Abstract
Arc welding is one of the most common forms of welding and includes the use of stainless steel electrodes that emit fumes containing chromium and nickel. Epidemological studies suggest a correlation between arc welding and adverse respiratory health effects. Studies evaluating the immunotoxic effects of welding fumes are limited due to the large number of variables associated with welding. This work investigates the immunotoxic effects of welding fumes by analyzing the in vivo and in vitro IgM response to a T-dependent antigen after welding fume exposure. Significant decreases in the total IgM activity/106 viable cells and total IgM activity/well were observed in splenocytes exposed to 5 μ g/ml of either total or soluble welding fumes. A significant reduction in the specific IgM activity in lung associated lymph node cells was also observed following four pharyngeal aspirations of 10 mg/kg total or soluble welding fumes to mice. Significant elevations in the absolute lymph node cell numbers for both B- and T-cells including the CD4+ and CD8+ subsets were observed. These results demonstrate that exposure to manual metal-stainless steel welding fumes is immunosuppressive in the presence of increased lymphoctye numbers in mice and raises concerns regarding the potential for adverse immunological effects to impact respiratory health in humans.
INTRODUCTION
An estimated 800,000 workers are employed worldwide as full-time welders and over 400,000 workers are estimated to be employed in the United States as welders, cutters, solderers, and brazers, while more than 1,000,000 perform welding intermittently as part of their work duties (Bureau of Labor, Citation1999). Studies have reported that up to 79% of welders experience some type of adverse health effect (Sferlazza and Beckett, Citation1991). The most common adverse respiratory effects identified in these welders include bronchitis, airway irritation, lung function changes, and increased susceptibility to infection. A possible increase in the incidence of lung cancer has also been suggested (Langard, Citation1993).
Welding provides a powerful manufacturing tool for the joining of metallic components. There are approximately 80 different types of welding and allied processes identified for commercial use with each method having its own particular metallurgical and operational advantages. This diversity presents unique health and safety issues associated with each type of welding fume (Villaume et al., Citation1979). Arc welding, the most common type of welding, joins pieces of metal that have been made liquid by heat produced as electricity passes from one electrical conductor to another. Extremely high temperatures in the arc will heat both the base metal pieces to be joined and a filler metal coming from a consumable electrode wire. Most of the materials in the welding fume come from the consumable electrode, which is partially volatilized in the welding process (Lockey et al., Citation1988).
Vaporized metals react with air and produce metal oxides which condense and form fumes consisting of particles that are of respirable size (Beckett et al., Citation1996). The chemical properties of welding fumes can be quite complex because different compositions of pure metals are unique to each type of welding fume. The uniqueness of each type of fume is attributed to the evaporation of different metals at different rates at elevated temperatures and vapor pressures (Lockey et al., Citation1988). Welding materials are alloy mixtures of metals characterized by different steels that may contain iron, manganese, silica, chromium, nickel, and others. Fumes generated from stainless steel (SS) electrodes contain approximately 20% chromium (Cr) with 10% nickel (Ni). Cr and Ni have been shown to be associated with increased airway sensitization (Hannu et al., Citation2005).
Epidemiological studies on welding fume exposure have been considered inconclusive due to the many factors that are involved (Division of Respiratory Disease Studies, Citation2002). These include: type of electrode used, composition of fume, concentrations of fume, duration of exposure, place of exposure and other underlying genetic and environmental factors. In studies evaluating the effects of welding fumes on lung function, it could not be determined if usual day-to-day welding exposure in the absence of an acute inhalation injury leads to severe or clinically apparent lung function impairment (Sferlazza and Beckett, Citation1991). Chemical, in particular metal fumes, irritation of the airway epithelium is a suspected cause of the increased incidence of respiratory infections (Kennedy, Citation1994). In response to this, the Norwegian Labor Inspection Authority issued a warning to physicians regarding the potentially lethal risk associated with pneumonia and the inhalation of fumes from thermal metal work (Wergeland and Iversen, Citation2001).
Animal studies investigating the effects of welding fumes on the immune system and respiratory defense mechanisms are limited. Several studies suggest that welding fume exposure increases susceptibility to infection (Antonini et al., Citation1997, Citation2004). Intratracheal (IT) exposure of rats to soluble manual metal arc-stainless steel welding fumes (MMA-SS) before pulmonary inoculation with Listeria monocytogenes severely damaged the lungs, increased animal mortality, suppressed bacterial killing by the macrophages and significantly impaired the clearance of the bacteria from the lungs. Additionally, it has been demonstrated that inhalation of fluoride (a component in welding electrode fluxes) suppresses lung antibacterial defense mechanisms in mice (Yamamoto et al., Citation2001). Further studies have demonstrated that Cr decreased the IgM plaque-forming cell (PFC) response to sheep red blood cells (SRBC) in freshwater catfish (Khangarot et al., Citation1999), while both Cr and Ni have been shown to induce Type I and Type IV hypersensitivity reactions (Hostynek Citation2002; Shrivastava et al., Citation2002).
The studies presented here were undertaken to evaluate the effects of MMA-SS on the murine systemic and pulmonary humoral immune responses. MMA-SS was selected as the test article for investigation since previous research has demonstrated decreased host resistance to bacteria following exposure to this fume (Antonini, Citation2003).
MATERIAL AND METHODS
Animals
Female B6C3F1 mice were purchased from Taconic (Hudson, NY) at 7–8 weeks of age. Upon arrival, the animals were allowed to acclimate for a minimum of 5 days in an environmentally controlled, barrier facility. The animals were housed 3–5 per cage in ventilated plastic shoe box cages with hardwood chip bedding, fed modified NIH-31 6% irradiated rodent diet (Harlan Teklad, item #7913), and given tap water from water bottles ad libitum. A standard light/dark cycle was maintained on 12-hour intervals. The room temperature was maintained between 65-78°F and the humidity between 20–60%. Cages were cleaned and sanitized weekly. The NIOSH Animal Research Program Facility is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International.
Welding Fume Samples
Total, soluble, and insoluble MMA-SS were examined in these experiments. The welding fumes were collected on 0.2 μ m Nuclepore filters (Nuclepore Co., Pleasanton, CA) using a vacuum pump on 8/28/2001 by Lincoln Electric (Cleveland, OH) using a flux-covered stainless steel E309-16 electrode (Antonini et al., Citation1996). The type of filter used did not allow for chemical or physically attachment of the particles so they were easily removed using a small brush and stored at room temperature in solid form. For the in vitro experiments, welding fume particles were diluted in Hanks balanced salt solution (HBSS, pH 7.0) to generate a 5 μ g/ml stock suspension. For the in vivo studies, welding fume particles were diluted in phosphate buffered saline (PBS) to generate a 15 mg/ml stock suspension. For certain experiments, the MMA-SS sample was further divided into its soluble and insoluble portions by first generating 2.5, 5, and 10 mg/ml suspensions from the 15 mg/ml welding fume stock. The particle suspensions were then incubated for 24 hr at 37°C before being centrifuged at 12,000×g for 30 min. The supernatant was then recovered and filtered through a 0.22 μ m filter (Millipore Corp., Bedford, MA) to generate the “soluble portion” used in the experiments. The pellet from the centrifugation was resuspended in PBS or HBSS to generate the “insoluble portion” used in the experiments. As the concentrations of the soluble and insoluble solutions are based upon the starting concentrations of their respective original total welding fume suspensions, they therefore represent equivalence values (i.e., 10 mg/kg soluble MMA-SS dose = soluble materials associated with that amount of total MMA-SS needed to dose at 10 mg/kg).
The mean diameters of the MMA-SS welding fume particles were analyzed using scanning electron microscopy and determined to be of respirable size (< 1 μ m). Analysis of the metal constituents present in the fumes using inductively coupled argon plasma atomic emission spectroscopy identified a composition of 41% Fe, 17% Mn, 29% Cr, and 3% Ni (Antonini et al., Citation2004). The remaining 10% is likely to be composed of nonmetallic and insoluble agents, including potassium and sodium salts and trace amounts of other metals. Characterization of the total metal content of MMA-SS by the method described above identified that the soluble portion contains 87% Cr and 11% Mn; there was no measurable amount of these metals in the insoluble portion. No measurable levels of organic materials have been identified in any of these welding fumes (Jenkins, Citation2005).
Mishell–Dutton Assay: Antibody response to SRBC
The in vitro IgM response to SRBC was analyzed using a modified version of the Mishell and Dutton (Mishell and Dutton, Citation1967) assay. Spleens from three mice were aseptically removed, pooled, and made into a single cell suspension by pressing the organs between the frosted ends of two microscopic slides. The splenocytes were washed once in HBSS and adjusted to 1 × 107 splenocytes/ml in complete culture media (1X RPMI, Cellgro, Kansas City, MO) containing 10% fetal calf serum (Hyclone, Logan, UT), 50 μM 2-mercaptoethanol (Sigma, St. Louis, MO) and 10 μ g/ml gentamicin (ICN, Costa Mesa, CA). Cells were aliquoted (500 μ l) into individual wells of a 48-well plate. MMA-SS welding fumes (total, soluble, and insoluble portions) were added at concentrations of 5, 2.5, and 1.25 μ g/ml in a 20 μ l volume.
These concentrations were selected based on the identification of the dose level of total MMA-SS welding fume where 50% of the experimental cells were viable after treatment in the absence of SRBC (LD50, 7.5 μ g/ml; data not shown). The welding fume suspensions were prepared fresh (as described in previous section) at the start of each experiment. HBSS was used as the vehicle and 7.5 μ l of a 50 mg/ml stock of asphalt fumes (generously donated by Heritage Research Group, Indianapolis, IN)—previously determined to be immunosuppressive—was used as the positive control (Diotte et al., Citation2002).
All cultures, except for a negative control, were immunized by adding 2.5 × 107 SRBC in a 40 μ l volume. All SRBC for these studies were drawn from a single donor animal (Lampire Laboratories, Pipersville, PA). Six wells were set up to examine each dose concentration, vehicle, and control. The cultures were incubated with rocking for 5 d at 37°C in a Mishell–Dutton box. The box was sealed with an atmosphere of 7% O2, 10% CO2 and 83% N2. After a 5-d incubation period, the Mishell–Dutton chamber was depressurized and the cells were mixed with a bulb pipet. Cells from each culture (50 μ l) were added to a test tube containing a 0.4 ml warm agar/dextran mixture (1.4% Bacto-Agar, DIFCO Laboratories, Detroit, MI; and 0.2% DEAE dextran, Sigma), 25 μ l of 1:1 ratio of SRBC suspension, and 25 μ l of 1:3 dilution (1 ml lyophilized) guinea pig complement (Cedarlane Laboratories, Hornby, Ontario).
Each sample was vortexed and 200 μ l of the mixture was pipetted into a petri dish, covered with a microscope coverslip (45 × 50 mm), and incubated at 37°C for 3 hr. After 3 hr, the plaques (representing antibody forming B-lymphocytes) were viewed and quantified. The results were expressed as specific activity (IgM PFC per 106 viable splenocytes) and total activity (IgM PFC per well), respectively. To identify the total number of splenocytes, 100 μ l of splenocytes were added to 10 ml of Isoton buffer (1:100) and then two drops of Zap-o-globin (Beckman Coulter, Fullerton, CA) were added to lyse red blood cells. The cells were then counted using a Coulter counter (Beckman Coulter). Cells were stained with propidium iodine (PI) and analyzed using a flow cytometer (Beckman Dickenson, San Jose, CA) to determine viability based on dye exclusion.
In Vivo Welding Fume Studies
All animals were randomly assigned to treatment groups, weighed, and individually identified using tattooing. A preliminary analysis of variance on body weights was performed to insure homogeneous distribution of animals across treatment groups. Mice were exposed to welding fume suspensions by pharyngeal aspiration as follows: under light isoflurane anesthesia (Abbott Laboratories, North Chicago, IL), mice were held vertically by their incisor teeth against an angled restraining device (Rao et al., Citation2003). The tongue was then gently extended with padded forceps to prevent swallowing, and the test solution was pipetted directly into the oropharynx. The tongue was maintained in extension until the MMA-SS welding fumes were aspirated into the lungs. The mice were exposed at several doses, up to a maximum of 30 mg/kg MMA-SS (total, soluble, or insoluble) for these experiments. Welding fumes or PBS vehicle were administered every 5th day over a 20-d period (a total of four times) for each mouse.
The welding fume suspensions were prepared fresh at the start of each experiment (see earlier section); after the initial preparation, aliquots of each working solution were prepared and stored at 4°C for use in all subsequent exposures. The dose of fume was selected based on a dose-response study that described the pneumotoxicity and pulmonary clearance of welding fumes in rats (Antonini et al., Citation1996). The vehicle control and each of the MMA-SS welding fume forms were instilled in a 50 μ l volume. Cyclophosphamide (CP) (Sigma), a well characterized immunosuppressant, was selected as the positive control and administered by intraperitoneal injection for the four consecutive days prior to sacrifice. 12 mg/kg/d of CP were used for the pulmonary lung associated lymph node (LALN) IgM studies and 25 mg/kg/d were used for the spleen IgM studies.
Toxicological Studies
The mice were sacrificed by CO2 asphyxiation, weighed, and examined for gross pathology at the end of the experiment. The following organs were removed, cleaned of connective tissue and weighed: liver, spleen, kidneys, thymus, and lungs.
IgM PFC Response to a T-Dependent Antigen, SRBC
The primary IgM response to SRBC was enumerated using a modified hemolytic plaque assay of Jerne and Nordin (Citation1963). Separate studies were done to examine the IgM PFC response after intravenous or pharyngeal challenge with SRBC after pretreatment with MMA-SS. Seven days prior to euthanasia, the mice were challenged by pharyngeal aspiration with 2 × 1010 SRBC suspended in a 50 μ l volume of sterile HBSS for the lymph nodes IgM studies. For spleen IgM studies, mice were immunized 4 d prior to sacrifice with 7.5 × 107 SRBC by intravenous injection in a 200 μ l volume. On the day of sacrifice, mice were euthanized by CO2 asphyxiation and spleen or LALN were removed.
Single cell suspensions of the organs from individual animals were prepared in HBSS by disrupting the lymph nodes or spleen between the frosted ends of microscopic slides. To identify the total number of lymph node or spleen cells, 20 μ l of cells were added to 10 ml of isoton buffer (1:500) and two drops of Zap-o-globin were added to lyse red blood cells. Cells were then counted using a Coulter counter. 1:30 and 1:120 dilutions of spleen cells and 1:4 or 1:8 dilutions of total lymph node cells were made. 100 μ l of the dilutions were added to a test tube containing a 0.5 ml warm agar/dextran mixture (0.5% Bacto-Agar, DIFCO; and 0.05% DEAE dextran, Sigma), 25 μ l of 1:1 ratio of SRBC suspension, and 25 μ l of 1:4 dilution (1 ml lyophilized) guinea pig complement (Cedarlane Labs). Each sample was vortexed, poured into a petri dish, covered with a microscope coverslip, and incubated 3 hr at 37°C. The plaques (representing antibody forming B-lymphocytes) were viewed and quantified after this incubation. Results were expressed as specific activity (IgM PFC per 106 LALN or spleen cells) and total activity (IgM PFC per LALN or spleen).
Flow Cytometric Analysis of the Lung Associated Lymph Node Cellular Subpopulations
B-Lymphocytes, T-lymphocytes, and CD4+ and CD8+ T-lymphocyte subsets of the LALN were enumerated as previously described (Yang et al., Citation2001). In brief, LALN cells were concentrated by centrifugation and resuspended in PBS (pH 7.4), containing 1% bovine serum albumin and 0.1% sodium azide, to a cell density of 1.5 106/ml. The cells were incubated with Fc Block (clone 2.4G2, Pharmingen, San Diego, CA) for 5 min to prevent nonspecific binding and then labeled with an appropriate monoclonal antibody (mAb) conjugated to a fluorescent probe for visualization, using flow cytometry. All antibodies were obtained from Pharmingen. B-Lymphocytes were enumerated using anti-mouse CD45R/B220 antibodies (clone RA3-6B2) conjugated to phycoerythrin (PE). Anti-mouse CD3 mAb (clone 145–2C11) conjugated to fluorescein isothiocyanate FITC was used to enumerate T-lymphocytes. For the T-lymphocyte subsets, the cells were identified by using anti-mouse CD4+ mAb (clone H129.19) conjugated to FITC, anti-mouse CD8+ mAb (clone 53–6.7) conjugated to PE, anti-mouse CD11b mAb (clone M1/70) conjugated to FITC, and anti-mouse NK1.1 mAb (clone PK136) conjugated to PE. An isotype control was used for each antibody.
Cell suspensions were incubated with labeling antibodies on ice, in the dark, for at least 30 min, washed, and then resuspended in 0.1 ml of a 1 μ g/ml solution of PI. After 5 min incubation with PI, cells were washed, resuspended in PBS, and enumerated using a FacsVantage Flow Cytometer (Becton Dickinson). PI-stained cells (dead cells) were eliminated from the analysis. The forward scatter threshold was set to eliminate cell debris. The results were expressed as the percentage of gated live cells with the corresponding cell surface marker and as the absolute numbers of cells calculated using the total numbers of LALN nucleated cells.
Statistical Analysis
The design structure of these experiments was a completely randomized design, and the treatment structure utilized a one-way layout with animals randomly assigned to a vehicle control, test article, or positive control group. Power analysis, based on pilot data, indicated that 6 animals per group provides greater than 80% power to detect a change of 30% in PFC/LALN or spleen and PFC/106 LALN cells or splenocytes from controls with a conservatively estimated standard deviation. Comparisons of endpoints between the control group and each treatment level were carried out using a one-way ANOVA with Dunnett's test (Dunnett and Crisafio, Citation1955). If the assumptions were not possibly met by parametric analysis, the nonparametric Kruskal–Wallis k-sample test (Kruskal and Wallis, 1952) was utilized followed by the Mann–Whitney U-test for pair-wise comparisons with the control. Linear trend analysis was performed to determine if welding fume exposure had a dose responsive effect of the IgM PFC response to SRBC. Differences were considered significant if p ≤ 0.05 as compared to the vehicle control.
RESULTS
Effect of MMA-SS on the In Vitro IgM Antibody Response to SRBC (Mishell–Dutton Culture)
Murine splenocytes were continuously exposed to total, soluble or insoluble MMA-SS welding fumes and SRBC in culture for a 5-d period. As shown in , exposure of splenocytes to total MMA-SS resulted in a decrease in the IgM PFC response to SRBC for both PFC/well and PFC/106 viable cells. The linear trend was determined to be dose responsive (data not shown). A significant decrease of the total IgM activity/106 viable cells (87%) and total IgM activity/well (73%) was observed in the splenocytes exposed to 5 μ g/ml of MMA-SS welding fumes when compared to the control cultures. The splenocytes exposed to asphalt fumes yielded an 88% decrease in the IgM antibody response/106 viable cells and a 73% decrease in the IgM PFC/well when compared to the control cultures. A significant increase in cell counts was observed after splenocytes were treated with 5 μ g/ml of total MMA-SS welding fumes.
TABLE 1A Effect of total MMA-SS on the PFC response to SRBC in splenocytes
The soluble and insoluble portions of MMA-SS were also examined in Mishell-Dutton cultures. As shown in , exposure of mice to the soluble portion of MMA-SS welding fumes resulted in a decrease in the IgM PFC response to SRBC for both PFC/well and PFC/106 viable cells. The linear trend was determined to be dose responsive (data not shown). A significant decrease (67%) of the total IgM activity/106 viable cells and total IgM activity/well (58%) was observed in the splenocytes exposed to 5 μ g/ml of soluble MMA-SS welding fumes when compared to the control cultures. All concentrations of soluble MMA-SS resulted in a significant reduction in the IgM PFC response/well. Cultures exposed to 1.25 μ g/ml and 2.5 μ g/ml MMA-SS also had a significant decrease in splenocyte number.
TABLE 1B Effect of soluble MMA-SS on the PFC response to SRBC in splenocytes
The cultured splenocytes exposed to asphalt fumes yielded a 90% decrease in the IgM antibody response/106 viable cells and an 86% decrease in the IgM PFC/well when compared to the control cultures. As shown in , there was no significant reduction in either the total IgM PFC response/106 viable cells or total IgM PFC/well for cultures exposed to any of the concentrations of the insoluble portion of MMA-SS. The cultured splenocytes exposed to asphalt fumes yielded a 96% decrease in the IgM antibody response/106 viable cells and a 64% decrease in the IgM PFC/well when compared to the control cultures.
TABLE 1C Effect of insoluble MMA-SS on the PFC response to SRBC in splenocytes
MMA-SS Toxicity
shows organ and body weights after exposure of mice to total MMA-SS by pharyngeal aspiration. No significant changes in body, spleen, thymus, kidney, and liver weights were observed after treatment with MMA-SS for any of the treatment groups compared to the vehicle. Through observation, as the animals did not manifest ruffled fur or seem lethargic, this indicates the dose was well tolerated. Lung weight and lung weight as a percent of body weight significantly increased in a dose-dependent manner after exposure to MMA-SS, indicating successful deposition of the welding fumes. No significant changes in lung or body weight were observed for the CP positive control group compared to the vehicle group although there were significant decreases in spleen and thymus weights as expected.
TABLE 2 Effect of MMA-SS on body weight (g) and organ weights (mg) in female B6C3F1 mice
IgM PFC Response to SRBC
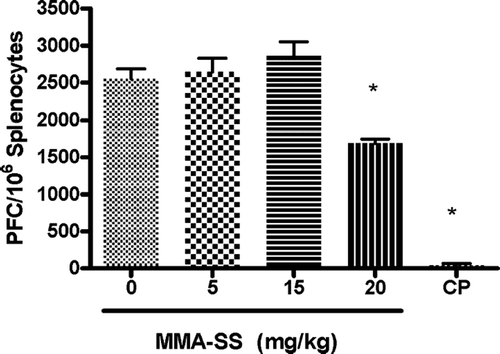
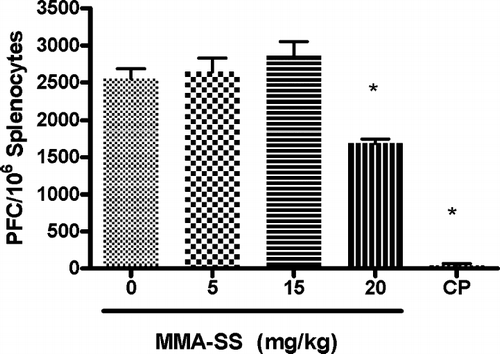
The splenic IgM response to SRBC after pretreatment with total MMA-SS was examined and the high concentration (20 mg/kg) were found to cause a significant reduction (32%) in the specific IgM/106 splenocyte response to SRBC when compared to vehicle (). These results demonstrate mild systemic immunosuppression after exposure to high concentrations of MMA-SS. To determine if there was a local pulmonary response to MMA-SS exposure, the IgM producing B-lymphocytes in the LALN were examined using a modified PFC assay to identify alterations in IgM levels after exposure to MMA-SS. The optimal concentration of SRBC and days required after immunization necessary for peak IgM levels in the LALN were previously determined (data not shown).
FIG. 1 Spleen IgM response to SRBC. The spleen IgM response is illustrated after pretreatment with MMA-SS. CP (25 mg/kg) was used as a positive control. Values represent the mean ± SE derived from six animals in each group. * p < 0.05 and ** p ≤ 0.01 vs. vehicle.
and illustrate the LALN IgM response to SRBC after pretreatment with total, soluble, and insoluble MMA-SS. shows a reduction in the specific LALN activity/106 cells after pretreatment with the total MMA-SS. There was a 45% and 58% reduction in the IgM response to SRBC after exposure to total MMA-SS for the 5 and 10 mg/kg groups, respectively, when compared to levels in the controls. shows a reduction in total LALN IgM activity after pretreatment with total MMA-SS reaching a 39% and 48% reduction for the for 5 and 10 mg/kg doses, respectively, when compared to levels in the controls. and show a reduction in the specific LALN IgM/106 cells and total LALN IgM activity after pretreatment with soluble MMA-SS.
FIG. 2 The specific LALN IgM response to SRBC. The specific LALN IgM response/106 cells to SRBC is illustrated after pretreatment with (A) total, (B) soluble and (C) insoluble MMA-SS. Values represent the mean ± SE derived from six animals in each group. CP (12.5 mg/kg) was used as a positive control. * p ≤ 0.05 and ** p ≤ 0.01 vs. vehicle.
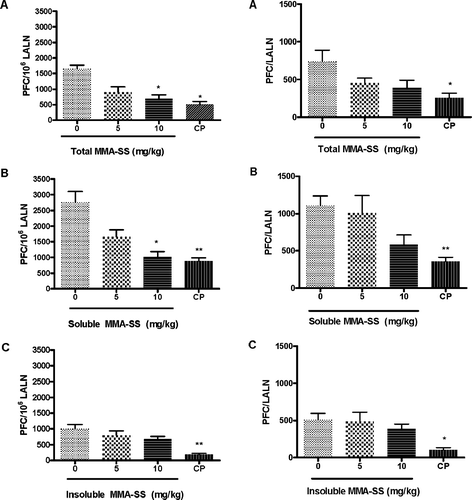
FIG. 3 The total LALN IgM response to SRBC. The LALN IgM response to SRBC after pretreatment with (A) total, (B) soluble and (C) insoluble MMA-SS is illustrated. Values represent the mean ± SE derived from six animals in each group. CP (12.5 mg/kg) was used as a positive control. * p ≤ 0.05 and ** p ≤ 0.01 vs. vehicle.
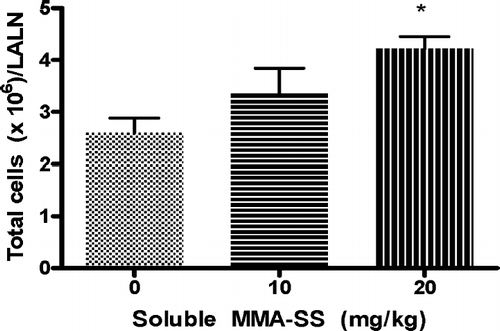
There was a 40% and 63% reduction (compared to controls) in the IgM/106 cells response to SRBC after exposure to soluble MMA-SS for the 5 and 10 mg/kg groups, respectively and a 48% reduction in the total LALN IgM response to SRBC for the 10 mg/kg animals. The linear trend after treatment with both total and soluble welding fumes (LALN IgM/106 cells and total LALN IgM activity) was determined to be dose responsive (data not shown). No significant differences in the IgM PFC/106 LALN cell response or the IgM/lymph node were observed between insoluble MMA-SS exposed groups and control for any of the concentrations examined ( and ). Cell counts of the LALN were found to be significantly increased after pretreatment with soluble MMA-SS ().
Immune Cell Profile of LALN after Soluble MMA-SS Exposure
The results of the LALN immune cell profile following exposure to the soluble MMA-SS portion are shown in . There was a significant elevation in the absolute cell numbers for both B- and T-lymphocytes including the CD4+ and CD8+ subsets. No change was observed in the percent lymphocyte population for any cell population tested. Natural killer and macrophage cells were detected at or below background levels (data not shown).
TABLE 3 Flow cytometric analysis of the LALN cell populations
DISCUSSION
These results indicate that exposures to total or soluble portions of MMA-SS are immunosuppressive in vivo and in vitro without causing a reduction in the percentage of T- or B-lymphocytes. To the contrary, absolute numbers of CD3+, both CD4+ and CD8+, and B220+ cell numbers were observed to increase following exposure to soluble MMA-SS. The PFC assay measures the complex immune response mediated by the combined actions of a number of cell types, including antigen-presenting cells, T-lymphocytes (required for the production and release of lymphokines and cell to cell contact) and B-lymphocytes. Chemically-induced changes in any of these cell types can result in a decrease in PFC activity (Anderson et al., Citation2006).
To try to explain the reasons for these findings, it was logical to first determine what were the major compositional differences between the total/soluble portions of MMA-SS and those of the insoluble portions that failed to show any immunosuppressive effects. Both Cr and Mn have been identified as the predominate metals in the soluble fraction of MMA-SS. In contrast, levels of each of these metals in the insoluble portions are low-to-below detection. It was thus apparent that it would be important to focus on the role that Cr, because it is found at much higher levels than even the Mn in the intact and soluble portions of MMA-SS, might have had in the observations in the current studies.
Hexavalent Cr (i.e., Cr[VI]) is widely used in industrial chemicals, extensively used in paints, metal finishes, stainless steel manufacturing, alloy cast irons, chrome and wood treatment. On the contrary, trivalent Cr (i.e., Cr[III]) salts such as chromium polynicotinate, chromium chloride and chromium picolinate (CrP) are used as micronutrients and nutritional supplements and have been demonstrated to exhibit a significant number of health benefits in animals and humans (Anderson, Citation2000). Chromium, in the soluble fraction of MMA-SS, exists in both trivalent Cr(III) and hexavalent Cr(VI) forms; it was recently determined there is ≈ 220 μ g of Cr(VI) per mg of welding fume suspension [Personal Communication from Mike Keane (NIOSH/HELD/EAB)]. Both Mn and Cr(VI) have been previously shown to be cytotoxic to lung epithelial cells at concentrations relevant to welding fume exposure, and the toxicity is associated with changes in intracellular signaling and inflammatory cytokine release (Pascal and Tessier, Citation2004).
The reduction of Cr(VI) to Cr(III) results in the formation of reactive intermediates that contribute to the cytotoxicity, genotoxicity and carcinogenicity of Cr(VI)-containing compounds. In vivo reduction of Cr(VI) to Cr(III) has been widely studied. Cr(VI) is reduced to Cr(III) in the epithelial lining fluid of the lungs by ascorbate and glutathione (Suzuki and Fukuda, Citation1990). If absorbed into the bloodstream, Cr(IV) readily enters red blood cells and undergoes intracellular reduction to Cr(III). Cr(III) is unable to cross the red blood cell membrane. Decreased availability of Cr(VI) in the bloodstream could be an explanation for why local immunosuppression was more severe than systemic immunosuppression.
Chromium is very toxic when introduced to the body by dermal and inhalation routes and has been found to cause lung cancer, nasal irritation, nasal ulcers, hypersensitivity reactions and contact dermatitis (Shrivastava et al., Citation2002). Inhalation of Cr has been found to cause enlargement, multinucleation or vacuolation, and accumulation of macrophages in the alveolar spaces as nodules. Increased doses of Cr(VI) have also been found to depress the phagocytic activity of alveolar macrophages (Glaser et al., Citation1985). Other studies have found that exposure of mice to MMA-SS resulted in enhanced levels of IL-10, which is involved in inhibiting macrophage function, after infection with Listeria monocytogenes (Antonini et al., Citation2004). Results from additional research indicate that Cr suppresses the IgM response to SRBC in freshwater catfish (Khangarot et al., Citation1999). A decreased function of the macrophage, acting as the antigen-presenting cell, could lead to a reduction in the IgM response to SRBC.
Chromium has also been identified to induce two types of hypersensitivity reactions: Type IV (delayed-type hypersensitivity) and Type I (IgE-mediated) hypersensitivity reactions (Bruynzeel et al., Citation1988; Kanerva et al., Citation2000). Exposure to sensitizers leads to proliferation of draining lymph node T- and B-lymphocyte populations. In an attempt to further investigate the increase in lymph node cell number, a modified local lymph node assay (LLNA) was conducted following exposure to the soluble portion of MMA-SS. Dermal application of the soluble portion of MMA-SS resulted in a significant increase in lymphocyte proliferation when compared to vehicle, resulting in an effective concentration (of 3%; EC3) value of 25 mg/kg (data not shown).
In summary, results from both in vivo and in vitro models demonstrate that stainless steel welding fume exposure is immunosuppressive and a component of the soluble portion of the welding fume, containing primarily Cr and Mn, is responsible for this effect. The studies presented here, in combination with data from the literature, support the hypothesis that exposure to MMA-SS results in a defect in cell function, with the macrophage as a likely target, rather than the inhibition of cell proliferation or the induction of apoptosis. Given the large number of people who frequently perform some degree of welding, these findings in animal models support the need for more extensive research into the adverse effect of welding fume exposure on human respiratory health.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
REFERENCES
- Anderson R. A. Chromium in the prevention and control of diabetes. Diabetes Metab. 2000; 26: 22–27
- Anderson S. E., Munson A. E., Meade B. J. Analysis of immunotoxicity by enumeration of antibody-producing B-cells. Current Protocols in Toxicology, J. S. Bus, L. G. Costa, E. Hodgson, D. A. Lawrence, D. Reed, Hoboken, NJ 2006
- Antonini J. M. Health effects of welding. CRC Crit. Rev. Toxicol. 2003; 33: 61–103
- Antonini J. M., Krishna Murthy G. G., Brain J. D. Responses to welding fumes:lung injury, inflammation, and the release of tumor necrosis factor-alpha and interleukin-1 beta. Exp. Lung Res. 1997; 23: 205–227
- Antonini J. M., Krishna Murthy G. G., Rogers R. A., Albert R., Ulrich G. D., Brain J. D. Pneumotoxicity and pulmonary clearance of different welding fumes after intratracheal instillation in the rat. Toxicol. Appl. Pharmacol. 1996; 140: 188–199
- Antonini J. M., Lawryk N. J., Murthy G. G., Brain J. D. Effect of welding fume solubility on lung macrophage viability and function in vitro. J. Toxicol. Environ. Health A 1999; 58: 343–363
- Antonini J. M., Taylor M. D., Millecchia L., Bebout A. R., Roberts J. R. Suppression in lung defense responses after bacterial infection in rats pretreated with different welding fumes. Toxicol. Appl. Pharmacol. 2004; 200: 206–218
- Beckett W. S., Pace P. E., Sferlazza S. J., Perlman G. D., Chen A. H., Xu X. P. Airway reactivity in welders: A controlled prospective cohort study. J. Occup. Environ. Med. 1996; 38: 1229–1238
- Bruynzeel D. P., Hennipman G., van Ketel W. G. Irritant contact dermatitis and chrome-passivated metal. Contact Derm. 1988; 19: 175–179
- Bureau of Labor. Occupational Employment Statistics: 1999 National Occupational Employment and Wage Estimates. 1999, Available at http://stats.bls.gov/oes/1999/oes514121.htm Accessed 6-24-05
- Diotte N. M., Munson A. E., Tomblyn S., Meade B. J. Asphalt fume-induced immunosuppresion in B6C3F1 female mice. Society of Toxicology Meeting. 2002, Abstract #133
- Division of Respiratory Disease Studies, N. 2002; 136, Work-Related Lung Disease Surveillance Report. Document No. 2003-111:1.21 p. 226, 1.58
- Dunnett C. W., Crisafio R. The operating characteristics of some official weight variation tests for tablets. J. Pharm. Pharmacol. 1955; 7: 314–327
- Glaser U., Hochrainer D. D., Kloppel H., Kuhnen H. Low level chromium (VI) inhalation effects on alveolar macrophages and immune functions in Wistar rats. Arch. Toxicol. 1985; 57: 250–256
- Hannu T., Piipari R. R., Kasurinen H., Keskinen H., Tuppurainen M., Tuomi T. Occupational asthma due to manual metal-arc welding of special stainless steels. Eur. Respir. J. 2005; 26: 736–739
- Hostynek J. J. Nickel-induced hypersensitivity:Etiology, immune reactions, prevention and therapy. Arch. Dermatol. Res. 2002; 294: 249–267
- Jenkins N. Chemical analysis of welding fume particles. Welding Res. 2005; 84: 87–93
- Jerne N. K., Nordin A. A. Plaque formation in agar by single antibody-producing cells. Science 1963; 140: 405
- Kanerva L., Jolanki R., Estlander T., Alanko K., Savela A. Incidence rates of occupational allergic contact dermatitis caused by metals. Am. J. Contact Derm. 2000; 11: 155–160
- Kennedy S. M. When is a disease occupational?. Lancet 1994; 344: 4–5
- Khangarot B. S., Rathore R. S., Tripathi D. M. Effects of chromium on humoral and cell-mediated immune responses and host resistance to disease in a freshwater catfish, Saccobranchus fossilis (Bloch). Ecotoxicol. Environ. Safety 1999; 43: 11–20
- Langard S. Role of chemical species and exposure characteristics in cancer among persons occupationally exposed to chromium compounds. Scand. J. Work Environ. Health 1993; 19(S1)81–89
- Lockey J. E., Schenker M. B., Howden D. G., Desmeules M. J., Saracci R., Sprince N. L., Harber P. I. Current issues in occupational lung disease. Am. Rev. Respir. Dis. 1988; 138: 1047–1050
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J. Exp. Med. 1967; 126: 423–442
- Pascal L. E., Tessier D. M. Cytotoxicity of chromium and manganese to lung epithelial cells in vitro. Toxicol. Lett. 2004; 147: 143–151
- Rao G. V., Tinkle S., Weissman D. N., Antonini J. M., Kashon M. L., Salmen R., Battelli L. A., Willard P. A., Hoover M. D., Hubbs A. F. Efficacy of a technique for exposing the mouse lung to particles aspirated from the pharynx. J. Toxicol. Environ. Health A 2003; 66: 1441–1452
- Sferlazza S. J., Beckett W. S. The respiratory health of welders. Am. Rev. Respir. Dis. 1991; 143: 1134–1148
- Shrivastava R., Upreti R. K., Seth P. K., Chaturvedi U. C. Effects of chromium on the immune system. FEMS Immunol. Med. Microbiol. 2002; 34: 1–7
- Suzuki Y., Fukuda K. Reduction of hexavalent chromium by ascorbic acid and glutathione with special reference to the rat lung. Arch. Toxicol. 1990; 64: 169–176
- Villaume J. E., Wasti K., Liss-Suter D., Hsiao S. Effects of Welding on Health. Miami, FL 1979
- Wergeland E., Iversen B. G. Deaths from pneumonia after welding. Scand. J. Work Environ. Health 2001; 27: 353
- Yamamoto S., Katagiri K., Ando M., Chen X. Q. Suppression of pulmonary antibacterial defenses mechanisms and lung damage in mice exposed to fluoride aerosol. J. Toxicol. Environ. Health A 2001; 62: 485–494
- Yang H. M., Antonini J. M., Barger M. W., Butterworth L., Roberts B. R., Ma J. K., Castranova V., Ma J. Y. Diesel exhaust particles suppress macrophage function and slow the pulmonary clearance of Listeria monocytogenes in rats. Environ. Health Perspect. 2001; 109: 515–521