic airway disease in adults via development of tolerance (Wang and McCusker, Citation2006). Thus, tolerance mechanisms may also be operating in limiting asthma symptoms in response to allergen challenges in adults. Clearly further studies are required to determine the mechanism of this decreased inflammatory response at high allergen doses in the Young/Adult treatment groups.
Studies of Cho et al. (Citation2005) demonstrated in adult female Balb/c mice that discontinuation of allergen challenge (secondary prevention) in conjunction with steroid treatment was beneficial in reducing airway inflammation and reducing collagen. Cho demonstrated that rigorous complete environmental control of exposure to a single allergen can effectively limit asthma symptoms. In reality, complete environmental elimination of allergen is not feasible. Our study employed a dose range of allergen exposure that spanned 4 log units. Dose reductions from 400 to at least 0.4 μ g/kg OVA were required to see a reduction in lung inflammation in young females. These are very large reductions in allergen load and may not be feasible with real life allergens such as dust mite, animal dander, and pollen. For example, in studies to actively control house dust mite levels in dust in the CAMP study, Der p 1 concentrations in dust in children's beds were reduced by at most 1 log unit, with an average reduction of 61% (From a maximum of ∼ 20 to 6–7 μ g/g allergen). With this reduction, no significant clinical benefit was noted (Marks et al., Citation2006). The Authors suggested that the lack of benefit of reducing allergen exposure was related to high initial allergen levels by international standards. However, two other international studies (Koopman et al., Citation2002; Woodcock et al., Citation2004) achieved even greater reductions of house dust mite allergen (as low as 0.2 μ g/g) in the active treatment group and also did not achieve clinical benefit. Thus, a 100-times reduction in house dust mite levels in humans resulted in no clear clinical benefit.
Major reductions in occupational allergens may be more feasible. For example, with the occupational allergen latex, a 100- to 1000-times reduction in latex allergen concentrations was achieved and was associated with reductions in sensitization of health care workers and a reduction in respiratory symptoms (Baur, Citation2003). The extent to which our findings regarding the dose-response to OVA in the guinea pig can be extrapolated to other allergens encountered by humans remains to be seen. However, our studies do provide a reference point for epidemiological and/or clinical investigations with other occupational or environmental allergens that are known to cause asthma in humans.
Our results indicate that adults sensitized when young had greater increases in inflammation than adults sensitized as adults. Studies in the female mouse by Gelfand et al. (Citation2004) had similar findings in that mice sensitized at 8 wk of age and challenged at 34 wk had greater increases in eosinophils in the BAL than mice sensitized at 30 wk and challenged at 34 wk. Thus delaying sensitization by limiting early exposure in children (primary prevention) may be very important to the success of secondary prevention for adults. If an individual is sensitized as a child, the utility of secondary prevention in adulthood may be quite limited. Certainly, the CAMP study (Marks et al., Citation2006) indicates that limiting allergen exposure in the young does not affect the prevalence of asthma in the young (like our Y/Y group). However, whether these same children will have fewer symptoms as adults in comparison to their control group is as yet unknown.
Sex differences in resident cell populations were evident in the guinea pig model. Consistent with studies of allergic inflammation in mice (Hayashi et al., Citation2003), the maximum absolute numbers of eosinophils after allergen challenge in female guinea pigs was greater than males. In pulmonary function, adult male guinea pigs started with a higher airway resistance than adult females, but had similar increases in airway hyperresponsiveness after antigen challenge. In young guinea pigs, airway hyperresponsiveness after antigen sensitization and challenge was apparent in the males, but not females. In a study of adult mice using endotoxin to cause airway hyperresponsiveness, males had significantly greater methacholine-induced increases in Rn, G and H after LPS treatment (Card et al., Citation2006). Baseline measurements of Rn, G and H were not different for mice of either sex. Certainly, sex and age differences are important to consider in assessing airway hyperresponsiveness and inflammation, and the contribution of age or sex may significantly vary with the environmental insult and the species.
OVA-specific IgG1 levels were measured in this study to indicate the degree of sensitization in the different age sex treatment groups. Guinea pigs sensitized intraperitoneally with OVA as in this study do not readily make IgE. In the guinea pig, both IgG1 and IgE are cytophilic antibodies and both are associated with allergic inflammation. The OVA specific IgG1 levels in the different treatment groups corresponded to the degree of airway hyperresponsiveness. Adult females had the most marked airway hyperresponsiveness and the highest OVA-specific IgG1 levels in serum, whereas young females did not exhibit significant airway hyperresponsiveness and had the lowest OVA-specific IgG1 levels. This is in contrast to eosinophilia where young females had large increases in eosinophils at the 400 μ g/kg challenge dose, yet had the lowest OVA-specific IgG1 levels and no evident airway hyperresponsiveness. Thus, the degree of OVA exposure in the young females was sufficient to result in inflammation but not sufficient to result in airway hyperresponsiveness. The OVA-specific IgG1 is likely not the only determinant of the magnitude of the allergic response. Our studies have indicated that IgG2 may also contribute to the allergic inflammatory response in the guinea pig (Fraser et al., Citation1998) and cellular immunity may also be a key component.
Age and sex clearly contribute to asthma heterogeneity, and future studies need to consider age and sex as important factors in determining treatment strategy. Allergen doses that result in maximal airway inflammation may variably cause changes in airway hyperresponsiveness depending on the age sex group. The dose-response relationship for a particular asthma endpoint differs with age and sex, both in the sensitization phase (Regal et al., Citation2006) as well as in the challenge phase. Events in the airway periphery may dominate in the adult asthmatic, whereas treatment of the inflammatory component of asthma may be more beneficial in children. Asthma heterogeneity likely contributes to the variable outcome of clinical studies depending on the asthma endpoint chosen. That is, strategies of primary and secondary prevention may be clinically successful when considering certain asthma endpoints but not others. In addition, our studies suggest that sensitization in childhood has long lasting ramifications for the elicitation of asthma symptoms as an adult so that primary prevention in children may be key to the success of secondary prevention strategies in the adult asthmatic.
ACKNOWLEDGMENTS
This work was supported by a grant from the U.S. Army Medical Research Acquisition Activity DAMD 17-02-1-0191. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office for this award. The animal husbandry provided by Jack Aldrich and Gail Boatman is gratefully acknowledged.
REFERENCES
- Arshad S. H. Primary prevention of asthma and allergy. J. Allergy Clin. Immunol. 2005; 116: 3–14
- Baur X. Are we closer to developing threshold limit values for allergens in the workplace. Ann. Allergy Asthma Immunol. 2003; 90: 11–18
- Bel E. H. Clinical phenotypes of asthma. Curr. Opin. Pulm. Med. 2004; 10: 44–50
- Card J. W., Carey M. A., Bradbury J. A., DeGraff L. M., Morgan D. L., Moorman M. P., Flake G. P., Zeldin D. C. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J. Immunol. 2006; 177: 621–630
- Chan-Yeung M., Ferguson A., Watson W., Dimich-Ward H., Rousseau R., Lilley M., DyBuncio A., Becker A. The Canadian childhood asthma primary prevention study: Outcomes at 7 years of age. J. Allergy Clin. Immunol. 2005; 116: 49–55
- Charles River Laboratories. Technical Bulletin, Volume 1, No. 1, 2nd Edition. Charles River Laboratories, Wilmington, MA 1999
- Cho J. Y., Miller M., McElwain K., McElwain S., Broide D. H. Combination of corticosteroid therapy and allergen avoidance reverses allergen-induced airway remodeling in mice. J. Allergy Clin. Immunol. 2005; 116: 1116–1122
- Custovic A., Green R., Fletcher A., Smith A., Pickering C. A., Chapman M. D., Woodcock A. Aerodynamic properties of the major dog allergen, Can f 1: Distribution in homes, concentration and particle size of allergen in the air. Am. J. Respir. Crit. Care Med. 1997; 155: 94–98
- Fraser D. G., Regal J. F., Arndt M. L. Trimellitic anhydride-induced allergic response in the lung: Role of the complement system in cellular changes. J. Pharmacol. Exp. Ther. 1995; 273: 793–801
- Fraser D. G., Graziano F. M., Larsen C. P., Regal J. F. The role of IgG1 and IgG2 in trimellitic anhydride-induced allergic response in the guinea pig lung. Toxicol. Appl. Pharmacol. 1998; 150: 218–227
- Gelfand E. W., Joetham A., Cui Z. H., Balhorn A., Takeda K., Taube C., Dakhama A. Induction and maintenance of airway responsiveness to allergen challenge are determined at the age of initial sensitization. J. Immunol. 2004; 173: 1298–1306
- Hanania N. A. Revisiting asthma control: How should it best be defined?. Pulm. Pharmacol. Ther. 2006, In Press
- Hayashi T., Adachi Y., Hasegawa K., Morimoto M. Less sensitivity for late airway inflammation in males than females in Balb/c mice. Scand. J. Immunol. 2003; 57: 562–567
- Heederik D., Venables K. M., Malmberg P., Hollander A., Karlsson A. S., Renstrom A., Doekes G., Nieuwenhijsen M., Gordon S. Exposure-response relationships for work-related sensitization in workers exposed to rat urinary allergens: Results from a pooled study. J. Allergy Clin. Immunol. 1999; 103: 678–684
- Jeal H., Draper A., Harris J., Newman Taylor A., Cullinan P., Jones M. Modified TH2 responses at high-dose exposures to allergen using an occupational model. Am. J. Respir. Crit. Care Med. 2006; 174: 21–25
- Jenkins H. A., Cherniack R., Szefler S. J., Covr R., Gelfand E. W., Spahn J. D. A comparison of the clinical characteristics of children and adults with severe asthma. Chest 2003; 124: 1318–1324
- King M. E., Mannino D. M., Holguin F. Risk factors for asthma incidence: A review of recent prospective evidence. Panminerva Med. 2004; 46: 97–111
- Koopman L. P., van Strien R. T., Kerkhof M., Wijga A., Smit H. A., de Jongste J. C., Gerritsen J., Aalberse R. C., Brunekreef B., Neijens H. J. Placebo-controlled trial of house dust mite impermeable mattress covers: Effect on symptoms in early childhood. Am. J. Respir. Crit. Care Med. 2002; 166: 307–313
- Krieger J. W., Takaro T. K., Song L., Weaver M. The Seattle-King County Healthy Homes Project: A randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. Am. J. Publ. Health 2005; 95: 652–659
- Larsen C. P., Regal J. F. Trimellitic anhydride-induced cellular infiltration into guinea pig lung varies with age but not gender. Int. Arch. Allergy Appl. Immunol. 2002; 127: 63–72
- Lowry O. J., Rosebrough N. J., Farr A. L., Randall P. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951; 193: 265–275
- Mapp C. E., Boschetto P., Maestrelli P., Fabbri L. M. Occupational asthma. Am. J. Respir. Crit. Care Med. 2005; 172: 280–305
- Marks G. B., Mihrshahi S., Kemp A. S., Tovey E. R., Webb K., Almqvist C., Ampon R. D., Crisafulli D., Belousova E. G., Mellis C. M., Peat J. K., Leeder S. R. Prevention of asthma during the first 5 years of life: A randomized controlled trial. J. Allergy Clin. Immunol. 2006; 118: 53–61
- Regal J. F., Fraser D. G., Weeks C. E., Greenberg N. A. Dietary phytoestrogens have anti-inflammatory activity in a guinea pig model of asthma. Proc. Soc. Exp. Biol. Med. 2000; 223: 372–378
- Regal J. F., Regal R. R., Meehan J. L., Mohrman M. E. Primary prevention of asthma: Age and sex influence sensitivity to allergen-induced airway inflammation and contribute to asthma heterogeneity in guinea pigs. Int. Arch. Allergy Immunol. 2006; 141: 241–256
- Simpson A., Custovic A. Allergen avoidance in the primary prevention of asthma. Curr. Opin. Allergy Clin. Immunol. 2004; 4: 45–51
- Wang Y., McCusker C. Neonatal exposure with LPS and/or allergen prevents experimental allergic airways disease: Development of tolerance using environmental antigens. J. Allergy Clin. Immunol. 2006; 118: 143–151
- Woodcock A., Forster L., Matthews E., Martin J., Letley L., Vickers M., Britton J., Strachan D., Howarth P., Altmann D., Frost C., Custovic A. Control of exposure to mite allergen and allergen-impermeable bed covers for adults with asthma. N. Engl. J. Med. 2003; 349: 221–232
- Woodcock A., Lowe L. A., Murray C. S., Simpson B. M., Pipis S. D., Kissen P., Simpson A., Custovic A. Early life environmental control: Effect on symptoms, sensitization, and lung function at age 3 years. Am. J. Respir. Crit. Care Med. 2004; 170: 433–439
